Abstract
Background:
Eliminating HIV vertical transmission (VT) and is a global priority. Estimates of paediatric HIV infections are commonly derived through mathematical models relying on rates of VT stratified by maternal immunological and treatment status from literature, namely the UNAIDS-supported Spectrum AIDS Impact Module (Spectrum-AIM) to assess progress towards eliminating VT. Default VT probabilities were last updated in 2018, since then there have been substantial changes to service delivery and ART regimens.
Methods:
We aimed to (1) update the systematic review of VT probabilities by maternal status compatible with Spectrum-AIM, (2) conduct a meta-regression to systematically pool studies to estimate VT probabilities with statistical uncertainty, and (3) assess determinants of VT, including maternal viral load. We searched PubMed, Embase, Global Health Database, WHO Global Index Medicus, CINAHL Complete, and Cochrane CENTRAL for peer-reviewed articles in English from all geographic regions with data on VT from randomized controlled trials, cohort studies, or observational studies. We excluded sources that did not stratify VT by maternal treatment or immunological status. We fit four meta-regression models to produce VT probability estimates compatible with stratifications used in Spectrum-AIM and assessed how updated VT probabilities estimated new paediatric infections compared to default parameters in Spectrum-AIM. We conducted subgroup analyses to assess how study inclusion affected model estimates. Finally, we fit a meta-regression model to assess ART class and initiation timing on viral load suppression at delivery.
Findings:
The updated systematic review identified 24 new studies published between January 2018 and February 2024. Combined with previous review data, 110 studies were included in the meta-regression analysis. Estimates were broadly consistent with previous reviews. For women not receiving PMTCT, the odds of perinatal transmission decreased by 0.20 (0.16–0.25) for each 100 mm3 increase in median CD4 of the study population. Among women on ART during pregnancy, each additional week on ART before delivery reduced the odds of VT by 5.6% (4.3%–6.8%). ART regimen class affected VT probability; the odds ratio of perinatal VT among WLHIV who initiated an INSTI-based regimen versus a NNRTI-based regimen 20 weeks before delivery was 0.355 (0.140–0.898). However, this effect was confounded by study region. Viral load suppression at delivery was significantly lower among women who started ART late during pregnancy (p=0.02), but did not significantly differ by ART class (p>0.05).
Interpretation:
Vertical transmission rates vary substantially according to maternal immunological stage, prophylactic regimen, and timing of treatment initiation. Time of initiation on ART before delivery was strongly associated with viral load suppression at delivery. Our estimates and their uncertainty can be used in Spectrum-AIM to produce estimates of paediatric incidence to inform funding and monitor progress towards eliminating VT.
Funding:
National Institutes of Health, UNAIDS, and the Medical Research Council
Introduction
Eliminating vertical transmission (VT) of HIV and addressing gaps in antiretroviral treatment coverage among children are global priorities.1 Most countries use the UNAIDS-supported Spectrum AIDS Impact module (Spectrum-AIM), to quantify the national HIV epidemic, including the number of children acquiring HIV infection through vertical transmission over time. Due to incomplete HIV testing among children, Spectrum-AIM models the final vertical transmission rate (proportion of HIV exposed infants infected by the end of breastfeeding annually) as the function of the number of pregnant women living with HIV (WLHIV) by immunological and treatment status,2 breastfeeding duration among WLHIV,3 and VT probability stratified by prevention of mother to child transmission (PMTCT) regimen and transmission timing (perinatal or breastfeeding).4 The final VT rate has declined over the past two decades commensurate with the scale-up of programmes to prevent VT.5 These programmes identify pregnant WLHIV during antenatal care (ANC) and initiate them on antiretroviral regimens according to World Health Organization (WHO) guidelines, which reflect the best practices to prevent HIV VT and preserve maternal health at the time of diagnosis.6–8
WHO-recommended and nationally implemented strategies to prevent VT have evolved over time.9–11 Initially strategies consisted of short-course antiretroviral regimens for pregnant women and prophylactic antiretroviral regimens administered to infants, and, more recently, immediate lifelong ART initiated at diagnosis, before or during pregnancy. To reflect evolving PMTCT guidelines before universal ART, Spectrum-AIM stratifies VT probabilities as follows: women with existing HIV infection who did not receive PMTCT, women who seroconvert during pregnancy or breastfeeding, women who received short course PMTCT (maternal single-dose Nevirapine (SDNVP),12 WHO 2006 dual ARV prophylaxis,13 Option A,6 and Option B6), and women on lifelong ART. Short course PMTCT options are defined in Supplementary Material S1. Women who discontinue ART before delivery are assigned the same VT probability as those without PMTCT to reflect rapid viral rebound after ART interruption.14
Spectrum-AIM’s default VT probabilities were initially estimated in a review conducted in 201215 and subsequently updated in 201516 and 201817 to reflect new empirical data on VT and the effects of newer PMTCT strategies. Most default VT probabilities in Spectrum-AIM are based on the weighted average of studies identified in the 2018 review (Supplementary Material, Table S1 and Table S2). Probability of VT for WLHIV not receiving PMTCT have not been updated since the initial 2012 systematic review.
In 2019, dolutegravir (DTG) became the recommended first-line ART for all PLHIV, including pregnant women.18 Viral suppression occurs more rapidly in PLHIV on DTG than PLHIV using previous first-line regimens, thus DTG has greater potential to reduce VT when initiated late in pregnancy.19,20 Since the 2018 systematic review, Universal Test and Treat and differentiated service delivery have increased the number of pregnant and breastfeeding women initiating ART early and remaining retained.21 While PMTCT guidelines have not changed since the 2015 recommendation of universal ART,8 innovations in ART formulations and service delivery models have improved HIV treatment effectiveness, including prevention of VT.
We conducted an updated systematic review of VT probabilities to assess evidence on VT probability published from 2018 to 2024, following recent biomedical and implementation innovations aimed at increasing viral suppression among PLHIV, including among pregnant and postpartum WLHIV. Combining data identified in previous reviews and our updated review, we use a meta-regression framework to estimate VT probability compatible with Spectrum-AIM. Finally, we assessed the association between ART initiation timing and ART regimen class and viral load suppression (VLS) at delivery. Updated VT probability estimates improve the evidence informing estimates of paediatric HIV infections to assess progress towards identify remaining gaps in eliminating HIV vertical transmission.
Methods
The objectives of this analysis were to (1) update the systematic review of VT probabilities by including studies published since 2018, (2) use meta-regression to derive pooled VT probabilities estimates using all studies identified across the 2012, 2015, 2018, and 2024 systematic reviews, and (3) assess ART class and time on ART before delivery as predictors of viral suppression at delivery. The systematic review and meta-analysis was pre-registered on PROSPERO (CRD: 42024511011) and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Supplementary Material Table S2.2.1 and Table S2.2.2).
The 2024 systematic review update
We systematically searched PubMed, Embase, Global Health Database, WHO Global Index Medicus, CINAHL Complete, and Cochrane CENTRAL for published literature with search term domains that mentioned “HIV”, “transmission”, “perinatal” and “breastfeeding periods”, and “infants born to women living with HIV” or related terms. The complete search strategies are detailed in Supplementary Material S2.3.22
Database search was completed on 8 February 2024 and included references published between 1 January 2018 and 8 February 2024. The systematic review was conducted and managed using Covidence Software.23 Citations were uploaded to Covidence and de-duplicated. Title and abstracts were screened for eligibility and full-text articles were reviewed for inclusion by two independent reviewers (MKW, MBu, MBa, SH, AR, MS), with conflicts resolved by a third reviewer or through consensus. Inclusion criteria was as follows: full-text articles published in English from all geographic regions with data on VT from randomized controlled trials, cohort studies, or observational studies. Data on VT needed to be stratified by maternal PMTCT regimen (or CD4 for women not receiving PMTCT); if pregnant WLHIV received ART during pregnancy, the timing of ART initiation (preconception or during pregnancy) was required. Measuring VT probability did not need to be the primary outcome of the study for inclusion. Cross-sectional, case-control, case series, case reports, commentaries, letters to editors, study protocols, grey literature, and non-human, animal studies study designs were excluded.
Included studies were independently extracted by MKW and another reviewer (MBu, MBa, SH, AR, or MS), resolving discrepancies through consensus. We extracted study details (e.g., author name(s), study title, publication year, geographic regions covered, study years) and VT details (e.g., HIV exposed infants who were tested for HIV, HIV positive infants, timing of vertical HIV transmission, and infant feeding patterns). Additionally, we extracted details on maternal PMTCT (timing of ART initiation and regimen) and immunological data (viral load or viral suppression and CD4 for women not receiving PMTCT). The list of variables extracted is in Supplementary Materials S2.4.
Meta-regression of HIV vertical transmission probabilities
Using studies identified from the previous and 2024 systematic reviews (Supplementary Material S3), we fit four meta-regression models to estimate VT probabilities stratified according to vertical transmission categories in the Spectrum-AIM.16 The four models estimated VT probability on the logit scale—the first among WLHIV not receiving PMTCT, the second among WLHIV who seroconverted during pregnancy or breastfeeding or received short course PMTCT regimens, the third for perinatal transmission among WLHIV receiving lifelong ART, and the fourth for monthly VT probability during breastfeeding among women receiving lifelong ART. We also assessed ART initiation timing and ART regimen class as predictors of viral suppression (<50 copies / mm) at delivery.
We used the same definitions of perinatal and breastfeeding transmission as the 2012, 2015, and 2018 systematic reviews (Supplementary Material S1).15 Perinatal VT was defined as HIV acquisition occurring before six weeks postpartum. For studies reporting transmission during breastfeeding, we converted cumulative acquisition probabilities to monthly probability for the period starting at the end of the perinatal period (6 weeks) and ending at the time of the last HIV test closest to the when women ceased breastfeeding. Most often this period spanned 1.5 and six months. Further details are in Supplementary Material S2.5 and S2.6.
Model one: VT probability from women not receiving PMTCT
Model one estimated VT among WLHIV not receiving PMTCT as a function of CD4 midpoint in the study population at baseline (Equation 1).
| Equation 1 |
Model one included fixed effects for perinatal and monthly breastfeeding transmission , a fixed effect for CD4 midpoint (, per 100 mm3 centred at 500 mm3), and a fixed effect for the interaction between CD4 midpoint and breastfeeding transmission . The CD4 midpoint was either the median CD4 of WLHIV not receiving PMTCT or the midpoint of a CD4 range in studies that reported VT by CD4 categories. More information on CD4 midpoint determination from each study and the sensitivity to this extraction is in Supplementary Material S5.1. Random effects were included for study and observation ( and , respectively).
Model two: VT probability from maternal seroconversion and short course PMTCT
Model two estimated VT probability among WLHIV who acquired HIV infection during pregnancy or breastfeeding or received short course PMTCT (Equation 2).
| Equation 2 |
Model two included fixed effects for the following categories () used in Spectrum-AIM: maternal seroconversion during pregnancy or breastfeeding, WLHIV receiving WHO 2006 dual ARV regimen, SDNVP, Option A, and Option B. For breastfeeding women receiving SDNVP, transmission rates were stratified by CD4 less 350 per cubic millimetre (CD <350) and CD4 ≥350. Random effects were included for study and observation ( and , respectively).
Model three: Perinatal transmission probability from women receiving ART by timing of initiation
Model three estimated perinatal transmission probabilities among women on lifelong ART by timing of maternal ART initiation (Equation 3).
| Equation 3 |
Model three included an intercept term , a fixed effect for weeks on ART during pregnancy before delivery (, centred on ART initiated 20 weeks before delivery), and a fixed effect for late ART initiation (, ART initiated less than four weeks before delivery). was preferentially extracted as the median weeks on ART before delivery. For studies that reported ART initiation during a range of gestational weeks, we extracted the midpoint of the range. Assumptions about weeks on ART before delivery are outlined in Supplementary Material S5.2. Random effects were included for study and observation ( and , respectively).
Model four: Monthly breastfeeding transmission from women receiving lifelong ART
Model four estimated monthly breastfeeding transmission probabilities by time of ART initiation (preconception or during pregnancy, Equation 4).
| Equation 4 |
For breastfeeding transmission, timing of ART initiation was classified as a binary covariate (preconception or during pregnancy) rather than continuous weeks before delivery (as in Model 3 for perinatal transmission) because timing of ART initiation during pregnancy is less directly related to viral suppression during breastfeeding period than viral suppression at delivery. This model included random effects on by observation . Study level random effects were not included because only two studies had multiple observations.
Effect of ART regimen class perinatal transmission probability and VLS at delivery
We modified model three (Equation 3) to include fixed effects for ART regimen class (NNRTI (reference), INSTI, PI, and miscellaneous). We evaluated geographic region as a confounder of this effect in Supplementary Material S5.3. Additionally, we used studies that reported the proportion of WLHIV with VLS (<50) at delivery, time on ART, and ART regimen class to assess determinants of proportion of WLHIV with VLS at delivery (Equation 5).
| Equation 5 |
This model included fixed effects for ART regimen class , timing of ART initiation (‘early’: before the second trimester and ‘late’: after the first trimester), and an interaction between ART class and timing. Random effects were included for study and observation ( and , respectively).
Implications of estimated VT probabilities for Spectrum-AIM’s estimates of paediatric HIV infections
We used predicted values from models 1–4 to produce VT probability parameters compatible with Spectrum-AIM transmission categories. For VT probabilities among untreated women stratified by CD4 categories <200, 200–349, and ≥350, model one predicted VT probabilities corresponding to CD4 midpoint values 100, 275, and 500 cells/mm3, respectively. Model two predicted VT probabilities for maternal seroconversion and short course PMTCT. For perinatal VT probabilities among women on ART <4 weeks, 4–39 weeks, and pre-conception, model three predicted probabilities corresponding to 2, 20, and 40 weeks on ART preconception, respectively. For VT during breastfeeding among WLHIV on ART, model 4 predicted VT probabilities corresponding to ART initiation before conception or during pregnancy.
Predicted transmission probabilities were input in Spectrum-AIM to calculate the number of paediatric infections in four countries (Rwanda, Malawi, Democratic Republic of Congo (DRC), and Burkina Faso) in years 2000, 2010, 2015, and 2023 using the Spectrum-AIM files published in 2023. Results were compared to the 2024 UNAIDS published HIV infections.5
All analyses were conducted in R 4.3.1.24 Meta-regression models were fit using glmmtmb.25 Data is available on request.
Results
Updated 2024 systematic review and previous searches
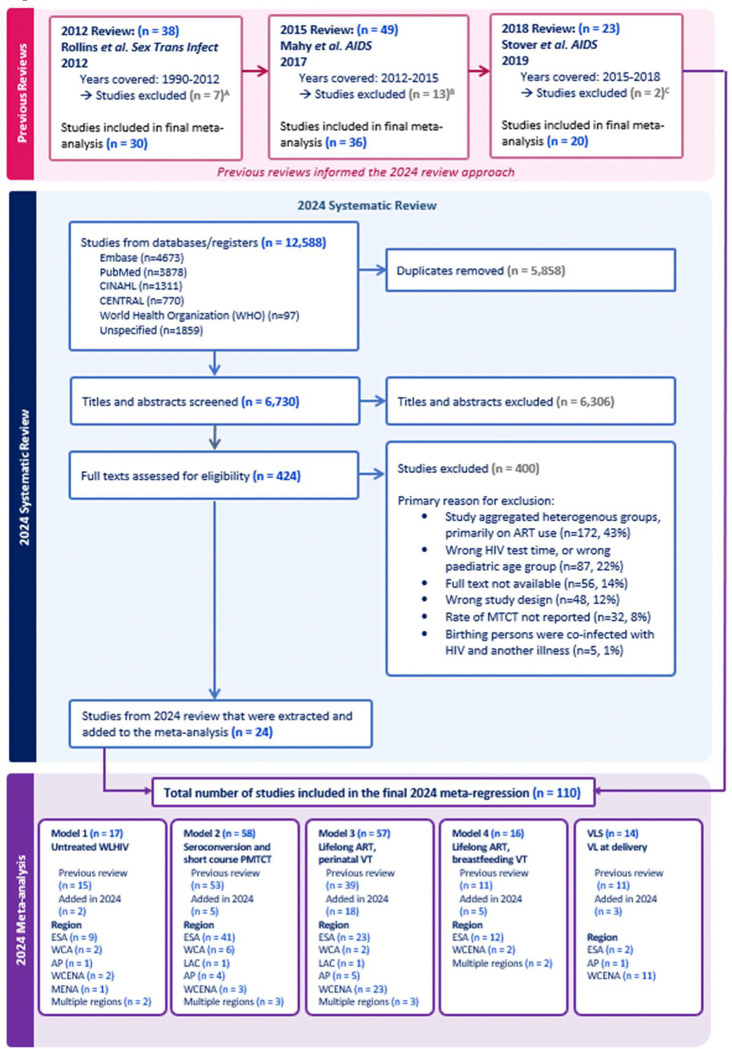
Our search identified 12,588 results, of which 6,730 were unique and underwent title and abstract screening (Figure 1). Among them, full-texts were reviewed for 424 studies and 400 were excluded; the most common reason for exclusion was aggregation of VT across distinct ART groups (43%, Figure 1). The remaining 24 studies were extracted and included in the final meta-regression. The 24 studies published from 2018 to 2024 were combined with 30 studies from the 2012 review, 36 from the 2015 review, and 20 from the 2018 review (Figure 1), yielding 110 studies included in the meta-regression analysis. All global regions were represented by at least one study, however most studies conducted in eastern and southern Africa (N=56, Supplementary Material Table S3). Studies were published between 1988 and 2023, with data collected between 1982 and 2022.
Figure 1.
PRISMA Flow Diagram for 2018–2024 review. Studies were excluded from previous reviews for the following reasons:
A Not peer-reviewed (3), duplicate data (3), aggregates heterogenous results (1)
B Not peer-reviewed (5), duplicate data (4), aggregates heterogeneous results (4)
C Not peer-reviewed (2)
Model one: VT probability from women not receiving PMTCT
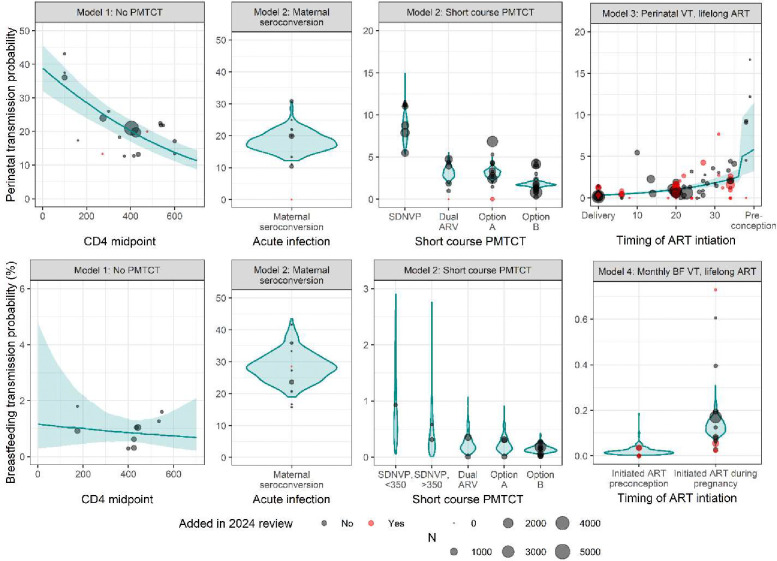
Model one included data from 17 studies on VT probability among women not receiving PMTCT, including two new studies published since the 2018 review (Figure 1; forest plots: Supplementary Material Figures S4.2.1 and S4.2.2). Observations from studies that reported VT stratified by CD4 range accounted for 80.2% of all observations. The odds of perinatal transmission decreased by 0.20 (0.16–0.25) for each 100 mm3 increase in median CD4 (Supplementary Material Table S4.1.1). When model one was fit to only studies that did not stratify VT by CD4 range, each 100 mm3 increase in median CD4 increased the odds of transmission by 1.4 times (1.0–1.8, Supplementary Material Table S5.1.1.2). This was driven by three studies that are not representative of the modern HIV epidemic and is corrected for through study-level random effects when model one is fit to all studies that describe VT among women not receiving PMTCT (Supplementary Material S5.1.1). The interaction between CD4 midpoint and breastfeeding transmission was not statistically significant (p=0.54, Supplementary Material Table S4.1.1). The perinatal transmission probabilities for women with CD4 less than 200, 200–350, and greater than 350 were 33.4% (27.8–39.0%), 25.1% (21.3–28.5%), and 16.7% (13.8–20.0%) respectively (Table 1). The monthly breastfeeding transmission probabilities were 1.07% (0.37–3.15%), 0.94% (0.43–1.72%), and 0.80% (0.43–1.35%) for the same CD4 categories (Table 2).
Table 1.
Perinatal vertical transmission probabilities
| PMTCT Regimen | Default Spectrum value (%) | Vertical transmission (%) | Percent change1 | Percent of pregnant WLHIV in each stratum | ||
|---|---|---|---|---|---|---|
| 20102 | 20152 | 20232 | ||||
| Model one: VTprobability from women not receiving PMTCT | ||||||
| [0–200) | 37.0 | 33.4 (27.8 – 39.0) | −10% | 41% | 20% | 18% |
| [200–350) | 27.0 | 25.1 (21.3 – 28.5) | −7% | |||
| >350 | 15.0 | 16.7 (13.8 – 20.0) | +11% | |||
| Model two: VT probability from maternal seroconversion or short course PMTCT | ||||||
| Infection | 18.1 | 18.0 (12.9 – 24.4) | −1% | |||
| SDNVP | 7.5 | 8.3 (6.1 – 11.6) | +11% | 11% | 1% | 0% |
| Dual ARV | 2.2 | 3.1 (2.1 – 4.8) | +41% | 15% | 0% | 0% |
| Option A | 4.1 | 3.1 (2.4 – 4.0) | −24% | 11% | 2% | 0% |
| Option B | 1.9 | 1.8 (1.4 – 2.3) | −5% | 3% | 4% | 0% |
| Model three: Perinatal transmission probability from women receiving ART by timing of initiation | ||||||
| Option B+, on ART <4 weeks | 8.2 | 5.2 (2.9 – 10.7) | −35% | 1% | 3% | 1% |
| Option B+, on ART 5–39 weeks | 1.4 | 1.0 (0.8 – 1.3) | −29% | 9% | 36% | 25% |
| Option B+, on ART preconception | 0.26 | 0.33 (0.23 – 0.48) | +27% | 9% | 34% | 56% |
Blue shading represents a percent decrease from the default value. Orange shading represents a percent increase from the default value.
The proportion of WLHIV who fall into each category each year globally
Table 2.
Monthly breastfeeding vertical transmission probabilities
| PMTCT Regimen | Default Spectrum value (%) | Vertical transmission (%) | Percent change1 | Percent of pregnant WLHIV in each stratum | ||
|---|---|---|---|---|---|---|
| 20102 | 20152 | 20232 | ||||
| Model one: VTprobability from women not receiving PMTCT | ||||||
| [0–200) | 0.89 | 1.07 (0.37 – 3.15) | +20% | 41% | 20% | 18% |
| [200–350) | 0.81 | 0.94 (0.52 – 1.72) | +16% | |||
| >350 | 0.51 | 0.80 (0.43 – 1.35) | +57% | |||
| Model two: VT probability from maternal seroconversion or short course PMTCT | ||||||
| Infection | 26.90 | 28.20 (20.61 – 38.42) | +5% | |||
| SDNVP, <350 | 0.99 | 0.74 (0.15 – 4.05) | −25% | 11% | 1% | 0% |
| SDNVP, >350 | 0.40 | 0.33 (0.04 – 1.68) | −18% | |||
| Dual ARV | 0.18 | 0.20 (0.06 – 0.66) | +11% | 15% | 0% | 0% |
| Option A | 0.20 | 0.20 (0.05 – 0.60) | 0% | 11% | 2% | 0% |
| Option B | 0.13 | 0.14 (0.07 – 0.29) | +8% | 3% | 4% | 0% |
| Model four: Monthly breastfeeding transmission from women receiving lifelong ART | ||||||
| Option B+, on ART <4 weeks | 0.20 | 0.13 (0.08 – 0.23) | −35% | 1% | 3% | 1% |
| Option B+, on ART 5–39 weeks | 0.11 | +18% | 9% | 36% | 25% | |
| Option B+, on ART preconception | 0.02 | 0.02 (0.00 – 0.06) | 0% | 9% | 34% | 56% |
Blue shading represents a percent decrease from the default value. Orange shading represents a percent increase from the default value.
The proportion of WLHIV who fall into each category each year globally
Model two: VT probability from maternal seroconversion or short course PMTCT
Model two included data from 58 studies, including four new studies from the updated systematic review (Figure 1; forest plots: Supplementary Material Figures S4.2.3–S4.2.13). Two new studies reported perinatal VT probability for women receiving Option A and two reported transmission from women who seroconverted, one during pregnancy and one during breastfeeding (Figure 2). Among women who seroconverted during pregnancy, perinatal transmission probability was 18.0% (12.9–24.4%, Table 1). Among women who seroconverted during breastfeeding, transmission probability was 28.20% (20.61–38.42%, Table 2). Among women who received short course PMTCT, the perinatal transmission probability was 8.3% (6.1–11.6%) for SDNVP, 3.1% (2.1–4.8%) for dual ARV, 3.1% (2.4–4.0%) for Option A, and 1.8% (1.4–2.3%) for Option B (Table 1). The monthly breastfeeding transmission probabilities were 0.74% (0.15–4.05%) and 0.33% (0.04–1.68%) for SDNVP among women with CD4 less than 350 and greater than or equal to 350 respectively, 0.20% (0.06–0.66%) for dual ARV, 0.20% (0.05–0.60%) for Option A, and 0.14% (0.07–0.29%) for Option B.
Figure 2.
Data used in models one through four and model estimates of VT probability. Point size reflects study size and colour indicates whether the study was added in the 2024 review. Blue violin plots are used for categorical models (model two and model four) and blue lines with ribbon are used for continuous models (model one and model three).
Model three: Perinatal transmission probability from women receiving lifelong ART by timing of initiation
Model three included 57 studies; 15 studies of which were identified in the 2024 systematic review (Figure 1 and Figure 2; forest plots: Supplementary Material Figure S4.2.14). Each additional week on ART before delivery reduced the odds of VT by 5.6% (4.3–6.8%, Supplementary Material Table S4.1.1). The odds ratio of perinatal transmission among WLHIV who initiate ART less than four weeks before delivery to those who initiated 20 weeks before delivery was 6.36 (3.82–8.58, Supplementary Material Table S4.1.1). When model three was fit to just studies that reported the median weeks on ART (rather than a range of weeks when ART was initiated) the same odds ratio was 12.1 (7.9–18.4, Supplementary Material S5.2). Perinatal transmission probability was 5.2% (2.9–10.7%) among women who initiated ART less than four weeks before delivery, 1.0% (0.8–1.3%) among women who initiated ART during pregnancy but before the final month, and 0.33% (0.23–0.48%) among women who initiated ART preconception (Table 1).
Model four: Monthly breastfeeding transmission from women receiving lifelong ART
Model four included 16 studies, five of which were identified in the 2024 systematic review (Figure 1, Supplementary Material Figure S4.2.15). The monthly breastfeeding transmission probability was 0.13% (0.08–0.23%) for women who initiated ART during pregnancy and 0.02 (0.00–0.06%) for women who initiated ART preconception (Table 3).
Effect of ART regimen class perinatal transmission probability and VLS at delivery
Using the 57 studies included in model three, we refit model three with fixed effects for ART regimen class. The odds ratio of perinatal VT among WLHIV who initiated an INSTI-based regimen versus a NNRTI-based regimen 20 weeks before delivery was 0.355 (0.140–0.898, Supplementary Material Table S4.1.2). When geographic region was added to the model, INSTI-based regimens did not have a significantly lower transmission rate than NNRTI-based regimens, suggesting that the effect of ART class on VT probability is confounded by study region (Supplementary Material S5.3).
Fourteen studies reported data on the proportion VL <50 copies/mL at delivery, three of which were identified in the 2024 systematic review (Figure 1). Most (11/14) studies were from Western and central Europe and North America. Probability of VLS at delivery was highest among WLHIV who initiated INSTI-based regimens before the second trimester (95.2% (87.7–98.5%), Supplementary Material Table S4.1.3), however there was no significant difference across ART classes. Probability of VLS among women who started ART before the second trimester was 90.7% (80.6–95.0%) for NNRTI, 82.6% (72.1–89.1%) for PI, and 90.6% (80.2–95.4%) for miscellaneous regimens. The probability of VLS among women who initiated ART after the first trimester was 40.4% (3.3–90.2%) for INSTI, 82.8% (70.2–90.4%) for NNRTI, 65.6% (51.7–76.5%) for PI, and for miscellaneous regimens.
Comparison with Spectrum-AIM default vertical transmission probabilities
Table 1 and Table 2 compare the predicted VT probabilities from the meta-regression models with current default probabilities in Spectrum-AIM used for 2024 UNAIDS global HIV estimates. Overall patterns were similar; the mean percent difference between default Spectrum-AIM VT probabilities and those estimated in this analysis was 2.5%. The monthly breastfeeding transmission probability from women with CD4 greater than 350 had the largest percent difference, our estimate was 57% higher than the default Spectrum-AIM value (Table 2). Updated perinatal transmission probabilities were on average lower (mean percent difference: 0.81% lower than default), whereas updated breastfeeding transmission probabilities were on average higher (mean percent difference: 5.5% higher).
Implications of estimated VT probabilities for Spectrum-AIM’s estimates of paediatric HIV infections
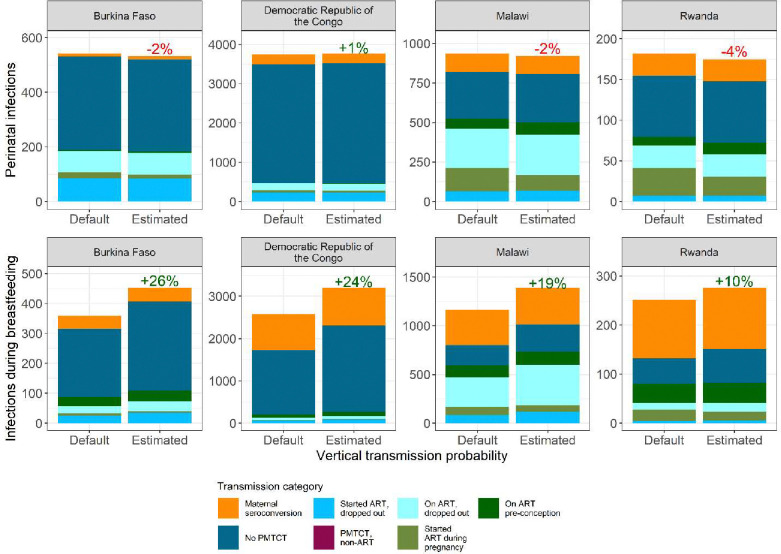
We applied the updated VT probabilities (Table 1 and Table 2) in Spectrum-AIM to estimate the number of paediatric infections in Rwanda, Malawi, DRC, and Burkina Faso in the years 2000, 2010, 2015, and 2023 (Supplementary Material S6). In 2023, estimated perinatal infections were slightly lower using the updated VT probabilities in all countries except DRC (Figure 3). Across years and locations on average, perinatal infections were 2.4% lower than perinatal infections estimated using the default VT probabilities. In 2023, estimated infections from breastfeeding were higher using the updated VT probabilities in all countries (Figure 3). Across years and locations on average, breastfeeding infections were 26.0% higher than breastfeeding infections estimated using the default VT probabilities. Increases in estimated infections during breastfeeding were largest in countries and years in which PMTCT coverage was low and ART interruption rates during pregnancy were high because updated breastfeeding transmission probabilities were most different among women who did not receive treatment (Table 2).
Figure 3.
Change in 2023 paediatric infections using the Spectrum-AIM default vs. estimated VT probabilities. Coloured percentage points represent the percent difference between the infections using the default vs estimated VT probabilities. Green percentages denote a percent increase and red percentages denote a percent decrease.
Discussion
Estimates of vertical transmission probability according to immunologic status, ARV-based preventive regimen, and timing of ART initiation are critical information for estimating children acquiring HIV infection and living with HIV and anticipated impact of efforts to eliminate vertical transmission. Since the last update of VT probabilities in 2018, Universal Test and Treat, differentiated service delivery, and DTG-based first-line regimens have expanded in aim of increasing the number of women receiving ART during pregnancy and remain retained through delivery26 and increasing VLS among WLHIV. Our analysis estimates lower vertical transmission probability among women initiating ART during pregnancy than previous studies. Additionally, we find that women receiving INSTI-based regimens have lower perinatal transmission probability than women receiving other classes of regimens, however this effect is not significant when accounting for global region. Finally, we find ART initiation before the second trimester is associated with VLS at delivery across ART classes.
The relative levels of our estimates of VT probability are like previous estimates— women not receiving PMTCT have the highest VT probability, short course PMTCT regimens reduced VT probability before universal treatment, and women on ART have the lowest VT probability. Our perinatal VT probability estimates were systematically slightly lower than the default Spectrum-AIM values. These changes are related to both new data from the updated systematic search and the meta-regression model (versus the weighted average method).15–17 The largest reduction from the default parameters was the estimate of perinatal transmission among WLHIV who initiate ART less than four weeks before delivery was 5.2% (2.9–10.7%), 35% lower than the current default value in Spectrum-AIM (8.2%). We show that this is due to more data on women starting ART during the third trimester and our model format but anticipate this will not have a large impact on Spectrum-AIM estimates as in 2023 only 1% of all pregnant WLHIV started ART less than four weeks before delivery. Our estimates of transmission probabilities during breastfeeding were systematically higher than the default Spectrum-AIM values. The largest differences occurred among women who did not receive any PMTCT. We did not identify any new studies that described breastfeeding transmission among women who did not receive PMTCT, so these differences are driven by the meta-regression model.
Our systematic review included randomized controlled trials, cohort studies, and other observational studies. Our global scope included heterogenous data that varied with respect to breastfeeding duration patterns for WLHIV, epidemic type, and ANC attendance.3,27,28 The exclusion of non-English publications and grey literature may limit our coverage of published VT probabilities. Our inclusion criteria required studies to disaggregate VT by PMTCT regimen and ART initiation timing. We excluded 170 studies (43% of all full texts) that did not specify this information. Despite these limitations, combining studies identified across four different reviews allowed us to estimate VT probability for various maternal immunological statuses and treatment regimens.
Our estimates rely on assumptions. For model one, we approximated a CD4 midpoint for studies that reported median CD4 or a CD4 range among women who did not receive PMTCT. A subgroup analysis showed that differences in these reporting methods were confounded by older studies, which represent early epidemic dynamics. These studies make up a small proportion of all studies used in model one, so we do not feel that these biased our results. Similarly, for women initiating ART before or during pregnancy we approximated a time on ART midpoint for all studies. Studies included in model three reported either median weeks on ART before delivery or a range of weeks during which WLHIV initiated ART during pregnancy. For the 26 studies that reported a range of weeks, we requested more precise information about the median weeks on ART but only received additional information for four studies. Excluding studies that reported a range of weeks would exclude 76% of all studies that report ART initiation occurring during the third trimester. Their inclusion is crucial for estimates of VT probability among women who initiate ART late in pregnancy.
Our VT probability estimates can be utilized in the Spectrum-AIM paediatric model and their uncertainties can be incorporated in Spectrum-AIM’s paediatric uncertainty analysis. Incorporating our VT probability estimates are unlikely to substantially change the number of perinatal infections but will result in more infections from breastfeeding transmission. The shift in infection timing to breastfeeding from perinatal has implications for HIV testing strategies. Retaining mother-infant pairs through the end of breastfeeding is essential to confirm final HIV outcomes. We anticipate that future studies will describe VT among women receiving DTG. We found that women who initiated INSTI-based regimens had the lowest VT probability compared to other regimen classes, however this was confounded by geographic region. Additionally, women on INSTI-based regimens had the highest probability of viral suppression, although differences across classes were not statistically significant, and data was limited on viral load suppression among women who initiated INSTI-based regimens late in pregnancy. Cohort studies have found no difference in vertical transmission and viral suppression among women on different ART regimens when ART is initiated early in or before pregnancy.29 To assess possible differences by ART class when ART is initiated late in pregnancy, an updated systematic review and meta-regression should be completed when more observational data is available among populations who initiated INSTI or DTG based regimens late in pregnancy.
Vertical transmission probability as a function of CD4 and maternal PMTCT are essential to model paediatric HIV burden in the Spectrum-AIM model. Improving treatment formulations, differentiated service delivery, and universal test and treat have all improved the proportion of WLHIV who are virally suppressed at delivery, which is associated with lower probability of VT. Updating VT parameters to reflect these changes allows for accurate estimation of paediatric HIV burden. Maintaining accurate parameterizations is important as these estimates inform funding for prevention and treatment and allow for monitoring progress towards the elimination of vertical HIV transmission.
Supplementary Material
Acknowledgements
The authors acknowledge and thank the UNAIDS Reference Group on HIV Estimates, Modelling, and Projections for suggestions to refine the analysis, Caitlin Dugdale and Andrea Ciaranello for insights on the viral load analysis, and Edmond Brewer and Megan Verma for assistance with initial title abstract screening.
Funding details:
This research was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number 1R01AI152721-01A1, UNAIDS, and the MRC Centre for Global Infectious Disease Analysis (reference MR/R015600/1), jointly funded by the UK Medical Research Council (MRC) and the UK Foreign, Commonwealth & Development Office (FCDO), under the MRC/FCDO Concordat agreement and is also part of the EDCTP2 programme supported by the European Union.
For the purpose of open access, the author has applied a ‘Creative Commons Attribution’ (CC BY) licence to any Author Accepted Manuscript version arising.
Footnotes
Conflicts of interest:
The authors declare no conflicts of interest. A preliminary analysis of this work was virtually presented in October 2024 to the UNAIDS Reference Group on HIV Estimates, Modelling, and Projections.
PROSPERO: CRD42024511011
References
- 1.World Health Organization. Global guidance on criteria and processes for validation: elimination of mother-to-child transmission of HIV and syphilis. World Health Organization, 2014. https://apps.who.int/iris/handle/10665/112858 (accessed Aug 17, 2022). [Google Scholar]
- 2.Stover J, Glaubius R. Methods and Assumptions for Estimating Key HIV Indicators in the UNAIDS Annual Estimates Process. JAIDS J Acquir Immune Defic Syndr 2024; 95: e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glaubius R, Stover J, Johnson LF, Mahiane SG, Mahy MI, Eaton JW. Differences in Breastfeeding Duration by Maternal HIV Status: A Pooled Analysis of Nationally Representative Surveys in Sub-Saharan Africa. JAIDS J Acquir Immune Defic Syndr 2024; 95: e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahy M, Marsh K, Sabin K, Wanyeki I, Daher J, Ghys PD. HIV estimates through 2018: data for decision-making. AIDS Lond Engl 2019; 33: S203–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.AIDSinfo | UNAIDS. https://aidsinfo.unaids.org/ (accessed March 1, 2023).
- 6.Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants: Recommendations for a Public Health Approach: 2010 Version. Geneva: World Health Organization, 2010. http://www.ncbi.nlm.nih.gov/books/NBK304944/ (accessed Nov 6, 2023). [PubMed] [Google Scholar]
- 7.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva: World Health Organization, 2013. https://iris.who.int/handle/10665/85321 (accessed Sept 3, 2024). [PubMed] [Google Scholar]
- 8.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach, 2nd ed. Geneva: World Health Organization, 2016. https://iris.who.int/handle/10665/208825 (accessed Sept 3, 2024). [PubMed] [Google Scholar]
- 9.Idele P, Hayashi C, Porth T, Mamahit A, Mahy M. Prevention of Mother-to-Child Transmission of HIV and Paediatric HIV Care and Treatment Monitoring: From Measuring Process to Impact and Elimination of Mother-to-Child Transmission of HIV. AIDS Behav 2017; 21: 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mwanza J, Kawonga M, Gray GE, Doherty T, Mutale W. Evolution of Prevention of Mother to Child transmission of HIV Policy in Zambia: Application of the Policy Triangle to Understand the Roles of Actors, Process and Power. Glob Public Health 2021; 17: 2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phanuphak N, Phanuphak P. History of the prevention of mother-to-child transmission of HIV in Thailand. J Virus Erad 2016; 2: 107–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lallemant M, Jourdain G, Coeur SL, et al. Single-Dose Perinatal Nevirapine plus Standard Zidovudine to Prevent Mother-to-Child Transmission of HIV-1 in Thailand. N Engl J Med 2004; 351: 217–28. [DOI] [PubMed] [Google Scholar]
- 13.Dao H, Mofenson LM, Ekpini R, et al. International recommendations on antiretroviral drugs for treatment of HIV-infected women and prevention of mother-to-child HIV transmission in resource-limited settings: 2006 update. Am J Obstet Gynecol 2007; 197: S42–55. [DOI] [PubMed] [Google Scholar]
- 14.Calin R, Hamimi C, Lambert-Niclot S, et al. Treatment interruption in chronically HIV-infected patients with an ultralow HIV reservoir. AIDS 2016; 30: 761–9. [DOI] [PubMed] [Google Scholar]
- 15.Rollins N, Mahy M, Becquet R, Kuhn L, Creek T, Mofenson L. Estimates of peripartum and postnatal mother-to-child transmission probabilities of HIV for use in Spectrum and other population-based models. Sex Transm Infect 2012; 88: i44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MAHY M, PENAZZATO M, CIARANELLO A, et al. Improving estimates of children living with HIV from the Spectrum AIDS Impact Model. AIDS Lond Engl 2017; 31: S13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stover J, Glaubius R, Mofenson L, et al. Updates to the Spectrum/AIM model for estimating key HIV indicators at national and subnational levels. AIDS 2019; 33: S227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Update of recommendations on first- and second-line antiretroviral regimens. https://www.who.int/publications/i/item/WHO-CDS-HIV-19.15 (accessed Sept 16, 2024).
- 19.Waitt C, Orrell C, Walimbwa S, et al. Safety and pharmacokinetics of dolutegravir in pregnant mothers with HIV infection and their neonates: A randomised trial (DolPHIN-1 study). PLOS Med 2019; 16: e1002895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walmsley S, Antela A, Clumeck N. Dolutegravir plus Abacavir–Lamivudine for the Treatment of HIV-1 Infection | New England Journal of Medicine. https://www-nejm-org.iclibezp1.cc.ic.ac.uk/doi/10.1056/NEJMoa1215541?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200www.ncbi.nlm.nih.gov (accessed Sept 16, 2024). [DOI] [PubMed] [Google Scholar]
- 21.Abrams EJ, Langwenya N, Gachuhi A, et al. Impact of universal antiretroviral therapy for pregnant and postpartum women on antiretroviral therapy uptake and retention. AIDS 2019; 33: 45. [DOI] [PubMed] [Google Scholar]
- 22.Louden DN. Rates of mother-to-child HIV transmission with new first-line antiretroviral therapies and associated viral suppression: updated systematic review and meta-regression. 2024; published online Feb 8. https://osf.io/uvnb9/ (accessed Nov 12, 2024).
- 23.Covidence systematic review software. 2024. www.covidence.org.
- 24.R Core Team. R: A Language and Environment for Statistical Computing. 2023. https://www.R-project.org/.
- 25.Brooks ME, Kristensen K, Benthem KJ van, et al. glmmTMB Balances Speed and Flexibility Among Packages for Zero-inflated Generalized Linear Mixed Modeling. R J 2017; 9: 378–400. [Google Scholar]
- 26.World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva: World Health Organization, 2015. https://iris.who.int/handle/10665/186275 (accessed Sept 16, 2024). [PubMed] [Google Scholar]
- 27.Mujumdar V, Berman D, Schafer KR. Reproduction and Fertility Beliefs, Perceptions, and Attitudes in People Living with HIV. AIDS Res Treat 2018; 2018: 5349793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kowalska JD, Pelchen-Matthews A, Ryom L, et al. Prevalence and outcomes of pregnancies in women with HIV over a 20-year period. AIDS 2021; 35: 2025. [DOI] [PubMed] [Google Scholar]
- 29.Davey S, Ajibola G, Maswabi K, et al. Mother-to-Child HIV Transmission With In Utero Dolutegravir vs. Efavirenz in Botswana. JAIDS J Acquir Immune Defic Syndr 2020; 84: 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.