Abstract
Albumin is the most abundant protein in the human body and is synthetized exclusively by the liver. Therefore, serum albumin levels are reduced in acute and/or chronic liver disease. In cirrhosis, low levels of albumin predict the outcome. In advanced cirrhosis, the quality of albumin is decreased due to high oxidative stress and a proinflammatory state. Therefore, the administration of i.v. albumin would seem to be of pathophysiological relevance and benefit. Yet, the questions that remain are who, when, how much, and how often. While albumin infusion is recommended after large-volume paracentesis, at diagnosis of spontaneous bacterial peritonitis, in acute kidney injury, and in hepatorenal syndrome, the amount and schedule of albumin to be administered require refinement, particularly given complications related to volume overload that have become increasingly apparent. Other indications for albumin such as infections other than spontaneous bacterial peritonitis, hyponatremia, HE, prevention of poor outcomes in hospitalized, and in outpatients with cirrhosis are still debated. The results of studies in these settings are either negative, controversial, or inconclusive. This sheds some doubts regarding the use of albumin as a “one size fits all” strategy. The indication and patient selection are crucial and not always intuitive. The amount and frequency also seem to play a role in the success or failure of albumin. This review will critically discuss the evidence and underline areas where there are indications for albumin use and others where evidence is still insufficient and will have to await the development/results of randomized controlled trials.
INTRODUCTION
The term “albumin” comes from the Latin “albus” which means “white.” It is the most abundant circulating protein and belongs to the group of globular proteins.1,2
Albumin is synthesized by hepatocytes. Therefore, any chronic illness that will result in hepatocellular dysfunction, mainly cirrhosis, will result in hypoalbuminemia.3,4 In patients with compensated cirrhosis, serum albumin levels can be entirely normal, however, with progression of disease, serum albumin levels decrease progressively. In fact, albumin is one of the 5 components of the Child-Turcotte-Pugh classification that stratifies patients with cirrhosis into 3 main prognostic categories: Child A (mainly compensated), Child B (mainly decompensated), and Child C (mainly “further” decompensated) each with lower serum albumin cutoffs.5
In compensated cirrhosis, the main mechanism leading to cirrhosis decompensation (ie, the development of ascites, variceal hemorrhage, or encephalopathy) is portal hypertension. This is due not only to an increase in intrahepatic resistance due to the distorted liver architecture but also because of an increase in portal blood flow. This increased flow is due to splanchnic vasodilatation that is a consequence of increased shear stress and bacterial translocation.6 A threshold portal pressure (as determined by a HVPG) ≥10 mm Hg identifies patients with compensated cirrhosis at risk for decompensation and this pressure threshold is now known as “clinically significant portal hypertension” (CSPH).7 However, even in this compensated stage, serum albumin (a reflection of liver function) is an important predictor of outcomes. In a secondary analysis of a prospective cohort study of patients with cirrhosis, serum albumin was shown to be the most important predictor of death in patients with compensated cirrhosis8; a serum albumin >4 g/dL in these patients identified those that would have a particularly good survival. Both in this cohort study7 and in the subgroup analysis of the “timolol” randomized controlled trial (RCT) that identified the HVPG as an important predictor of clinical decompensation,7 serum albumin was identified as being an independent predictor of decompensation at a cutoff of level of 4 g/dL. In fact, the addition of albumin to the Fibrosis-4 (FIB-4) score has been identified as predicting the presence of CSPH.9 More recently, in a large retrospective cohort study of patients with NAFLD, serum albumin was once more found to be an independent predictor of decompensation in those with compensated cirrhosis.10
In decompensated cirrhosis, serum albumin has also been shown to predict survival, although in this setting other parameters such as the Model for End-Stage Liver Disease (MELD) score have a greater predictive value.8 In fact, the MELD score has been recently recalibrated (MELD 3.0) to include serum albumin as one of the prognostic parameters in decompensated cirrhosis.11 It is important to emphasize that “natural”: serum albumin is the one that is of prognostic value as it reflects liver function. The exogenous administration of albumin, while improving some of the complications of cirrhosis (as will be shown below), may alter serum albumin levels so that it will no longer reflect liver function. The increasing use of i.v. albumin as a therapeutic strategy will thus represent an important confounder in any prognostic score that includes serum albumin.
The decompensated stage of cirrhosis is followed by a stage of “further decompensation” defined as the development of a second decompensating event or the development of refractory ascites, recurrent variceal hemorrhage, recurrent encephalopathy, or hepatorenal syndrome (HRS-AKI).12 In the decompensated stage, there is also a prothrombotic state that leads to complications such as PVT.12 The main driver of further decompensation is a systemic inflammatory state that worsens vasodilatation and may lead to acute-on-chronic liver failure (ACLF), an acute inflammatory state often precipitated by bacterial infections and that is associated with the highest mortality.13 The inflammatory state associated with decompensated cirrhosis can lead to hepatic reprioritization of protein synthesis resulting in lower serum concentrations of albumin and prealbumin and this is augmented by a redistribution of serum proteins because of an increase in capillary permeability.4 It is in a subset of patients with further decompensation and/or ACLF where i.v. administration of albumin has been shown to be an important therapeutic strategy.
Albumin administration after large-volume paracentesis (LVP) (>5 L) and in spontaneous bacterial peritonitis (SBP) has been recommended by both the American Association for the Study of Liver Diseases (AASLD)12 and the European Association for the Study of the Liver (EASL) guidelines, as is the use of albumin together with vasoconstrictors in the management of HRS-AKI.12,14,15 Other uses of albumin are controversial and require further research before they can be widely recommended.16–18
In a recent European survey19 completed by 101 hepatologists practicing at 86 centers (75% academic hospitals), the vast majority (93%–97%) of participants used i.v. albumin for guideline-recommended settings, specifically, after LVP for the prevention of post–paracentesis circulatory dysfunction (PCD), prevention of renal failure after SBP, and diagnosis and management of HRS-AKI. However, up to a third of practitioners from tertiary and university hospitals admitted to using albumin in settings where albumin use is not recommended, specifically non-SBP infections, severe hyponatremia, HE, and long-term use for the treatment of ascites. Interestingly and inexplicably, half of the respondents would recommend i.v. albumin solely for hypoalbuminemia. In addition, even for recommended indications, doses used were not the ones recommended based on clinical trials. This mirrors the reality of clinical medicine when the label of the medication is broad and where costs and perceived benefits of a drug have an influence on practice, even in the absence of supportive evidence.
The survey also revealed conceptual differences regarding the mechanism of action of albumin in the management of the complications of cirrhosis. While some considered the beneficial effect of albumin was due to its oncotic properties associated with volume expansion, others attributed the beneficial effects of albumin to nononcotic properties such as antioxidant and scavenging activities, binding and transport of toxins, regulation of endothelial function as well as modulation of inflammatory/immune responses, endothelial function, and coagulation.
Conceptual differences, together with the fact that albumin may lead to volume overload and respiratory insufficiency and, by being solely derived from human sources, is an expensive product, have made the use of albumin in cirrhosis a controversial matter that requires further research.16–18 While in the case of pharmaceutical agents, particularly those that are expensive and/or associated with risk, there is a “label” that defines indications and restrictions in use, in the case of human serum albumin the label indicates it for “restoration and maintenance of circulating blood volume, where volume deficiency has been demonstrated and use of colloid is appropriate.” This label is quite broad and nonspecific, allowing for the use of albumin for practically every patient with decompensated cirrhosis and for every complication of cirrhosis, even for those for which there is no evidence of efficacy and, therefore, for which there are no formal recommendations for its use by medical societies. Still, insurance companies and other stakeholders worldwide consider the recommendations put forward by scientific societies and may restrict its use for only certain settings, although there is not as strict a control as there is for expensive pharmaceutical agents.14,15
The aim of this review is to discuss the settings in decompensated cirrhosis in which albumin use is not controversial, the settings that remain controversial and future directions to resolve these controversies (Table 1).
TABLE 1.
Use of albumin in decompensated cirrhosis: potential benefits (pros) and disadvantages (cons) as well as areas of uncertainty
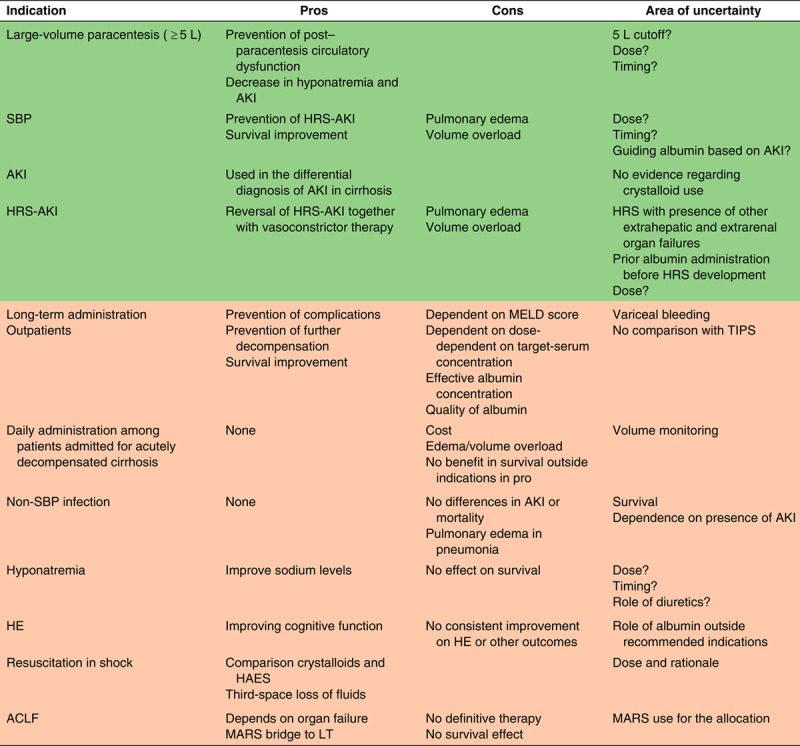
Abbreviations: ACLF, acute-on-chronic liver failure; AKI, acute kidney injury; HRS, hepatorenal syndrome; LT, liver transplantation; MELD, Model for End-Stage Liver Disease; SBP, spontaneous bacterial peritonitis.
Areas in green denote settings with which albumin use is recommended per society guidelines. Areas in orange are settings where albumin use is not recommended.
Mechanisms of action of albumin and pathophysiological considerations in cirrhosis
Albumin has many functions as depicted in Figure 1 that can potentially be beneficial in decompensated cirrhosis. Albumin levels are dependent on different factors. The half-life of albumin is around 20 days, while albumin undergoes a continuous renewal by hepatocytes at the rate of ∼10–15 g/d.20–22 Recent studies have suggested that endothelial cells and immune cells can uptake albumin.16,17,23 In addition to oncotic and binding properties, albumin also interacts with different cells and may act as an anti-inflammatory on immune cells, antithrombotic on platelets, and may modify endothelial cells and stabilize blood vessels.16,21
FIGURE 1.
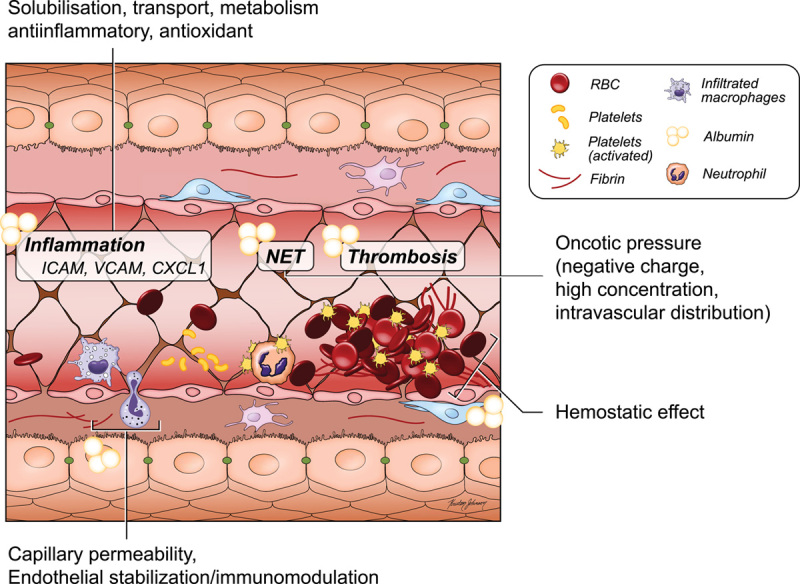
Molecular mechanisms of action of albumin in cirrhosis. Abbreviation: CXCL1, C-X-C motif chemokine ligand 1; ICAM, intracellular cell adhesion molecule; NET, neutrophil extracellular traps; RBC, red blood cell; VCAM, vascular cell-adhesion molecule 1.
Oncotic pressure
The main function of albumin in the maintenance of oncotic pressure was described almost a century ago.24 Albumin is 66 kDa large and between 584 and 590 amino acids long and demonstrates a good binding capacity for water, which renders it suitable as a solvent. In addition, albumin has a pH around 4.6.21,25 These 2 properties, together with its molecular mass and the negative net charge at physiological pH, attract positively charged ions into the intravascular compartment leading to the maintenance of oncotic pressure and thereby plasma expansion.16
The administration of i.v. albumin in patients with cirrhosis has been shown to prevent acute kidney injury (AKI) after LVP (the so-called post-PCD) and in those with spontaneous bacterial peritonitis.26,27 In both instances, the pathophysiology of AKI is worsening of the vasodilatory state of cirrhosis that leads to a decreased effective arterial blood volume (EABV) and kidney hypoperfusion6,28,29 (Figure 2). In these settings, it appears that maintenance of oncotic pressure and plasma expansion are the main mechanism by which albumin prevents AKI.
FIGURE 2.
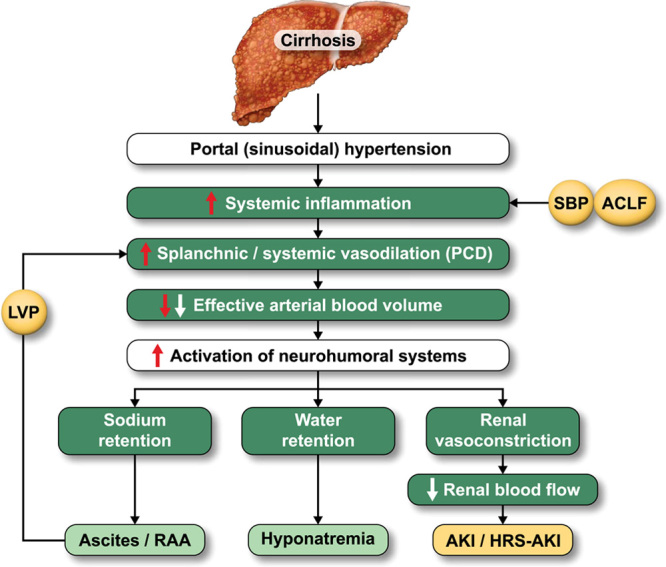
Mechanistic vision of albumin in the development of complications in cirrhosis. Abbreviations: ACLF, acute-on-chronic liver failure; AKI, acute kidney injury; HRS, hepatorenal syndrome; LVP, large-volume paracentesis; RAA, renin-angiotensin-aldosterone; SBP, spontaneous bacterial peritonitis.
Antioxidant and binding abilities
In addition to regulating oncotic pressure, albumin has the ability of a “sponge” to bind to many endogenous and exogenous substances, including different radicals (reactive oxygen species, hydroxyl, etc.), thereby ameliorating the deleterious effect that these substances have on the organism.16,30 It must be mentioned that, in situations of high oxidative stress or in the presence of other toxins as present in decompensated cirrhosis, the quality of albumin is reduced because of posttranscriptional abnormalities of the molecule, such as cysteinylation or sulfinylation of the cystein-34 residue, truncations at the amino terminus or carboxy terminus, and glycosylation,17,31 which is compounded by low albumin levels due to liver dysfunction.32–34
Endothelial stabilization and coagulation
Albumin plays a role in endothelial stabilization, as well as in decreasing endothelial permeability probably by means of its interactions with interstitial matrix.35 In diseases with higher endothelial leakage, higher albumin levels are needed since albumin is uptaken by cells especially after the degradation or oxidation of albumin. The antioxidant properties of albumin protect endothelium and improve vascular integrity.36 In addition, hyperaggregation of platelets related to hypoalbuminemia may lead to procoagulant tendency, which has been shown to occur in kidney disease37 and can be extrapolated to cirrhosis.38,39 Finally, the interaction of albumin with nitric oxide and eicosanoids promotes vasodilation.40–42 In cirrhosis, both vascular dysfunction and coagulation system play a pathogenic role, therefore low albumin levels aggravate the outcome in these patients as reflected in the Child-Pugh score.5
Anti-inflammatory
Albumin influences several immunological pathways. In experimental studies, human albumin inhibits TNFα-mediated inflammation and monocyte adherence to endothelial cells.43 In patients with infections, albumin administration decreases circulating inflammatory biomarkers.44 Another study demonstrated that albumin could bind and neutralize proinflammatory lipids (prostaglandin E2), and could improve anti-infective B-cell function.45,46 Finally, albumin reprograms immune cells toward an anti-inflammatory phenotype.23 Systemic inflammation is the key pathogenetic step in the development of organ dysfunction and failure in decompensated cirrhosis.36,47 Low levels and quality of albumin in cirrhosis cannot counteract the gradual increase of systemic inflammation and cannot halt the vicious cycle of complications.13,48,49 Although not proven, exogenous albumin administration could theoretically restore the equilibrium of proinflammation and anti-inflammation in cirrhosis.
Recommended indications of albumin
In the following paragraphs, we describe the clinical settings in which there is evidence for or against the use of albumin in patients with cirrhosis and the resulting recommendations that are endorsed by national and international societies (Figure 2).
Albumin use after LVP
In cirrhosis, the regulation of oncotic pressure and plasma expansion is extremely important, especially in patients who undergo LVP. This procedure is associated with the so-called post-PCD, a vasodilatory state that leads to a reduction in EABV which represents the part of the intravascular arterial compartment that effectively perfuses tissues. This reduction in EABV leads to the activation of neurohumoral systems (renin-angiotensin-aldosterone system and sympathetic nervous system) and a subsequent decrease in renal perfusion leading to prerenal AKI.50 The use of volume expanders like i.v. albumin, by increasing EABV, should therefore prevent the development of PCD.
LVP is a local therapy recommended in patients with cirrhosis and ascites who are no longer responding to diuretics.14,15 In the mid-1980s, a study showed that LVP could be performed safely without significant effects on systemic hemodynamics, serum electrolytes, or renal function.51 However, in this study only 5 L of ascites were removed and paracentesis was only performed once. In addition, more subtle markers of EABV such as plasma renin activity and aldosterone levels were not assessed. Other trials in which larger volumes were removed showed significant increases in these markers 24–48 hours after paracentesis.28,52,53
In a seminal RCT by Gines et al,26 patients undergoing LVP were randomized to i.v. albumin versus no albumin and showed that patients randomized to albumin developed significantly less hyponatremia and renal dysfunction as well as a lower rate of “post-PCD.” This entity has been defined as an increase in plasma renin activity on the sixth day after paracentesis (time at which renin activity peaks) and, although it is not associated with abnormalities in routine blood tests such as blood urea nitrogen (BUN), creatinine, or electrolytes, patients who develop PCD have a higher rate of ascites re-accumulation and, more importantly, a decreased survival compared with patients who do not develop PCD.29 Two other RCTs have shown a decrease in PCD with albumin infusion.28,29
In a study comparing albumin to the synthetic plasma expanders, dextran-70 and polygeline, it was found that for LVPs of <5 L the incidence of PCD was similar in all patients, regardless of the type of plasma expander used.29 However, if >5 L of ascites were removed, the incidence of PCD among patients who were given albumin was 18% compared with 34% and 38% for patients given dextran-70 and polygeline, respectively.29 A subsequent meta-analysis that included this and 16 additional trials including 1225 patients showed that, compared with alternative treatments (dextran, gelatin, hydroxyethyl starch, and hypertonic saline), albumin reduced the incidence of PCD, and hyponatremia and mortality.54 Albumin is thought to be a more effective volume expander than others because of its longer half-life and greater oncotic property. Of note, a more recent Cochrane systematic meta-analysis showed no differences in mortality, AKI, or hyponatremia after LVP in patients receiving any plasma expander versus no plasma expander, however, albumin was grouped together with other nonalbumin plasma expanders.55
Synthetic crystalloids may be substituted for albumin if <5 L of ascitic fluid are removed. In fact, the use of a plasma volume expander may not be necessary at all in this setting29 and is not recommended unless there is another reason for volume expansion (eg, presence of AKI).
Although the use of albumin in the setting of LVP (>5 L) is not questionable, there are several points that require discussion. On one hand, the dose of albumin (6–8 g/L of ascites removed) was established empirically. A RCT in which a dose of albumin of 4 g/L of ascites removed was compared with 8 g/L after LVP (>5 L of ascites removed), showed no differences in the incidence of PCD, hyponatremia, renal dysfunction, recurrence of ascites, or survival.56 In fact, one could argue that the dose of albumin should be individualized based on factors such as “dry” body mass index, volume status, and/or presence of concomitant AKI.
On the other hand, the indication to administer albumin only after 5 L or more of ascites are removed is based on 2 proof-of-concept small studies that demonstrated a lack of deleterious hemodynamic effects up to 48 hours after a single 5 L paracentesis.13,51 One would wonder whether doing daily paracenteses of <5 L would forego the need for albumin. The RCT by Gines et al26 suggests that this is not the case since patients randomized to daily LVPs of 4–6 L who received 40 g of albumin with each paracentesis still had better outcomes than those randomized to not receiving albumin.26 As mentioned previously, in the meta-analysis comparing albumin to plasma volume expanders,54 for LVPs <5 L there were no differences in outcomes (mainly PCD) between albumin and plasma volume expanders which indicates that some volume expansion is necessary even with small volumes of ascites removed and it would make rational sense to recommend albumin in patients with AKI. In fact, a recent study showed that, in patients with ACLF per APASL criteria, characterized by jaundice and ascites, paracenteses <5 L were associated with the development of PCD and had a lower survival, both of which were improved by the administration of albumin.57 This is not surprising, as patients with increased systemic inflammation, the hallmark of ACLF, are more vasodilated and are therefore more prone to develop PCD.58 Therefore, in the presence of ACLF (by any definition) albumin administration would be recommended with paracenteses of any volume.
In this era of personalized medicine, biomarkers should be developed before, during, or after paracentesis that would guide the decision to administer albumin (or not) and to determine the best dose for each specific case.
Since vasodilation is the main pathogenic mechanism underlying PCD, the use of vasoconstrictors has also been considered in this setting. Five small trials comparing albumin versus different vasoconstrictors (terlipressin, norepinephrine, or midodrine) after LVP were analyzed in the aforementioned meta-analysis,54 showing no difference between the 2 groups. Another small, placebo-controlled trial compared albumin versus octreotide plus midodrine in patients undergoing LVP and showed no differences in PCD or recurrence of ascites but more AKI in the vasoconstrictor group.59
Until more evidence is gathered, society guidelines state that LVP is the first-line treatment for refractory ascites and that albumin infusion at the time of LVP of >5 L is recommended to mitigate the risk of PCD. The recommended dose of albumin replacement, based on expert opinion, is 6–8 g for every liter of ascites removed.14,15
Albumin use in SBP
Up to one third of patients with SBP will develop AKI and this is the most important predictor of mortality in these patients.60 The mechanism of AKI in SBP is systemic inflammation from infection leading to vasodilatation and a decrease in EABV. This is supported by finding of increased plasma renin activity and development of prerenal kidney dysfunction in patients with bacterial infection (not only SBP and not restricted to patients with cirrhosis), particularly with increasing severity.61
Despite clearance of infection, patients with SBP may develop progressive AKI and even HRS-AKI leading to death.27 Therefore, it is essential to prevent/treat AKI as soon as the diagnosis of SBP is established. Treatment is focused on expanding the EABV with plasma expanders such as albumin.27 In patients with SBP, albumin administration has been shown to be associated with an improvement in both vasodilatation and cardiac function.62
In a landmark multicenter randomized nonblinded study by Sort et al27 performed in patients with uncomplicated SBP, patients randomized to cefotaxime plus albumin had a significantly lower rate of AKI (10%) compared with those randomized to cefotaxime alone (33%) and they also had a lower in-hospital mortality (10% vs. 29%). Importantly, patients with serum bilirubin >4 mg/dL and evidence of AKI at baseline (creatinine >1.0 mg/dL and BUN >30 mg/dL) were most likely to benefit from albumin therapy.27
A recent meta-analysis of 5 RCTs of albumin in patients with SBP,63 that included the study by Sort and colleagues, confirmed a lower rate of renal impairment in albumin versus control groups (9% vs. 26%) and lower rates of 30-day mortality in the albumin group (14% vs. 30% in control), confirming results from a previous meta-analysis.64 All studies were unblinded. Control groups in 4 of the trials did not receive albumin and in one of them, albumin was compared with hydroxyethyl starch.65 Except for one, all studies used the empirical arbitrary dose of albumin recommended in the Sort and colleagues’ study, that is 1.5 mg/kg of body weight on day 1 (within 6 h of randomization) and 1 g/kg of body weight on day 3). Interestingly in one study from Taiwan,42 where only 30 g of albumin is covered by the National Health Insurance, patients randomized to albumin received 10 g/d from days 1 to 3. The study was underpowered (only 15 patients in each group) but showed a lower rate renal dysfunction (7% vs. 20%) and in-hospital mortality (27% vs. 40%) in the albumin group.
This begs the question on whether the doses recommended should be lowered. Using the recommended doses in the study by Sort and colleagues, a 75 kg patient would receive 105 g on day 1 and 70 g on day 3. These doses are larger than those recommended after LVP or for AKI and could be even larger with increasing numbers of obese patients with cirrhosis secondary to NASH. It would not be surprising to see an even greater increase in complications due to volume overload in these patients, who are more likely to have cardiovascular disease. As for LVP, a personalized approach to dosing albumin in patients with SBP should be based on daily clinical assessment of volume status and kidney function rather than on a preestablished dose/schedule.
Regarding alternatives to albumin in this setting, the already mentioned nonblinded randomized study comparing hydroxyethyl starch to albumin in 20 patients with SBP showed albumin to be superior in improving systemic hemodynamics.65
Because society guidelines are tied to evidence obtained from RCTs, recommendations are that patients with SBP should be treated with IV albumin (1.5 g/kg on day 1 and 1 g/kg on day 3) in addition to antibiotics.11,12 It is specified that patients with AKI and/or jaundice at the time of diagnosis of SBP are more likely to benefit from albumin.14,15 In addition, following the recommendations for the use of albumin in AKI (that involve lower doses of albumin as described below) would also be considered appropriate.14,15
Albumin use in HRS-AKI
AKI occurs in up to 50% of hospitalized patients with cirrhosis. The most common cause of AKI in cirrhosis is renal hypoperfusion (prerenal) from hypovolemia (eg, overdiuresis, diarrhea from lactulose), followed by acute tubular necrosis, an intrarenal type of injury, followed by HRS-AKI and with urinary obstruction (eg, prostate hypertrophy) being the least common.66,67
AKI is defined as an increase in serum creatinine (SCr) ≥0.3 mg/dL from baseline within 48 hours or an increase in SCr ≥1.5 times the baseline level that is known or presumed to have occurred within the prior 7 days or a urine output <0.5 mL/kg/h over a period of 6 hours (this assumes that patient has a urinary catheter). Volume expansion is a cornerstone in the diagnostic and therapeutic algorithm of AKI in cirrhosis. After workup to determine the type of AKI and discontinuation of diuretics, nephrotoxins, and other drugs that may affect kidney perfusion, volume resuscitation is recommended for a trial period of 24–48 hours. Guidance documents recommend using 5% albumin or crystalloids initially if the patient is clinically volume depleted with further albumin challenge in patients in whom creatinine does not improve or worsens.14,15,67 However, it is important to exercise caution when fluids or albumin are administered in patients with AKI to avoid the development of fluid overload and pulmonary edema.
The pathophysiology of HRS is the extreme of the spectrum of abnormalities that lead to cirrhotic ascites, with maximal peripheral vasodilatation and maximal activation of the renin-angiotensin-aldosterone system, leading to renal vasoconstriction, a further decrease in renal perfusion pressure and subsequently filtration rate and urine output.61 Previously, the acute form of HRS was called HRS-1 and was defined as a doubling of initial SCr to a level >2.5 mg/dL in <2 weeks. For greater clarity and to align with AKI criteria as defined by consensus,68 HRS-1 was renamed as HRS-AKI and defined in the same manner as AKI, based on changes in SCr rather than absolute threshold values.69
Because vasodilatation (with a consequent reduction in EABV and decreased renal perfusion pressure) is the main pathogenic mechanism in HRS-AKI, the mainstay of management consists of the use of vasoconstrictors with terlipressin being the one most used. Changes in mean arterial pressure induced by vasoconstrictors have been shown to correlate with changes in SCr.70,71 Albumin has been administered concomitant to vasoconstrictors in HRS-AKI with the hypothesis that increasing intravascular volume would further increase EABV and renal perfusion pressure.
The benefit of adding albumin to vasoconstrictor therapy in HRS-AKI has only been examined in one small nonrandomized proof-of-concept study that compared terlipressin alone versus terlipressin plus albumin. It showed that the reversal of HRS (decrease in SCr to a level <1.5 mg/dL) was more likely to occur with combination therapy (77%) than with terlipressin alone (25%) and was associated with improvement in plasma renin activity and surrogates of kidney function.72 The recommended dose of albumin has varied widely, but a common approach is to give 100 mg the first day (in 4 divided doses) followed by 20–40 g/d thereafter. A dose-response relationship between infused albumin and survival in patients with HRS-AKI was suggested by a meta-analysis of 19 clinical studies although it may have been confounded by higher cumulative doses of albumin in patients surviving longer.73 A meta-analysis of 9 RCTs including a total of 473 patients with HRS-AKI (defined by doubling of SCr to >2.5 mg/dL) showed that terlipressin plus albumin was associated with a beneficial effect on the reversal of HRS-AKI and mortality when compared with placebo.74 In the most recent, largest pivotal RCT of terlipressin plus albumin versus placebo plus albumin in the management of HRS-AKI (not included in the meta-analysis), the CONFIRM trial, HRS-AKI reversal occurred in a significantly higher proportion of patients randomized to terlipressin plus albumin.75 However, deaths from cardiopulmonary complications, mainly pulmonary edema, occurred more frequently in patients who received terlipressin and albumin compared with those in the placebo group.75,76 Serum albumin levels at the time of randomization were already high (3.7 in the terlipressin group and 4.0 in the placebo group) and during the trial, subjects received more i.v. albumin (199 g in the terlipressin group; 240 g in the placebo group). Therefore, it is plausible that albumin infusion was a major contributor to these cardiopulmonary complications which had already been described in the meta-analysis and occurred particularly in patients with ACLF grade 3.74
Notably, none of the RCTs of terlipressin versus placebo have defined HRS-AKI using the most current definition. In the CONFIRM trial, HRS-AKI was defined as a doubling of SCr to a level >2.25 mg/dL) and patients had a mean creatinine of 3.5 mg/dL at randomization.75 With the new definition, lower baseline SCr levels could meet HRS-AKI criteria and it is likely that a shorter duration of vasoconstrictor/albumin therapy would be required, particularly since the definition of reversal of HRS-AKI now only requires a return of SCr to a level within 0.3 mg/dL of baseline (pre-AKI). Society guidelines recommend the use of vasoconstrictor drugs in combination with albumin as the treatment of choice for HRS-AKI, with terlipressin being the preferred drug, administered either as i.v. bolus or continuous i.v. infusion.14,15 Both guidelines recommend that patients should be closely monitored for the possible development of side effects of vasoconstrictors and albumin, including ischemic complications and pulmonary edema.14,15
Further possible and controversial indications of albumin
Because of the many recommended indications of albumin and its purported benefits beyond volume expansion, many practitioners have considered this as a “golden bullet,” the answer to every complication of cirrhosis.19 This has led to the widespread use of albumin for indications for which there is a lack of strong scientific evidence or even for indications for which evidence discourages the use of albumin. These indications and the rationale for the use of albumin in them are outlined in the following section.
Infections other than SBP
While, as shown above, the use of albumin in patients with SBP is recommended by international society guidelines, benefits of the use of albumin in patients with a non-SBP infection are not clear. One would assume that, worsening systemic inflammation associated with any bacterial infection, would lead to worsening of vasodilation, renal vasoconstriction, AKI, and a higher mortality. However, patients with non-SBP infections have better outcomes than those with SBP. An important distinction is that, while all patients with SBP have, by definition, ascites, those with non-SBP infection do not necessarily have ascites. That is, the hemodynamic alterations that lead to ascites formation make patients with ascites more prone to develop further vasodilatation and consequently kidney injury and death.
In fact, in the first randomized non–placebo-controlled study of albumin in non-SBP infections by Guevara et al,77 with the most common infections being pneumonia and urinary tract infection, there were no differences in 30-day mortality (primary outcome) or development of AKI between patients who received albumin and those who did not, although in post hoc multivariable analyses, albumin was an independent predictive factor of survival. Notably, 25%–30% of the patients did not have ascites.77
In another larger randomized non–placebo-controlled study by Thevenot et al78 including patients with a higher MELD score and non-SBP infection (also predominantly pneumonia and urinary tract infection) with 13% having severe sepsis, albumin use was not associated with a significant benefit in either the 3-month development of renal dysfunction (primary outcome) or in 3-month survival (secondary endpoint). As in the study by Guevara and colleagues, a third of the patients did not have ascites. Of note, pulmonary edema developed in 8 of 96 patients in the albumin group of whom 2 died, leading to the premature discontinuation of the study. Investigators cautioned against the use of albumin in the “sickest cirrhotic patients.”78
A third multicenter study (INFECIR-2) was recently performed in a study population that was more similar to the Guevara et al’s study.79 The study did not show significant differences in in-hospital and 90-day mortality rates between treatment arms, confirming the results of the prior RCTs.79 In fact, the trial had to be terminated prematurely when less than a third of the planned number of patients had been enrolled because of lack of effect on survival and, as in the 2 other studies, because of a greater incidence of pulmonary edema in the albumin treatment group. In post hoc analyses, albumin prevented secondary bacterial infection, decreased inflammatory biomarkers, and improved circulatory dysfunction.79
The dose of albumin used in the 3 trials are those recommended for SBP that, as mentioned above, were empirical: 1.5 g/kg of body weight on day 1 and 1 g/kg of body weight on day 3. Since the 3 trials had pulmonary edema as the main complication it is obvious that doses of albumin recommended are too high and should not be weight-based. This would be particularly relevant for patients with NASH, the currently most common etiology of cirrhosis in the United States, where obesity is more prevalent compared with other etiologies. Importantly, patients with pneumonia were the most prone to develop pulmonary edema in these trials and special attention should be placed when volume expanding these patients independent of the indication.
Based on 3 negative RCTs and a meta-analysis,80 one cannot recommend albumin in the management of infections other than SBP, in a similar way as for SBP. There may be a subgroup of these patients that may benefit from albumin use and these may be those with AKI, for whom albumin is already recommended at lower doses (see above).
Hyponatremia
As shown in Figure 2, hyponatremia in patients with cirrhosis and ascites is also the result of inflammation and the hemodynamic abnormalities that lead not only to the secretion of kidney vasoconstrictive factors (eg, renin and angiotensin) but also to the secretion of antidiuretic hormone. Therefore, albumin administration has a rationale in the management of hyponatremia in decompensated cirrhosis.
In a proof-of-concept study, i.v. albumin led to resolution of hyponatremia in 3 patients with cirrhosis and hyponatremia while it was unsuccessful in one patient with acute liver failure.81 In a large cohort study of hospitalized patients with cirrhosis enrolled prospectively in the North American Consortium for the Study of End-Stage Liver Disease (NACSELD) database, patients who had received i.v. albumin had a higher sodium level and a small, albeit statistically significantly, rate of hyponatremia resolution (69% vs. 61%, p = 0.008) compared with those who did not.82
There are no fully published randomized controlled studies analyzing the effect of albumin on hyponatremia (as primary outcome) and therefore a recommendation to use albumin in this setting cannot be made. New or ongoing well-designed studies (eg, ALBUCAT NCT03941405) should address the topic of hyponatremia since it is an important predictor of mortality in patients on the waitlist for liver transplantation.
HE
Decreased brain perfusion, similar to that occurring in the kidney, seems to be one of the mechanisms in the pathogenesis of overt HE (OHE) in cirrhosis.48 In addition, systemic inflammation may also play a role in its pathogenesis.48 Albumin, by binding neuroinflammatory toxins and by improving circulatory dysfunction could have a beneficial effect on HE. Importantly, one must not forget evidence from over a century ago pointing toward ammonia as a major player in the pathogenesis of OHE.83 Overdiuresis is a common precipitant of OHE in cirrhosis lead to an increase in BUN which in turn leads to an increase in the synthesis of ammonia. Therefore, in the presence of volume contraction and increased BUN, volume repletion with crystalloids should be the main strategy in treating OHE.
Interestingly, in a proof-of-concept small study of 15 patients with alcohol-associated cirrhosis and diuretic-induced OHE (grade 2–4), volume expansion with albumin in 8 patients led to significant improvement in HE, while in another 7 patients that received colloid this improvement was not observed even though both treatments led to a similar reduction in plasma ammonia concentration. Because plasma malondialdehyde, a marker of oxidative stress, was reduced only in the group treated with albumin, the authors conclude that the benefit observed with albumin could have been due to binding of reactive oxygen species by albumin.84
Another similar study in which patients with cirrhosis and an acute episode of OHE, mostly due to infection or overdiuresis, were randomized to albumin (n = 26) or isotonic saline (n = 30) showed no differences in the percentage of patients who had resolved OHE at day 4 between groups.85 Because in this trial there was evidence of better survival at day 90 after albumin administration, a subsequent trial of albumin versus isotonic saline in patients with HE grade 2 or higher with a primary outcome of death at 90 days was performed. Only 82 (out of 116 planned patients) could be enrolled and, although post hoc analysis showed some benefits in clinical outcome, the primary results were negative.86
Regarding the prevention of HE, in a study that included 23 patients receiving albumin after placement of the TIPS, no beneficial effect regarding the development of post-TIPS OHE was observed when albumin was compared with historical controls.87
Although not directly addressing the treatment of OHE, a recent double-blind, placebo-controlled trial of 48 outpatients with prior OHE and current covert HE, albumin infusions over 5 weeks improved cognitive function and psychosocial quality of life.88
Based on a few small studies performed to date, evidence is lacking to support the use of albumin in the management or prevention of OHE. Additional studies are warranted and should probably be focused on the management of OHE not responding to standard therapy with volume expansion (in diuresis-induced OHE) and/or ammonia-reducing strategies that constitute the standard-of-care.
Albumin for resuscitation, septic shock, and ACLF
Albumin has been used for the treatment of shock in patients with and without cirrhosis.89,90 A recent study demonstrated that a 5% albumin infusion performed better than saline solution in reversing sepsis-induced hypotension in patients with cirrhosis.91 Older studies have demonstrated that this strategy may even be cost-effective.92 Nevertheless, data are scarce even though in clinical practice albumin is widely used for resuscitation. Important questions remain including whether 5% or 20% albumin would be preferred, the specific treatment regime, whether it should be first-line treatment or whether its use should be restricted to patients who do not respond to saline solution or crystalloid, etc. Until these questions are clarified, the use of albumin as sole/primary volume expansion management of shock will be up to the intensive care specialist. In the general intensive care unit population and based on the Surviving Sepsis Campaign guidelines,93 it is suggested that albumin be used in patients with sepsis or septic shock who have received large volumes of crystalloids rather than continuing with crystalloids alone. These recommendations are based on very large clinical trials in which patients with cirrhosis are, unfortunately, underrepresented.
Treatment of albumin in patients with ACLF will depend on the type of “organ failure (OF)” that is present in each patient. The 6 OFs that define ACLF are kidney, liver (bilirubin), brain (encephalopathy), coagulation system, lung, and cardiovascular (hypotension).14
The most common OF in ACLF is AKI and, given that this is a complication of cirrhosis for which albumin administration is recommended, it is clear that albumin would be indicated in the presence of AKI at the stage and at doses recommended for AKI without ACLF. As mentioned above, in patients with ACLF-3 and HRS-AKI, terlipressin (and therefore albumin) is not or only cautiously recommended as these patients are more prone to develop cardiopulmonary complications.75 Fluid management is particularly challenging in patients with ACLF who have multiple OFs. In this setting, careful assessment and monitoring of volume status (clinical, point of care ultrasonography) should be implemented to tailor albumin/vasoconstrictor use.94
An interesting concept is to use the properties of albumin beyond volume expansion in the setting of albumin dialysis extracorporeal devices such as MARS or Prometheus as well as plasma exchange that would avoid volume overload.44,95 These machines would be useful to bridge these very sick patients to liver transplantation and/or to make them more suitable candidates for transplant.95,96 Larger studies (eg, APACHE-trial NCT03702920) are necessary to further investigate the effectiveness of these devices in patients with ACLF.
Albumin in the prevention of relevant outcomes in hospitalized patients with cirrhosis and ascites
The ATTIRE study, that included inpatients with decompensated cirrhosis, hypothesized that albumin infusion would prevent bacterial infection, AKI, and/or death.97 This was an open-label randomized trial that included 777 patients hospitalized with a decompensating event, mostly new or worsening ascites. Patients were randomized to either standard-of-care (albumin administration for recommended indications: LVP, SBP, AKI, HRS-AKI) or to albumin infusions aimed at maintaining a serum albumin level ≥3.0 g/L throughout hospitalization. The study was negative as it failed to find differences in the composite primary outcome between daily albumin and standard-of-care (30% in each group experienced the primary outcome). In addition, the experimental group received 10 times the amount of albumin that the standard-of-care group received and, consequently, had more severe serious adverse events, especially pulmonary edema. Again, although experimentally these investigators had shown that albumin could improve immunity by improving B-cell function45 and macrophage-mediated hepatic inflammation,46 the lack of efficacy of albumin in the ATTIRE trial indicates that in these patients the severity of disease is more relevant than the experimentally demonstrated potential benefits of albumin. Importantly, the ATTIRE study demonstrates that targeting albumin administration to reach predefined serum albumin levels is not appropriate for clinical practice and can lead to volume overload.
An important conclusion derived from most studies regarding albumin infusion is that albumin administration should be monitored not based on serum albumin levels but on volume repletion status. Future studies in inpatients should explore noninvasive methods to evaluate volume repletion so that albumin dosing could be used in a rational way.
Albumin in the prevention of relevant outcomes in outpatients with cirrhosis and ascites Infection
Another controversial use of albumin is its long-term use in outpatients with ascites. An initial proof-of-concept study in 45 patients suggested that twice weekly albumin infusions were associated with an improved survival, compared with historical controls.98 Subsequently, 3 RCTs have analyzed the effect of chronic weekly administration of albumin in patients with cirrhosis and ascites on survival (primary outcome).99–101 The first RCT, performed in patients with new onset ascites99 showed an improvement in survival and a lower recurrence of ascites with i.v. albumin (25 g/wk in the first year and 25 g every 2 wk thereafter). The second, the ANSWER trial, was a large multicenter Italian open-label study that compared weekly i.v. albumin infusions versus standard-of-care in patients with a median MELD of 12 in whom ascites persisted despite diuretic therapy but who did not meet criteria for refractory ascites.100 The primary outcome, 18-month mortality, was significantly lower in patients randomized to albumin and was associated with a significant decrease in the number of paracenteses required and, importantly, with a reduction of other complications of ascites such as refractory ascites, hyponatremia, and HRS-AKI. Unfortunately, the study was not placebo-controlled and patients randomized to albumin received a loading dose of 40 g twice weekly for 2 weeks and then 40 g weekly thereafter. The lack of a placebo arm is associated with inherent biases including the intensity of medical supervision and the subjectivity of some of the outcomes such as the need for LVP.100 Still, this study involved >400 patients with a MELD score between 12 and 13, with the vast majority being Child B. The albumin effect was specially reflected in a lower rate of infection and renal dysfunction. In a recent post hoc analysis of this study, the authors demonstrated that patients reaching on-treatment serum albumin level of 4 g/dL or greater 1 month after randomization was had a lower 18-month mortality.102 In addition, baseline serum albumin levels and MELD score could predict whether this goal albumin level would be achievable. That is, the sicker the patient, the more difficult it will be to reach these targets and the less the patient will respond to albumin.
In contrast, a better-designed, placebo-controlled double-blind randomized study comparing albumin plus midrodrine versus a double placebo in patients with ascites and a median MELD score of 16 showed no differences in mortality or other complications of ascites (MATCH).101 They could demonstrate improvement in hemodynamics as evidenced by decreased aldosterone and noradrenaline levels as well as improvement in hyponatremia, but these effects were not sufficient to affect the primary outcome at 12 months.
The exact population that will benefit from long-term outpatient administration of albumin remains to be determined. Other issues such as cost of albumin and travel to receive i.v. albumin, loss of working days, as well as the logistics of albumin administration in the outpatient clinic would have to be taken into account. Future RCTs (eg, PRECIOSA-study NCT03451292) may give more guidance regarding the role of long-term albumin administration in patients after an acute decompensation, while stratification based on biomarkers (eg, Alb-Trial NCT05056220) may identify patients that most benefit from this therapy.
CONCLUSION AND FUTURE DIRECTIONS
Intravenous albumin use in cirrhosis has been an important therapeutic advance in the management of many aspects of decompensated cirrhosis (Table 1). However, as we have shown, it is not the panacea in this setting and its future use requires refinement of the indications and doses as well as the identification of targets/markers by which to monitor and guide its use to avoid serious adverse events that have arisen over time with its more liberal and widespread use.
Another issue in the interpretation of the results is that the population of patients included in the different trials is often heterogeneous, making it difficult to compare results among trials and to draw firm conclusions. Therefore, there is still a clear need for “well-designed” trials.
The usefulness of albumin relates to its 2 main properties, first, by improving the clinical phenotype (eg, AKI, shock) of the patient with decompensated cirrhosis through its oncotic and consequent volume expansion properties, and by modifying triggers of decompensation (eg, systemic inflammation, oxidative stress).
While for the purposes of volume expansion, a sufficient (albeit, not excessive) amount of albumin is required, for the second, the quality of albumin and its concentration may be more important. Thereby, the use of 5%, 20%, or 40% albumin solutions of albumin may have to be chosen depending on the desired effect. For example, in patients with ACLF, one may even consider not infusing albumin but using extracorporeal treatments of plasma using albumin properties.
Albumin should only be used in the different settings for which it is currently recommended by society guidelines with doses and target levels (if any) tailored to the severity of liver disease and to cardiopulmonary status. Data from quality studies are required before recommendations can be extended to other settings, in the knowledge that performing such trials will be challenging given the “noise” that widespread use of albumin will introduce. Ideally, placebo-controlled multicenter trials and/or careful hemodynamic/mechanistic studies should be performed to establish patient population criteria and dosages.
Importantly, while serum albumin levels are good biomarkers of outcomes in patients with cirrhosis, they are not good biomarkers of response to albumin therapy. Biomarkers of response to albumin are required in clinical practice and consist of those that will reflect intravascular volume repletion and those that will evaluate the quality of albumin on its capacity to bind toxic metabolites (eg, Alb-Trial NCT05056220).
In the clinical setting, attention will have to be paid to the logistics of albumin administration, whether it is in the hospital, day clinic, ambulatory services, or in the patient’s home, particularly if longer term use in an outpatient setting is anticipated. The issue of its high cost will need to be redefined and the development of flexible concentrations per unit or even the use of synthetic albumin or recombinant albumin would be beneficial to the field.
Other qualities of albumin that would need to be further explored would include its capacity to bind drugs and transport them to the site of action, even into specific cells. In this setting, albumin would act as a “spacer” allowing the prolongation of circulation times of specific molecules,103,104 particularly small molecules that inhibit powerful kinases, such as ROCK (rho-associated protein kinase) that would be transported to their final destination and slowly released from albumin.103,104 The same may be true for cancer drugs or other inflammatory diseases.
The field of therapeutics in decompensated cirrhosis has advanced enormously with the goal of preventing/treating further decompensation and ACLF and optimizing or even preventing the need for liver transplantation, particularly once the etiological factor can be eliminated. In summary, there is hope for hepatology.
Acknowledgments
FUNDING INFORMATION
Jonel Trebicka is supported by German Research Foundation (DFG) project ID 403224013—SFB 1382 (A09), by the German Federal Ministry of Education and Research (BMBF) for the DEEP-HCC project and by the Hessian Ministry of Higher Education, Research and the Arts (HMWK) for the ENABLE and ACLF-I cluster projects. Jonel Trebicka is supported by the MICROB-PREDICT (project ID 825694), DECISION (project ID 847949), and IHMCSA (project ID 964590) projects have received funding from the European Union’s Horizon 2020 research and innovation program. Guadalupe Garcia-Tsao is supported by NIH P30 DK34989.
CONFLICTS OF INTEREST
Jonel Trebicka consults and is on the speakers’ bureau for Alexion, CSL Behring, and Grifols. He consults for Boehringer Ingelheim, Genfit, Mallickrodt, and Versantis. He is on the speakers’ bureau for Falk and Gore. Guadalupe Garcia-Tsao has no conflicts to report.
Footnotes
Abbreviations: AASLD, American Association for the Study of Liver Diseases; ACLF, acute-on-chronic liver failure; AKI, acute kidney injury; APASL, Asian Pacific Association for the Study of Liver; BUN, blood urea nitrogen; CSPH, clinically significant portal hypertension; EABV, effective arterial blood volume; EASL, European Association for the Study of the Liver; HRS, hepatorenal syndrome; LT, liver transplantation; LVP, large-volume paracentesis; MELD, Model for End-Stage Liver Disease; NACSELD, North American Consortium for the Study of End-Stage Liver Disease; OF, organ failure; OHE, overt HE; PCD, paracentesis circulatory dysfunction; RAA, renin-angiotensin-aldosterone; RBC, red blood cell; RCT, randomized controlled trial; SBP, spontaneous bacterial peritonitis; SCr, serum creatinine.
Contributor Information
Jonel Trebicka, Email: Jonel.Trebicka@ukmuenster.de.
Guadalupe Garcia-Tsao, Email: guadalupe.garcia-tsao@yale.edu.
REFERENCES
- 1.Fasano M, Curry S, Terreno E, Galliano M, Fanali G, Narciso P, et al. The extraordinary ligand binding properties of human serum albumin. IUBMB Life. 2005;57:787–796. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Martinez R, Caraceni P, Bernardi M, Gines P, Arroyo V, Jalan R. Albumin: pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology. 2013;58:1836–1846. [DOI] [PubMed] [Google Scholar]
- 3.Gortzen J, Schierwagen R, Bierwolf J, Klein S, Uschner FE, van der Ven PF, et al. Interplay of matrix stiffness and c-SRC in hepatic fibrosis. Front Physiol. 2015;6:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans DC, Corkins MR, Malone A, Miller S, Mogensen KM, Guenter P, et al. The use of visceral proteins as nutrition markers: an ASPEN Position Paper. Nutr Clin Pract. 2021;36:22–28. [DOI] [PubMed] [Google Scholar]
- 5.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. [DOI] [PubMed] [Google Scholar]
- 6.Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodes J. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151–1157. [DOI] [PubMed] [Google Scholar]
- 7.Ripoll C, Groszmann R, Garcia-Tsao G, Grace N, Burroughs A, Planas R, et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481–488. [DOI] [PubMed] [Google Scholar]
- 8.Ripoll C, Bari K, Garcia-Tsao G. Serum albumin can identify patients with compensated cirrhosis with a good prognosis. J Clin Gastroenterol. 2015;49:613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rabiee A, Deng Y, Ciarleglio M, Chan JL, Pons M, Genesca J, et al. Noninvasive predictors of clinically significant portal hypertension in NASH cirrhosis: validation of ANTICIPATE models and development of a lab-based model. Hepatol Commun. 2022;6:3324–3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen AM, Therneau TM, Ahmed OT, Gidener T, Mara KC, Larson JJ, et al. Clinical course of non-alcoholic fatty liver disease and the implications for clinical trial design. J Hepatol. 2022;77:1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim WR, Mannalithara A, Heimbach JK, Kamath PS, Asrani SK, Biggins SW, et al. MELD 3.0: The model for end-stage liver disease updated for the modern era. Gastroenterology. 2021;161:1887–1895 e1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C, Baveno VIIF, et al. Renewing consensus in portal hypertension. J Hepatol. 2022;76:959–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arroyo V, Angeli P, Moreau R, Jalan R, Claria J, Trebicka J, et al. The systemic inflammation hypothesis: towards a new paradigm of acute decompensation and multiorgan failure in cirrhosis. J Hepatol. 2021;74:670–685. [DOI] [PubMed] [Google Scholar]
- 14.European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018;69:406-460. [DOI] [PubMed] [Google Scholar]
- 15.Biggins SW, Angeli P, Garcia-Tsao G, Gines P, Ling SC, Nadim MK, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014–1048. [DOI] [PubMed] [Google Scholar]
- 16.Bernardi M, Angeli P, Claria J, Moreau R, Gines P, Jalan R, et al. Albumin in decompensated cirrhosis: new concepts and perspectives. Gut. 2020;69:1127–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jagdish RK, Maras JS, Sarin SK. Albumin in advanced liver diseases: The good and bad of a drug! Hepatology. 2021;74:2848–2862. [DOI] [PubMed] [Google Scholar]
- 18.Trebicka J. Role of albumin in the treatment of decompensated liver cirrhosis. Curr Opin Gastroenterol. 2022;38:200–205. [DOI] [PubMed] [Google Scholar]
- 19.Caraceni P, Pavesi M, Baldassarre M, Bernardi M, Arroyo V. The use of human albumin in patients with cirrhosis: A European survey. Expert Rev Gastroenterol Hepatol. 2018;12:625–632. [DOI] [PubMed] [Google Scholar]
- 20.Beeken WL, Volwiler W, Goldsworthy PD, Garby LE, Reynolds WE, Stogsdill R, et al. Studies of I-131-albumin catabolism and distribution in normal young male adults. J Clin Invest. 1962;41:1312–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peters T, Jr. Serum albumin. Adv Protein Chem. 1985;37:161–245. [DOI] [PubMed] [Google Scholar]
- 22.Quinlan GJ, Martin GS, Evans TW. Albumin: Biochemical properties and therapeutic potential. Hepatology. 2005;41:1211–1219. [DOI] [PubMed] [Google Scholar]
- 23.Casulleras M, Flores-Costa R, Duran-Guell M, Alcaraz-Quiles J, Sanz S, Titos E, et al. Albumin internalizes and inhibits endosomal TLR signaling in leukocytes from patients with decompensated cirrhosis. Sci Transl Med. 2020;12:eaax5135. [DOI] [PubMed] [Google Scholar]
- 24.Wells HS, Youmans JB, Miller DG. A formula and nomogram for the estimation of the osmotic pressure of colloids from the albumin and total protein concentrations of human blood sera. J Clin Invest. 1933;12:1103–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaudhury C, Mehnaz S, Robinson JM, Hayton WL, Pearl DK, Roopenian DC, et al. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J Exp Med. 2003;197:315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gines P, Tito L, Arroyo V, Planas R, Panes J, Viver J, et al. Randomized comparative study of therapeutic paracentesis with and without intravenous albumin in cirrhosis. Gastroenterology. 1988;94:1493–1502. [DOI] [PubMed] [Google Scholar]
- 27.Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403–409. [DOI] [PubMed] [Google Scholar]
- 28.Luca A, Garcia-Pagan JC, Bosch J, Feu F, Jimenez W, Gines A, et al. Beneficial effects of intravenous albumin infusion on the hemodynamic and humoral changes after total paracentesis. Hepatology. 1995;22:753–758. [PubMed] [Google Scholar]
- 29.Gines A, Fernandez-Esparrach G, Monescillo A, Vila C, Domenech E, Abecasis R, et al. Randomized trial comparing albumin, dextran 70, and polygeline in cirrhotic patients with ascites treated by paracentesis. Gastroenterology. 1996;111:1002–1010. [DOI] [PubMed] [Google Scholar]
- 30.Naldi M, Baldassarre M, Domenicali M, Bartolini M, Caraceni P. Structural and functional integrity of human serum albumin: Analytical approaches and clinical relevance in patients with liver cirrhosis. J Pharm Biomed Anal. 2017;144:138–153. [DOI] [PubMed] [Google Scholar]
- 31.Bernardi M, Caraceni P. Novel perspectives in the management of decompensated cirrhosis. Nat Rev Gastroenterol Hepatol. 2018;15:753–764. [DOI] [PubMed] [Google Scholar]
- 32.Oettl K, Birner-Gruenberger R, Spindelboeck W, Stueger HP, Dorn L, Stadlbauer V, et al. Oxidative albumin damage in chronic liver failure: Relation to albumin binding capacity, liver dysfunction and survival. J Hepatol. 2013;59:978–983. [DOI] [PubMed] [Google Scholar]
- 33.Domenicali M, Baldassarre M, Giannone FA, Naldi M, Mastroroberto M, Biselli M, et al. Posttranscriptional changes of serum albumin: Clinical and prognostic significance in hospitalized patients with cirrhosis. Hepatology. 2014;60:1851–1860. [DOI] [PubMed] [Google Scholar]
- 34.Baldassarre M, Domenicali M, Naldi M, Laggetta M, Giannone FA, Biselli M, et al. Albumin homodimers in patients with cirrhosis: Clinical and prognostic relevance of a novel identified structural alteration of the molecule. Sci Rep. 2016;6:35987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiao R, Siflinger-Birnboim A, Lum H, Tiruppathi C, Malik AB. Albumin and Ricinus communis agglutinin decrease endothelial permeability via interactions with matrix. Am J Physiol. 1993;265:C439–C446. [DOI] [PubMed] [Google Scholar]
- 36.Iwakiri Y, Trebicka J. Portal hypertension in cirrhosis: pathophysiological mechanisms and therapy. JHEP Rep. 2021;3:100316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lang JD, Jr, Figueroa M, Chumley P, Aslan M, Hurt J, Tarpey MM, et al. Albumin and hydroxyethyl starch modulate oxidative inflammatory injury to vascular endothelium. Anesthesiology. 2004;100:51–58. [DOI] [PubMed] [Google Scholar]
- 38.Praktiknjo M, Trebicka J, Carnevale R, Pastori D, Queck A, Ettorre E, et al. Von Willebrand and factor VIII portosystemic circulation gradient in cirrhosis: Implications for portal vein thrombosis. Clin Transl Gastroenterol. 2020;11:e00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Queck A, Carnevale R, Uschner FE, Schierwagen R, Klein S, Jansen C, et al. Role of portal venous platelet activation in patients with decompensated cirrhosis and TIPS. Gut. 2020;69:1535–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tobias MD, Wambold D, Pilla MA, Greer F. Differential effects of serial hemodilution with hydroxyethyl starch, albumin, and 0.9% saline on whole blood coagulation. J Clin Anesth. 1998;10:366–371. [DOI] [PubMed] [Google Scholar]
- 41.Kim SB, Chi HS, Park JS, Hong CD, Yang WS. Effect of increasing serum albumin on plasma D-dimer, von Willebrand factor, and platelet aggregation in CAPD patients. Am J Kidney Dis. 1999;33:312–317. [DOI] [PubMed] [Google Scholar]
- 42.Chen TA, Tsao YC, Chen A, Lo GH, Lin CK, Yu HC, et al. Effect of intravenous albumin on endotoxin removal, cytokines, and nitric oxide production in patients with cirrhosis and spontaneous bacterial peritonitis. Scand J Gastroenterol. 2009;44:619–625. [DOI] [PubMed] [Google Scholar]
- 43.Duran-Guell M, Flores-Costa R, Casulleras M, Lopez-Vicario C, Titos E, Diaz A, et al. Albumin protects the liver from tumor necrosis factor alpha-induced immunopathology. FASEB J. 2021;35:e21365. [DOI] [PubMed] [Google Scholar]
- 44.Fernandez J, Claria J, Amoros A, Aguilar F, Castro M, Casulleras M, et al. Effects of albumin treatment on systemic and portal hemodynamics and systemic inflammation in patients with decompensated cirrhosis. Gastroenterology. 2019;157:149–162. [DOI] [PubMed] [Google Scholar]
- 45.O’Brien AJ, Fullerton JN, Massey KA, Auld G, Sewell G, James S, et al. Immunosuppression in acutely decompensated cirrhosis is mediated by prostaglandin E2. Nat Med. 2014;20:518–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.China L, Freemantle N, Forrest E, Kallis Y, Ryder SD, Wright G, et al. Targeted albumin therapy does not improve short-term outcome in hyponatremic patients hospitalized with complications of cirrhosis: data from the ATTIRE Trial. Am J Gastroenterol. 2021;116:2292–2295. [DOI] [PubMed] [Google Scholar]
- 47.Trebicka J, Reiberger T, Laleman W. Gut-liver axis links portal hypertension to acute-on-chronic liver failure. Visc Med. 2018;34:270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trebicka J. Predisposing factors in acute-on-chronic liver failure. Semin Liver Dis. 2016;36:167–173. [DOI] [PubMed] [Google Scholar]
- 49.Trebicka J, Amoros A, Pitarch C, Titos E, Alcaraz-Quiles J, Schierwagen R, et al. Addressing profiles of systemic inflammation across the different clinical phenotypes of acutely decompensated cirrhosis. Front Immunol. 2019;10:476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruiz-del-Arbol L, Monescillo A, Jimenez W, Garcia-Plaza A, Arroyo V, Rodes J. Paracentesis-induced circulatory dysfunction: mechanism and effect on hepatic hemodynamics in cirrhosis. Gastroenterology. 1997;113:579–586. [DOI] [PubMed] [Google Scholar]
- 51.Kao HW, Rakov NE, Savage E, Reynolds TB. The effect of large volume paracentesis on plasma volume—a cause of hypovolemia? Hepatology. 1985;5:403–407. [DOI] [PubMed] [Google Scholar]
- 52.Simon DM, McCain JR, Bonkovsky HL, Wells JO, Hartle DK, Galambos JT. Effects of therapeutic paracentesis on systemic and hepatic hemodynamics and on renal and hormonal function. Hepatology. 1987;7:423–429. [DOI] [PubMed] [Google Scholar]
- 53.Panos MZ, Moore K, Vlavianos P, Chambers JB, Anderson JV, Gimson AE, et al. Single, total paracentesis for tense ascites: Sequential hemodynamic changes and right atrial size. Hepatology. 1990;11:662–667. [DOI] [PubMed] [Google Scholar]
- 54.Bernardi M, Caraceni P, Navickis RJ, Wilkes MM. Albumin infusion in patients undergoing large-volume paracentesis: A meta-analysis of randomized trials. Hepatology. 2012;55:1172–1181. [DOI] [PubMed] [Google Scholar]
- 55.Simonetti RG, Perricone G, Nikolova D, Bjelakovic G, Gluud C. Plasma expanders for people with cirrhosis and large ascites treated with abdominal paracentesis. Cochrane Database Syst Rev. 2019;6:CD004039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alessandria C, Elia C, Mezzabotta L, Risso A, Andrealli A, Spandre M, et al. Prevention of paracentesis-induced circulatory dysfunction in cirrhosis: Standard vs half albumin doses. A prospective, randomized, unblinded pilot study. Dig Liver Dis. 2011;43:881–886. [DOI] [PubMed] [Google Scholar]
- 57.Arora V, Vijayaraghavan R, Maiwall R, Sahney A, Thomas SS, Ali R, et al. Paracentesis-induced circulatory dysfunction with modest-volume paracentesis is partly ameliorated by albumin infusion in acute-on-chronic liver failure. Hepatology. 2020;72:1043–1055. [DOI] [PubMed] [Google Scholar]
- 58.Arroyo V, Moreau R, Jalan R. Acute-on-chronic liver failure. N Engl J Med. 2020;382:2137–2145. [DOI] [PubMed] [Google Scholar]
- 59.Bari K, Minano C, Shea M, Inayat IB, Hashem HJ, Gilles H, et al. The combination of octreotide and midodrine is not superior to albumin in preventing recurrence of ascites after large-volume paracentesis. Clin Gastroenterol Hepatol. 2012;10:1169–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Follo A, Llovet JM, Navasa M, Planas R, Forns X, Francitorra A, et al. Renal impairment after spontaneous bacterial peritonitis in cirrhosis: incidence, clinical course, predictive factors and prognosis. Hepatology. 1994;20:1495–1501. [DOI] [PubMed] [Google Scholar]
- 61.Wong F. Recent advances in our understanding of hepatorenal syndrome. Nat Rev Gastroenterol Hepatol. 2012;9:382–391. [DOI] [PubMed] [Google Scholar]
- 62.Fernandez J, Navasa M, Garcia-Pagan JC, J GA, Jimenez W, Bosch J, et al. Effect of intravenous albumin on systemic and hepatic hemodynamics and vasoactive neurohormonal systems in patients with cirrhosis and spontaneous bacterial peritonitis. J Hepatol. 2004;41:384–390. [DOI] [PubMed] [Google Scholar]
- 63.Batool S, Waheed MD, Vuthaluru K, Jaffar T, Garlapati SKP, Bseiso O, et al. Efficacy of intravenous albumin for spontaneous bacterial peritonitis infection among patients with cirrhosis: a meta-analysis of randomized control trials. Cureus. 2022;14:e33124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salerno F, Navickis RJ, Wilkes MM. Albumin infusion improves outcomes of patients with spontaneous bacterial peritonitis: a meta-analysis of randomized trials. Clin Gastroenterol Hepatol. 2013;11:123–130.e1. [DOI] [PubMed] [Google Scholar]
- 65.Fernandez J, Monteagudo J, Bargallo X, Jimenez W, Bosch J, Arroyo V, et al. A randomized unblinded pilot study comparing albumin versus hydroxyethyl starch in spontaneous bacterial peritonitis. Hepatology. 2005;42:627–634. [DOI] [PubMed] [Google Scholar]
- 66.Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology. 2008;48:2064–2077. [DOI] [PubMed] [Google Scholar]
- 67.Nadim MK, Garcia-Tsao G. Acute kidney injury in patients with cirrhosis. N Engl J Med. 2023;388:733–745. [DOI] [PubMed] [Google Scholar]
- 68.Nadim MK, Kellum JA, Davenport A, Wong F, Davis C, Pannu N, et al. Hepatorenal syndrome: the 8th International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2012;16:R23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Angeli P, Garcia-Tsao G, Nadim MK, Parikh CR. News in pathophysiology, definition and classification of hepatorenal syndrome: a step beyond the International Club of Ascites (ICA) consensus document. J Hepatol. 2019;71:811–822. [DOI] [PubMed] [Google Scholar]
- 70.Velez JC, Nietert PJ. Therapeutic response to vasoconstrictors in hepatorenal syndrome parallels increase in mean arterial pressure: a pooled analysis of clinical trials. Am J Kidney Dis. 2011;58:928–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Velez JCQ, Karakala N, Tayebi K, Wickman TJ, Mohamed M, Kovacic RA, et al. Responsiveness to vasoconstrictor therapy in hepatorenal syndrome type 1. Kidney360. 2023;4:e448–e456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ortega R, Gines P, Uriz J, Cardenas A, Calahorra B, De Las Heras D, et al. Terlipressin therapy with and without albumin for patients with hepatorenal syndrome: results of a prospective, nonrandomized study. Hepatology. 2002;36:941–948. [DOI] [PubMed] [Google Scholar]
- 73.Salerno F, Navickis RJ, Wilkes MM. Albumin treatment regimen for type 1 hepatorenal syndrome: a dose-response meta-analysis. BMC Gastroenterol. 2015;15:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Allegretti AS, Israelsen M, Krag A, Jovani M, Goldin AH, Schulman AR, et al. Terlipressin versus placebo or no intervention for people with cirrhosis and hepatorenal syndrome. Cochrane Database Syst Rev. 2017;6:CD005162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wong F, Pappas SC, Curry MP, Reddy KR, Rubin RA, Porayko MK, et al. Terlipressin plus albumin for the treatment of type 1 hepatorenal syndrome. N Engl J Med. 2021;384:818–828. [DOI] [PubMed] [Google Scholar]
- 76.Wong F, Pappas SC, Reddy KR, Vargas H, Curry MP, Sanyal A, et al. Terlipressin use and respiratory failure in patients with hepatorenal syndrome type 1 and severe acute-on-chronic liver failure. Aliment Pharmacol Ther. 2022;56:1284–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guevara M, Terra C, Nazar A, Sola E, Fernandez J, Pavesi M, et al. Albumin for bacterial infections other than spontaneous bacterial peritonitis in cirrhosis. A randomized, controlled study. J Hepatol. 2012;57:759–765. [DOI] [PubMed] [Google Scholar]
- 78.Thevenot T, Bureau C, Oberti F, Anty R, Louvet A, Plessier A, et al. Effect of albumin in cirrhotic patients with infection other than spontaneous bacterial peritonitis. A randomized trial. J Hepatol. 2015;62:822–830. [DOI] [PubMed] [Google Scholar]
- 79.Fernandez J, Angeli P, Trebicka J, Merli M, Gustot T, Alessandria C, et al. Efficacy of albumin treatment for patients with cirrhosis and infections unrelated to spontaneous bacterial peritonitis. Clin Gastroenterol Hepatol. 2020;18:963–973.e14. [DOI] [PubMed] [Google Scholar]
- 80.Wong YJ, Qiu TY, Tam YC, Mohan BP, Gallegos-Orozco JF, Adler DG. Efficacy and safety of IV albumin for non-spontaneous bacterial peritonitis infection among patients with cirrhosis: a systematic review and meta-analysis. Dig Liver Dis. 2020;52:1137–1142. [DOI] [PubMed] [Google Scholar]
- 81.McCormick PA, Mistry P, Kaye G, Burroughs AK, McIntyre N. Intravenous albumin infusion is an effective therapy for hyponatraemia in cirrhotic patients with ascites. Gut. 1990;31:204–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bajaj JS, Tandon P, O’Leary JG, Biggins SW, Wong F, Kamath PS, et al. The impact of albumin use on resolution of hyponatremia in hospitalized patients with cirrhosis. Am J Gastroenterol. 2018;113:1339. [DOI] [PubMed] [Google Scholar]
- 83.Butterworth RF. Pathophysiology of hepatic encephalopathy: A new look at ammonia. Metab Brain Dis. 2002;17:221–227. [DOI] [PubMed] [Google Scholar]
- 84.Jalan R, Kapoor D. Reversal of diuretic-induced hepatic encephalopathy with infusion of albumin but not colloid. Clin Sci (Lond). 2004;106:467–474. [DOI] [PubMed] [Google Scholar]
- 85.Simon-Talero M, Garcia-Martinez R, Torrens M, Augustin S, Gomez S, Pereira G, et al. Effects of intravenous albumin in patients with cirrhosis and episodic hepatic encephalopathy: A randomized double-blind study. J Hepatol. 2013;59:1184–1192. [DOI] [PubMed] [Google Scholar]
- 86.Ventura-Cots M, Simon-Talero M, Poca M, Ariza X, Masnou H, Sanchez J, et al. Effects of albumin on survival after a hepatic encephalopathy episode: Randomized double-blind trial and meta-analysis. J Clin Med. 2021;10:4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Riggio O, Nardelli S, Pasquale C, Pentassuglio I, Gioia S, Onori E, et al. No effect of albumin infusion on the prevention of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Metab Brain Dis. 2016;31:1275–1281. [DOI] [PubMed] [Google Scholar]
- 88.Fagan A, Gavis EA, Gallagher ML, Mousel T, Davis B, Puri P, et al. A double-blind randomized placebo-controlled trial of albumin in outpatients with hepatic encephalopathy: HEAL study. J Hepatol. 2023;78:312–321. [DOI] [PubMed] [Google Scholar]
- 89.Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R, et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247–2256. [DOI] [PubMed] [Google Scholar]
- 90.Vincent JL, De Backer D, Wiedermann CJ. Fluid management in sepsis: the potential beneficial effects of albumin. J Crit Care. 2016;35:161–167. [DOI] [PubMed] [Google Scholar]
- 91.Philips CA, Maiwall R, Sharma MK, Jindal A, Choudhury AK, Kumar G, et al. Comparison of 5% human albumin and normal saline for fluid resuscitation in sepsis induced hypotension among patients with cirrhosis (FRISC study): a randomized controlled trial. Hepatol Int. 2021;15:983–994. [DOI] [PubMed] [Google Scholar]
- 92.Guidet B, Mosqueda GJ, Priol G, Aegerter P. The COASST study: Cost-effectiveness of albumin in severe sepsis and septic shock. J Crit Care. 2007;22:197–203. [DOI] [PubMed] [Google Scholar]
- 93.Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Intensive Care Med. 2004;30:536–555. [DOI] [PubMed] [Google Scholar]
- 94.Koratala A, Ronco C, Kazory A. Albumin infusion in patients with cirrhosis: time for POCUS-enhanced physical examination. Cardiorenal Med. 2021;11:161–165. [DOI] [PubMed] [Google Scholar]
- 95.Banares R, Ibanez-Samaniego L, Torner JM, Pavesi M, Olmedo C, Catalina MV, et al. Meta-analysis of individual patient data of albumin dialysis in acute-on-chronic liver failure: Focus on treatment intensity. Therap Adv Gastroenterol. 2019;12:1756284819879565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Artzner T, Bernal W, Belli LS, Conti S, Cortesi PA, Sacleux SC, et al. Location and allocation: Inequity of access to liver transplantation for patients with severe acute-on-chronic liver failure in Europe. Liver Transpl. 2022;28:1429–1440. [DOI] [PubMed] [Google Scholar]
- 97.China L, Freemantle N, Forrest E, Kallis Y, Ryder SD, Wright G, et al. A randomized trial of albumin infusions in hospitalized patients with cirrhosis. N Engl J Med. 2021;384:808–817. [DOI] [PubMed] [Google Scholar]
- 98.Di Pascoli M, Fasolato S, Piano S, Bolognesi M, Angeli P. Long-term administration of human albumin improves survival in patients with cirrhosis and refractory ascites. Liver Int. 2019;39:98–105. [DOI] [PubMed] [Google Scholar]
- 99.Romanelli RG, La Villa G, Barletta G, Vizzutti F, Lanini F, Arena U, et al. Long-term albumin infusion improves survival in patients with cirrhosis and ascites: An unblinded randomized trial. World J Gastroenterol. 2006;12:1403–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Caraceni P, Riggio O, Angeli P, Alessandria C, Neri S, Foschi FG, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391:2417–2429. [DOI] [PubMed] [Google Scholar]
- 101.Sola E, Sole C, Simon-Talero M, Martin-Llahi M, Castellote J, Garcia-Martinez R, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol. 2018;69:1250–1259. [DOI] [PubMed] [Google Scholar]
- 102.Caraceni P, Tufoni M, Zaccherini G, Riggio O, Angeli P, Alessandria C, et al. On-treatment serum albumin level can guide long-term treatment in patients with cirrhosis and uncomplicated ascites. J Hepatol. 2021;74:340–349. [DOI] [PubMed] [Google Scholar]
- 103.Klein S, Van Beuge MM, Granzow M, Beljaars L, Schierwagen R, Kilic S, Heidari I, et al. HSC-specific inhibition of Rho-kinase reduces portal pressure in cirrhotic rats without major systemic effects. J Hepatol. 2012;57:1220–1227. [DOI] [PubMed] [Google Scholar]
- 104.Klein S, Frohn F, Magdaleno F, Reker-Smit C, Schierwagen R, Schierwagen I, Uschner FE, et al. Rho-kinase inhibitor coupled to peptide-modified albumin carrier reduces portal pressure and increases renal perfusion in cirrhotic rats. Sci Rep. 2019;9:2256. [DOI] [PMC free article] [PubMed] [Google Scholar]


