Abstract
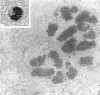
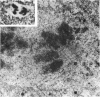
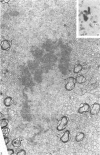
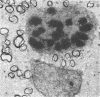
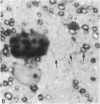
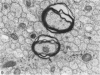
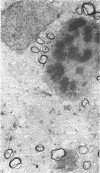
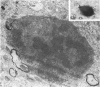
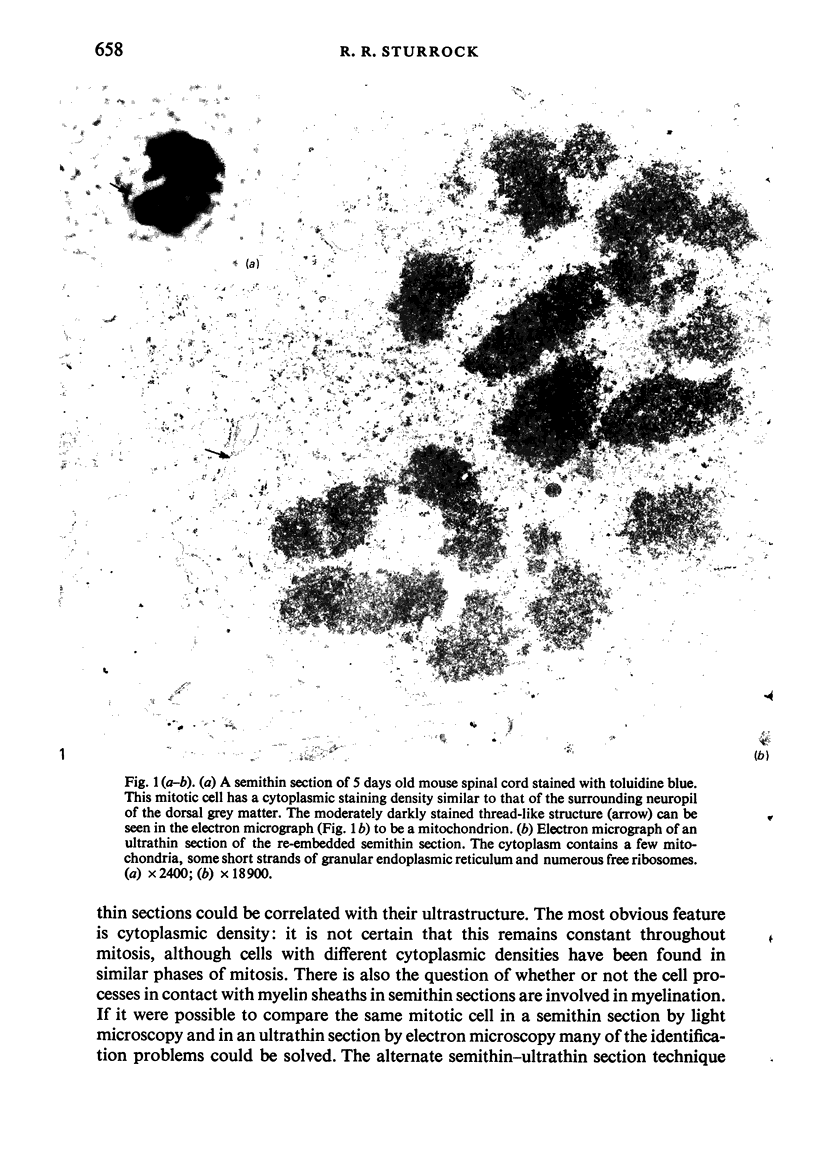
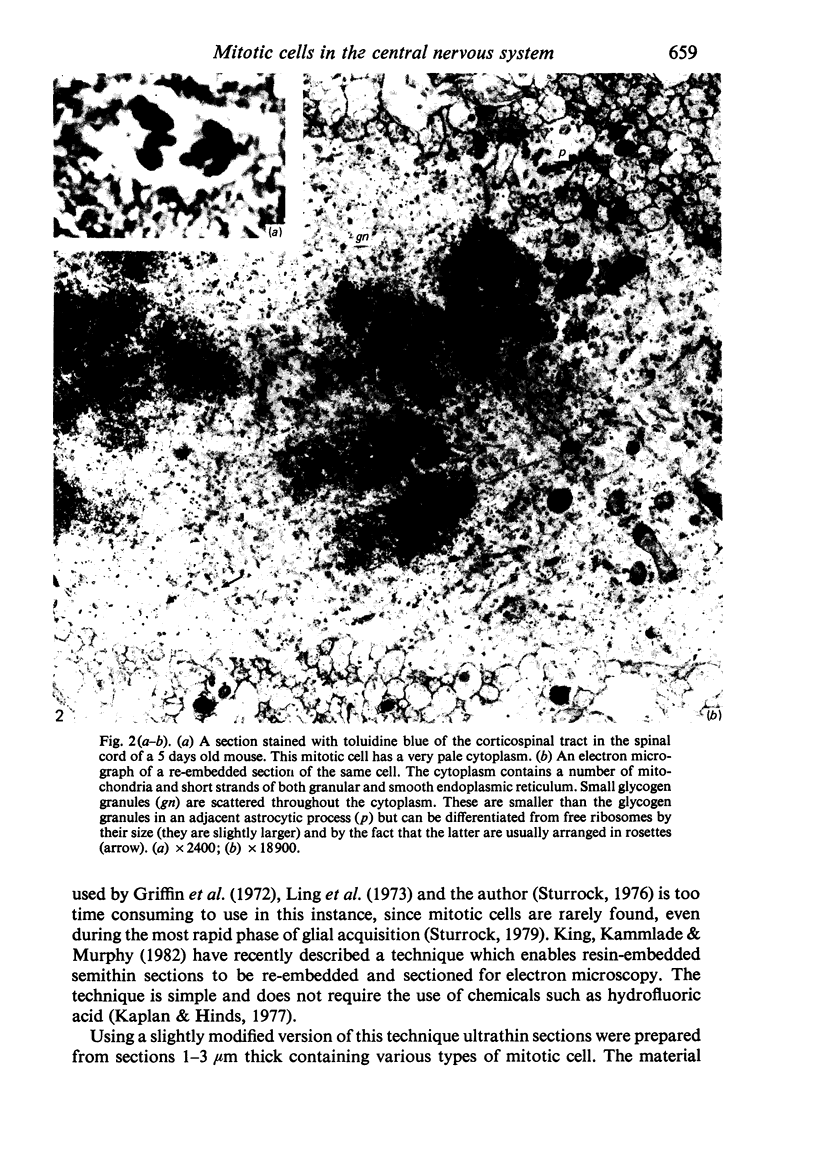
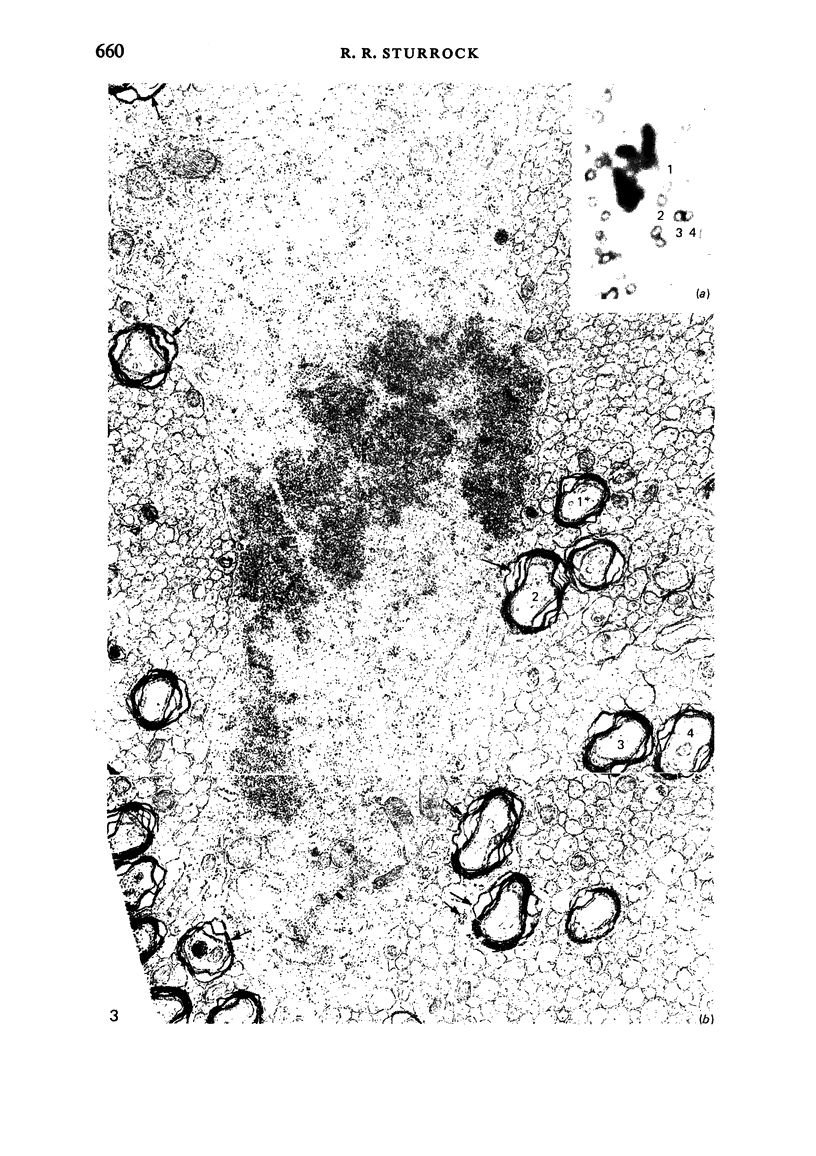
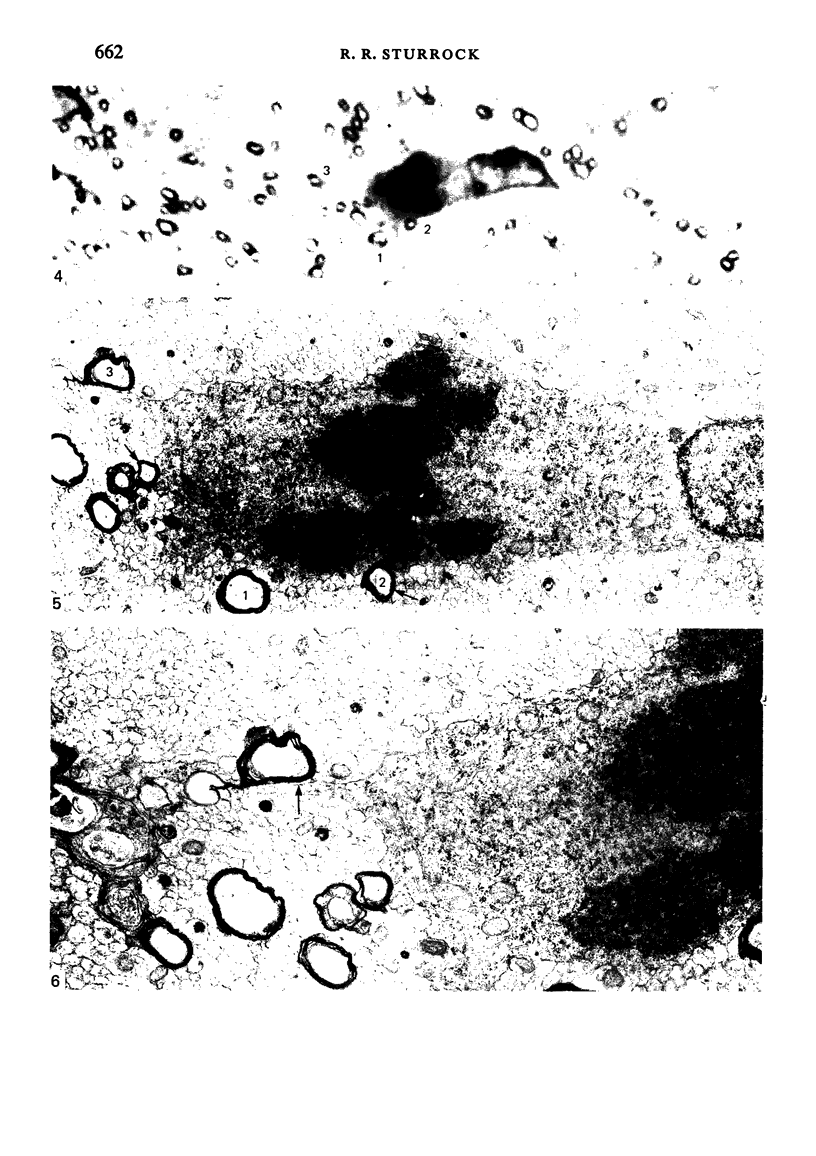
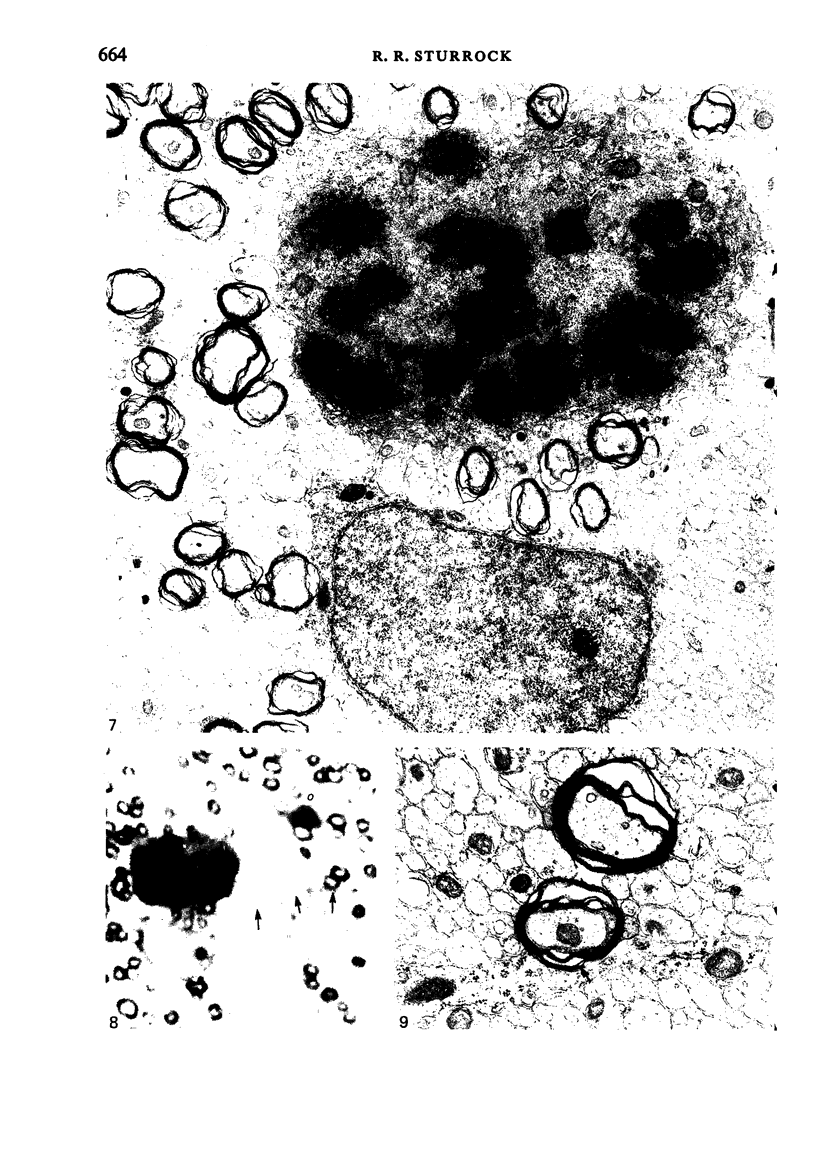
Semithin (1-3 micron) sections of 5 days postnatal mouse spinal cord and 17 days postnatal mouse corpus callosum and hippocampal commissure were examined to find examples of mitotic glial cells. The sections were re-embedded for electron microscopy and the structure of the mitotic cells by light and electron microscopy was compared. The most commonly found type of mitotic cell had a cytoplasmic staining density similar to, or slightly darker than, the surrounding neuropil. This was seen electron microscopically to be an undifferentiated cell, similar to mitotic cells described by other authors in the subependymal layer. This cell was classified as a glioblast. Mitotic cells with cytoplasm which appeared unstained in semithin sections were identified as partially, or fully, differentiated astrocytes. Mitotic cells with processes in contact with myelin sheaths had a wide variety of cytoplasmic staining densities in semithin sections. Electron microscopy confirmed that these cells were involved in myelination and it is possible that the mitotic cells compared to the light, medium and dark varieties of oligodendrocytes.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUNGE R. P., GLASS P. M. SOME OBSERVATIONS ON MYELIN-GLIAL RELATIONSHIPS AND ON THE ETIOLOGY OF THE CEREBROSPINAL FLUID EXCHANGE LESION. Ann N Y Acad Sci. 1965 Mar 31;122:15–28. doi: 10.1111/j.1749-6632.1965.tb20188.x. [DOI] [PubMed] [Google Scholar]
- Bignami A., Dahl D. Astrocyte-specific protein and neuroglial differentiation. An immunofluorescence study with antibodies to the glial fibrillary acidic protein. J Comp Neurol. 1974 Jan 1;153(1):27–38. doi: 10.1002/cne.901530104. [DOI] [PubMed] [Google Scholar]
- Blakemore W. F. The ultrastructure of the subependymal plate in the rat. J Anat. 1969 May;104(Pt 3):423–433. [PMC free article] [PubMed] [Google Scholar]
- Bologa L., Bisconte J. C., Joubert R., Margules S., Herschkowitz N. Proliferative activity and characteristics of immunocytochemically identified oligodendrocytes in embryonic mouse brain cell cultures. Exp Brain Res. 1983;50(1):84–90. doi: 10.1007/BF00238234. [DOI] [PubMed] [Google Scholar]
- Bunge R. P. Glial cells and the central myelin sheath. Physiol Rev. 1968 Jan;48(1):197–251. doi: 10.1152/physrev.1968.48.1.197. [DOI] [PubMed] [Google Scholar]
- Erlandson R. A., de Harven E. The ultrastructure of synchronized HeLa cells. J Cell Sci. 1971 Mar;8(2):353–397. doi: 10.1242/jcs.8.2.353. [DOI] [PubMed] [Google Scholar]
- Griffin R., Illis L. S., Mitchell J. Identification of neuroglia by light and electronmicroscopy. Acta Neuropathol. 1972;22(1):7–12. doi: 10.1007/BF00687546. [DOI] [PubMed] [Google Scholar]
- Imamoto K., Paterson J. A., Leblond C. P. Radioautographic investigation of gliogenesis in the corpus callosum of young rats. I. Sequential changes in oligodendrocytes. J Comp Neurol. 1978 Jul 1;180(1):115-28, 132-7. doi: 10.1002/cne.901800108. [DOI] [PubMed] [Google Scholar]
- Jokelainen P. T. The ultrastructure and spatial organization of the metaphase kinetochore in mitotic rat cells. J Ultrastruct Res. 1967 Jul;19(1):19–44. doi: 10.1016/s0022-5320(67)80058-3. [DOI] [PubMed] [Google Scholar]
- Kaplan M. S., Hinds J. W. Gliogenesis of astrocytes and oligodendrocytes in the neocortical grey and white matter of the adult rat: electron microscopic analysis of light radioautographs. J Comp Neurol. 1980 Oct 1;193(3):711–727. doi: 10.1002/cne.901930309. [DOI] [PubMed] [Google Scholar]
- Kaplan M. S., Hinds J. W. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977 Sep 9;197(4308):1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- King D. G., Kammlade N., Murphy J. A simple device to help re-embed thick plastic sections. Stain Technol. 1982 Sep;57(5):307–310. doi: 10.3109/10520298209066728. [DOI] [PubMed] [Google Scholar]
- Korr H. Proliferation of different cell types in the brain of senile mice autoradiographic studies with 3H- and 14C-thymidine. Exp Brain Res. 1982;Suppl 5:51–57. doi: 10.1007/978-3-642-68507-1_8. [DOI] [PubMed] [Google Scholar]
- Korr H. Proliferation of different cell types in the brain. Adv Anat Embryol Cell Biol. 1980;61:1–72. doi: 10.1007/978-3-642-67577-5. [DOI] [PubMed] [Google Scholar]
- Korr H., Schilling W. D., Schultze B., Maurer W. Autoradiographic studies of glial proliferation in different areas of the brain of the 14-day-old rat. Cell Tissue Kinet. 1983 Jul;16(4):393–413. [PubMed] [Google Scholar]
- Leblond C. P. The life history of cells in renewing systems. Am J Anat. 1981 Feb;160(2):114–158. doi: 10.1002/aja.1001600202. [DOI] [PubMed] [Google Scholar]
- Matthews M. A., Duncan D. A quantitative study of morphological changes accompanying the initiation and progress of myelin production in the dorsal funiculus of the rat spinal cord. J Comp Neurol. 1971 May;142(1):1–22. doi: 10.1002/cne.901420102. [DOI] [PubMed] [Google Scholar]
- Mori S., Leblond C. P. Electron microscopic features and proliferation of astrocytes in the corpus callosum of the rat. J Comp Neurol. 1969 Oct;137(2):197–225. doi: 10.1002/cne.901370206. [DOI] [PubMed] [Google Scholar]
- Mori S., Leblond C. P. Electron microscopic identification of three classes of oligodendrocytes and a preliminary study of their proliferative activity in the corpus callosum of young rats. J Comp Neurol. 1970 May;139(1):1–28. doi: 10.1002/cne.901390102. [DOI] [PubMed] [Google Scholar]
- Paterson J. A. Dividing and newly produced cells in the corpus callosum of adult mouse cerebrum as detected by light microscopic radioautography. Anat Anz. 1983;153(2):149–168. [PubMed] [Google Scholar]
- Privat A., Leblond C. P. The subependymal layer and neighboring region in the brain of the young rat. J Comp Neurol. 1972 Nov;146(3):277–302. doi: 10.1002/cne.901460302. [DOI] [PubMed] [Google Scholar]
- Privat A., Valat J., Fulcrand J. Proliferation of neuroglial cell lines in the degenerating optic nerve of young rats. A radioautographic study. J Neuropathol Exp Neurol. 1981 Jan;40(1):46–60. [PubMed] [Google Scholar]
- ROBBINS E., GONATAS N. K. THE ULTRASTRUCTURE OF A MAMMALIAN CELL DURING THE MITOTIC CYCLE. J Cell Biol. 1964 Jun;21:429–463. doi: 10.1083/jcb.21.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel G., Sensenbrenner M., Labourdette G., Wittendorp-Rechenmann E., Pettmann B., Nussbaum J. L. An immunohistochemical study of two myelin-specific proteins in enriched oligodendroglial cell cultures combined with an autoradiographic investigation using [3H]thymidine. Brain Res. 1983 Jun;284(2-3):193–204. doi: 10.1016/0165-3806(83)90004-4. [DOI] [PubMed] [Google Scholar]
- Schonbach J., Hu K. H., Friede R. L. Cellular and chemical changes during myelination: histologic, autoradiographic, histochemical and biochemical data on myelination in the pyramidal tract and corpus callosum of rat. J Comp Neurol. 1968 Sep;134(1):21–36. doi: 10.1002/cne.901340104. [DOI] [PubMed] [Google Scholar]
- Skoff R. P. The fine structure of pulse labeled (3-H-thymidine cells) in degenerating rat optic nerve. J Comp Neurol. 1975 Jun 15;161(4):595–611. doi: 10.1002/cne.901610408. [DOI] [PubMed] [Google Scholar]
- Sturrock R. R. A developmental study of the mouse neostriatum. J Anat. 1980 Mar;130(Pt 2):243–261. [PMC free article] [PubMed] [Google Scholar]
- Sturrock R. R. A light and electron microscopic study of proliferation and maturation of fibrous astrocytes in the optic nerve of the human embryo. J Anat. 1975 Apr;119(Pt 2):223–234. [PMC free article] [PubMed] [Google Scholar]
- Sturrock R. R. A quantitative lifespan study of changes in cell number, cell division and cell death in various regions of the mouse forebrain. Neuropathol Appl Neurobiol. 1979 Nov-Dec;5(6):433–456. doi: 10.1111/j.1365-2990.1979.tb00642.x. [DOI] [PubMed] [Google Scholar]
- Sturrock R. R. Electron microscopic evidence for mitotic division of oligodendrocytes. J Anat. 1981 May;132(Pt 3):429–432. [PMC free article] [PubMed] [Google Scholar]
- Sturrock R. R. Gliogenesis in the prenatal rabbit spinal cord. J Anat. 1982 Jun;134(Pt 4):771–793. [PMC free article] [PubMed] [Google Scholar]
- Sturrock R. R. Histogenesis of the anterior limb of the anterior commissure of the mouse brain. 3. An electron microscopic study of gliogenesis. J Anat. 1974 Feb;117(Pt 1):37–53. [PMC free article] [PubMed] [Google Scholar]
- Sturrock R. R. Identification of mitotic oligodendrocytes in semithin sections of the developing mouse corpus callosum and hippocampal commissure. J Anat. 1983 Aug;137(Pt 1):47–55. [PMC free article] [PubMed] [Google Scholar]
- Sturrock R. R. Light microscopic identification of immature glial cells in semithin sections of the developing mouse corpus callosum. J Anat. 1976 Dec;122(Pt 3):521–537. [PMC free article] [PubMed] [Google Scholar]
- Sturrock R. R., Smart I. H. A morphological study of the mouse subependymal layer from embryonic life to old age. J Anat. 1980 Mar;130(Pt 2):391–415. [PMC free article] [PubMed] [Google Scholar]