Abstract
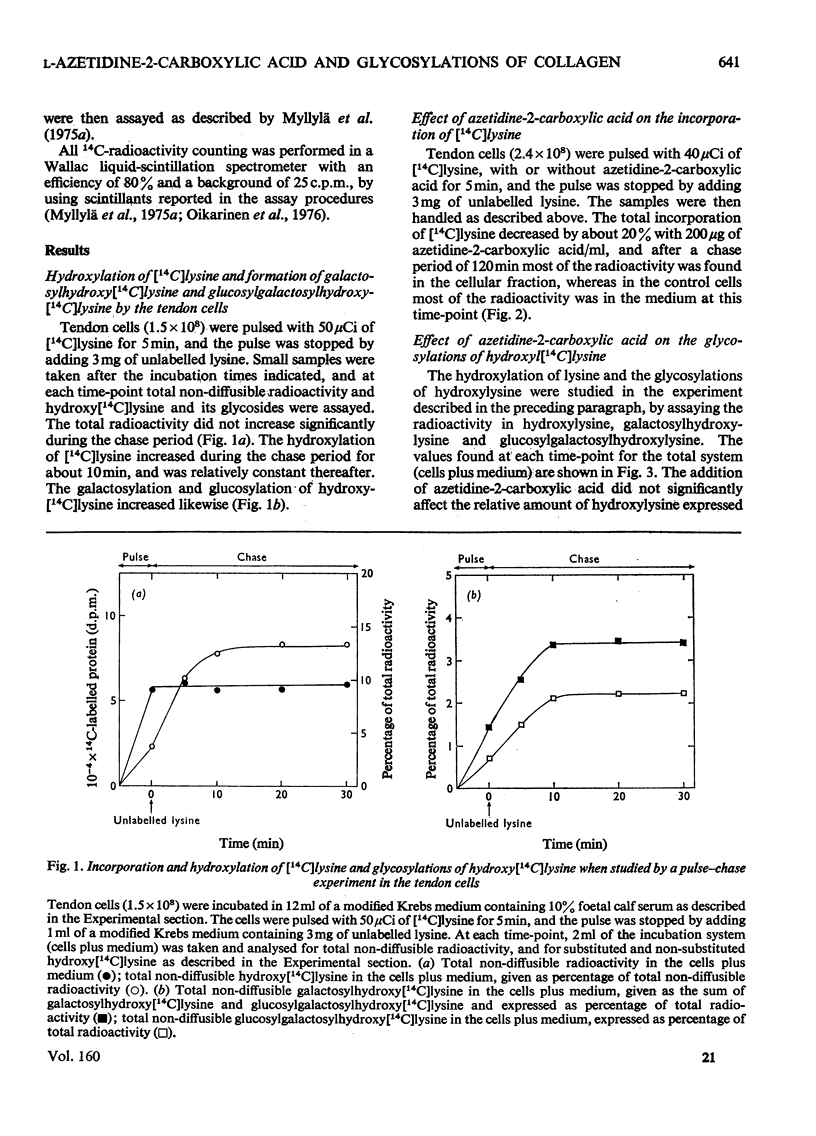
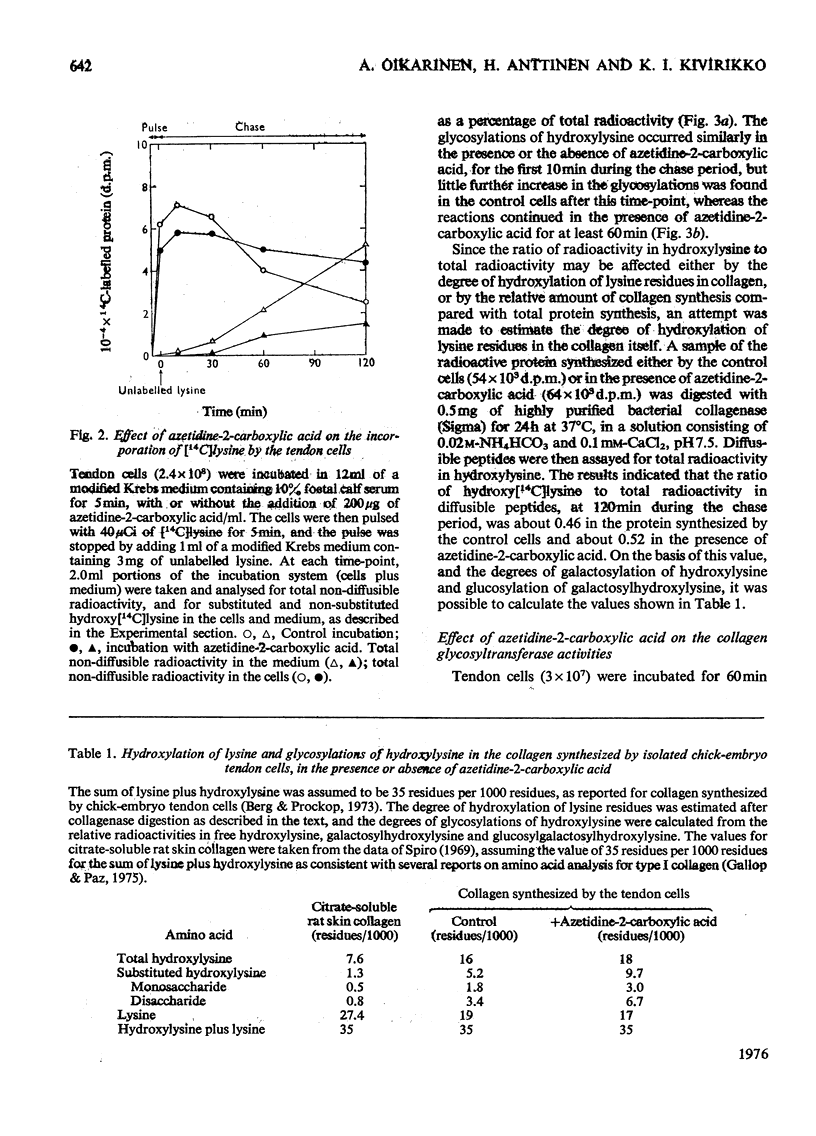
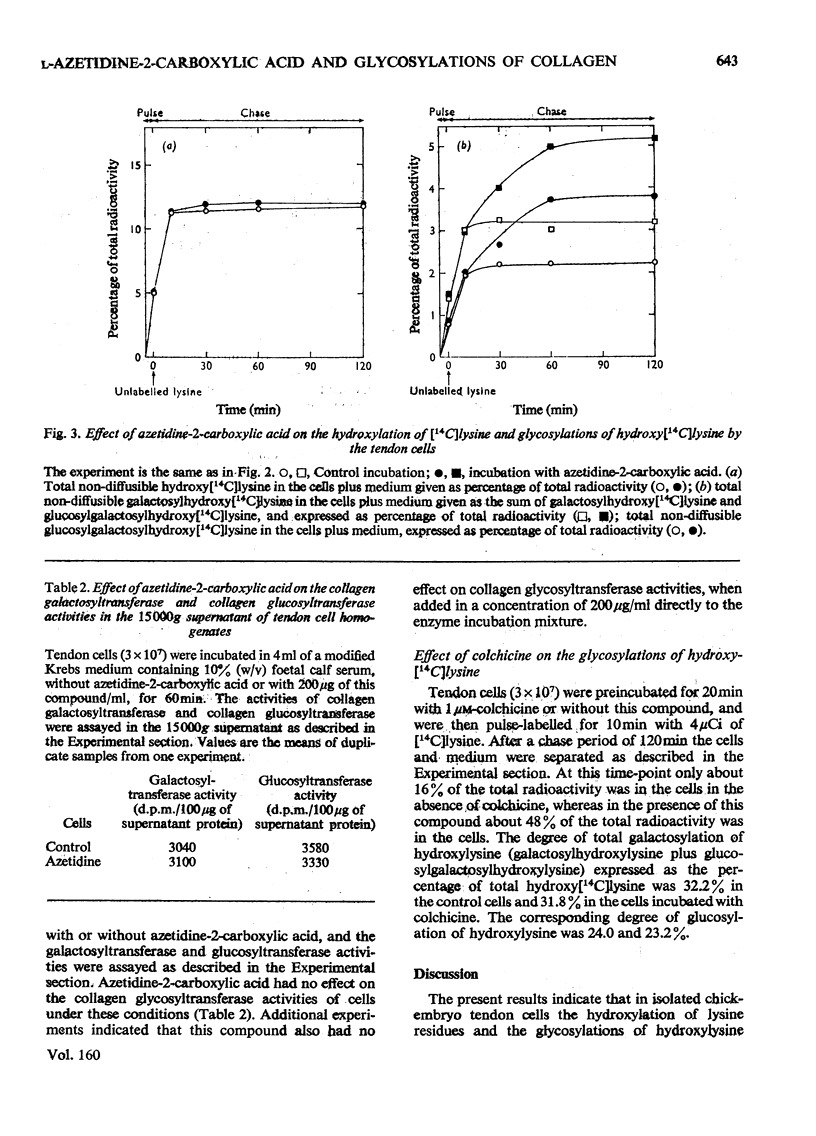
The glycosylations of hydroxylysine during collagen biosynthesis in isolated chick-embryo tendon cells were studied by using pulse-chase labelling experiments with [14C]-lysine. The hydroxylation of lysine and the glycosylations of hydroxylysine continued after a 5 min pulse label for up to about 10 min during the chase period. These data differ from those obtained previously in isolated chick-embryo cartilage cells, in which, after a similar 5 min pulse label, these reactions continued during the chase period for up to about 20 min. The collagen synthesized by the isolated chick-embryo tendon cells differed markedly from the type I collagen of adult tissues in its degree of hydroxylation of lysine residues and glycosylations of hydroxylysine residues. When the isolated tendon cells were incubated in the presence of L-azetidine-2-carboxylic acid, the degree of glycosylations of hydroxylysine during the first 10 min of the chase period was identical with that in cells incubated without thcarboxylic acid for at least 60 min, whereas no additional glycosylations took place in the control cells after the 10 min time-point. As a consequence, the collagen synthesized in the presence of this compound contained more carbohydrate than did the collagen synthesized by the control cells. Additional experiments indicated that azetidine-2-carboxylic acid did not increase the collagen glycosyltransferase activities in the tendon cells or the rate of glycosylation reactions when added directly to the enzyme incubation mixture. Control experiments with colchicine indicated that the delay in the rate of collagen secretion, which was observed in the presence of azetidine-2-carboxylic acid, did not in itself affect the degree of glycosylations of collagen. The results thus suggest that the increased glycosylations were due to inhibition of the collagen triple-helix formation, which is known to occur in the presence of azetidine-2-carboxylic acid.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anttinen H., Orava S., Ryhänen L., Kivirikko K. I. Assay of protocollagen lysyl hydroxylase activity in the skin of human subjects and changes in the activity with age. Clin Chim Acta. 1973 Aug 30;47(2):289–294. doi: 10.1016/0009-8981(73)90326-4. [DOI] [PubMed] [Google Scholar]
- Askenasi R. A new rapid method for measuring hydroxylysine and its glycosides in hydrolysates and physiological fluids. Biochim Biophys Acta. 1973 Apr 28;304(2):375–383. doi: 10.1016/0304-4165(73)90256-0. [DOI] [PubMed] [Google Scholar]
- Barnes M. J., Constable B. J., Morton L. F., Royce P. M. Age-related variations in hydroxylation of lysine and proline in collagen. Biochem J. 1974 May;139(2):461–468. doi: 10.1042/bj1390461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. A., Prockop D. J. Purification of (14C) protocollagen and its hydroxylation by prolyl-hydroxylase. Biochemistry. 1973 Aug 28;12(18):3395–3401. doi: 10.1021/bi00742a005. [DOI] [PubMed] [Google Scholar]
- Bornstein P. The biosynthesis of collagen. Annu Rev Biochem. 1974;43(0):567–603. doi: 10.1146/annurev.bi.43.070174.003031. [DOI] [PubMed] [Google Scholar]
- Brownell A. G., Veis A. The intracellular location of the glycosylation of hydroxylysine of collagen. Biochem Biophys Res Commun. 1975 Mar 17;63(2):371–377. doi: 10.1016/0006-291x(75)90698-1. [DOI] [PubMed] [Google Scholar]
- Dehm P., Prockop D. J. Time lag in the secretion of collagen by matrix-free tendon cells and inhibition of the secretory process by colchicine and vinblastine. Biochim Biophys Acta. 1972 Apr 21;264(2):375–382. doi: 10.1016/0304-4165(72)90302-9. [DOI] [PubMed] [Google Scholar]
- Diegelmann R. F., Peterkofsky B. Inhibition of collagen secretion from bone and cultured fibroblasts by microtubular disruptive drugs. Proc Natl Acad Sci U S A. 1972 Apr;69(4):892–896. doi: 10.1073/pnas.69.4.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich H. P., Ross R., Bornstein P. Effects of antimicrotubular agents on the secretion of collagen. A biochemical and morphological study. J Cell Biol. 1974 Aug;62(2):390–405. doi: 10.1083/jcb.62.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallop P. M., Paz M. A. Posttranslational protein modifications, with special attention to collagen and elastin. Physiol Rev. 1975 Jul;55(3):418–487. doi: 10.1152/physrev.1975.55.3.418. [DOI] [PubMed] [Google Scholar]
- Harwood R., Bhalla A. K., Grant M. E., Jackson D. S. The synthesis and secretion of cartilage procollagen. Biochem J. 1975 Apr;148(1):129–138. doi: 10.1042/bj1480129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood R., Grant M. E., Jackson D. S. Studies on the glycosylation of hydroxylysine residues during collagen biosynthesis and the subcellular localization of collagen galactosyltransferase and collagen glucosyltransferase in tendon and cartilage cells. Biochem J. 1975 Nov;152(2):291–302. doi: 10.1042/bj1520291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood R., Grant M. E., Jackson D. S. The route of secretion of procollagen. The influence of alphaalpha'-bipyridyl, colchicine and antimycin A on the secretory process in embryonic-chick tendon and cartilage cells. Biochem J. 1976 Apr 15;156(1):81–90. doi: 10.1042/bj1560081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez S. A., Harsch M., Murphy L., Rosenbloom J. Effects of temperature on conformation, hydroxylation, and secretion of chick tendon procollagen. J Biol Chem. 1974 Jul 25;249(14):4480–4486. [PubMed] [Google Scholar]
- Jimenez S., Rosenbloom J. Decreased thermal stability of collagens containing analogs of proline or lysine. Arch Biochem Biophys. 1974 Aug;163(2):459–465. doi: 10.1016/0003-9861(74)90502-5. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Risteli L. Biosynthesis of collagen and its alterations in pathological states. Med Biol. 1976 Jun;54(3):159–186. [PubMed] [Google Scholar]
- Kivirikko K. I., Ryhänen L., Anttinen H., Bornstein P., Prockop D. J. Further hydroxylation of lysyl residues in collagen by protocollagen lysyl hydroxylase in vitro. Biochemistry. 1973 Nov 20;12(24):4966–4971. doi: 10.1021/bi00748a023. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Shudo K., Sakakibara S., Prockop D. J. Studies on protocollagen lysine hydroxylase. Hydroxylation of synthetic peptides and the stoichiometric decarboxylation of -ketoglutarate. Biochemistry. 1972 Jan 4;11(1):122–129. doi: 10.1021/bi00751a021. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Martin G. R., Byers P. H., Piez K. A. Procollagen. Adv Enzymol Relat Areas Mol Biol. 1975;42:167–191. doi: 10.1002/9780470122877.ch3. [DOI] [PubMed] [Google Scholar]
- Murai A., Miyahara T., Shiozawa S. Age-related variations in glycosylation of hydroxylysine in human and rat skin collagens. Biochim Biophys Acta. 1975 Oct 9;404(2):345–348. doi: 10.1016/0304-4165(75)90343-8. [DOI] [PubMed] [Google Scholar]
- Myllylä R., Risteli L., Kivirikko K. I. Assay of collagen-galactosyltransferase and collagen-glucosyltransferase activities and preliminary characterization of enzymic reactions with transferases from chick-embryo cartilage. Eur J Biochem. 1975 Apr 1;52(3):401–410. doi: 10.1111/j.1432-1033.1975.tb04008.x. [DOI] [PubMed] [Google Scholar]
- Myllylä R., Risteli L., Kivirikko K. I. Glucosylation of galactosylhydroxylysyl residues in collagen in vitro by collagen glucosyltransferase. Inhibition by triple-helical conformation of the substrate. Eur J Biochem. 1975 Oct 15;58(2):517–521. doi: 10.1111/j.1432-1033.1975.tb02400.x. [DOI] [PubMed] [Google Scholar]
- Oikarinen A., Anttinen H., Kivirikko K. I. Hydroxylation of lysine and glycosylation of hydroxylysine during collagen biosynthesis in isolated chick-embryo cartilage cells. Biochem J. 1976 Jun 15;156(3):545–551. doi: 10.1042/bj1560545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen B. R., Prockop D. J. Ferritin-conjugated antibodies used for labeling of organelles involved in the cellular synthesis and transport of procollagen. Proc Natl Acad Sci U S A. 1974 May;71(5):2033–2037. doi: 10.1073/pnas.71.5.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risteli J., Kivirikko K. I. Activities of prolyl hydroxylase, lysyl hydroxylase, collagen galactosyltransferase and collagen glucosyltransferase in the liver of rats with hepatic injury. Biochem J. 1974 Oct;144(1):115–122. doi: 10.1042/bj1440115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risteli L., Myllyä R., Kivirikko K. I. Partial purification and characterization of collagen galactosyltransferase from chick embryos. Biochem J. 1976 Apr 1;155(1):145–153. doi: 10.1042/bj1550145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryhänen L., Kivirikko K. I. Developmental changes in protocollagen lysyl hydroxylase activity in the chick embryo. Biochim Biophys Acta. 1974 Mar 20;343(1):121–128. doi: 10.1016/0304-4165(74)90243-8. [DOI] [PubMed] [Google Scholar]
- Ryhänen L., Kivirikko K. I. Hydroxylation of lysyl residues in native and denatured protocollagen by protocollagen lysyl hydroxylase in vitro. Biochim Biophys Acta. 1974 Mar 20;343(1):129–137. doi: 10.1016/0304-4165(74)90244-x. [DOI] [PubMed] [Google Scholar]
- Schofield J. D., Uitto J., Prockop D. J. Formation of interchain disulfide bonds and helical structure during biosynthesis of procollagen by embryonic tendon cells. Biochemistry. 1974 Apr 23;13(9):1801–1806. doi: 10.1021/bi00706a004. [DOI] [PubMed] [Google Scholar]
- Spiro R. G. Characterization and quantitative determination of the hydroxylysine-linked carbohydrate units of several collagens. J Biol Chem. 1969 Feb 25;244(4):602–612. [PubMed] [Google Scholar]
- Uitto J., Hoffman H., Prockop D. J. Retention of nonhelical procollagen containing cis-hydroxyproline in rough endoplasmic reticulum. Science. 1975 Dec 19;190(4220):1202–1204. doi: 10.1126/science.1198105. [DOI] [PubMed] [Google Scholar]
- Uitto J., Prockop D. J. Hydroxylation of peptide-bound proline and lysine before and after chain completion of the polypeptide chains of procollagen. Arch Biochem Biophys. 1974 Sep;164(1):210–217. doi: 10.1016/0003-9861(74)90024-1. [DOI] [PubMed] [Google Scholar]
- Uitto J., Prockop D. J. Rate of helix formation by intracellular procollagen and protocollagen. Evidence for a role for disulfide bonds. Biochem Biophys Res Commun. 1973 Dec 10;55(3):904–911. doi: 10.1016/0006-291x(73)91229-1. [DOI] [PubMed] [Google Scholar]
- Uitto J., Prockop D. J. Synthesis and secretion of under-hydroxylated procollagen at various temperatures by cells subject to temporary anoxia. Biochem Biophys Res Commun. 1974 Sep 9;60(1):414–423. doi: 10.1016/0006-291x(74)90220-4. [DOI] [PubMed] [Google Scholar]


