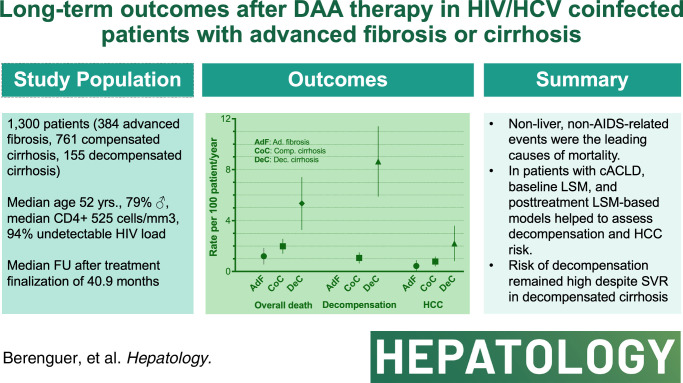
Abstract
Background and Aims:
We assessed long-term clinical outcomes and prognostic factors for liver disease progression after sustained viral response with direct-acting antivirals in patients coinfected with HIV/HCV with advanced fibrosis or cirrhosis.
Approach and Results:
A total of 1300 patients who achieved sustained viral response with direct-acting antivirals from 2014 to 2017 in Spain were included: 1145 with compensated advanced chronic liver disease (384 advanced fibrosis and 761 compensated cirrhosis) and 155 with decompensated cirrhosis. The median follow-up was 40.9 months. Overall, 85 deaths occurred, 61 due to non-liver non-AIDS–related causes that were the leading cause of death across all stages of liver disease. The incidence (95% CI) of decompensation per 100 person-years (py) was 0 in patients with advanced fibrosis, 1.01 (0.68–1.51) in patients with compensated cirrhosis, and 8.35 (6.05–11.53) in patients with decompensated cirrhosis. The incidence (95% CI) of HCC per 100 py was 0.34 (0.13–0.91) in patients with advanced fibrosis, 0.73 (0.45–1.18) in patients with compensated cirrhosis, and 1.92 (1.00–3.70) per 100 py in patients with decompensated cirrhosis. Prognostic factors for decompensation in patients with compensated advanced chronic liver disease included serum albumin, liver stiffness measurement (LSM), and fibrosis 4. In this population, LSM and LSM-based posttreatment risk stratification models showed their predictive ability for decompensation and HCC.
Conclusions:
Non-liver non-AIDS–related events were the leading causes of morbidity and mortality after direct-acting antiviral cure among coinfected patients with advanced fibrosis/cirrhosis. Among those with compensated advanced chronic liver disease, baseline LSM and posttreatment LSM-based models helped to assess decompensation and HCC risk.
INTRODUCTION
Over the last decade, the introduction of all-oral direct-acting antivirals (DAAs) against the HCV has revolutionized the treatment of hepatitis C.1 The high effectiveness, safety, and lack of contraindications for DAA have improved clinical outcomes for patients with hepatitis C, including those with compensated and decompensated cirrhosis.2–5 Broad access to DAA has also contributed dramatically to reducing the burden of HCV-related liver disease,6 significantly affecting the evolution of the number and results of liver transplantation due to this indication.7,8
DAA therapy has had a significant impact on persons with HIV (PWH) coinfected with HCV, a population group difficult to treat in the interferon plus ribavirin era1,9 with sustained viral response (SVR) rates over 90% in real-world practice.10,11 Despite all the progress, the burden of HCV-related liver disease among PWH will persist in the years to come, as a substantial number of those with chronic hepatitis C clearing the infection with anti-HCV therapy have cirrhosis.12 Studies in the interferon era confirmed the clinical benefits of SVR in patients coinfected with HIV/HCV, including a reduction in liver-related complications and mortality13 and a decrease in HIV progression and mortality not related to liver disease.14 However, information on the long-term effects of SVR following DAAs in PWH, particularly in those with more severe liver disease, is limited.15
We aimed to assess the long-term clinical outcomes and prognostic factors for liver disease progression after HCV clearance in a large cohort of patients coinfected with HIV/HCV with advanced fibrosis or cirrhosis who achieved SVR following oral DAA therapy.
METHODS
Design and study population
This multicenter retrospective study was carried out by the AIDS Study Group (Grupo de Estudio del SIDA [GeSIDA]) of the Spanish Society of Infectious Diseases and Clinical Microbiology (Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica [SEIMC]). It included previously untreated and anti-HCV therapy–experienced patients coinfected with HIV/HCV with advanced fibrosis or cirrhosis who achieved SVR following all-oral DAA therapy from 2014 to 2017 in 21 centers across 5 regions in Spain (GeSIDA 10318-Study). Patients were included in 3 prospective observational cohort studies, 2 of which were conducted by GeSIDA16,17 and 1 within the Madrid Coinfection Registry (Madrid-CoRe).10 The censoring date of the study was December 31, 2019.
Investigations
We leveraged the databases from the 3 studies to obtain baseline data for the working database for this new investigation. New fields for follow-up data have been added as needed. The electronic case report form was built using REDCap electronic data capture tools18 and hosted by the SEIMC/GeSIDA Foundation.
Baseline variables included demographics, height and weight, clinical data on HIV and its treatment, HCV-related liver disease, several comorbid conditions, current smoking, high alcohol intake, and whether the participants were on methadone maintenance programs (see below). Laboratory parameters included complete blood counts, international normalized ratio, biochemical parameters (serum lipids, alanine aminotransferase, aspartate aminotransferase, serum albumin concentration, and serum creatinine concentration), HCV-RNA quantification, HCV genotype, HBsAg, cluster of differentiation 4 (CD4) + T lymphocyte count, and HIV-RNA load. Liver fibrosis was estimated by liver stiffness measurement (LSM) assessed by transient elastography and the fibrosis 4 (FIB-4) index.19 Other noninvasive biomarkers include the triglyceride and glucose index, a screening index for insulin resistance and NAFLD,20 and the hepatic steatosis index, which is another screening index for NAFLD.21 Furthermore, LSM and various laboratory parameters were recorded 1 year (±2 mo) following treatment completion to evaluate posttreatment LSM-based risk stratification models developed in patients with compensated advanced chronic liver disease (cACLD) who had undergone HCV treatment with DAAs.
Patients underwent follow-up visits at least twice a year with surveillance for HCC using abdominal ultrasound. Interim and final data monitoring was performed by clinical research monitors from the SEIMC/GeSIDA Foundation to ensure data consistency with medical records. The study was conducted in accordance with the Declarations of Helsinki and Istanbul and was approved by the ethics committee of the Hospital General Universitario Gregorio Marañón, which waived informed consent for the collection of clinical data (FHG-AAD-2018-01). All the processes satisfied the local data confidentiality requirements.
Definitions
SVR was defined as undetectable serum HCV-RNA level 12 weeks after discontinuing anti-HCV therapy. Advanced fibrosis was defined by bridging fibrosis in liver biopsy or an LSM value >9.9 and ≤12.5 kPa. Liver cirrhosis was defined by liver biopsy, an LSM >12.5 kPa, or clinical-biological or imaging-compatible parameters. Liver decompensation was defined by a history of clinically detectable ascites, variceal bleeding, or portosystemic encephalopathy.22 To encompass advanced fibrosis and compensated cirrhosis, we used the term cACLD.23 HCC was defined using imaging criteria (CT or magnetic resonance) defined by the European Association for the Study of the Liver (EASL) and the European Organization for Research and Treatment of Cancer (EORTC) guidelines.24
Non-liver non-AIDS–related comorbidities were defined according to the Spanish AIDS Research Network (CoRIS) criteria, as described elsewhere.25 These included arterial hypertension, hyperlipidemia, diabetes mellitus, obesity, ischemic cardiovascular disease (myocardial infarction, angina, stroke, peripheral artery disease, and mesenteric artery ischemia), heart failure, chronic kidney disease, bone events (fractures and avascular necrosis), and biopsy-confirmed non-liver–related non-AIDS–related cancer. Metabolic syndrome was assessed according to the American Heart Association/National Heart, Lung, and Blood Institute (AHA/NHLBI),26 while a high intake of alcohol was defined as the consumption of more than 50 g of alcohol per day for at least 12 months.
Investigators classified the cause of death at each center according to the following criteria: (a) liver-related death when the train of events that ended in death was caused by liver decompensation or HCC, (b) AIDS-related death when death was directly related to an AIDS-defining condition, and (c) non-liver non-AIDS–related death when the 2 previous criteria were not met.
Study outcomes
We first analyzed overall and cause-specific mortality in the entire study cohort. Next, we analyzed other outcomes separately for patients with compensated and decompensated liver disease owing to their differing natural histories and prognostic factors.27 The primary study outcomes for patients with cACLD were decompensation and HCC. The primary study outcome for patients with decompensated cirrhosis was the first episode of further decompensation defined according to the Baveno II consensus statement.22 The secondary outcomes for all patients included new AIDS-defining conditions and incident non-liver non-AIDS–related events.
Statistics
Each participant was followed from the date of finalization of the DAA therapy to the last follow-up visit, death, or administrative censoring date, whichever occurred first. Descriptive statistics are expressed as absolute numbers, percentages, medians, and IQRs. Differences between groups were analyzed using the chi-square test, t test, or Mann-Whitney test, as appropriate. Normality was analyzed using the Kolmogorov-Smirnov test. We calculated the frequency and incidence rate of events per 100 person-years (100-py) of follow-up for different liver disease stages. When assessing non-liver, non-AIDS–related events, those with a history of a specific event at baseline were excluded from the population at risk when considering the incidence of such events. We used Poisson regression to calculate the incidence rate ratio and 95% CI of clinical events between categories of liver disease. When assessing the study outcomes of patients with decompensated cirrhosis, we categorized them according to the type of prior decompensation: ascites, variceal hemorrhage (with or without ascites), and portosystemic encephalopathy (with or without ascites or variceal hemorrhage).
Multivariable Fine-Grey competing-risk regression analyses, with multiple imputations by chained equations for missing data, were used to assess the effect of the independent variables on the outcomes. In patients with cACLD, competing events for the analysis of decompensation included death and HCC, as it can precipitate decompensation,22 whereas the competing event was death while for the examination of HCC. In patients with decompensated cirrhosis, competing events were death and liver transplantation. In patients with cACLD, the independent variables analyzed included liver disease stage (advanced fibrosis or compensated cirrhosis), sex, age, current smoking, high alcohol intake, the presence of metabolic syndrome, serum albumin concentration, CD4+ T-cell count, liver stiffness, FIB-4, and triglyceride and glucose index. In patients with decompensated cirrhosis, the independent variables analyzed included the type of decompensation before the initiation of DAA therapy, sex, age, albumin concentration, the Model for End-Stage Liver Disease score, and platelet count. The selection of potential predictor variables was based on the underlying conceptual framework, and they were included in the multivariable models regardless of statistical significance.
To assess the performance of pretreatment LSM in predicting liver decompensation among patients with cACLD, we used time-dependent receiver operating characteristic (t-ROC) curves for censored event times and applied the inverse probability of censoring weighting estimators to address competing risks.28 In the absence of a parallel metric to Youden’s index for determining the optimal cutoff value within the context of t-ROC curves for censored event times with competing risks, various cutoff values for LSM were systematically explored. To rule out decompensation, we identified the highest LSM cutoff value associated with the maximum negative predictive value over a 42-month follow-up period.
In addition, 4 posttreatment LSM-based risk stratification models developed in patients with cACLD who had undergone HCV treatment with DAAs were assessed. The model by Semmler et al29 was based on posttreatment LSM and platelet count that was developed to estimate the probability of clinically significant portal hypertension and to evaluate the risk of decompensation, and 3 models that were developed to assess the risk of HCC: the model by Pons et al30 based on posttreatment LSM and albumin concentration, Semmler et al31 based on age, alcohol consumption, posttreatment LSM, and albumin concentration, and Alonso Lopez et al32 based on baseline LSM, the percentage of reduction in LSM at 1-year posttreatment (1-year DeltaLSM), and albumin concentration. To achieve this, the incidence rate of events for distinct risk categories defined by these indices was computed. A detailed description of the calculation of posttreatment LSM-based risk stratification models is provided in Supplemental Table S1, http://links.lww.com/HEP/I336.
Statistical analyses were performed using the Stata software (version 15.0; Stata Corporation). We used R version 4.3.2 (R Core Team, 2023) for calculations and graphical representations of t-ROC curves, leveraging the “timeROC” package.
RESULTS
Study cohort
A total of 1300 patients were included in the study, 384 of whom had advanced fibrosis (29.5%), 761 had compensated cirrhosis (58.5%), and 155 had decompensated cirrhosis (11.9%). Their characteristics before initiating DAA therapy, categorized by liver disease stage, are shown in Table 1. In brief, 79.2% of patients were male, the median age was 51.9 years, 88.9% had acquired HIV by injection drug use, 40.4% had prior AIDS-defining conditions, 97.9% were on antiretroviral therapy, the median CD4 cell count was 525 cells/mm3, and 94.0% had an HIV viral load lower than 50 copies per mL. Fifty-two percent of patients were naïve to anti-HCV in 60.2%, was the most frequent DAA regimen. The median baseline liver stiffness and FIB-4 values were 15.8 kPa (IQR: 11.8–26.0) and 2.84 (IQR: 1.83–5.13), respectively. Current smoking and a high alcohol intake were registered in 71.5% and 14.5% of patients, respectively. In addition, 21.4% of the patients were on methadone maintenance programs. The most frequent comorbidities were arterial hypertension (23.3%), hyperlipidemia (18.2%), diabetes mellitus (13.4%), and obesity (10.1%). Metabolic syndrome was detected in 17.1% of the participants.
TABLE 1.
Baseline characteristics of 1300 patients coinfected with HIV/HCV with advanced fibrosis/cirrhosis with sustained viral response following all-oral direct-acting antiviral therapy against HCV
| Characteristic | Advanced fibrosis (N=384) | Compensated cirrhosis (N=761) | Decompensated cirrhosis (N=155) | Total (N=1300) |
|---|---|---|---|---|
| Demographics and anthropometry | ||||
| Sex, male, N/total N (%) | 303/383 (79.1) | 621/761 (81.6) | 105/155 (67.7)a b | 1029/1299 (79.2) |
| Age, median (IQR), y | 51.6 (48.3–54.6) | 51.9 (48.9–55.1) | 52.4 (49.3–55.2) | 51.9 (48.9–55) |
| White race, N/total N (%) | 362/364 (99.5) | 712/717 (99.3) | 149/149 (100) | 1223/1230 (99.4) |
| BMI, median (IQR), kg/m2 | 23.6 (20.9–26.8) | 24.1 (21.3–26.7) | 23.9 (21.9–27.1) | 24 (21.2–26.8) |
| HIV data | ||||
| HIV acquired by IDU, N/total N (%) | 328/375 (87.5) | 668/742 (90) | 132/152 (86.8) | 1128/1269 (88.9) |
| Prior AIDS defining conditions, N/total N (%) | 152/369 (41.2) | 296/740 (40) | 61/152 (40.1) | 509/1261 (40.4) |
| Antiretroviral therapy, N/total N (%) | 375/384 (97.7) | 744/761 (97.8) | 154/155 (99.4) | 1273/1300 (97.9) |
| CD4+ T-cell count, median (IQR) | 613 (406–827) | 518 (319–729)a | 356 (209–573)a b | 525 (324–742) |
| HIV RNA <50 copies/mL, N/total N (%) | 318/334 (95.2) | 631/681 (92.7) | 136/139 (97.8) | 1085/1154 (94.0) |
| Liver disease data | ||||
| HBsAg positive, N/total N (%) | 9/340 (2.6) | 9/684 (1.3) | 3/144 (2.1) | 21/1168 (1.8) |
| HCV-RNA genotypes, N/total N (%) | 384/384 (100) | 759/761 (99.9) | 155/155 (100) | 1298/1300 (99.8) |
| 1a | 165 (43.0) | 301 (39.7) | 63 (40.6) | 529 (40.8) |
| 1b | 54 (14.1) | 130 (17.1) | 23 (14.8) | 207 (15.9) |
| 1 non-subtyped | 9 (2.3) | 16 (2.1) | 6 (3.9) | 31 (2.4) |
| 2 | 3 (0.8) | 6 (0.8) | 2 (1.3) | 11 (0.8) |
| 3 | 66 (17.2) | 151 (19.9) | 27 (17.4) | 244 (18.8) |
| 4 | 80 (20.8) | 145 (19.1) | 30 (19.4) | 255 (19.6) |
| Mixed | 7 (1.8) | 10 (1.3) | 4 (2.6) | 21 (1.6) |
| Prior liver transplantation, N/with data (%) | 2/384 (0.5) | 5/758 (0.7) | 4/155 (2.6) | 11/1297 (0.8) |
| Naïve for anti-HCV treatment, N/with data (%) | 238/384 (62.0) | 412/761 (54.1)a | 67/155 (43.2)a b | 717/1300 (55.2) |
| Anti-HCV treatment regimen, N/total N (%) | ||||
| Sofosbuvir/ledipasvir | 225/384 (58.6) | 491/761 (64.5) | 67/155 (43.2)a b | 783/1300 (60.2) |
| Sofosbuvir and daclatasvir | 63/384 (16.4) | 112/761 (14.7) | 49/155 (31.6)a b | 224/1300 (17.2) |
| Ombitasvir/paritaprevir/ritonavir and dasabuvir | 59/384 (15.4) | 86/761 (11.3) | 2/155 (1.3)a b | 147/1300 (11.3) |
| Other direct-acting antivirals | 37/384 (9.6) | 72/761 (9.5) | 37/155 (23.9)a b | 146/1300 (11.2) |
| Liver stiffness, median (IQR), kPa | 10.7 (10.1–11.8) | 19.5 (14.6–28.4)a | 28 (19 – 45.0)a b | 15.8 (11.8–26.0) |
| Fibrosis-4 index (FIB4), median (IQR) | 1.90 (1.36–2.69) | 3.22 (2.05–5.64)a | 5.52 (3.27–8.81)a b | 2.84 (1.83–5.13) |
| Triglyceride and glucose index (TyG), median (IQR) | 4.66 (4.50–4.87) | 4.70 (4.53–4.89) | 4.64 (4.45–4.83) | 4.68 (4.52–4.87) |
| Hepatic steatosis index (HSI), median (IQR) | 34 (30.3–39.1) | 33.7 (29.4–36.9) | 31.4 (28.7–36.2) | 33.5 (29.4–37.4) |
| Current substance use | ||||
| Smoking, N/with data (%) | 245/348 (70.4) | 522/724 (72.1) | 100/141 (70.9) | 867/1213 (71.5) |
| Alcohol abuse, N/with data (%) | 36/299 (12.0) | 108/651 (16.6) | 14/138 (10.1) | 158/1088 (14.5) |
| Methadone use, N/with data (%) | 73/363 (20.1) | 162/745 (21.7) | 35/155 (22.6) | 270/1263 (21.4) |
| Comorbid conditions, N/with data (%) | ||||
| Arterial hypertension | 81/382 (21.2) | 186/756 (24.6) | 34/154 (22.1) | 301/1292 (23.3) |
| Hyperlipidemia | 72/380 (18.9) | 138/751 (18.4) | 24/155 (15.5) | 234/1286 (18.2) |
| Metabolic syndrome | 56/384 (14.6) | 139/761 (18.3) | 27/155 (17.4) | 222/1300 (17.1) |
| Diabetes mellitus | 32/383 (8.4) | 111/757 (14.7)a | 31/155 (20.0)a | 174/1295 (13.4) |
| Obesity | 23/250 (9.2) | 49/522 (9.4) | 17/111 (15.3) | 89/883 (10.1) |
| Bone fractures or avascular necrosis | 37/380 (9.7) | 58/744 (7.8) | 20/151 (13.2) | 115/1275 (9.0) |
| Ischemic cardiovascular diseasec | 25/382 (6.5) | 52/751 (6.9) | 7/154 (4.5) | 84/1287 (6.5) |
| Chronic kidney disease | 24/381 (6.3) | 43/755 (5.7) | 17/155 (11.0) | 84/1291 (6.5) |
| NLR-NAR cancer (active) | 11/378 (2.9) | 36/737 (4.9) | 9/154 (5.8) | 56/1269 (4.4) |
| Heart failure | 5/379 (1.3) | 13/749 (1.7) | 5/154 (3.2) | 23/1282 (1.8) |
| Laboratory results, median (IQR) | ||||
| Platelet count×109 per liter | 168 (130–216) | 128.5 (88–172)a | 80 (55–114)a b | 136 (92–182) |
| ALT, IU/L | 55 (36–90) | 66 (42–105)a | 42 (29–74)a b | 61 (37–97) |
| AST, IU/L | 47.5 (33–67) | 66 (43–102)a | 58 (36–92)a | 59 (38–92) |
| Albumin, g/dL | 4.3 (4.1–4.5) | 4.2 (3.8–4.41)a | 3.7 (3.3–4.1)a b | 4.2 (3.8–4.5) |
| Total bilirubin, mg/dL | 0.6 (0.4–0.8) | 0.8 (0.56–1.16)a | 1.0 (0.7–1.7)a b | 0.7 (0.5–1.1) |
| INR | 1.00 (0.98–1.06) | 1.06 (1–1.14)a | 1.17 (1.09–1.28)a b | 1.05 (1–1.13) |
| Creatinine, mg/dL | 0.87 (0.76–1.01) | 0.85 (0.74–0.98) | 0.84 (0.71–1.02) | 0.85 (0.75–1) |
| Triglycerides, mg/dL | 117 (88–171) | 122 (90–167) | 103 (75–152)a b | 118 (88–167) |
| Total cholesterol, mg/dL | 164 (138–189) | 158 (134–181) | 144.5 (121–176)a b | 159 (133–183) |
| LDL cholesterol, mg/dL | 91 (70–113) | 86 (64.4–107) | 79.5 (56–106.5)a | 87 (65–109) |
| HDL cholesterol, mg/dL | 43 (35–54) | 42 (33–54.9) | 43.4 (32.25–55) | 42.3 (34–54.9) |
Statistically significant differences with F3.
Statistically significant differences with F4 comp.
Myocardial infarction, angina, cerebrovascular disease, and peripheral artery ischemic disease.
Abbreviations: CD4, cluster of differentiation 4; NLR-NAR, non-liver-related non-AIDS-related.
Statistically significant differences between the groups were found for age, CD4+ T-cell count, history of prior anti-HCV treatment, DAA regimens employed, liver stiffness and FIB-4 values, diabetes mellitus, and most laboratory parameters analyzed.
The median follow-up time after the completion of DAA therapy was 40.9 (34.5–45.1) months. Eighty-nine patients were lost to follow-up for an incidence rate of 2.16 (95% CI: 1.76–2.66) per 100-py (Table 2).
TABLE 2.
Frequency and incidence rate of losses to follow-up and events in 1300 patients coinfected with HIV/HCV with advanced fibrosis and cirrhosis and sustained viral response with all-oral direct-acting antivirals
| Event by liver-disease categories | Number at risk | Person-years of follow-up | Number of events | Incidence rate of events per 100 PY (95% CI) |
|---|---|---|---|---|
| Lost to follow-up | ||||
| All patients | 1300 | 4121 | 89 | 2.16 (1.76–2.66) |
| Advanced fibrosis | 384 | 1190 | 28 | 2.35 (1.62–3.41) |
| Compensated cirrhosis | 761 | 2439 | 55 | 2.26 (1.73–2.94) |
| Decompensated cirrhosis | 155 | 492 | 6 | 1.22 (0.55–2.72) |
| Mortality | ||||
| Overall mortality | ||||
| All patients | 1300 | 4121 | 85 | 2.06 (1.67–2.55) |
| Advanced fibrosis | 384 | 1190 | 13 | 1.09 (0.63–1.88) |
| Compensated cirrhosis | 761 | 2439 | 47 | 1.93 (1.45–2.57) |
| Decompensated cirrhosis | 155 | 492 | 25 | 5.08 (3.43–7.52) |
| Non-liver non-AIDS–related mortality | ||||
| All patients | 1300 | 4121 | 61 | 1.48 (1.15–1.90) |
| Advanced fibrosis | 384 | 1190 | 12 | 1.00 (0.57–1.78) |
| Compensated cirrhosis | 761 | 2439 | 34 | 1.39 (0.99–1.95) |
| Decompensated cirrhosis | 155 | 492 | 15 | 3.05 (1.84–5.06) |
| Liver-related mortality | ||||
| All patients | 1300 | 4121 | 24 | 0.58 (0.39–0.87) |
| Advanced fibrosis | 384 | 1190 | 1 | 0.08 (0.01–0.60) |
| Compensated cirrhosis | 761 | 2439 | 13 | 0.53 (0.31–0.92) |
| Decompensated cirrhosis | 155 | 492 | 10 | 2.03 (1.09–3.78) |
| AIDS-related mortality | ||||
| All patients | 1300 | 4121 | 0 | – |
| Liver-related events | ||||
| Liver decompensation | ||||
| All patients | 1266 | 3996 | 61 | 1.53 (1.19–1.96) |
| Advanced fibrosis | 374 | 1181 | 0 | – |
| Compensated cirrhosis | 737 | 2373 | 24 | 1.01 (0.68–1.51) |
| Decompensated cirrhosis | 155 | 443 | 37 | 8.35 (6.05–11.53) |
| HCC | ||||
| All patients | 1236 | 3967 | 30 | 0.76 (0.53–1.08) |
| Advanced fibrosis | 372 | 1171 | 4 | 0.34 (0.13–0.91) |
| Compensated cirrhosis | 721 | 2328 | 17 | 0.73 (0.45–1.18) |
| Decompensated cirrhosis | 143 | 468 | 9 | 1.92 (1.00–3.70) |
| AIDS-defining eventsa | ||||
| All patients | 1251 | 4040 | 12 | 0.30 (1.17–0.52) |
| Advanced fibrosis | 372 | 1168 | 2 | 0.17 (0.04–0.69) |
| Compensated cirrhosis | 732 | 2387 | 7 | 0.29 (0.14–0.62) |
| Decompensated cirrhosis | 147 | 485 | 3 | 0.62 (0.20–1.92) |
| Non-liver non-AIDS–related eventsa | ||||
| Ischemic cardiovascular event | ||||
| All patients | 1265 | 4003 | 44 | 1.10 (0.82–1.48) |
| Advanced fibrosis | 376 | 1167 | 6 | 0.51 (0.23–1.15) |
| Compensated cirrhosis | 740 | 2364 | 30 | 1.27 (0.89–1.82) |
| Decompensated cirrhosis | 149 | 473 | 8 | 1.69 (0.85–3.38) |
| Heart failure | ||||
| All patients | 1236 | 3987 | 14 | 0.35 (0.21–0.59) |
| Advanced fibrosis | 370 | 1165 | 3 | 0.26 (0.08–0.80) |
| Compensated cirrhosis | 723 | 2357 | 5 | 0.21 (0.09–0.51) |
| Decompensated cirrhosis | 143 | 465 | 6 | 1.29 (0.58–2.88) |
| Chronic renal failure | ||||
| All patients | 1181 | 3776 | 27 | 0.72 (0.49–1.04) |
| Advanced fibrosis | 350 | 1097 | 7 | 0.64 (0.30–1.34) |
| Compensated cirrhosis | 699 | 2247 | 18 | 0.80 (0.51–1.27) |
| Decompensated cirrhosis | 132 | 431 | 2 | 0.46 (0.12–1.85) |
| Bone event | ||||
| All patients | 1124 | 3599 | 32 | 0.89 (0.63–1.26) |
| Advanced fibrosis | 330 | 1039 | 6 | 0.58 (0.26–1.29) |
| Compensated cirrhosis | 666 | 2146 | 21 | 0.98 (0.64–1.50) |
| Decompensated cirrhosis | 128 | 415 | 5 | 1.21 (0.50–2.90) |
| Diabetes mellitus | ||||
| All patients | 1092 | 3476 | 41 | 1.18 (0.87–1.60) |
| Advanced fibrosis | 343 | 1077 | 7 | 0.65 (0.31–1.36) |
| Compensated cirrhosis | 630 | 2018 | 28 | 1.39 (0.96–2.01) |
| Decompensated cirrhosis | 119 | 382 | 6 | 1.57 (0.71–3.50) |
| Non-liver non-AIDS–related cancer | ||||
| All patients | 1202 | 3841 | 49 | 1.28 (0.96–1.69) |
| Advanced fibrosis | 365 | 1154 | 10 | 0.87 (0.47–1.61) |
| Compensated cirrhosis | 698 | 2235 | 34 | 1.52 (1.09–2.13) |
| Decompensated cirrhosis | 139 | 452 | 5 | 1.11 (0.46–2.66) |
When assessing new AIDS-related events, those with prior AIDS were excluded from the analysis. When assessing non-liver non-AIDS–related events, those with a history of the event at baseline were excluded from the population at risk when considering the incidence of events.
Mortality
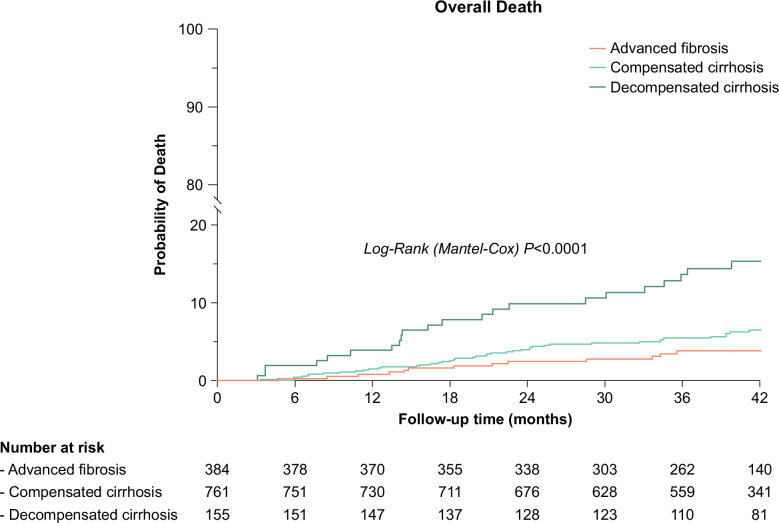
Overall, 85 patients died during the study period, 61 due to non-liver non-AIDS–related events, and 24 due to a liver-related event, with no AIDS-related deaths identified in the entire cohort. Non-liver non-AIDS–related deaths outnumbered liver-related deaths across all liver disease stages (Table 2). The relative risk of overall, liver-related, and non-liver non-AIDS–related mortality was significantly higher for decompensated cirrhosis versus advanced fibrosis and decompensated versus compensated cirrhosis. However, no statistically significant differences in relative risk or overall and cause-specific mortality were found between compensated cirrhosis and advanced fibrosis (Supplemental Table S2, http://links.lww.com/HEP/I336). Kaplan-Meier and cumulative incidence plots of overall death over 3½ years of follow-up are shown in Figure 1.
FIGURE 1.
Kaplan-Meier plots of overall death over 3½ years of follow-up among 1300 patients coinfected with HIV/HCV with advanced fibrosis or cirrhosis following DAA-induced SVR. Abbreviations: DAA, direct-acting antiviral; SVR, sustained viral response.
Clinical events and prognostic factors in patients with cACLD
Table 2 shows the number of clinical events and their corresponding incidence rates. Notably, no instances of liver decompensation were observed in patients with advanced fibrosis, while 24 patients (3.3%) with compensated cirrhosis experienced decompensation, resulting in an incidence rate of 1.01 (95% CI: 0.68–1.51) per 100-py. Detailed information regarding the different types of liver-related, AIDS-related, and non-liver non-AIDS–related events in patients with cACLD during follow-up is shown in Supplemental Table S3, http://links.lww.com/HEP/I336.
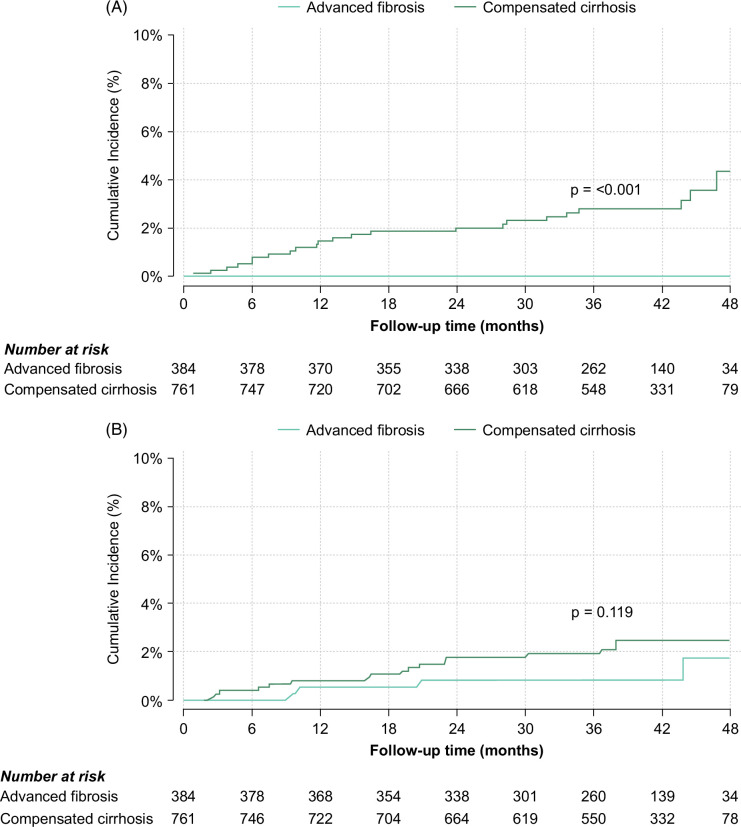
The median time to decompensation following the completion of therapy among patients with compensated cirrhosis was 13.9 (6.7–32.8) months. The cumulative incidence plots of liver decompensation in patients with compensated cirrhosis and advanced fibrosis are shown in Figure 2A.
FIGURE 2.
Cumulative incidence plots of liver decompensation (A) and HCC (B) for patients with compensated cirrhosis and advanced fibrosis. p values were calculated using the Gray tests.
Newly diagnosed HCC was observed in 4 (0.3%) patients with advanced fibrosis and 17 (2.4%) patients with compensated cirrhosis, resulting in incidence rates of 0.34 (95% CI: 0.13–0.91) and 0.73 (95% CI: 0.45–1.18) per 100-py, respectively. The median time to HCC diagnosis after the end of therapy was 15.6 (IQR: 9.9–32.4) months for the former and 19.3 (IQR: 7.6–23.1) months for the latter. The cumulative incidence plots of HCC over 3½ years of follow-up for patients with compensated cirrhosis and advanced fibrosis are shown in Figure 2B.
The most frequent non-liver non-AIDS–related events during follow-up among the patients with cACLD were non-liver non-AIDS–related cancer, ischemic cardiovascular events, and diabetes mellitus. The relative risks of clinical events in patients with compensated cirrhosis and advanced fibrosis are shown in Table 3. No statistically significant differences were observed between the 2 groups regarding the risk of HCC, new AIDS-defining events, or most non-liver non-AIDS–related events. However, a notable exception was ischemic cardiovascular events, where individuals with compensated cirrhosis exhibited a 2.47-fold higher relative risk (95% CI: 1.03–5.93) compared to those with advanced fibrosis.
TABLE 3.
Incidence rate ratio of clinical events according to the severity of liver disease among 1145 patients coinfected with HIV/HCV with advanced fibrosis or compensated cirrhosis and sustained viral response with direct-acting antivirals (Poisson regression)
| Compensated cirrhosis vs. advanced fibrosis incidence rate ratio (95% CI) | |
|---|---|
| Liver-related events | |
| Liver decompensation | NA |
| HCC | 2.14 (0.72–6.35) |
| New AIDS-defining event | 1.71 (0.36–8.25) |
| Non-liver–related non-AIDS–related events | |
| Ischemic cardiovascular event | 2.47 (1.03–5.93) |
| Heart failure | 0.82 (0.20–3.45) |
| Chronic renal failure | 1.26 (0.52–3.01) |
| Bone events | 1.69 (0.68–4.20) |
| Diabetes mellitus | 2.13 (0.93–4.89) |
| Non-liver non-AIDS–related cancer | 1.75 (0.87–3.55) |
Abbreviation: NA, not assessable due to the absence of events among patients with advanced fibrosis.
Baseline factors associated with decompensation in patients with cACLD are summarized in Table 4. Serum albumin concentration (adjusted subhazard ratio [aSHR] [95% CI]: 0.51 [0.28–0.94] per g/L increase) and LSM (aSHR [95% CI]: 1.05 [1.03–1.07] per kPa increase), and FIB-4 (aSHR [95% CI]: 1.04 [1.00–1.09] per unit increase) were independently associated with decompensation risk.
TABLE 4.
Multivariable Fine-Grey regression analysis evaluating baseline factors associated with liver decompensation and with HCC among 1145 patients coinfected with HIV/HCV with compensated advanced chronic liver disease and SVR following all oral DAA therapy
| Liver decompensationa | HCCb | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Nc | Adjusted sHR | 95% CI | p | Adjusted sHR | 95% CI | p |
| Liver-disease stagesd | 1145 | ||||||
| Advanced fibrosis | 384 | — | Reference | ||||
| Compensated cirrhosis | 761 | — | — | — | 1.43 | 0.45–4.55 | 0.539 |
| Demographics and clinical variables | |||||||
| Sex, malee | 1144 | 2.20 | 0.41–11.69 | 0.361 | — | — | — |
| Age (per 10 years) | 1145 | 0.71 | 0.32–1.55 | 0.387 | 1.52 | 0.81–2.85 | 0.187 |
| High intake of alcohol | 950 | 0.99 | 0.30–3.30 | 0.984 | 0.71 | 0.16–3.19 | 0.655 |
| Metabolic syndrome | 1145 | 0.40 | 0.10–1.59 | 0.192 | 0.49 | 0.11–2.15 | 0.344 |
| Laboratory parameters and noninvasive indexes | |||||||
| Serum albumin (per mg/dL) | 1057 | 0.51 | 0.28–0.94 | 0.030 | 0.56 | 0.36–0.87 | 0.009 |
| CD4+ cell count (per 100 cells/mm3) | 1143 | 0.95 | 0.80–1.13 | 0.546 | 0.95 | 0.81–1.11 | 0.533 |
| Liver stiffness measurement (per kPa) | 1132 | 1.05 | 1.03–1.07 | <0.001 | 1.01 | 0.98–1.03 | 0.515 |
| FIB-4 index (per unit) | 1064 | 1.04 | 1.00–1.09 | 0.043 | 1.03 | 0.97–1.09 | 0.354 |
| TyG index (per unit) | 1087 | 1.42 | 0.19–10.48 | 0.729 | 1.01 | 0.16–6.25 | 0.991 |
A total of 24 patients experienced decompensation during follow-up.
A total of 21 patients developed HCC during follow-up.
Number of patients with the variable available; values were imputed when the variable was unavailable.
sHR of decompensation could not be estimated due to the absence of cases of decompensation among patients with advanced fibrosis.
sHR of HCC for sex could not be determined due to the absence of cases among females.
Abbreviations: CD4, cluster of differentiation 4; DAA, direct-acting antivirals; FIB-4, fibrosis 4; sHR, sub hazard ratio; SVR, sustained viral response; TyG, triglyceride and glucose index.
The median (IQR) LSM for patients without decompensation or competitive events (N=1045), those with a competitive event (N=63), and those with decompensation (N=24) were 14.4 (11.5–22.0), 17.1 (12.2–27.0), and 36.9 (23.2–64.5) kPa, respectively. The utility of LSM as a predictive tool for decompensation over 42 weeks after therapy assessed using t-ROC curves, and accounting for competing risks is shown in Supplemental Table S4, http://links.lww.com/HEP/I336 and Supplemental Figure S1, http://links.lww.com/HEP/I336. In brief, the areas under the t-ROC curves for LSM predicting decompensation at 12, 24, and 42 months were 82.7%, 85.2%, and 84.0%, respectively, with a cutoff of 13.5 kPa exhibiting a 100% negative predictive value at different time points up to 42 months of follow-up (Supplemental Table S5, http://links.lww.com/HEP/I336).
The risk of decompensation assessed 1 year after therapy using the posttreatment LSM/platelet count criterion developed by Semmler and colleagues among 631 patients is summarized in Table 5. The incidence rate of decompensation per 100-py was null among the 206 patients who were categorized as low risk (LSM <12 kPa and platelet count >150×109 platelets/L). For patients in the gray zone or at high risk (LSM >25 kPa), the incidence rates of decompensation were 0.36 (0.12–1.13) and 1.70 (0.55–5.28) per 100-py, respectively.
TABLE 5.
Risk of decompensation and risk of developing HCC according to posttreatment risk stratification models assessed 1 year after the finalization of DAA therapy among patients with cACLD
| Posttreatment risk stratification indexesa | Patients at risk | Person-years of follow-up | Patients with competitive events | Patients with event | Incidence of event 100-PY (95% CI) |
|---|---|---|---|---|---|
| Liver decompensation | |||||
| LSM/platelet count (Semmler et al29) | |||||
| LSM<12 kPa and PLT>150×109/L | 206 | 455.0 | 4 | 0 | 0 |
| Gray-zone | 349 | 825.3 | 12 | 3 | 0.36 (0.12–1.13) |
| LSM >25 kPa | 76 | 176.3 | 5 | 3 | 1.70 (0.55–5.28) |
| Total | 631 | 1456.6 | 21 | 6 | 0.41 (0.19–0.92) |
| Hepatocellular carcinoma | |||||
| LSM/albumin (Pons et al30) | |||||
| Low risk | 441 | 1006.8 | 8 | 2 | 0.20 (0.05–0.79) |
| High risk | 178 | 421.5 | 5 | 6 | 1.42 (0.64–3.17) |
| Total | 619 | 1428.3 | 13 | 8 | 0.56 (0.28–1.12) |
| Age/alcohol/LSM/albumin (Semmler et al31) | |||||
| Low risk | 539 | 1235.8 | 10 | 5 | 0.40 (0.17–0.97) |
| High risk | 80 | 192.5 | 3 | 3 | 1.56 (0.50–4.83) |
| Total | 619 | 1428.3 | 13 | 8 | 0.56 (0.28–1.12) |
| LSM/1yDelta-LSM/albumin (Alonso Lopez et al32) | |||||
| Low risk | 182 | 411.5 | 3 | 0 | 0 |
| Intermediate risk | 215 | 487.6 | 4 | 1 | 0.21 (0.03–1.46) |
| High risk | 149 | 356.6 | 3 | 4 | 1.12 (0.42–2.99) |
| Very high risk | 72 | 171.9 | 0 | 3 | 1.74 (0.56–5.41) |
| Total | 618 | 1427.6 | 10 | 8 | 0.56 (0.28–1.12) |
A detailed description of the calculation of the posttreatment LSM-based risk stratification models is provided in Supplemental Table S1, http://links.lww.com/HEP/I336.
Abbreviations: 1yDelta-LSM, percentage of reduction in LSM at 1 year posttreatment; cACLD, compensated advanced chronic liver disease; DAA, direct-acting antivirals; LSM, liver stiffness measurement; PY, patient-years.
Serum albumin concentration (aSHR [95% CI]: 0.56 [0.36–0.87] per g/L increase) was the sole factor independently associated with HCC risk (Table 6). The risk of developing HCC, assessed 1 year after therapy using 3 post-SVR HCC risk stratification models, is summarized in Table 5. The incidence rates of HCC (95% CI) per 100-py of follow-up among patients in the low-risk category in the models by Pons et al,30 Semmler et al,31 and Alonso Lopez et al32 were 0.20 (0.05–0.79), 0.40 (0.17–0.97), and 0, respectively.
TABLE 6.
Multivariable Fine-Grey regression analysis evaluating factors associated with further liver decompensation in 155 HIV/HCV-coinfected patients with decompensated cirrhosis and SVR following all oral DAA therapy
| Further liver decompensationa | ||||
|---|---|---|---|---|
| Variable | Nb | SHR | 95% CI | p |
| Type of previous decompensation | 155 | |||
| Ascites (only) | 77 | Reference | — | — |
| Hemorrhage (with or without ascites) | 30 | 1.73 | 0.80–3.74 | 0.166 |
| Portosystemic encephalopathy | 48 | 0.79 | 0.33–1.84 | 0.579 |
| Sex, male | 155 | 2.95 | 1.24–7.03 | 0.015 |
| Age (y) | 155 | 1.32 | 0.69–2.54 | 0.400 |
| Serum albumin (g/dL) | 146 | 0.77 | 0.42–1.38 | 0.376 |
| MELD score | 136 | 1.03 | 0.94–1.14 | 0.503 |
| Platelets (per 104/μL) | 154 | 1.04 | 0.96–1.13 | 0.285 |
A total of 37 patients experienced further decompensation during follow-up.
Number of patients with the variable available; values were imputed when the variable was unavailable.
Abbreviations: DAA, direct-acting antiviral; SVR, sustained viral response.
Clinical events and prognostic factors in patients with decompensated cirrhosis
Before the initiation of DAA therapy, 219 episodes of liver decompensation occurred among 155 patients with decompensated cirrhosis, including ascites (N=128), portosystemic encephalopathy (N=48), and variceal bleeding (N=43). Detailed information about the different types of liver-related, AIDS-related, and non-liver non-AIDS–related events in patients with decompensated cirrhosis during follow-up are shown in Supplemental Table S3, http://links.lww.com/HEP/I336. During follow-up, 37 patients (23.9%) developed 48 new episodes indicating further decompensation, including ascites (N=20), variceal bleeding (N=14), and portosystemic encephalopathy (N=14). In addition, 9 patients (6.3%) were diagnosed with de novo HCC. As shown in Table 2, the incidence rates of further decompensation and HCC for patients with decompensated cirrhosis were 8.35 (95% CI: 6.05–11.53) and 1.92 (95% CI: 1.00–3.70) per 100-py. Table 2 also shows the frequency and incidence rates of AIDS-defining and non-liver non-AIDS–related events among patients with decompensated cirrhosis.
The prognostic factors of further liver decompensation in patients with decompensated liver disease are shown in Table 6. Male sex (aSHR [95% CI]: 2.95 [1.24–7.03]) was the only factor independently associated with further decompensation.
DISCUSSION
This study provides a comprehensive analysis of mortality, clinical events, and prognostic factors in a cohort of 1300 patients coinfected with HIV/HCV with advanced fibrosis or cirrhosis over a median follow-up of almost 3½ years after HCV clearance following all oral DAA therapy. Non-liver–related and non-AIDS–related conditions were the leading cause of death across all stages of liver disease. Among patients with cACLD, baseline LSM and LSM-based posttreatment risk stratification models showed predictive abilities. Among patients with decompensated cirrhosis, the risk of further decompensation remains high despite HCV clearance.
The liver-related mortality and HCC rates observed in individuals with cACLD in our cohort were comparable to those reported after HCV clearance with DAAs in patients without HIV who had similar liver disease stages and follow-up durations.33,34 This suggests that the long-term benefits of successful DAA therapy in altering the natural course of liver disease among individuals with chronic hepatitis C and cACLD are not compromised by well-controlled HIV infection. Notably, there were no AIDS-related deaths in our study population, most of whom were on antiretroviral therapy and had fully suppressed HIV viremia.
Furthermore, we found that non-liver–related and non-AIDS–related mortality exceeded liver-related mortality across all liver disease stages. This observation aligns with other reports assessing the impact of DAAs on mortality in individuals with and without HIV,35 highlighting the influence of concurrent health risk behaviors and comorbidities on mortality in patients with chronic hepatitis C.36 In our cohort, all-cause mortality rates were higher among patients with cACLD than among those described after HCV clearance with DAAs in patients without HIV who had similar liver disease stages.33 This discrepancy must be considered in the context of a higher burden of aging-related comorbidities, coinfections, and substance use disorders among people living with HIV than among age-matched and sex-matched individuals without HIV.37 Notably, cancer, ischemic cardiovascular events, and diabetes mellitus were the most frequently observed non-liver, non-AIDS–related events in our cohort, with no significant differences in the incidence rate ratio between patients with advanced fibrosis and those with compensated cirrhosis, except for ischemic cardiovascular events, which exhibited a significantly higher risk in patients with compensated cirrhosis than in those with advanced fibrosis. This finding should be considered taking into account that the prevalence of diabetes mellitus, a common comorbidity among patients with cirrhosis38 and a recognized risk factor for cardiovascular disease, was significantly higher among patients with compensated cirrhosis than among those with advanced fibrosis.
Significantly, we did not observe substantial differences in the overall or cause-specific mortality risk between patients with advanced fibrosis and those with compensated cirrhosis. However, the risk of HCC was somewhat higher among individuals with cirrhosis than among those with advanced fibrosis, with the latter group experiencing no liver decompensation during the follow-up.
Serum albumin concentration, LSM, and FIB-4 index emerged as the only factors independently associated with the risk of decompensation among patients with cACLD. No significant associations with decompensation were found for demographics, CD4+ cell counts, high alcohol intake, or metabolic-related variables. However, it should be noted that our cohort displayed a high degree of homogeneity regarding these variables, as patients were predominantly males in their late forties and early fifties, receiving antiretroviral therapy with full suppression of HIV viremia, high CD4+ T cell counts, and low prevalence of harmful alcohol consumption and obesity.
To assess the performance of LSM before the initiation of DAA therapy in predicting the risk of decompensation among patients with cACLD, we used t-ROC curves for censored event times, accounting for competing risks. Our results demonstrated good discrimination of pre-therapy LSM for this purpose, with a consistent 100% negative predictive value over 3½ of follow-up for a cutoff value of 13.5 kPa. In line with the growing body of evidence supporting the utility of LSM-based noninvasive indexes in prognosticating patients with cACLD following HCV eradication with DAAs, we evaluated the index developed by Semmler et al.29 This index, utilizing LSM and platelet count at the time of SVR, aimed to predict the likelihood of clinically significant portal hypertension and the risk of decompensation after HCV cure with DAA therapy. Our assessment of 631 patients, with both parameters recorded 1 year after completing therapy, showed that none of those categorized as low risk (LSM <12 kPa and platelet count >150×10^9/L) experienced decompensation during follow-up. This supports the current recommendation that patients who are at low-risk, in the absence of cofactors, may be exempted from ongoing surveillance for portal hypertension, including LSM and endoscopy.22
During the follow-up period, we documented 4 incident cases of HCC in patients with advanced fibrosis and 17 in those with compensated cirrhosis, yielding incidence rates of 0.37 and 0.73 events per 100-py, respectively. A recent systematic review and meta-analysis reported HCC incidence rates following HCV cure with DAAs of 0.63 per 100-py for patients with advanced fibrosis and 2.99 per 100-py for patients with patients with cirrhosis (without differentiation between compensated and decompensated disease), with values ranging from 0.32 to 11.47 across the 31 individual studies analyzed.33 In our cohort, the upper limits of the CI for the incidence rate of HCC in patients with advanced fibrosis and compensated cirrhosis were 0.91 and 1.18 per 100-py, respectively. Incidence rates below the threshold of >1.32 per 100-py were proposed for cost-effective HCC screening in patients with DAA-cured HCV by employing current screening methodologies.34
Among the pretreatment variables, serum albumin concentration was found to be independently associated with HCC risk. In addition, we assessed 3 LSM-based post-SVR HCC risk stratification models in a group of 618/619 patients with LSM determined 1 year after the end of treatment and free of HCC at the same time point.30–32 These selection criteria were adopted, as 2 of the mentioned studies30,32 did, to preclude including patients with malignant transformation before clinical recognition. All 3 indices could categorize patients according to their risk of developing HCC and identify individuals with incidence rates above the cost-effective HCC screening threshold. Notably, the model developed by Alonso-Lopez et al32 showed a lower incidence of HCC in the low-risk group (0 per 100-py), suggesting a potentially better ability to rule out HCC during follow-up in this population.
The advent of all-oral DAA therapy has enabled HCV cure in patients with HCV-related decompensated liver disease, both with and without HIV.5,10,39,40 However, it is worth noting that the success rates are slightly lower in individuals with decompensated liver disease than in those with compensated disease.10,40 Although DAA-induced viral clearance in patients with HCV-related decompensated cirrhosis has been associated with short-term improvements in liver function,39,40 it has not been linked to reduced disease progression, even in patients with sustained improvements in liver function, as demonstrated in a large multinational observational study.4 Among the 115 patients with decompensated cirrhosis in our cohort, nearly a quarter experienced further episodes of decompensation despite HCV clearance. These findings are concordant with the observations that in patients with HCV-related decompensated cirrhosis, the disappearance of clinically significant portal hypertension is rarely, if ever, achieved17,41 and underscores that they remain at a high risk of experiencing further decompensation or developing HCC, which may ultimately necessitate liver transplantation.
Our study had several limitations and strengths worth noting. The main limitation stems from the retrospective evaluation of the mortality and clinical events. However, the patient’s scheduled follow-up in the participating centers may have lessened their relevance. The study was also limited by missing observations, which were partially mitigated by implementing a strategy for imputing missing values. A major limitation, shared with many past studies, is the limited follow-up duration (median 3½ years), which hinders the assessment of the long-term risk of HCC development after SVR with DAAs, which may be observed beyond 5 years. A significant strength of our study was the large cohort of participants with comprehensive and prospectively captured baseline information. All patients were treated within a relatively short timeframe, decreasing the potential impact of changes in clinical management practices over time. Finally, interim and final independent monitoring was carried out, to ensure the reliability of our data set.
This study has important practical implications. First, it highlights the critical role of age-related comorbidities among patients coinfected with HIV/HCV with cACLD as they emerge as the principal determinants of morbidity and mortality after DAA treatment. In addition, our study provides valuable insights into the prognostication and risk stratification of decompensation and HCC in patients with cACLD following HCV eradication with DAAs. Furthermore, it supports the use of LSM-based noninvasive indexes to assess risk and guide targeted surveillance strategies. Finally, our study reaffirms that patients with HCV-related decompensated cirrhosis continue to face a substantial risk of further decompensation or HCC despite achieving HCV clearance. Although our study sheds crucial light on these critical aspects, there are still critical knowledge gaps, particularly in identifying specific subgroups of PWH and cACLD who may remain at negligible risk of developing HCC and can safely forgo HCC screening. This question demands further research to better guide clinical decision-making and improve outcomes for PWH with cACLD.
Supplementary Material
AUTHOR CONTRIBUTIONS
Juan Berenguer and Juan González-García conceptualized and designed the study with later input from Rafael Bañares. Teresa Aldámiz-Echevarría, Víctor Hontañón, Chiara Fanciulli, Carmen Quereda, Carmen Busca, Lourdes Domínguez, Cristina Hernández, Jorge Vergas, Gabriel Gaspar, Lucio J. García-Fraile, and Cristina Díez contributed substantially to data acquisition. José M. Bellón and Marta De Miguel curated the databases and managed the global data collection. José M. Bellón, Juan Berenguer, Rafael Bañares, and Juan González-García analyzed and interpreted the data. Juan Berenguer drafted the manuscript and all authors critically reviewed the manuscript. All authors were responsible for the final decision to submit for publication and have seen and approved the manuscript.
ACKNOWLEDGMENTS
The authors are indebted to the patients who participated in this research. Several centers participating in this study are supported by CIBER—Consorcio Centro de Investigación Biomédica en Red (grant numbers: CB21/13/00039, CB21/13/00044, CB21/13/00086, CB21/13/00091, CB21/13/00126, and CB06/04/0082), Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación and Unión Europea—NextGenerationEU. Chiara Fanciulli is a Rio-Hortega researcher supported and funded by the ISCIII (grant number: CM20/00086). The members of the GeSIDA-10318 Study Group are: Hospital General Universitario Gregorio Marañón, Madrid: Teresa Aldámiz-Echevarría, Chiara Fanciulli, Cristina Díez, L. Pérez-Latorre, F. Tejerina, A. Carrero, P. Miralles, J.C. López, F. Parras, B. Padilla, Gutiérrez, M. Ramírez, S. Carretero, José M Bellón, and Juan Berenguer. Hospital Universitario La Paz, Madrid: V. Hontañón, J.R. Arribas, M.L. Montes, I. Bernardino, J.F. Pascual, F. Zamora, J.M. Peña, F. Arnalich, M. Díaz, and Juan González-García. Hospital Universitari Vall d’Hebron, Barcelona: E. Van den Eynde, M. Pérez, E. Ribera, and M. Crespo. Hospital Universitario Príncipe de Asturias, Alcalá de Henares: A. Arranz, E. Casas, J. de Miguel, S. Schroeder, and J. Sanz. Hospital Donostia, San Sebastián: M.J. Bustinduy, J.A. Iribarren, F. Rodríguez-Arrondo, and M.A. Von-Wichmann. Hospital Universitario de La Princesa, Madrid: J. Sanz and I. Santos. Hospital Clínico San Carlos, Madrid: J. Vergas and M.J. Téllez. Hospital Clínico Universitario, Valencia: A. Ferrer and M.J. Galindo. Hospital Universitario Ramón y Cajal, Madrid: J.L. Casado, F. Dronda, A. Moreno, M.J. Pérez-Elías, M.A. Sanfrutos, S. Moreno, and C. Quereda. Hospital General Universitario, Valencia: L. Ortiz and E. Ortega. Fundación SEIMC-GESIDA, Madrid: M. Yllescas, P. Crespo, E. Aznar, Marta De Miguel, and H. Esteban.
FUNDING INFORMATION
This study was supported by grants from the Instituto de Salud Carlos III (PI17/00657 to Juan Berenguer and PI17/00903 to Juan González-García) and GLD14-00279 from the GILEAD Fellowship Program (Spain) to Juan Berenguer.
CONFLICTS OF INTEREST
Juan Berenguer advises, is on the speakers’ bureau, and received grants from Gilead, MSD, and ViiV Healthcare. He advises and is on the speakers’ bureau for AbbVie, GlaxoSmithKline, and Janssen. Teresa Aldámiz-Echevarría advises and is on the speakers’ bureau for Gilead, Janssen, MSD, and ViiV Healthcare. Chiara Fanciulli is employed by Gilead, Janssen, MSD, and ViiV Healthcare. Jorge Vergas advises and is on the speakers’ bureau for Janssen and ViiV Healthcare. He is on the speakers’ bureau for Gilead. Rafael Bañares advises and is on the speakers’ bureau for AbbVie, Gore, and Gilead. Juan González-García advises, is on the speakers’ bureau, and received grants from Gilead, MSD, and ViiV Healthcare. He advises and is on the speakers’ bureau for Janssen. The remaining authors have no conflicts to report.
Footnotes
Abbreviations: 1-year DeltaLSM, percentage of reduction in LSM at one-year posttreatment; AHA, American Heart Association; aSHR, adjusted subhazard ratio; cACLD, compensated advanced chronic liver disease; CD4, cluster of differentiation 4; CoRIS, Cohort of the Spanish AIDS Research Network; DAA, direct-acting antivirals; EASL, European Association for the Study of the Liver; EORTC, European Organization for Research and Treatment of Cancer; FIB-4, fibrosis 4; GeSIDA, Grupo de Estudio del SIDA (AIDS Study Group); LSM, liver stiffness measurement; Madrid-CoRe, Madrid Coinfection Registry; NHLBI, National Heart, Lung, and Blood Institute; PWH, persons with HIV; py, person-years; SEIMC Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (Spanish Society of Infectious Diseases and Clinical Microbiology); SVR, sustained viral response; t-ROC, time-dependent receiver operating characteristics.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.hepjournal.com.
Contributor Information
Juan Berenguer, Email: jbb4@me.com.
Víctor Hontañón, Email: victor.hontanon@gmail.com.
Chiara Fanciulli, Email: fanciulli.chiara@gmail.com.
Carmen Quereda, Email: cqueredar.hrc@salud.madrid.org.
Carmen Busca, Email: carmen.busca@gmail.com.
Lourdes Domínguez, Email: loudguez@gmail.com.
Cristina Hernández, Email: cris.hg.86@gmail.com.
Jorge Vergas, Email: jorgevergas@me.com.
Gabriel Gaspar, Email: g.gaspar.av@gmail.es.
Lucio J. García-Fraile, Email: lucio.garciafraile@gmail.com.
Cristina Díez, Email: crispu82@gmail.com.
Marta De Miguel, Email: mdemiguel@f-sg.org.
Rafael Bañares, Email: rbanares@ucm.es.
REFERENCES
- 1.Liang TJ, Ghany MG. Therapy of hepatitis C—Back to the future. N Engl J Med. 2014;370:2043–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carrat F, Fontaine H, Dorival C, Simony M, Diallo A, Hezode C, et al. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: A prospective cohort study. Lancet. 2019;393:1453–1464. [DOI] [PubMed] [Google Scholar]
- 3.Backus LI, Belperio PS, Shahoumian TA, Mole LA. Impact of sustained virologic response with direct-acting antiviral treatment on mortality in patients with advanced liver disease. Hepatology. 2019;69:487–497. [DOI] [PubMed] [Google Scholar]
- 4.Krassenburg LAP, Maan R, Ramji A, Manns MP, Cornberg M, Wedemeyer H, et al. Clinical outcomes following DAA therapy in patients with HCV-related cirrhosis depend on disease severity. J Hepatol. 2021;74:1053–1063. [DOI] [PubMed] [Google Scholar]
- 5.Cheung MCM, Walker AJ, Hudson BE, Verma S, McLauchlan J, Mutimer DJ, et al. Outcomes after successful direct-acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;65:741–747. [DOI] [PubMed] [Google Scholar]
- 6.Alavi M, Law MG, Valerio H, Grebely J, Amin J, Hajarizadeh B, et al. Declining hepatitis C virus-related liver disease burden in the direct-acting antiviral therapy era in New South Wales, Australia. J Hepatol. 2019;71:281–288. [DOI] [PubMed] [Google Scholar]
- 7.Belli LS, Perricone G, Adam R, Cortesi PA, Strazzabosco M, Facchetti R, et al. Impact of DAAs on liver transplantation: Major effects on the evolution of indications and results. An ELITA study based on the ELTR registry. J Hepatol. 2018;69:810–817. [DOI] [PubMed] [Google Scholar]
- 8.Flemming JA, Kim WR, Brosgart CL, Terrault NA. Reduction in liver transplant wait-listing in the era of direct-acting antiviral therapy. Hepatology. 2017;65:804–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sikavi C, Chen PH, Lee AD, Saab EG, Choi G, Saab S. Hepatitis C and human immunodeficiency virus coinfection in the era of direct-acting antiviral agents: No longer a difficult-to-treat population. Hepatology. 2018;67:847–857. [DOI] [PubMed] [Google Scholar]
- 10.Berenguer J, Gil-Martin A, Jarrin I, Moreno A, Dominguez L, Montes M, et al. All-oral direct-acting antiviral therapy against hepatitis C virus (HCV) in human immunodeficiency virus/HCV-coinfected subjects in real-world practice: Madrid coinfection registry findings. Hepatology. 2018;68:32–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amele S, Peters L, Rodger A, Lundgren J, Rockstroh J, Matulionyte R, et al. Effectiveness and safety of interferon-free direct-acting antiviral hepatitis C virus therapy in HIV/hepatitis C virus coinfected individuals: Results from a pan-European study. J Acquir Immune Defic Syndr. 2021;86:248–257. [DOI] [PubMed] [Google Scholar]
- 12.Fanciulli C, Berenguer J, Busca C, Vivancos MJ, Tellez MJ, Dominguez L, et al. Epidemiological trends of HIV/HCV coinfection in Spain, 2015-2019. HIV Med. 2022;23:705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berenguer J, Alvarez-Pellicer J, Martin PM, Lopez-Aldeguer J, Von-Wichmann MA, Quereda C, et al. Sustained virological response to interferon plus ribavirin reduces liver-related complications and mortality in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology. 2009;50:407–413. [DOI] [PubMed] [Google Scholar]
- 14.Berenguer J, Rodriguez E, Miralles P, Von Wichmann MA, Lopez-Aldeguer J, Mallolas J, et al. Sustained virological response to interferon plus ribavirin reduces non-liver-related mortality in patients coinfected with HIV and hepatitis C virus. Clin Infect Dis. 2012;55:728–736. [DOI] [PubMed] [Google Scholar]
- 15.Chalouni M, Wittkop L, Bani-Sadr F, Lacombe K, Esterle L, Gilbert C, et al. Risk of severe clinical events after sustained virological response following direct-acting antiviral therapy in HIV and hepatitis C virus coinfected participants. HIV Med. 2021;22:791–804. [DOI] [PubMed] [Google Scholar]
- 16.Carrero A, Berenguer J, Hontanon V, Navarro J, Hernandez-Quero J, Galindo MJ, et al. Effects of eradication of HCV on cardiovascular risk and preclinical atherosclerosis in HIV/HCV-coinfected patients. J Acquir Immune Defic Syndr. 2020;83:292–300. [DOI] [PubMed] [Google Scholar]
- 17.Diez C, Berenguer J, Ibanez-Samaniego L, Llop E, Perez-Latorre L, Catalina MV, et al. Persistence of clinically significant portal hypertension after eradication of hepatitis C virus in patients with advanced cirrhosis. Clin Infect Dis. 2020;71:2726–2729. [DOI] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. [DOI] [PubMed] [Google Scholar]
- 20.Simental-Mendia LE, Rodriguez-Moran M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6:299–304. [DOI] [PubMed] [Google Scholar]
- 21.Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42:503–508. [DOI] [PubMed] [Google Scholar]
- 22.de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C, Baveno VIIF, et al. Renewing consensus in portal hypertension. J Hepatol. 2022;76:959–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Franchis R. on behalf of Baveno VF. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743–752. [DOI] [PubMed] [Google Scholar]
- 24.EASL-EORTC . EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. [DOI] [PubMed] [Google Scholar]
- 25.Berenguer J, Rodriguez-Castellano E, Carrero A, Von Wichmann MA, Montero M, Galindo MJ, et al. Eradication of hepatitis C virus and non-liver-related non-acquired immune deficiency syndrome-related events in human immunodeficiency virus/hepatitis C virus coinfection. Hepatology. 2017;66:344–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. [DOI] [PubMed] [Google Scholar]
- 27.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: A systematic review of 118 studies. J Hepatol. 2006;44:217–231. [DOI] [PubMed] [Google Scholar]
- 28.Blanche P, Dartigues JF, Jacqmin-Gadda H. Estimating and comparing time-dependent areas under receiver operating characteristic curves for censored event times with competing risks. Stat Med. 2013;32:5381–5397. [DOI] [PubMed] [Google Scholar]
- 29.Semmler G, Lens S, Meyer EL, Baiges A, Alvardo-Tapias E, Llop E, et al. Non-invasive tests for clinically significant portal hypertension after HCV cure. J Hepatol. 2022;77:1573–1585. [DOI] [PubMed] [Google Scholar]
- 30.Pons M, Rodriguez-Tajes S, Esteban JI, Marino Z, Vargas V, Lens S, et al. Non-invasive prediction of liver-related events in patients with HCV-associated compensated advanced chronic liver disease after oral antivirals. J Hepatol. 2020;72:472–480. [DOI] [PubMed] [Google Scholar]
- 31.Semmler G, Meyer EL, Kozbial K, Schwabl P, Hametner-Schreil S, Zanetto A, et al. HCC risk stratification after cure of hepatitis C in patients with compensated advanced chronic liver disease. J Hepatol. 2022;76:812–821. [DOI] [PubMed] [Google Scholar]
- 32.Alonso Lopez S, Manzano ML, Gea F, Gutierrez ML, Ahumada AM, Devesa MJ, et al. A model based on noninvasive markers predicts very low hepatocellular carcinoma risk after viral response in hepatitis C virus-advanced fibrosis. Hepatology. 2020;72:1924–1934. [DOI] [PubMed] [Google Scholar]
- 33.Kim NJ, Vutien P, Cleveland E, Cravero A, Ioannou GN. Fibrosis stage-specific incidence of hepatocellular cancer after hepatitis C cure with direct-acting antivirals: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2023;21:1723–1738.e1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farhang Zangneh H, Wong WWL, Sander B, Bell CM, Mumtaz K, Kowgier M, et al. Cost effectiveness of hepatocellular carcinoma surveillance after a sustained virologic response to therapy in patients with hepatitis C virus infection and advanced fibrosis. Clin Gastroenterol Hepatol. 2019;17:1840–1849.e1816. [DOI] [PubMed] [Google Scholar]
- 35.Requena MB, Grabar S, Lanoy E, Pialoux G, Billaud E, Duvivier C, et al. Mortality in HCV-cured versus HCV-uninfected people with HIV: A matched analysis in the ANRS CO4 FHDH cohort. AIDS. 2023;37:1297–1306. [DOI] [PubMed] [Google Scholar]
- 36.Innes H, McAuley A, Alavi M, Valerio H, Goldberg D, Hutchinson SJ. The contribution of health risk behaviors to excess mortality in American adults with chronic hepatitis C: A population cohort-study. Hepatology. 2018;67:97–107. [DOI] [PubMed] [Google Scholar]
- 37.Verheij E, Boyd A, Wit FW, Verboeket SO, Verburgh ML, van der Valk M, et al. Long-term evolution of comorbidities and their disease burden in individuals with and without HIV as they age: Analysis of the prospective AGE(h)IV cohort study. Lancet HIV. 2023;10:e164–e174. [DOI] [PubMed] [Google Scholar]
- 38.Garcia-Compean D, Jaquez-Quintana JO, Gonzalez-Gonzalez JA, Maldonado-Garza H. Liver cirrhosis and diabetes: Risk factors, pathophysiology, clinical implications and management. World J Gastroenterol. 2009;15:280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foster GR, Irving WL, Cheung MC, Walker AJ, Hudson BE, Verma S, et al. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;64:1224–1231. [DOI] [PubMed] [Google Scholar]
- 40.Fernandez Carrillo C, Lens S, Llop E, Pascasio JM, Crespo J, Arenas J, et al. Treatment of hepatitis C virus infection in patients with cirrhosis and predictive value of model for end-stage liver disease: Analysis of data from the Hepa-C registry. Hepatology. 2017;65:1810–1822. [DOI] [PubMed] [Google Scholar]
- 41.Lens S, Alvarado-Tapias E, Mariño Z, Londoño MC, LLop E, Martinez J, et al. Effects of all-oral anti-viral therapy on HVPG and systemic hemodynamics in patients with hepatitis C virus-associated cirrhosis. Gastroenterology. 2017;153:1273–1283.e1271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.