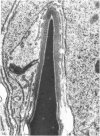
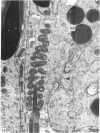
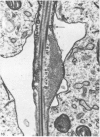
Abstract
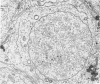
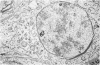
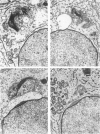
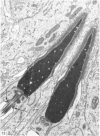
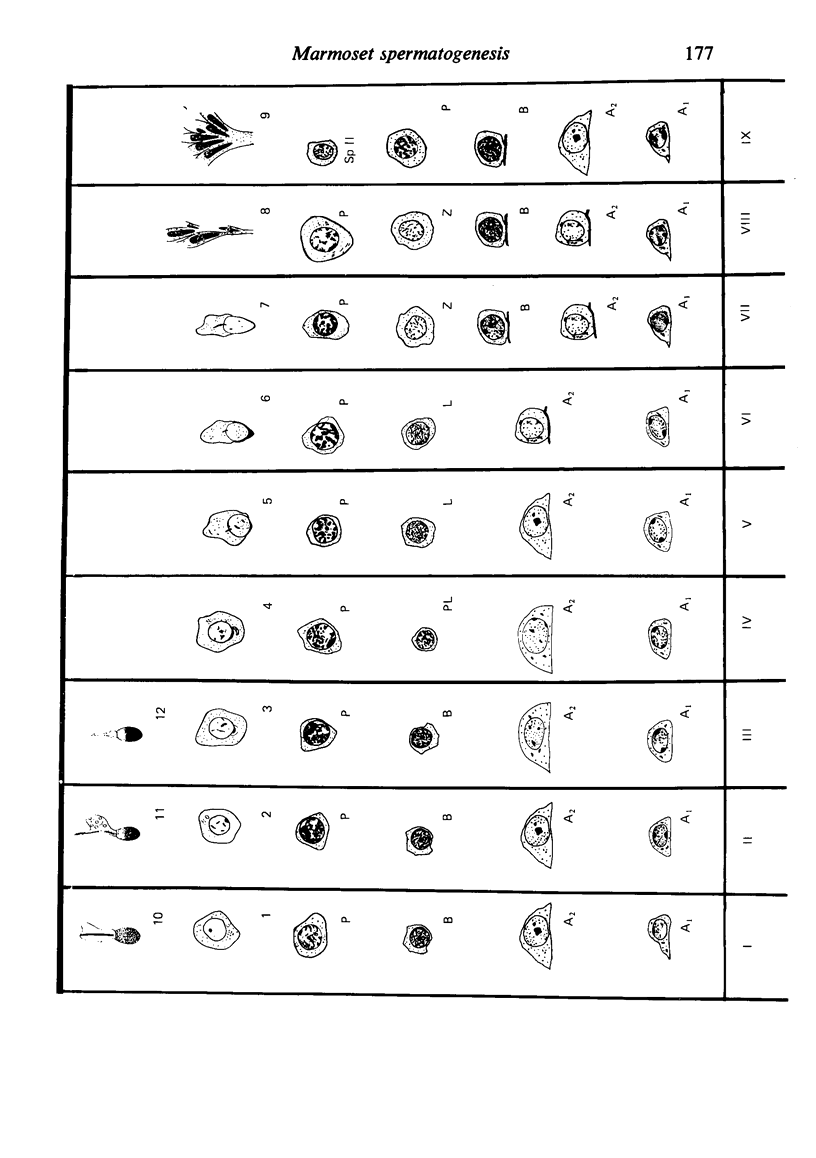
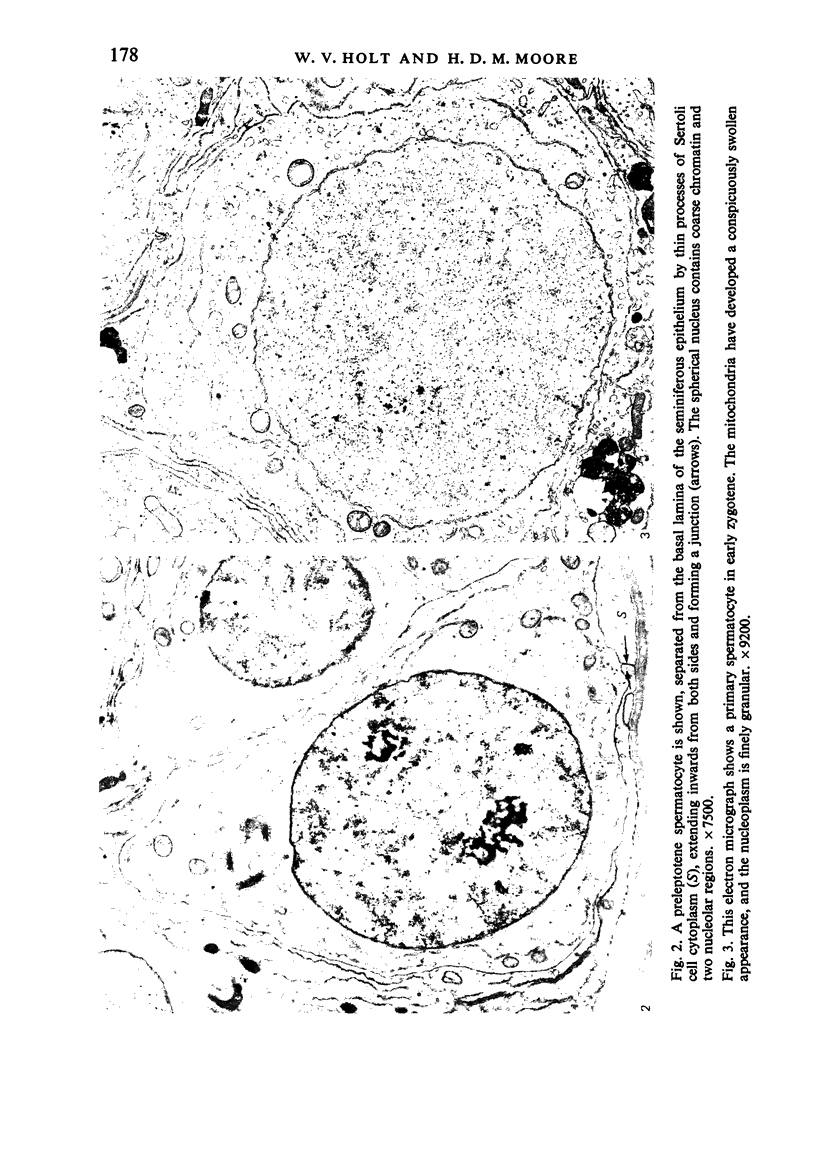
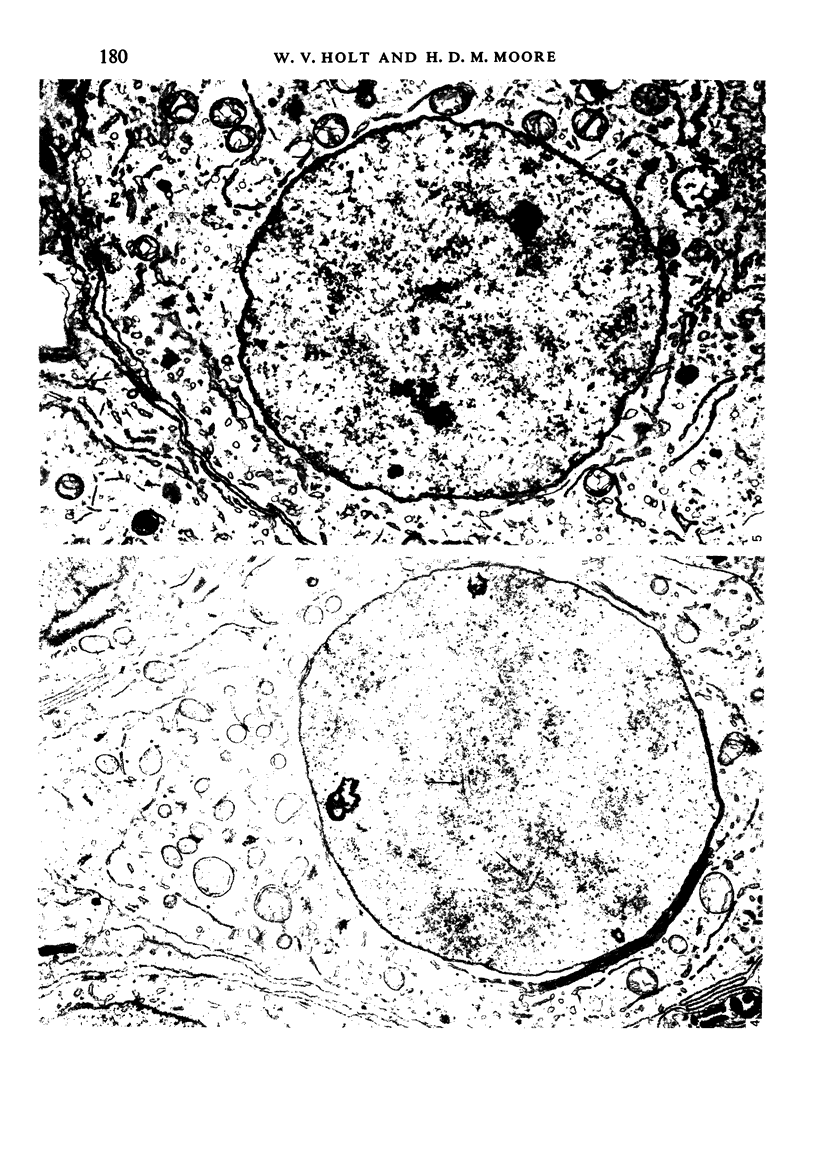
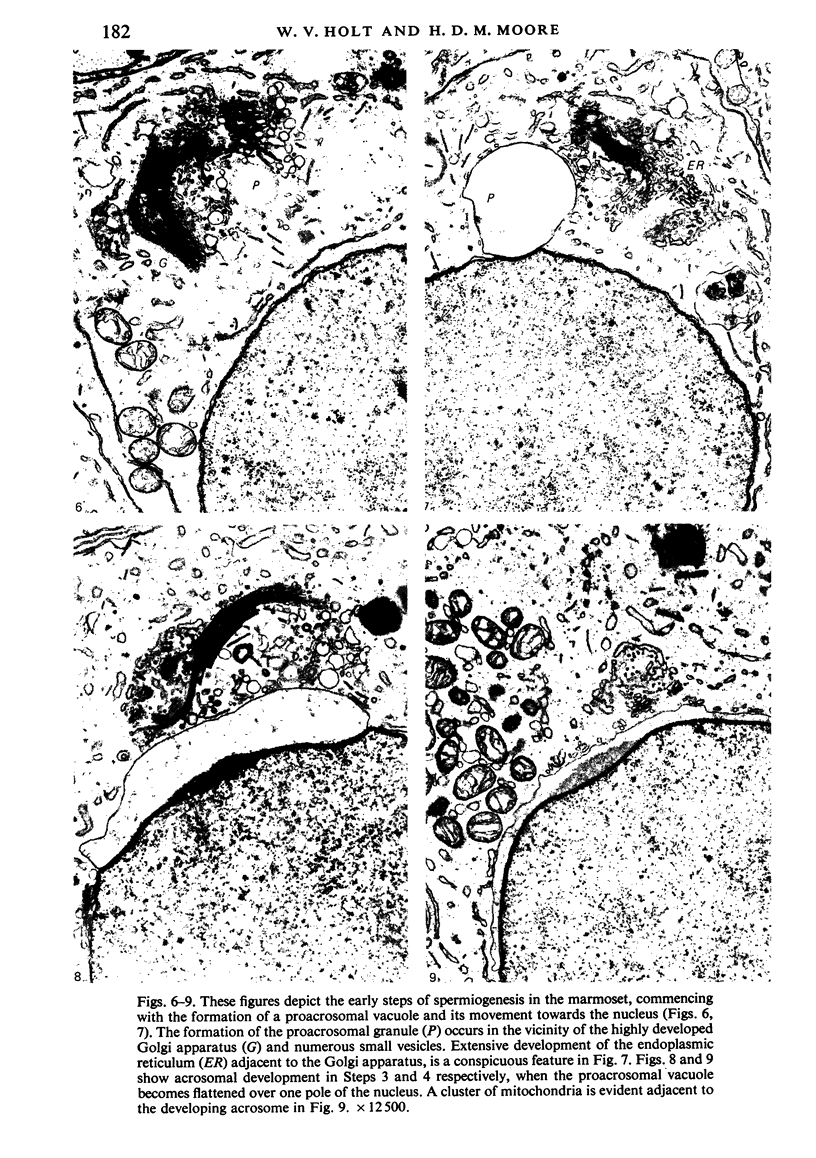
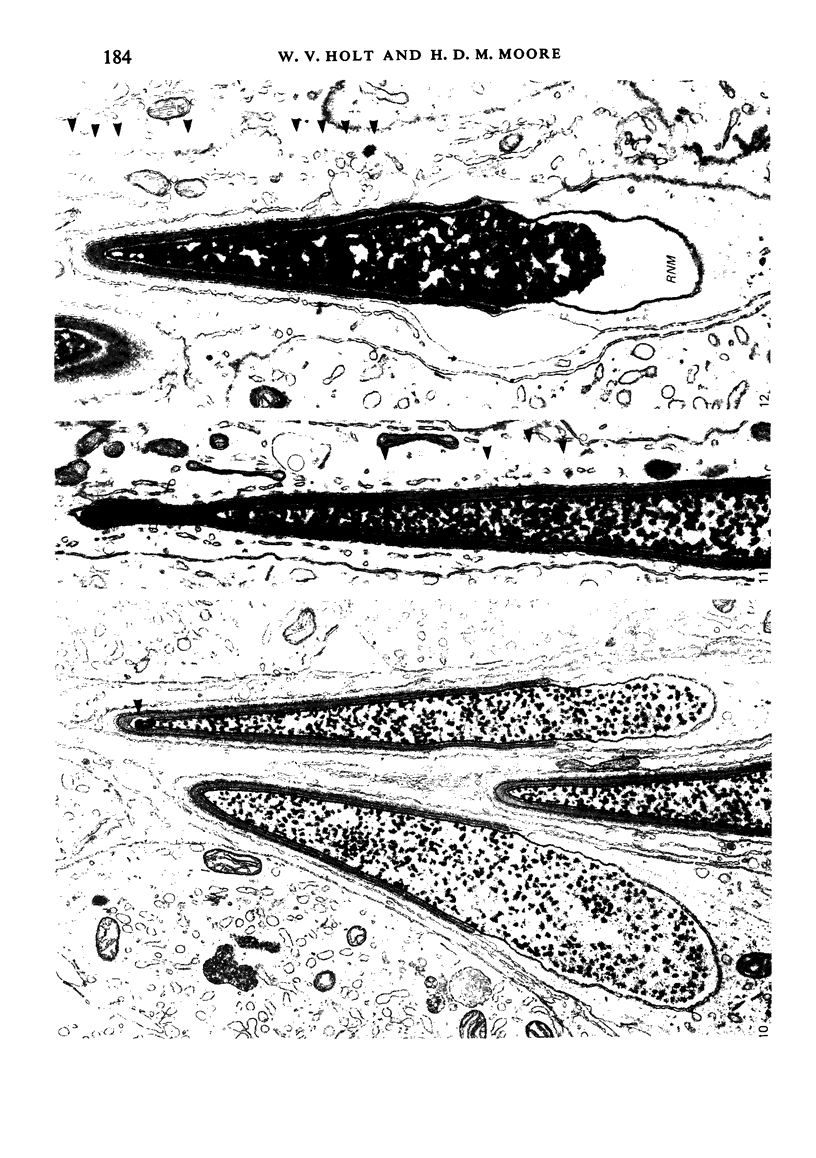
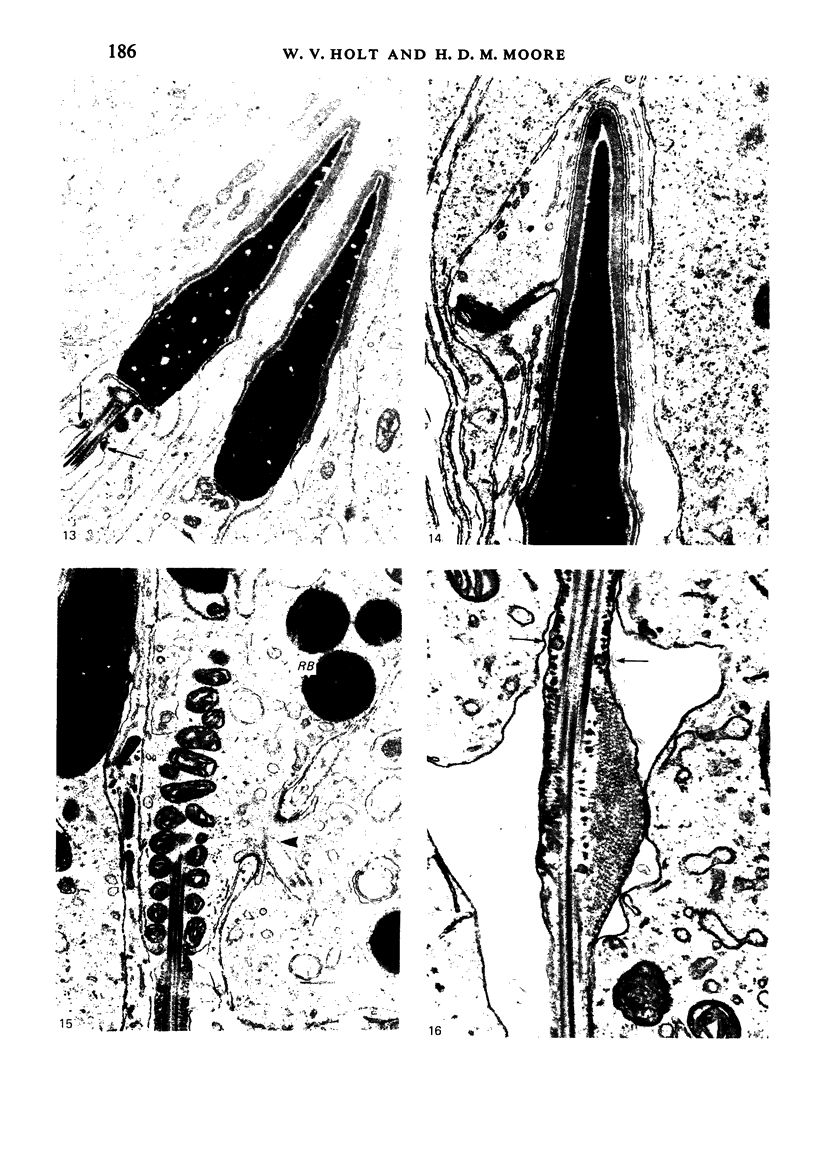
The pattern of normal spermatogenesis in the common marmoset, Callithrix jacchus, is described and a system classifying the spermatogenic cycle into nine successive stages is presented. In contrast to most previous studies of spermatogenesis, the classification system developed in this study depends more upon the recognition of characteristic cell associations rather than upon identification of particular steps of spermatid development. The structurally simple acrosome of the marmoset spermatozoon displays insufficiently clear morphological changes during spermatid elongation to allow this to be used as a key to spermatogenic classification. Ultrastructural aspects of spermatogenesis in the marmoset are also described; in general, these correspond to similar descriptions of this process in other primates.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barr A. B. Timing of spermatogenesis in four nonhuman primate species. Fertil Steril. 1973 May;24(5):381–389. [PubMed] [Google Scholar]
- Berndston W. E., Desjardins C. The cycle of the seminiferous epithelium and spermatogenesis in the bovine testis. Am J Anat. 1974 Jun;140(2):167–179. doi: 10.1002/aja.1001400204. [DOI] [PubMed] [Google Scholar]
- CLERMONT Y., LEBLOND C. P. Differentiation and renewal of spermatogonia in the monkey, Macacus rhesus. Am J Anat. 1959 Mar;104:237–273. doi: 10.1002/aja.1001040204. [DOI] [PubMed] [Google Scholar]
- Cavicchia J. C., Dym M. Ultrastructural characteristics of monkey spermatogonia and preleptotene spermatocytes. Biol Reprod. 1978 Mar;18(2):219–228. doi: 10.1095/biolreprod18.2.219. [DOI] [PubMed] [Google Scholar]
- Chowdhury A. K., Steinberger E. A study of germ cell morphology and duration of spermatogenic cycle in the baboon, Papio anubis. Anat Rec. 1976 Jun;185(2):155–169. doi: 10.1002/ar.1091850204. [DOI] [PubMed] [Google Scholar]
- Clermont Y., Antar M. Duration of the cycle of the seminiferous epithelium and the spermatogonial renewal in the monkey Macaca arctoides. Am J Anat. 1973 Feb;136(2):153–165. doi: 10.1002/aja.1001360204. [DOI] [PubMed] [Google Scholar]
- Clermont Y. Two classes of spermatogonial stem cells in the monkey (Cercopithecus aethiops). Am J Anat. 1969 Sep;126(1):57–71. doi: 10.1002/aja.1001260106. [DOI] [PubMed] [Google Scholar]
- Courtens J. L. Release of carbohydrate-containing vesicles by the developing acrosomes of ram spermatids--relation to morphometric changes of the acrosome. J Ultrastruct Res. 1978 Nov;65(2):182–189. doi: 10.1016/s0022-5320(78)90055-2. [DOI] [PubMed] [Google Scholar]
- Dym M., Cavicchia J. C. Functional morphology of the testis. Biol Reprod. 1978 Feb;18(1):1–15. doi: 10.1095/biolreprod18.1.1. [DOI] [PubMed] [Google Scholar]
- Dym M., Cavicchia J. C. Further observations on the blood-testis barrier in monkeys. Biol Reprod. 1977 Oct;17(3):390–403. doi: 10.1095/biolreprod17.3.390. [DOI] [PubMed] [Google Scholar]
- Dym M. The fine structure of the monkey (Macaca) Sertoli cell and its role in maintaining the blood-testis barrier. Anat Rec. 1973 Apr;175(4):639–656. doi: 10.1002/ar.1091750402. [DOI] [PubMed] [Google Scholar]
- Franklin L. E. Formation of the redundant nuclear envelope in monkey spermatids. Anat Rec. 1968 Jun;161(2):149–161. doi: 10.1002/ar.1091610202. [DOI] [PubMed] [Google Scholar]
- Johnson O. W., Buss I. O. The testis of the African elephant (Loxodonta africana). I. Histological features. J Reprod Fertil. 1967 Feb;13(1):11–21. doi: 10.1530/jrf.0.0130011. [DOI] [PubMed] [Google Scholar]
- Mollenhauer H. H., Morré D. J. Polyribosomes associated with forming acrosome membranes in guinea pig spermatids. Science. 1978 Apr 7;200(4337):85–86. doi: 10.1126/science.635579. [DOI] [PubMed] [Google Scholar]
- Nistal M., Paniagua R., Esponda P. Development of the endoplasmic reticulum during human spermatogenesis. Acta Anat (Basel) 1980;108(2):238–249. doi: 10.1159/000145305. [DOI] [PubMed] [Google Scholar]
- Pedersen H. Microtubules in the spermatid of the rabbit. Z Zellforsch Mikrosk Anat. 1969;98(1):148–156. doi: 10.1007/BF00344514. [DOI] [PubMed] [Google Scholar]
- Rattner J. B., Brinkley B. R. Ultrastructure of mammalian spermiogenesis. I. A tubular complex in developing sperm of the cottontop marmoset Sequinus oedipus. J Ultrastruct Res. 1970 Aug;32(3):316–322. doi: 10.1016/s0022-5320(70)80012-0. [DOI] [PubMed] [Google Scholar]
- Russell L. D. Further observations on tubulobulbar complexes formed by late spermatids and Sertoli cells in the rat testis. Anat Rec. 1979 Jun;194(2):213–232. doi: 10.1002/ar.1091940204. [DOI] [PubMed] [Google Scholar]
- Russell L. D. Spermatid-Sertoli tubulobulbar complexes as devices for elimination of cytoplasm from the head region late spermatids of the rat. Anat Rec. 1979 Jun;194(2):233–246. doi: 10.1002/ar.1091940205. [DOI] [PubMed] [Google Scholar]
- Russell L., Clermont Y. Anchoring device between Sertoli cells and late spermatids in rat seminiferous tubules. Anat Rec. 1976 Jul;185(3):259–278. doi: 10.1002/ar.1091850302. [DOI] [PubMed] [Google Scholar]
- Russell L. Observations on rat Sertoli ectoplasmic ('junctional') specializations in their association with germ cells of the rat testis. Tissue Cell. 1977;9(3):475–498. doi: 10.1016/0040-8166(77)90007-6. [DOI] [PubMed] [Google Scholar]
- Smith F. E., Berlin J. D. Cytoplasmic annulate lamellae in human spermatogenesis. Cell Tissue Res. 1977 Jan 12;176(2):235–242. doi: 10.1007/BF00229464. [DOI] [PubMed] [Google Scholar]
- Swierstra E. E., Gebauer M. R., Pickett B. W. Reproductive physiology of the stallion. I. Spermatogenesis and testis composition. J Reprod Fertil. 1974 Sep;40(1):113–123. doi: 10.1530/jrf.0.0400113. [DOI] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartenberg H., Holstein A. F. Morphology of the "spindle-shaped body" in the developing tail of human spermatids. Cell Tissue Res. 1975 Jun 24;159(4):435–443. doi: 10.1007/BF00221701. [DOI] [PubMed] [Google Scholar]