Transthyretin amyloid cardiomyopathy (ATTR-CM) is an increasingly recognized cause of heart failure. Acoramidis, a high-affinity TTR (transthyretin) stabilizer, inhibits tetrameric TTR dissociation, which can lead to amyloid fibrils. ATTRibute-CM (a phase 3, multicenter, double-blind trial) randomized participants with ATTR-CM to receive acoramidis or placebo (2:1). It met its primary hierarchical end point of mortality, cardiovascular-related hospitalization, change in NT-proBNP (N-terminal pro-B-type natriuretic peptide), and 6-minute walk test (P<0.0001).1
Cardiac magnetic resonance (CMR) with extracellular volume (ECV) mapping has proven utility in tracking response to treatment in ATTR-CM by assessing changes in cardiac structure, function, and amyloid burden.2 Eligible ATTRibute-CM participants were invited to enroll in the preplanned, exploratory CMR substudy (end points: change in left ventricular [LV] mass, biventricular structure and function, and myocardial tissue characterization, including ECV mapping). ATTRibute-CM was approved by the research ethics committee, and all participants provided written informed consent. After the initial CMR scan, participants underwent scans at month 12, month 24, and month 30 (M30). M30 results are reported, as treatment effects accrue progressively to match with the ATTRibute-CM efficacy results.
Amyloid regression was defined as an absolute reduction in ECV ≥5%, progression as an absolute increase in ECV ≥5%, and stable if ECV change was <5%.3 The 5% threshold is an established independent prognosticator in light chain amyloid cardiomyopathy, and its robustness and applicability in ATTR-CM are supported by emergent data.
Fifty-two participants (47 male; mean age, 74.3 years) were enrolled. Eight had variant ATTR-CM (V122I=6; T60A=2); the remainder had wild-type ATTR-CM. Twenty-six of 41 acoramidis recipients and 5 of 11 receiving placebo completed M30 scans. Baseline characteristics were comparable. Mortality was higher in placebo recipients (n=4/11; 36%) versus acoramidis (n=5/41; 12%). Before M30, 8 participants (acoramidis =7/26) received cardiac device implantations and 4 discontinued (acoramidis =3; placebo =1); thus, they were excluded. Three participants underwent M30 CMR without ECV due to exclusionary renal impairment (acoramidis =1) and technical difficulties (acoramidis =1; placebo =1).
Acoramidis recipients demonstrated favorable trends in CMR parameters versus placebo. In acoramidis recipients, mean indexed LV mass remained stable through M30, with a mean (SD) reduction of 2.0 (10.54) g/m2 by M30. Conversely, indexed LV mass increased by 5.6 (8.26) g/m2 in placebo recipients.
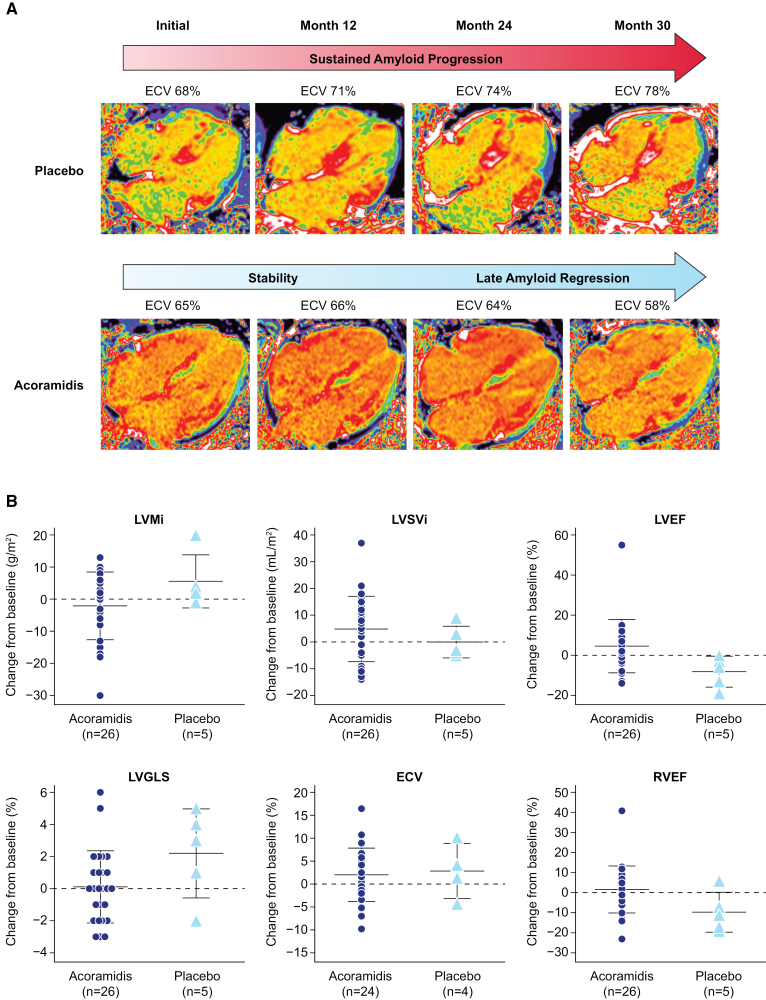
Parameters of biventricular function showed favorable changes through M30 in acoramidis recipients versus placebo (Figure). LV ejection fraction improved from a baseline mean (SD) of 50.7 (12.33)% by 4.6 (13.32)% in acoramidis recipients; in placebo recipients, LV ejection fraction declined from baseline 50.5 (12.05)% by 8.2 (7.73)%. Indexed LV stroke volume improved in acoramidis recipients with a median (interquartile range) increase of 6.5 (18.0) mL/m2, whereas in placebo, indexed LV stroke volume declined by 3.0 (7.0) mL/m2. LV global longitudinal strain was stable at M30 with acoramidis, with an absolute mean (SD) change from baseline of 0.1 (2.25)% and deteriorated in placebo recipients by 2.2 (2.78)%. Right ventricular parameters improved by M30 in acoramidis recipients: right ventricular ejection fraction increased by an absolute mean (SD) 1.8 (11.73)% and deteriorated by 9.6 (9.94)% with placebo; indexed right ventricular stroke volume improved by mean (SD) 4.5 (12.07) mL/m2 and remained relatively unchanged in placebo recipients with a change of 0.8 (6.76) mL/m2. ECV at baseline was mean (SD) 61.5 (8.06)% in acoramidis recipients versus 63.8 (7.87)% in placebo. Absolute mean (SD) change from baseline in ECV at M30 was similar in both groups, with acoramidis recipients increasing by 2.0 (5.84)% and placebo by 2.9 (6.03)%. Myocardial contraction fraction4 was similar in both groups at baseline (mean [SD]; acoramidis, 34.6 [10.58] and placebo, 35.9 [13.19]). At M30, myocardial contraction fraction absolute mean (SD) change from baseline improved in acoramidis recipients (29.9 [76.92%]) versus placebo (−1.9 [27.46%]). Amyloid regression was observed in 3/24 (12.5%) acoramidis recipients; no placebo recipients demonstrated regression (illustrative examples in the Figure).
Figure.
Change in extracellular volume (ECV) and cardiac magnetic resonance (CMR) parameters over time. A, Illustrative examples of 2 participants within the CMR substudy. Top, Serial 4-chamber ECV maps from a participant who received a placebo. Sustained amyloid progression and increase in cardiac amyloid burden consistent with the natural history of transthyretin amyloid cardiomyopathy are seen in the placebo participant, with ECV rising from 68% at initial CMR to 78% at month 30 (M30). Bottom, A participant receiving acoramidis who initially demonstrated stable cardiac amyloid burden, followed by late amyloid regression at M30 with an observed reduction of ECV from 65% at initial CMR to 58% at M30. B, Scatter plots represent a change from baseline at M30 for selected CMR parameters (indexed left ventricular mass [LVMi], indexed left ventricular stroke volume [LVSVi], left ventricular ejection fraction [LVEF], left ventricular global longitudinal strain [LVGLS], ECV, and right ventricular ejection fraction [RVEF]) for each participant. Each symbol represents data for individual participants, and horizontal bars represent the mean and SD for acoramidis and placebo groups. Favorable trends were noted across parameters in participants receiving acoramidis (n=26 for all parameters other than ECV; n=24 for ECV) relative to their placebo counterparts (n=5 for all parameters other than ECV; n=4 for ECV). CMR scans were acquired on a 1.5T scanner (MAGNETOM Aera, Siemens Healthcare, Germany) using a standardized protocol comprising cine imaging with steady-state-free-precession sequence and late gadolinium enhancement imaging. Native T1 measurement (comprising 3 short axes and a 4-chamber map) was performed with the modified look-locker inversion (MOLLI) recovery sequence. Fifteen minutes following the administration of a 0.1-mmol/kg gadolinium meglumine bolus (Dotarem, Guebet, France), T1 mapping was acquired using the same slice locations with the MOLLI sequence, and inline ECV maps were automatically generated using hematocrit. Image analysis was performed blinded to other trial data. ECV values were measured by drawing a region of interest in the basal-mid septum on 4-chamber maps. Myocardial contraction fraction (%) was calculated based on Shimada et al,4 as (left ventricular end-diastolic volume−left ventricular end-systolic volume)/left ventricular myocardial volume×100.4 ECV maps are acquired by isolating a signal to the myocardial extracellular space by using the precontrast and postcontrast T1 maps in conjunction with the serum hematocrit; due to varying serum hematocrit values between patients, the myocardial blood pool may appear visually different between patients with similar myocardial ECV values. CMR image quality and volumetric data may be impacted by several factors, including, but not limited to, arrhythmia, breathing artifacts, and unintentional patient movement.
Findings are reported descriptively due to the small sample size. Participants without ECV mapping may have led to an underestimation of ECV differences. Serial data were only available from participants with follow-up imaging visits, potentially adding a survival bias. The extent of improvement observed in acoramidis recipients relative to placebo may have been underestimated, as a higher proportion of nonsurviving placebo participants may have exhibited accelerated amyloid accumulation with associated deterioration in myocardial function if they survived longer.
This is the first longitudinal CMR evaluation included within a phase 3 ATTR-CM trial. Results demonstrate that acoramidis trended toward improving or stabilizing structural and biventricular functional CMR parameters versus placebo, where the myocardial structure and function remained stable or deteriorated. LV ejection fraction, indexed LV stroke volume, and global longitudinal strain have prognostic significance in ATTR-CM, with deterioration in strain negatively impacting survival.5 Parameters of right ventricular systolic function also stabilized or improved with acoramidis. By M30, 12.5% of acoramidis recipients demonstrated amyloid regression, which is inconsistent with the natural history of ATTR-CM. This suggests that TTR stabilization with acoramidis may allow the rate of innate amyloid clearance mechanisms to exceed the rate of amyloid formation, enabling cardiac remodeling and functional recovery.3 These results further inform the mechanism underlying the clinical benefits of acoramidis treatment.
Data supporting these findings are available from the study sponsor upon reasonable request.
ARTICLE INFORMATION
Acknowledgments
The authors thank the investigators of the ATTRibute-CM (a phase 3, multicenter, double-blind trial) cardiac magnetic resonance substudy. The sponsor of the study and investigators also thank the patients and their families for their participation in and support of this study. Editorial support was provided by Shweta Rane of BridgeBio Pharma Inc.
Sources of Funding
This study was sponsored by Eidos Therapeutics, an affiliate of BridgeBio Pharma Inc (San Francisco, CA). Editorial support was provided by ApotheCom (San Francisco) and Syneos Health funded by BridgeBio Pharma Inc.
Disclosures
Y. Razvi received consulting fees from BridgeBio Pharma Inc. Dr Judge received grants and funding for the trial, travel expenses, and consultancy fees from Pfizer; received consultancy fees from Alnylam, Blade Therapeutics, and GlaxoSmithKline; and received clinical trial funding from Array Biopharma and Eidos Therapeutics (a subsidiary of BridgeBio Pharma Inc). Dr Gillmore received grants, consultancy fees, and speaker fees from Alnylam Pharmaceuticals and consultancy fees from AstraZeneca, ATTRalus, Eidos Therapeutics (a subsidiary of BridgeBio Pharma Inc), Intellia Therapeutics, Ionis Pharmaceuticals Inc, and Pfizer Inc. Drs Du, Tamby, Castaño, Siddhanti, Katz, and Fox are employees and stockholders of BridgeBio Pharma Inc. Dr Fontana received consulting fees from Pfizer, Akcea, Ionis, Alnylam, Alexion, AstraZeneca, Eidos Therapeutics (a subsidiary of BridgeBio Pharma Inc), Intellia, Janssen Global Services LLC, and Novo Nordisk and research grants from Pfizer and Eidos Therapeutics. Drs Martinez-Naharro, Venneri, Kellman, and Taubel and A. Ioannou, R. Patel, and L. Edwards report no conflicts.
Footnotes
The ATTRibute-CM (NCT03860935) trial was conducted in accordance with the International Council for Harmonization, Good Clinical Practice, and Declaration of Helsinki. All participants provided written informed consent. Requests for access to data will be evaluated by the sponsor and can be submitted via medinfo@bridgebio.com. Access will be provided contingent upon the approval of a research proposal and the execution of a data-sharing agreement.
This work was presented as an abstract at the American College of Cardiology Annual Meeting, Atlanta, GA, April 6–8, 2024.
For Sources of Funding and Disclosures, see page 1204.
Contributor Information
Yousuf Razvi, Email: yousuf.razvi@nhs.net.
Ana Martinez-Naharro, Email: anamartinez.naharro@nhs.net.
Adam Ioannou, Email: adam.castano@gmail.com.
Lucia Venneri, Email: lucia.venneri@nhs.net.
Julian D. Gillmore, Email: j.gillmore@ucl.ac.uk.
Peter Kellman, Email: kellmanp@nhlbi.nih.gov.
Laura Edwards, Email: l.edwards@richmondpharmacology.com.
Jorg Taubel, Email: j.taubel@richmondpharmacology.com.
Jing Du, Email: Jing.Du@bridgebio.com.
Jean-François Tamby, Email: JF.Tamby@bridgebio.com.
Adam Castaño, Email: adam.castano@gmail.com.
Suresh Siddhanti, Email: suresh.siddhanti@bridgebio.com.
Jonathan C. Fox, Email: Jf@bridgebio.com.
References
- 1.Gillmore JD, Judge DP, Cappelli F, Fontana M, Garcia-Pavia P, Gibbs S, Grogan M, Hanna M, Hoffman J, Masri A, et al. ; ATTRibute-CM Investigators. Efficacy and safety of acoramidis in transthyretin amyloid cardiomyopathy. N Engl J Med. 2024;390:132–142. doi: 10.1056/NEJMoa2305434 [DOI] [PubMed] [Google Scholar]
- 2.Fontana M, Martinez-Naharro A, Chacko L, Rowczenio D, Gilbertson JA, Whelan CJ, Strehina S, Lane T, Moon J, Hutt DF, et al. Reduction in CMR derived extracellular volume with patisiran indicates cardiac amyloid regression. JACC Cardiovasc Imaging. 2021;14:189–199. doi: 10.1016/j.jcmg.2020.07.043 [DOI] [PubMed] [Google Scholar]
- 3.Martinez-Naharro A, Patel R, Kotecha T, Karia N, Ioannou A, Petrie A, Chacko LA, Razvi Y, Ravichandran S, Brown J, et al. Cardiovascular magnetic resonance in light-chain amyloidosis to guide treatment. Eur Heart J. 2022;43:4722–4735. doi: 10.1093/eurheartj/ehac363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimada YJ, Hoeger CW, Latif F, Takayama H, Ginns J, Maurer MS. Myocardial contraction fraction predicts cardiovascular events in patients with hypertrophic cardiomyopathy and normal ejection fraction. J Card Fail. 2019;25:450–456. doi: 10.1016/j.cardfail.2019.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chacko L, Martone R, Bandera F, Lane T, Martinez-Naharro A, Boldrini M, Rezk T, Whelan C, Quarta C, Rowczenio D, et al. Echocardiographic phenotype and prognosis in transthyretin cardiac amyloidosis. Eur Heart J. 2020;41:1439–1447. doi: 10.1093/eurheartj/ehz905 [DOI] [PubMed] [Google Scholar]