Abstract
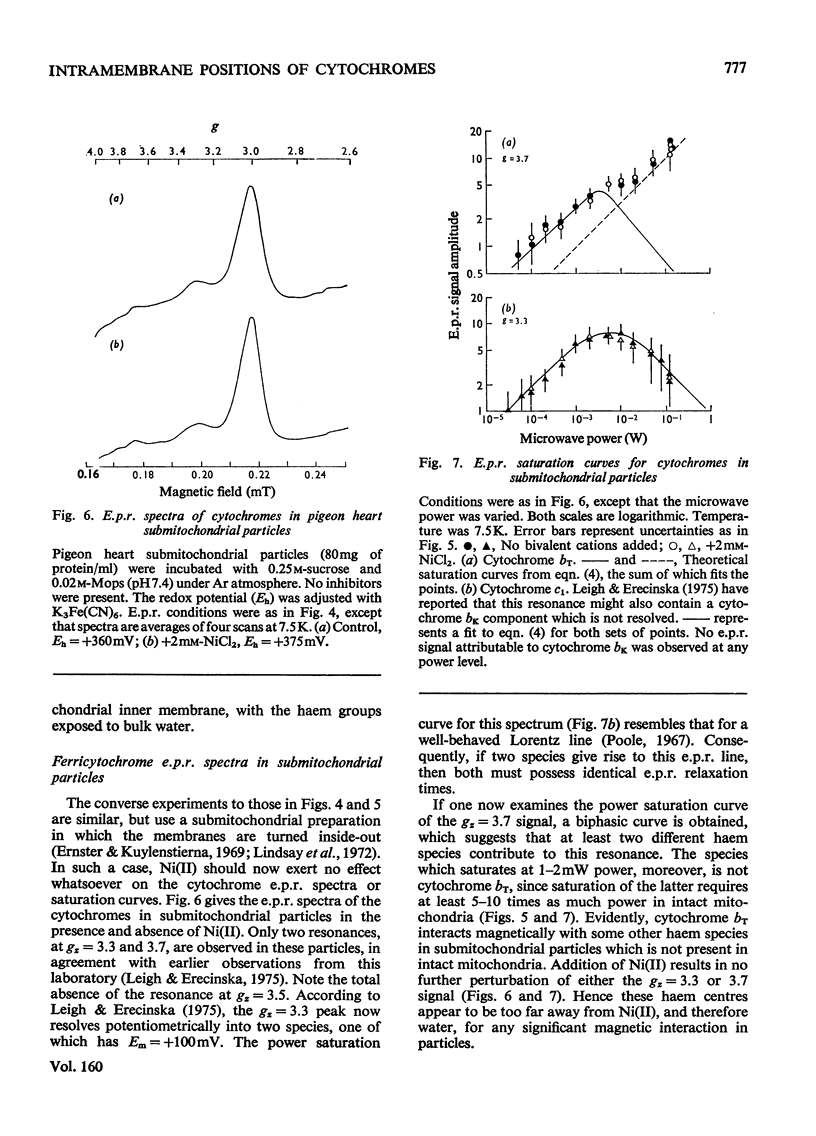
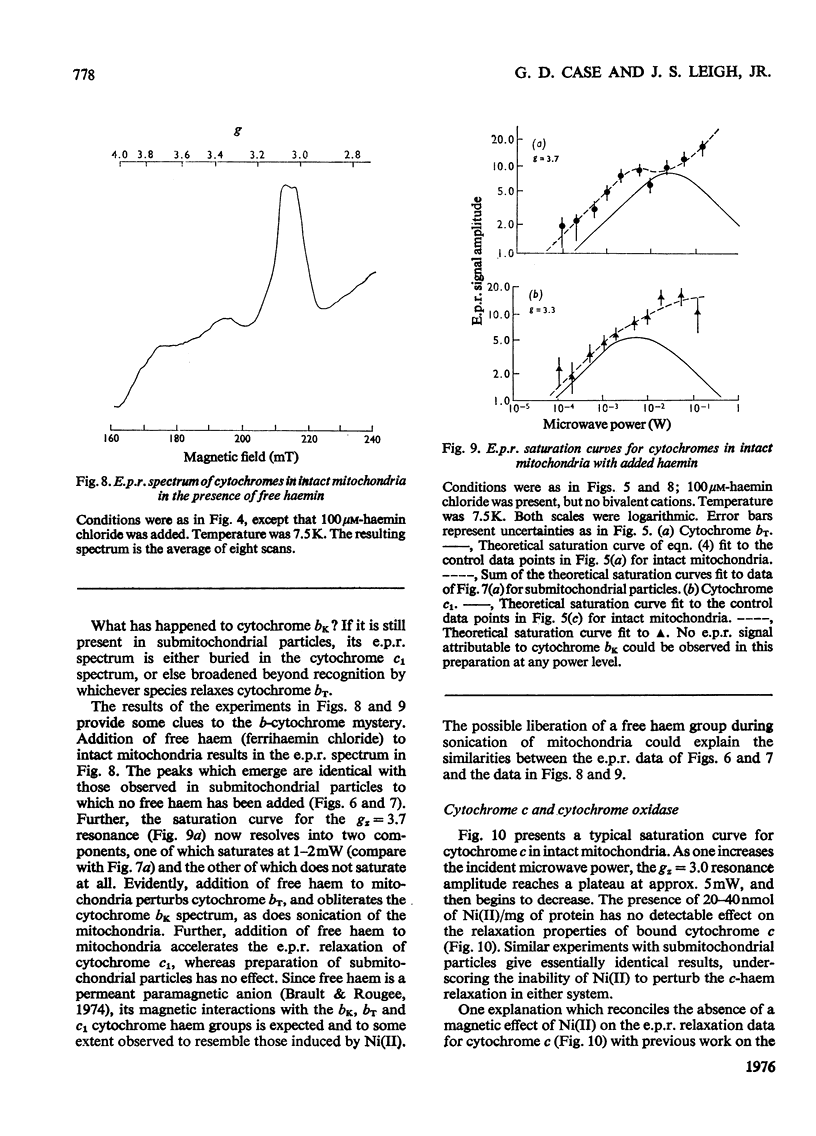
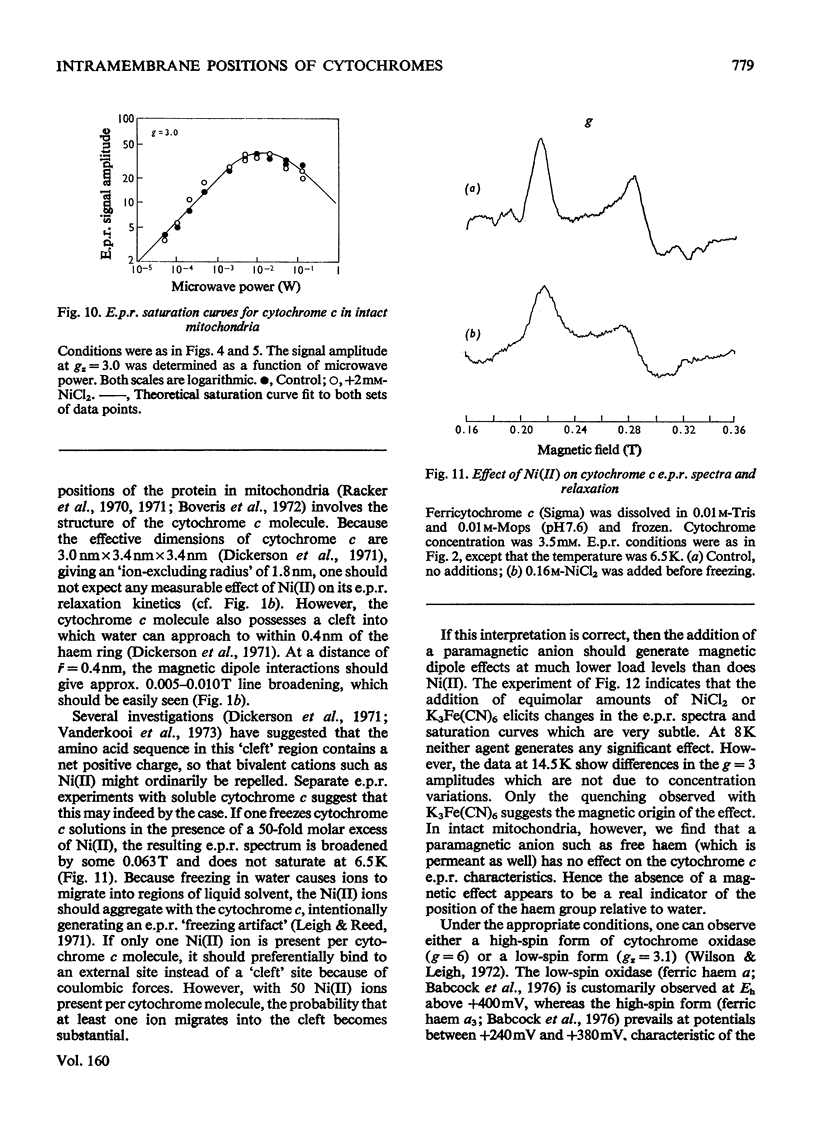
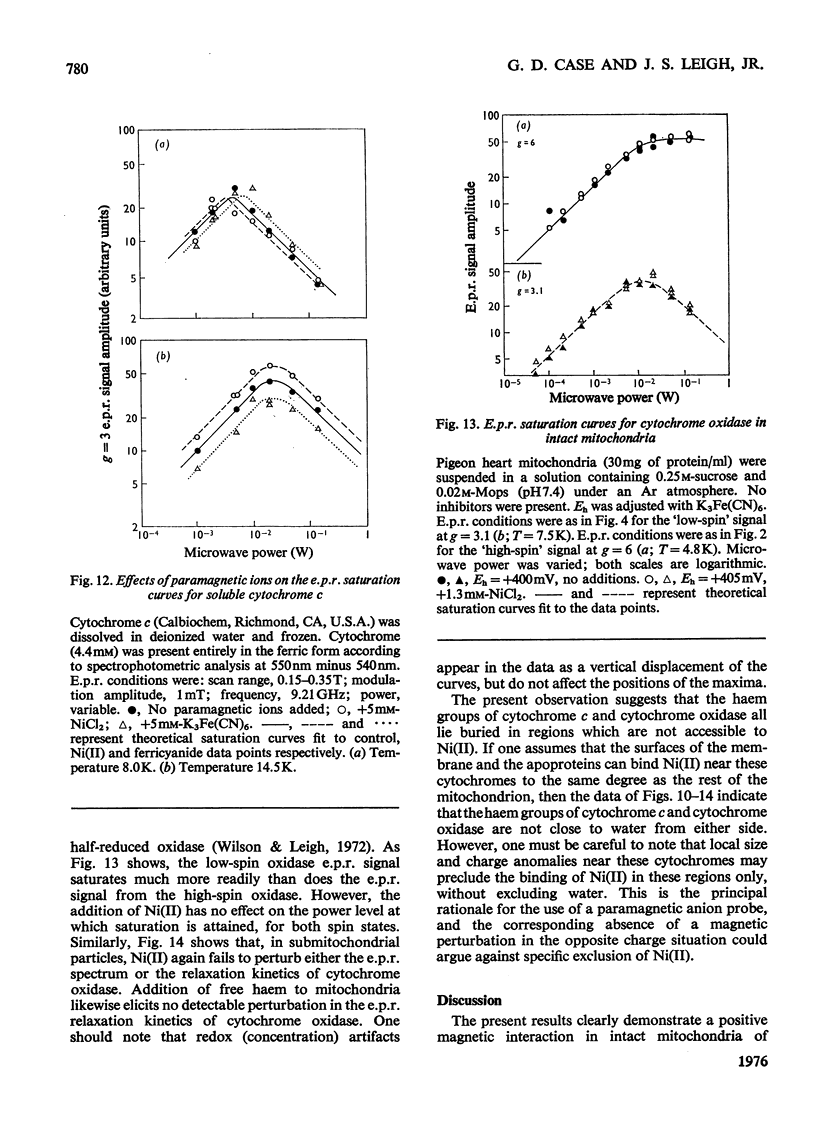
E.p.r.(electron-paramagnetic-resonance) spectra of the ferricytochromes were studied in normal and 'nickel-plated' pigeon heart mitochondria and pigeon heart submitochondrial particles. NiCL2 added to either mitochondria or particles was bound completely to the membranes, but none was transported across the vesicles. Hence, any perturbations of the haem e.p.r. spectra by Ni(II) should occur only for those cytochromes in close proximity to the exterior surface. Whenever Ni(II) can approach to within 1 nm of cytochrome haem. the consequent acceleration of the haem e.p.r. relaxation kinetics should elicit dipolar line broadening. Relaxation acceleration should also increase the incident power level required to saturate the haem e.p.r. signal. In pigeon heart mitochondria, at least three e.p.r. resonances, attributable in part to cytochromes c1, bK and br, are observed at gz=3.3 resonance. In these submitochondrial particles, the peak at gz=3.5 is missing, and the resonance at gz=3.6 resolves into two components, neither of which is sensitive to added Ni(ii). Addition of free haemin (ferric, a paramagnetic anion) to intact mitochondria elicits the same e.p.r. signal changes as does a preparation of submitochondrial particles. Saturation curves for cytochrome oxidase obtained for e.p.r. spectra of the high-spin form (g = 6) and the low-spin form (gz=3.1) also reveal no effect of Ni(II) on the haem e.p.r. relaxation in either mitochondria or inverted submitochondrial particles. Further, Ni(II) fails to alter the spectra or saturation properties of cytochrome c in either mitochondria or submitochondrial particles therefrom. Only with a 50-fold molar excess of Ni(II) can one accelerate the e.p.r. relaxation of cytochrome c in aqueous solution, although other more subtle types of magnetic interactions may occur between the cytochrome and either Ni(II) or ferricyanide. Addition of haemin to mitochondria likewise failed to alter the e.p.r. characteristics of either cytochrome c or cytochrome oxidase. The present observations strongly suggest that cytochromes bK, br and c1 reside on the exterior surface of the inner mitochondrial membrane. On the other hand, we find no positive evidence for the location of cytochrome c or cytochrome oxidase haem groups within 1 nm of either membrane surface. Because of possible shielding effects from the protein moieties, however, we cannot unequivocally assign the location of the haem groups to the membrane interior. The present results are not inconsistent with the observations of other investigators who used different techniques. However, it is clear that any model of energy coupling in mitochondrial oxidative phosphorylation must account for the positioning of all the b-c cytochrome haem groups on the outside.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albracht S. P. Some new paramagnetic centers in submitochondrial particles detectable by EPR spectroscopy. Biochim Biophys Acta. 1974 May 22;347(2):183–192. doi: 10.1016/0005-2728(74)90043-7. [DOI] [PubMed] [Google Scholar]
- Arion W. J., Wright B. J. Preservation of energy coupling in submitochondrial particles during extraction and reinsertion of cytochrome C. Biochem Biophys Res Commun. 1970 Aug 11;40(3):594–599. doi: 10.1016/0006-291x(70)90944-7. [DOI] [PubMed] [Google Scholar]
- Astle L., Cooper C. Relationship of sidedness of mitochondrial inner membrane vesicles to their enzymic properties. Biochemistry. 1974 Jan 1;13(1):154–160. doi: 10.1021/bi00698a024. [DOI] [PubMed] [Google Scholar]
- Ausländer W., Junge W. The electric generator in the photosynthesis of green plants. II. Kinetic correlation between protolytic reactions and redox reactions. Biochim Biophys Acta. 1974 Aug 23;357(2):285–298. doi: 10.1016/0005-2728(74)90067-x. [DOI] [PubMed] [Google Scholar]
- Beinert H., Ackrell B. A., Kearney E. B., Singer T. P. Iron-sulfur components of succinate dehydrogenase: stoichiometry and kinetic behavior in activated preparations. Eur J Biochem. 1975 May;54(1):185–194. doi: 10.1111/j.1432-1033.1975.tb04128.x. [DOI] [PubMed] [Google Scholar]
- Boveris A., Oshino N., Chance B. The cellular production of hydrogen peroxide. Biochem J. 1972 Jul;128(3):617–630. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault D., Rougee M. Ferrous porphyrins in organic solvents. II. Optical spectra and paramagnetic susceptibilities. Biochemistry. 1974 Oct 22;13(22):4598–4602. doi: 10.1021/bi00719a020. [DOI] [PubMed] [Google Scholar]
- CRANE F. L., GLENN J. L., GREEN D. E. Studies on the electron transfer system. IV. The electron transfer particle. Biochim Biophys Acta. 1956 Dec;22(3):475–487. doi: 10.1016/0006-3002(56)90058-0. [DOI] [PubMed] [Google Scholar]
- Case G. D. Magnetic resonance studies on the mitochondrial divalent cation carrier. Biochim Biophys Acta. 1975 Jan 14;375(1):69–86. doi: 10.1016/0005-2736(75)90073-5. [DOI] [PubMed] [Google Scholar]
- Case G. D., Ohnishi T., Leigh J. S., Jr Intramitochondrial positions of ubiquinone and iron-sulphur centres determined by dipolar interactions with paramagnetic ions. Biochem J. 1976 Dec 15;160(3):785–795. doi: 10.1042/bj1600785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Crofts A. R., Nishimura M., Price B. Fast membrane H+ binding in the light-activated state of Chromatium chromatophores. Eur J Biochem. 1970 Apr;13(2):364–374. doi: 10.1111/j.1432-1033.1970.tb00938.x. [DOI] [PubMed] [Google Scholar]
- Chance B., Erecińska M., Lee C. P. Localization of cytochromes in intact and fragmented mitochondrial membranes. Proc Natl Acad Sci U S A. 1970 Jul;66(3):928–935. doi: 10.1073/pnas.66.3.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Mela L. Energy-linked changes of hydrogen ion concentration in submitochondrial particles. J Biol Chem. 1967 Mar 10;242(5):830–844. [PubMed] [Google Scholar]
- Dervartanian D. V., Albracht S. P., Berden J. A., van Gelder B. F., Slater E. C. The EPR spectrum of isolated complex 3. Biochim Biophys Acta. 1973 Feb 22;292(2):496–501. doi: 10.1016/0005-2728(73)90055-8. [DOI] [PubMed] [Google Scholar]
- Dickerson R. E., Takano T., Eisenberg D., Kallai O. B., Samson L., Cooper A., Margoliash E. Ferricytochrome c. I. General features of the horse and bonito proteins at 2.8 A resolution. J Biol Chem. 1971 Mar 10;246(5):1511–1535. [PubMed] [Google Scholar]
- Dutton P. L., Wilson D. F., Lee C. P. Oxidation-reduction potentials of cytochromes in mitochondria. Biochemistry. 1970 Dec 22;9(26):5077–5082. doi: 10.1021/bi00828a006. [DOI] [PubMed] [Google Scholar]
- Dutton P. L., Wilson D. F. Redox potentiometry in mitochondrial and photosynthetic bioenergetics. Biochim Biophys Acta. 1974 Oct 31;346(2):165–212. doi: 10.1016/0304-4173(74)90008-1. [DOI] [PubMed] [Google Scholar]
- Eytan G. D., Carroll R. C., Schatz G., Racker E. Arrangement of the subunits in solubilized and membrane-bound cytochrome c oxidase from bovine heart. J Biol Chem. 1975 Nov 25;250(22):8598–8603. [PubMed] [Google Scholar]
- Fleischer S., Fleischer B., Stoeckenius W. Fine structure of lipid-depleted mitochondria. J Cell Biol. 1967 Jan;32(1):193–208. doi: 10.1083/jcb.32.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORNALL A. G., BARDAWILL C. J., DAVID M. M. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949 Feb;177(2):751–766. [PubMed] [Google Scholar]
- Green D. E., Ji S. The electromechanochemical model of mitochondrial structure and function. J Bioenerg. 1972 May;3(1):159–202. doi: 10.1007/BF01516006. [DOI] [PubMed] [Google Scholar]
- Green D. E. The electromechanochemical model for energy coupling in mitochondria. Biochim Biophys Acta. 1974 Apr 30;346(1):27–78. doi: 10.1016/0304-4173(74)90011-1. [DOI] [PubMed] [Google Scholar]
- Grinius L. L., Guds T. I., Skulachev V. P. Arrangement of the electric potential-generating redox chain in the mitochondrial membrane. J Bioenerg. 1971 May;2(2):101–113. doi: 10.1007/BF01648925. [DOI] [PubMed] [Google Scholar]
- Gutman M., Singer T. P., Beinert H. Relation of the respiratory chain-linked reduced nicotinamide--adenine dinucleotide dehydrogenase to energy-coupling site 1. Biochemistry. 1972 Feb 15;11(4):556–562. doi: 10.1021/bi00754a012. [DOI] [PubMed] [Google Scholar]
- Harmon H. J., Hall J. D., Crane F. L. Structure of mitochondrial cristae membranes. Biochim Biophys Acta. 1974 Sep 16;344(2):119–155. doi: 10.1016/0304-4157(74)90002-1. [DOI] [PubMed] [Google Scholar]
- Jackson J. B., Crofts A. R., von Stedingk L. V. Ion transport induced by light and antibiotics IN CHROMATOPHORES FROM Rhodospirillum rubrum. Eur J Biochem. 1968 Oct 17;6(1):41–54. doi: 10.1111/j.1432-1033.1968.tb00417.x. [DOI] [PubMed] [Google Scholar]
- Jagendorf A. T., Uribe E. ATP formation caused by acid-base transition of spinach chloroplasts. Proc Natl Acad Sci U S A. 1966 Jan;55(1):170–177. doi: 10.1073/pnas.55.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge W., DeVault D. Symmetry, orientation and rotational mobility in the a3 heme of cytochrome c oxidase in the inner membrane of mitochondria. Biochim Biophys Acta. 1975 Dec 11;408(3):200–214. doi: 10.1016/0005-2728(75)90123-1. [DOI] [PubMed] [Google Scholar]
- Junge W., Witt H. T. On the ion transport system of photosynthesis--investigations on a molecular level. Z Naturforsch B. 1968 Feb;23(2):244–254. doi: 10.1515/znb-1968-0222. [DOI] [PubMed] [Google Scholar]
- Kalina M., Plapinger R. E., Hoshino Y., Seligman A. M. Nonosmiophilic tetrazolium salts that yield osmiophilic, lipophobic formazans for ultrastructural localization of dehydrogenase activity. J Histochem Cytochem. 1972 Sep;20(9):685–695. doi: 10.1177/20.9.685. [DOI] [PubMed] [Google Scholar]
- Klingenberg M. Localization of the glycerol-phosphate dehydrogenase in the outer phase of the mitochondrial inner membrane. Eur J Biochem. 1970 Apr;13(2):247–252. doi: 10.1111/j.1432-1033.1970.tb00924.x. [DOI] [PubMed] [Google Scholar]
- Lehninger A. L., Carafoli E., Rossi C. S. Energy-linked ion movements in mitochondrial systems. Adv Enzymol Relat Areas Mol Biol. 1967;29:259–320. doi: 10.1002/9780470122747.ch6. [DOI] [PubMed] [Google Scholar]
- Leigh J. S., Jr, Erecinska M. Thermodynamic and EPR characterization of mitochondrial succinate-cytochrome c reductase-phospholipid complexes. Biochim Biophys Acta. 1975 Apr 14;387(1):95–106. doi: 10.1016/0005-2728(75)90054-7. [DOI] [PubMed] [Google Scholar]
- Lindsay J. G., Dutton P. L., Wilson D. F. Energy-dependent effects on the oxidation-reduction midpoint potentials of the b and c cytochromes in phosphorylating submitochondrial particles from pigeon heart. Biochemistry. 1972 May 9;11(10):1937–1942. doi: 10.1021/bi00760a031. [DOI] [PubMed] [Google Scholar]
- Mela L., Chance B. Spectrophotometric measurements of the kinetics of Ca2+ and Mn2+ accumulation in mitochondria. Biochemistry. 1968 Nov;7(11):4059–4063. doi: 10.1021/bi00851a038. [DOI] [PubMed] [Google Scholar]
- Mildvan A. S., Cohn M. Aspects of enzyme mechanisms studies by nuclear spin relazation induced by paramagnetic probes. Adv Enzymol Relat Areas Mol Biol. 1970;33:1–70. doi: 10.1002/9780470122785.ch1. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Estimation of membrane potential and pH difference across the cristae membrane of rat liver mitochondria. Eur J Biochem. 1969 Feb;7(4):471–484. doi: 10.1111/j.1432-1033.1969.tb19633.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Evidence discriminating between the chemical and the chemiosmotic mechanisms of electron transport phosphorylation. Nature. 1965 Dec 18;208(5016):1205–1206. doi: 10.1038/2081205a0. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Protonmotive redox mechanism of the cytochrome b-c1 complex in the respiratory chain: protonmotive ubiquinone cycle. FEBS Lett. 1975 Aug 1;56(1):1–6. doi: 10.1016/0014-5793(75)80098-6. [DOI] [PubMed] [Google Scholar]
- Ohnishi T. Studies on the mechanism of site I energy conservation. Eur J Biochem. 1976 Apr 15;64(1):91–103. doi: 10.1111/j.1432-1033.1976.tb10277.x. [DOI] [PubMed] [Google Scholar]
- Ohnishi T. Thermodynamic and EPR characterization of iron-sulfur centers in the NADH-ubiquinone segment of the mitochondrial respiratory chain in pigeon heart. Biochim Biophys Acta. 1975 Jun 17;387(3):475–490. doi: 10.1016/0005-2728(75)90087-0. [DOI] [PubMed] [Google Scholar]
- Onishi T., Leigh J. S., Ragan C. I., Racker E. Low temperature electron paramagnetic resonance studies on iron-sulfur centers in cardiac NADH dehydrogenase. Biochem Biophys Res Commun. 1974 Feb 4;56(3):775–782. doi: 10.1016/0006-291x(74)90672-x. [DOI] [PubMed] [Google Scholar]
- Onishi T., Leigh J. S., Winter D. B., Lim J., King T. E. EPR studies on two ferredoxin-type iron-sulfur centers in reconstitutively active, inactive, and reactivated soluble succinate dehydrogenases. Biochem Biophys Res Commun. 1974 Dec 11;61(3):1026–1035. doi: 10.1016/0006-291x(74)90258-7. [DOI] [PubMed] [Google Scholar]
- Onishi T. Mechanism of electron transport and energy conservation in the site I region of the respiratory chain. Biochim Biophys Acta. 1973 Dec 7;301(2):105–128. [PubMed] [Google Scholar]
- Orme-Johnson N. R., Hansen R. E., Beinert H. Electron paramagnetic resonance-detectable electron acceptors in beef heart mitochondria. Reduced diphosphopyridine nucleotide ubiquinone reductase segment of the electron transfer system. J Biol Chem. 1974 Mar 25;249(6):1922–1927. [PubMed] [Google Scholar]
- Orme-Johnson N. R., Hansen R. E., Beinert H. Electron paramagnetic resonance-detectable electron acceptors in beef heart mitochondria. Ubihydroquinone-cytochrome c reductase segment of the electron transfer system and complex mitochondrial fragments. J Biol Chem. 1974 Mar 25;249(6):1928–1939. [PubMed] [Google Scholar]
- Reuben J., Cohn M. Magnetic resonance studies of manganese (II) binding sites of pyruvate kinase. Temperature effects and frequency dependence of proton relaxation rates of water. J Biol Chem. 1970 Dec 25;245(24):6539–6546. [PubMed] [Google Scholar]
- Romslo I., Flatmark T. Energy-dependent accumulation of iron by isolated rat liver mitochondria. I. General features. Biochim Biophys Acta. 1973 Apr 27;305(1):29–40. doi: 10.1016/0005-2728(73)90228-4. [DOI] [PubMed] [Google Scholar]
- Scarpa A., Graziotti P. Mechanisms for intracellular calcium regulation in heart. I. Stopped-flow measurements of Ca++ uptake by cardiac mitochondria. J Gen Physiol. 1973 Dec;62(6):756–772. doi: 10.1085/jgp.62.6.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa A. Spectrophotometric measurement of calcium by murexide. Methods Enzymol. 1972;24:343–351. doi: 10.1016/0076-6879(72)24082-4. [DOI] [PubMed] [Google Scholar]
- Schneider D. L., Kagawa Y., Racker E. Chemical modification of the inner mitochondrial membrane. J Biol Chem. 1972 Jun 25;247(12):4074–4079. [PubMed] [Google Scholar]
- Scholes C. P., Isaacson R. A., Feher G. Determination of the zero-field splitting of Fe 3+ in heme proteins from the temperature dependence of the spin-lattice relaxation rate. Biochim Biophys Acta. 1971 Jul 20;244(1):206–210. doi: 10.1016/0304-4165(71)90138-3. [DOI] [PubMed] [Google Scholar]
- Tinberg H. M., Melnick R. L., Maguire J., Packer L. Studies on mitochondrial proteins. II. Localization of components in the inner membrane: labeling with diazobenzenesulfonate, a non-penetrating probe. Biochim Biophys Acta. 1974 Apr 12;345(1):118–128. doi: 10.1016/0005-2736(74)90251-x. [DOI] [PubMed] [Google Scholar]
- Vanderkooi G. Molecular architecture of biological membranes. Ann N Y Acad Sci. 1972 Jun 20;195:6–15. [PubMed] [Google Scholar]
- Vanderkooi J., Erecińska M., Chance B. Cytochrome c interaction with membranes. II. Comparative study of the interaction of c cytochromes with the mitochondrial membrane. Arch Biochem Biophys. 1973 Aug;157(2):531–540. doi: 10.1016/0003-9861(73)90672-3. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Dutton P. L. Energy dependent changes in the oxidation-reduction potential of cytochrome b. Biochem Biophys Res Commun. 1970 Apr 8;39(1):59–64. doi: 10.1016/0006-291x(70)90757-6. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Leigh J. S., Jr Heme-heme interaction in cytochrome c oxidase in situ as measured by EPR spectroscopy. Arch Biochem Biophys. 1972 May;150(1):154–163. doi: 10.1016/0003-9861(72)90022-7. [DOI] [PubMed] [Google Scholar]
- von Jagow G., Klingenberg M. Pathways of hydrogen in mitochondria of Saccharomyces carlsbergensis. Eur J Biochem. 1970 Feb;12(3):583–592. doi: 10.1111/j.1432-1033.1970.tb00890.x. [DOI] [PubMed] [Google Scholar]


