Abstract
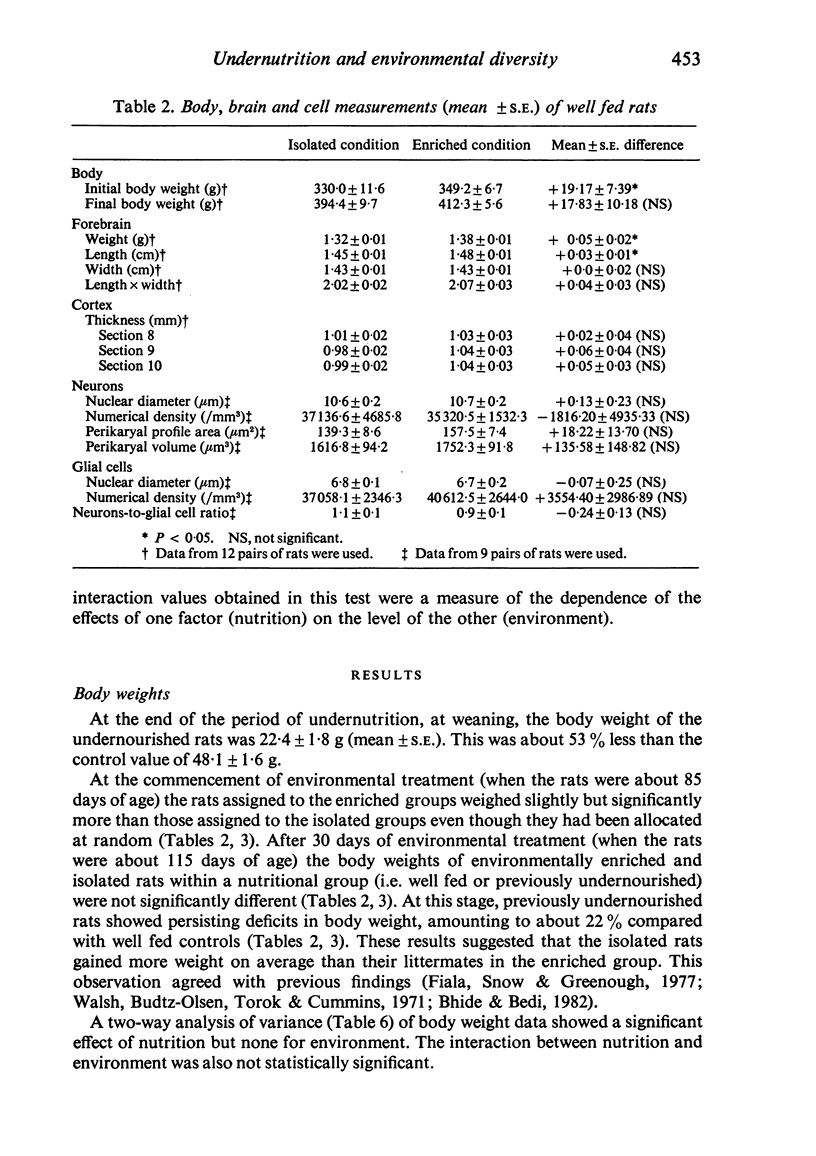
Black and white hooded Lister rats were undernourished from the sixteenth day of gestation until 25 postnatal days of age. Around 85 days of age, 12 previously undernourished male rats were assigned to an 'enriched environmental condition' and 12 to an 'isolated environmental condition'. Well fed controls were similarly assigned. After 30 days in these environmental conditions all rats were killed by perfusion with 2% buffered glutaraldehyde. Body and forebrain weights and forebrain lengths and widths were determined for each animal. Cortical depths were measured from sections through the left occipital cortical region. Neuronal and glial cell nuclear diameters and numerical densities as well as neuronal perikaryal volumes were determined from sections through the right visual cortex. In both well fed and previously undernourished groups, the environmentally enriched rats had heavier forebrains and greater forebrain lengths compared to isolated rats. There were no significant differences between enriched and isolated rats in forebrain width or cortical depth measurements in either nutritional group. In both the well fed and previously undernourished groups there were no consistently significant differences between enriched and isolated rats in any of the measurements on neurons and glial cells. Two-way analysis of variance tests on combined data from both nutritional groups indicated significant effects of environment on forebrain weight, forebrain length and on cortical depth in one of the three sections studied (section 10). Nutrition had a significant effect on body weight, forebrain weight and forebrain width. The interaction between nutrition and environment was not statistically significant for any of the measurements carried out.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALTMAN J., DAS G. D. AUTORADIOGRAPHIC EXAMINATION OF THE EFFECTS OF ENRICHED ENVIRONMENT ON THE RATE OF GLIAL MULTIPLICATION IN THE ADULT RAT BRAIN. Nature. 1964 Dec 19;204:1161–1163. doi: 10.1038/2041161a0. [DOI] [PubMed] [Google Scholar]
- BENNETT E. L., DIAMOND M. C., KRECH D., ROSENZWEIG M. R. CHEMICAL AND ANATOMICAL PLASTICITY BRAIN. Science. 1964 Oct 30;146(3644):610–619. doi: 10.1126/science.146.3644.610. [DOI] [PubMed] [Google Scholar]
- Bedi K. S., Hall R., Davies C. A., Dobbing J. A stereological analysis of the cerebellar granule and Purkinje cells of 30-day-old and adult rats undernourished during early postnatal life. J Comp Neurol. 1980 Oct 15;193(4):863–870. doi: 10.1002/cne.901930404. [DOI] [PubMed] [Google Scholar]
- Bedi K. S., Thomas Y. M., Davies C. A., Dobbing J. Synapse-to-neuron ratios of the frontal and cerebellar cortex of 30-day-old and adult rats undernourished during early postnatal life. J Comp Neurol. 1980 Sep 1;193(1):49–56. doi: 10.1002/cne.901930104. [DOI] [PubMed] [Google Scholar]
- Bhide P. G., Bedi K. S. The effects of environmental diversity on well-fed and previously undernourished rats: I. Body and brain measurements. J Comp Neurol. 1982 Jun 1;207(4):403–409. doi: 10.1002/cne.902070411. [DOI] [PubMed] [Google Scholar]
- Cummins R. A., Livesey P. J. Enrichment-isolation, cortex length and the rank order effect. Brain Res. 1979 Dec 7;178(1):89–98. doi: 10.1016/0006-8993(79)90089-1. [DOI] [PubMed] [Google Scholar]
- Cummins R. A., Walsh R. N., Budtz-Olsen O. E., Konstantinos T., Horsfall C. R. Environmentally-induced changes in the brains of elderly rats. Nature. 1973 Jun 29;243(5409):516–518. doi: 10.1038/243516a0. [DOI] [PubMed] [Google Scholar]
- DIAMOND M. C., KRECH D., ROSENZWEIG M. R. THE EFFECTS OF AN ENRICHED ENVIRONMENT ON THE HISTOLOGY OF THE RAT CEREBRAL CORTEX. J Comp Neurol. 1964 Aug;123:111–120. doi: 10.1002/cne.901230110. [DOI] [PubMed] [Google Scholar]
- Das Gupta V., Cadwallader D. E. Acid dye method for the analysis of thiamine. J Pharm Sci. 1968 Jan;57(1):112–117. doi: 10.1002/jps.2600570122. [DOI] [PubMed] [Google Scholar]
- Diamond M. C., Johnson R. E., Ingham C., Rosenzweig M. R., Bennett E. L. Effects of differential experience on neuronal nuclear and perikarya dimensions in the rat cerebral cortex. Behav Biol. 1975 Sep;15(1):107–111. doi: 10.1016/s0091-6773(75)92144-6. [DOI] [PubMed] [Google Scholar]
- Diamond M. C., Law F., Rhodes H., Lindner B., Rosenzweig M. R., Krech D., Bennett E. L. Increases in cortical depth and glia numbers in rats subjected to enriched environment. J Comp Neurol. 1966 Sep;128(1):117–126. doi: 10.1002/cne.901280110. [DOI] [PubMed] [Google Scholar]
- Fiala B., Snow F. M., Greenough W. T. "Impoverished" rats weigh more than "enriched" rats because they eat more. Dev Psychobiol. 1977 Nov;10(6):537–541. doi: 10.1002/dev.420100607. [DOI] [PubMed] [Google Scholar]
- Kaplan M. S., Hinds J. W. Gliogenesis of astrocytes and oligodendrocytes in the neocortical grey and white matter of the adult rat: electron microscopic analysis of light radioautographs. J Comp Neurol. 1980 Oct 1;193(3):711–727. doi: 10.1002/cne.901930309. [DOI] [PubMed] [Google Scholar]
- Katz H. B., Davies C. A. The effects of early-life undernutrition and subsequent environment on morphological parameters of the rat brain. Behav Brain Res. 1982 May;5(1):53–64. doi: 10.1016/0166-4328(82)90090-0. [DOI] [PubMed] [Google Scholar]
- Katz H. B., Davies C. A. The separate and combined effects of early undernutrition and environmental complexity at different ages on cerebral measures in rats. Dev Psychobiol. 1983 Jan;16(1):47–58. doi: 10.1002/dev.420160106. [DOI] [PubMed] [Google Scholar]
- Ling E. A., Paterson J. A., Privat A., Mori S., Leblond C. P. Investigation of glial cells in semithin sections. I. Identification of glial cells in the brain of young rats. J Comp Neurol. 1973 May 1;149(1):43–71. doi: 10.1002/cne.901490104. [DOI] [PubMed] [Google Scholar]
- Malkasian D. R., Diamond M. C. The effects of environmental manipulation on the morphology of the neonate rat brain. Int J Neurosci. 1971 Nov;2(4):161–169. doi: 10.3109/00207457109146998. [DOI] [PubMed] [Google Scholar]
- Mayhew T. M., Cruz L. M. Stereological correction procedures for estimating true volume proportions from biased samples. J Microsc. 1973 Dec;99(3):287–299. doi: 10.1111/j.1365-2818.1973.tb04628.x. [DOI] [PubMed] [Google Scholar]
- ROSENZWEIG M. R., KRECH D., BENNETT E. L., DIAMOND M. C. Effects of environmental complexity and training on brain chemistry and anatomy: a replication and extension. J Comp Physiol Psychol. 1962 Aug;55:429–437. doi: 10.1037/h0041137. [DOI] [PubMed] [Google Scholar]
- Rosenzweig M. R., Bennett E. L. Effects of differential environments on brain weights and enzyme activities in gerbils, rats, and mice. Dev Psychobiol. 1969;2(2):87–95. doi: 10.1002/dev.420020208. [DOI] [PubMed] [Google Scholar]
- Siassi F., Siassi B. Differential effects of protein-calorie restriction and subsequent repletion on neuronal and nonneuronal components of cerebral cortex in newborn rats. J Nutr. 1973 Nov;103(11):1625–1633. doi: 10.1093/jn/103.11.1625. [DOI] [PubMed] [Google Scholar]
- Szeligo F., Leblond C. P. Response of the three main types of glial cells of cortex and corpus callosum in rats handled during suckling or exposed to enriched, control and impoverished environments following weaning. J Comp Neurol. 1977 Mar 15;172(2):247–263. doi: 10.1002/cne.901720205. [DOI] [PubMed] [Google Scholar]
- Walsh R. N., Budtz-Olsen O. E., Torok A., Cummins R. A. Environmentally induced changes in the dimensions of the rat cerebrum. Dev Psychobiol. 1971;4(2):115–122. doi: 10.1002/dev.420040203. [DOI] [PubMed] [Google Scholar]
- Warren M. A., Bedi K. S. Synapse-to-neuron ratios in the visual cortex of adult rats undernourished from about birth until 100 days of age. J Comp Neurol. 1982 Sep 1;210(1):59–64. doi: 10.1002/cne.902100107. [DOI] [PubMed] [Google Scholar]
- Weibel E. R. Stereological principles for morphometry in electron microscopic cytology. Int Rev Cytol. 1969;26:235–302. doi: 10.1016/s0074-7696(08)61637-x. [DOI] [PubMed] [Google Scholar]


