Abstract
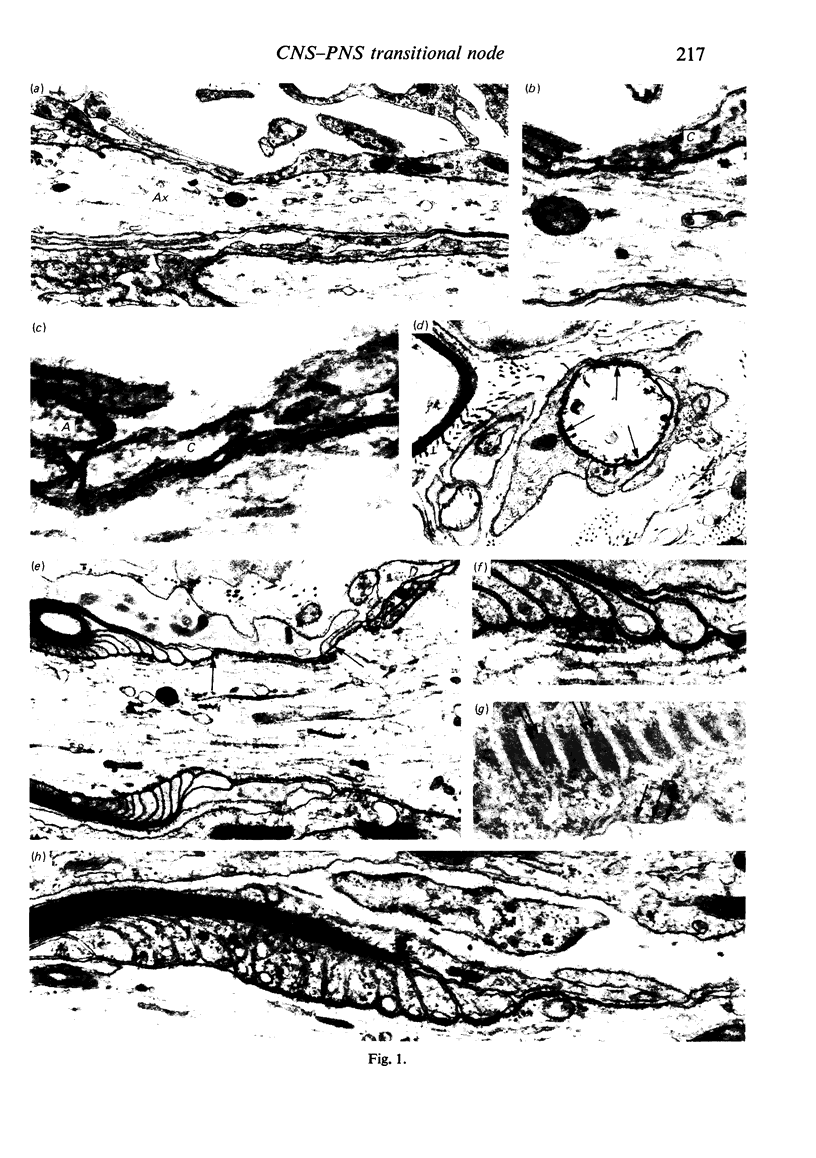
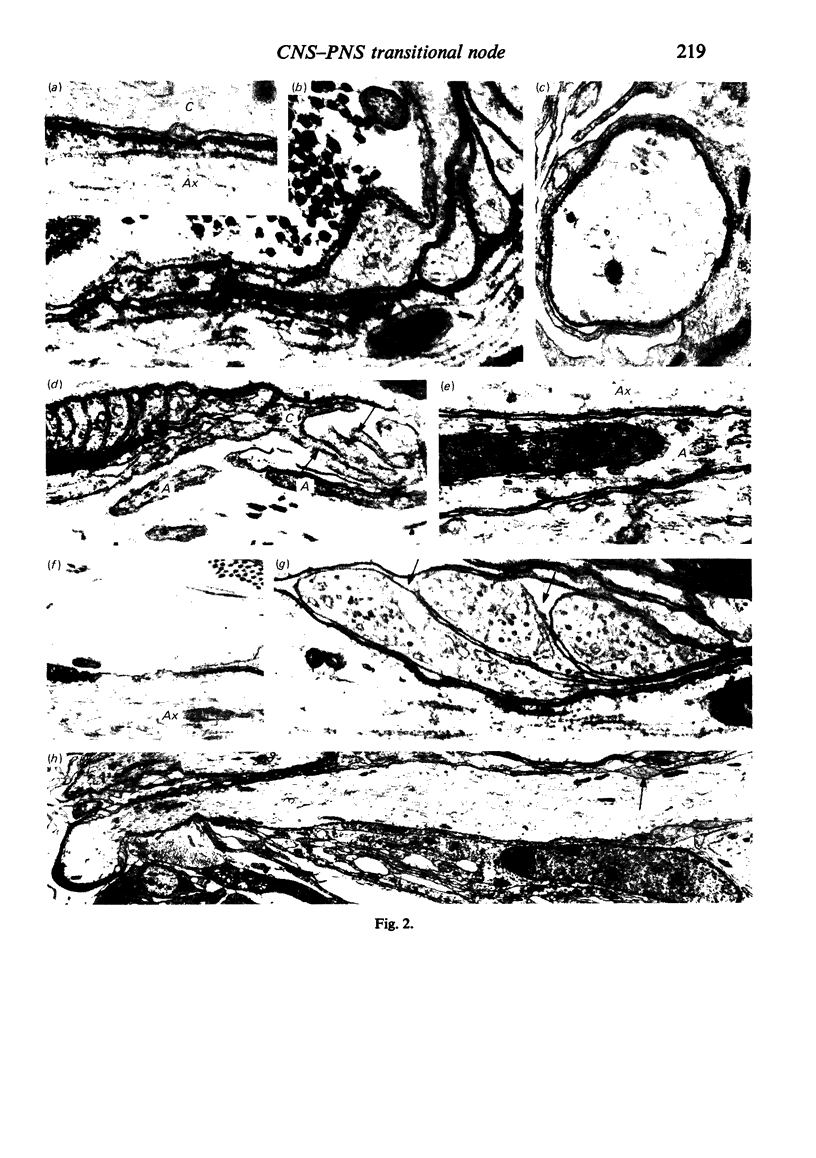
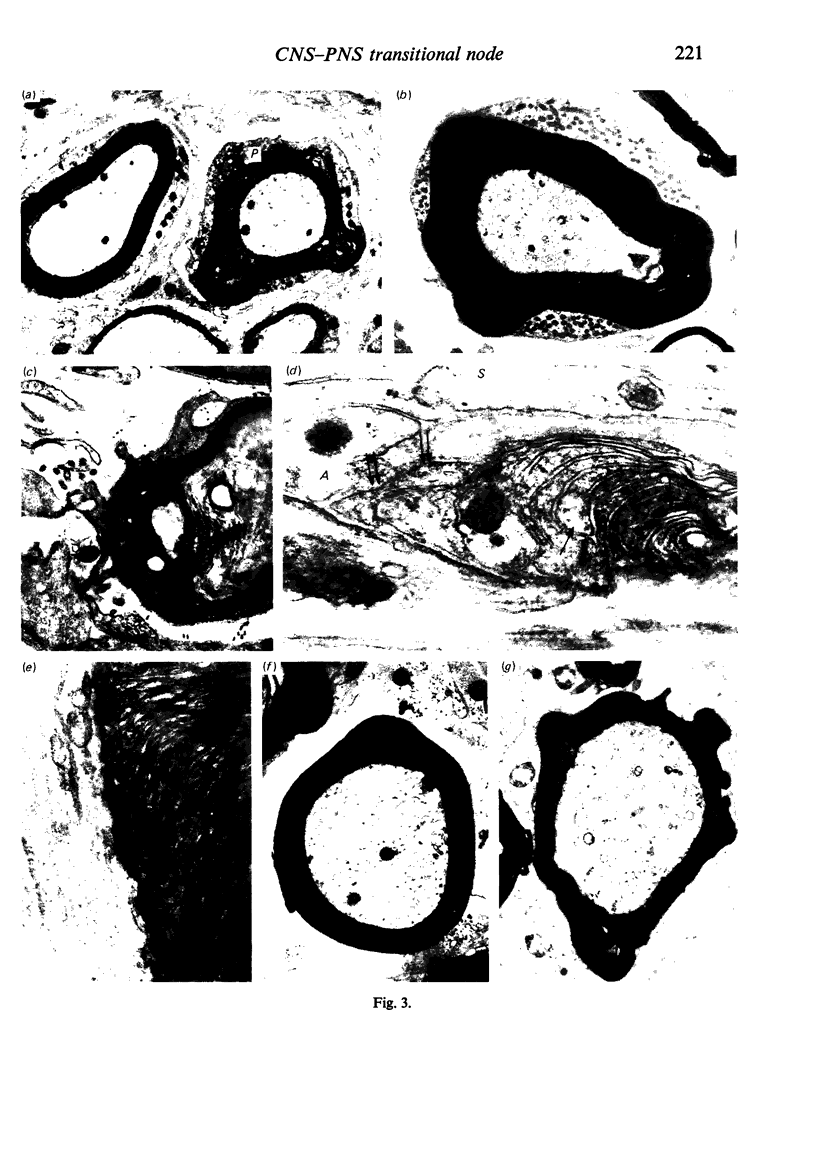
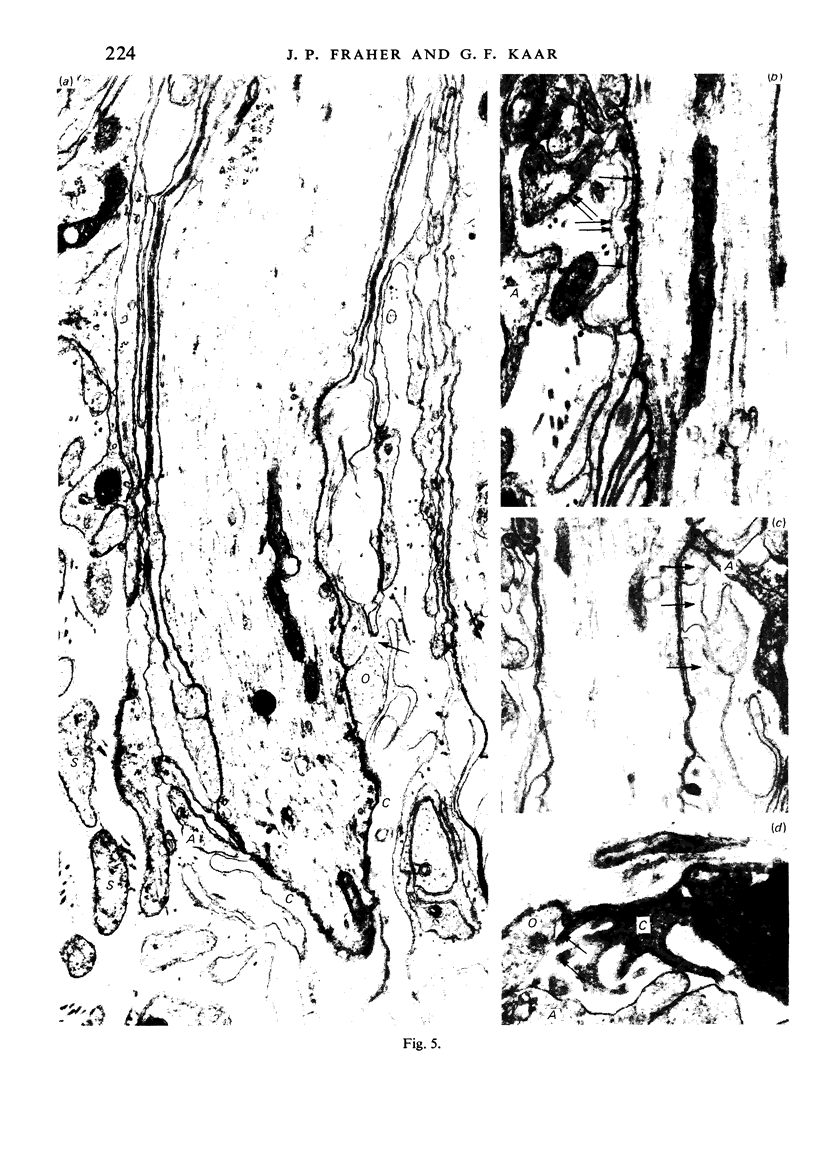
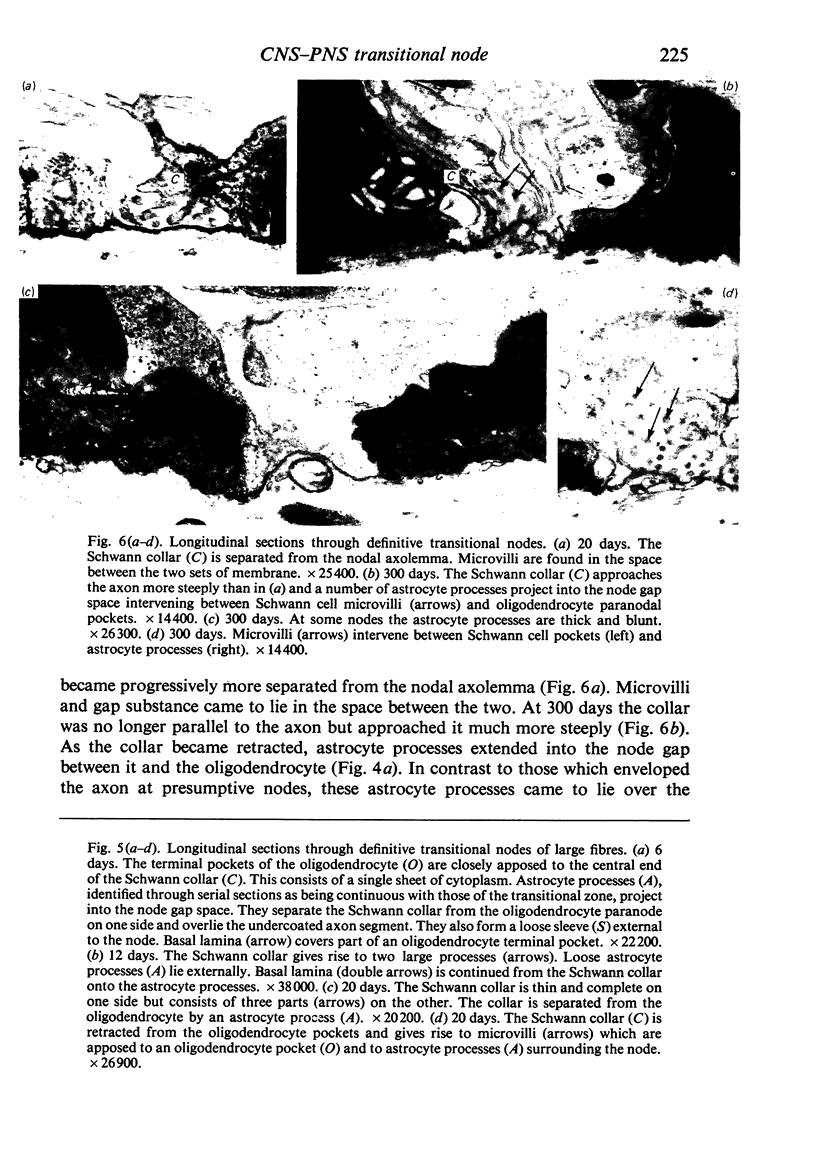
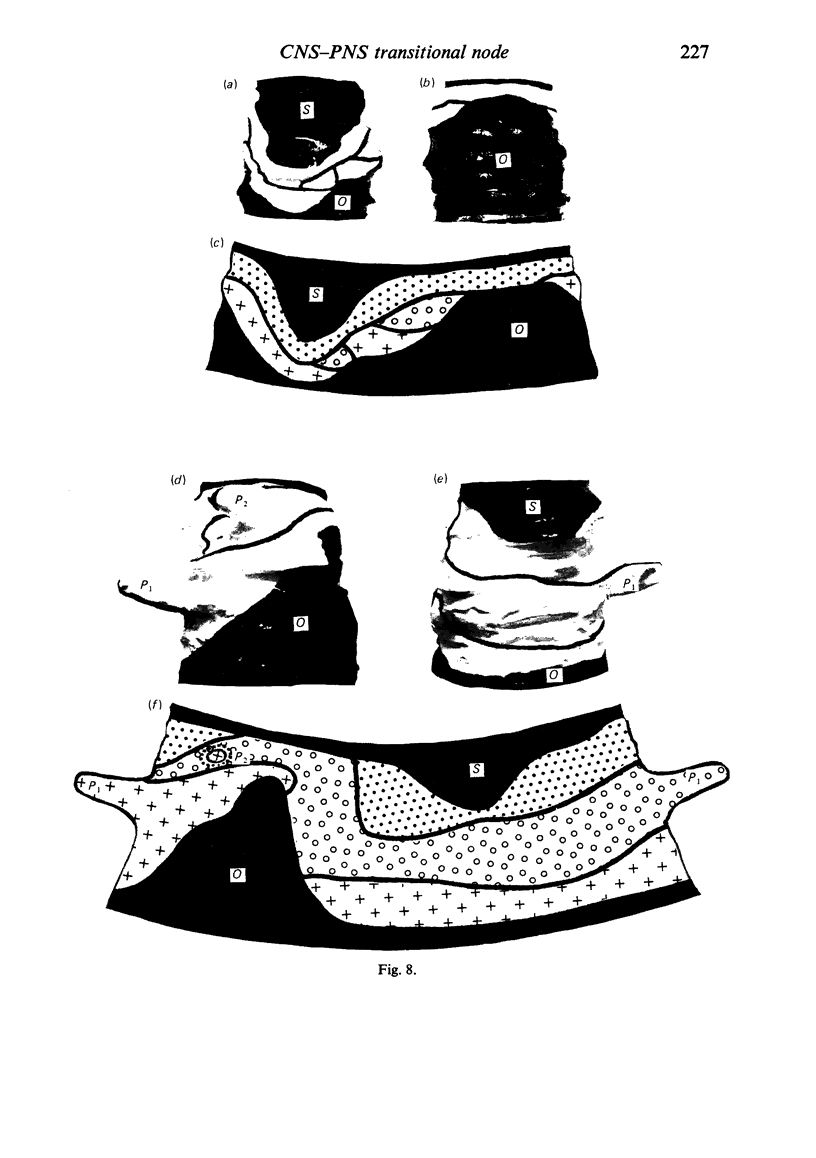
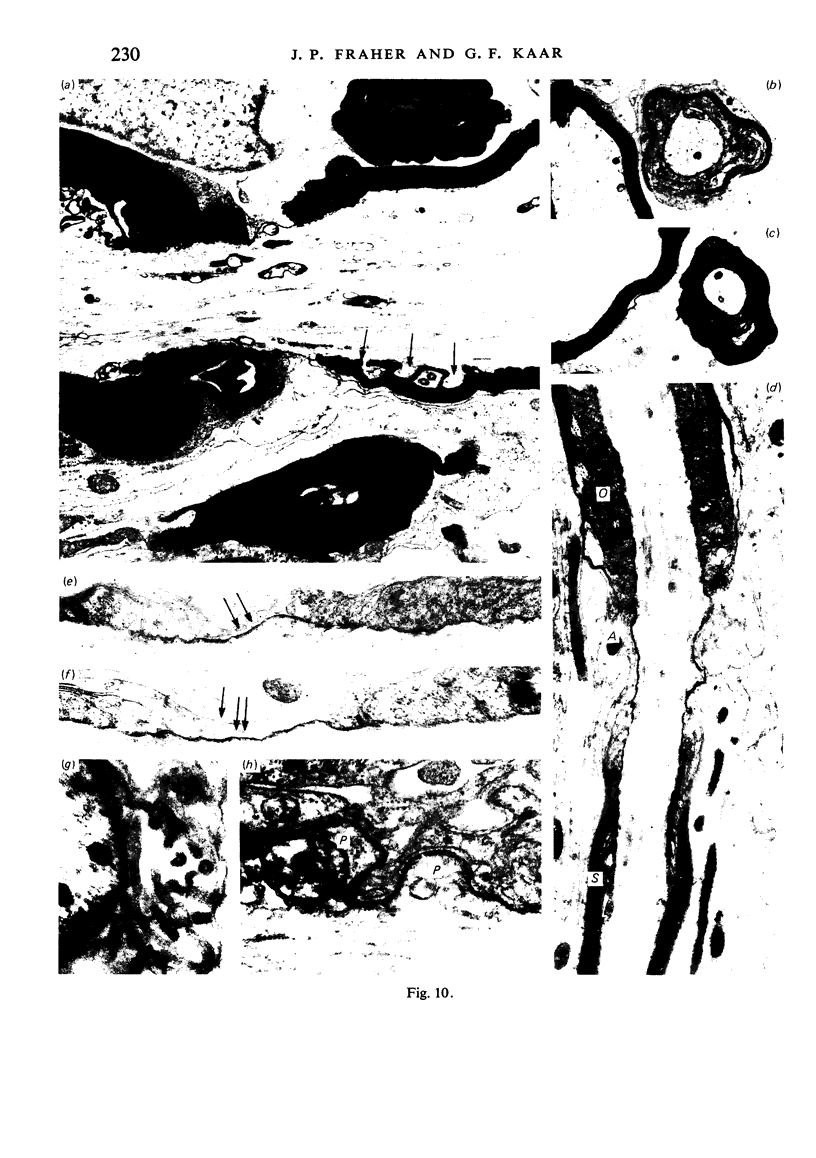
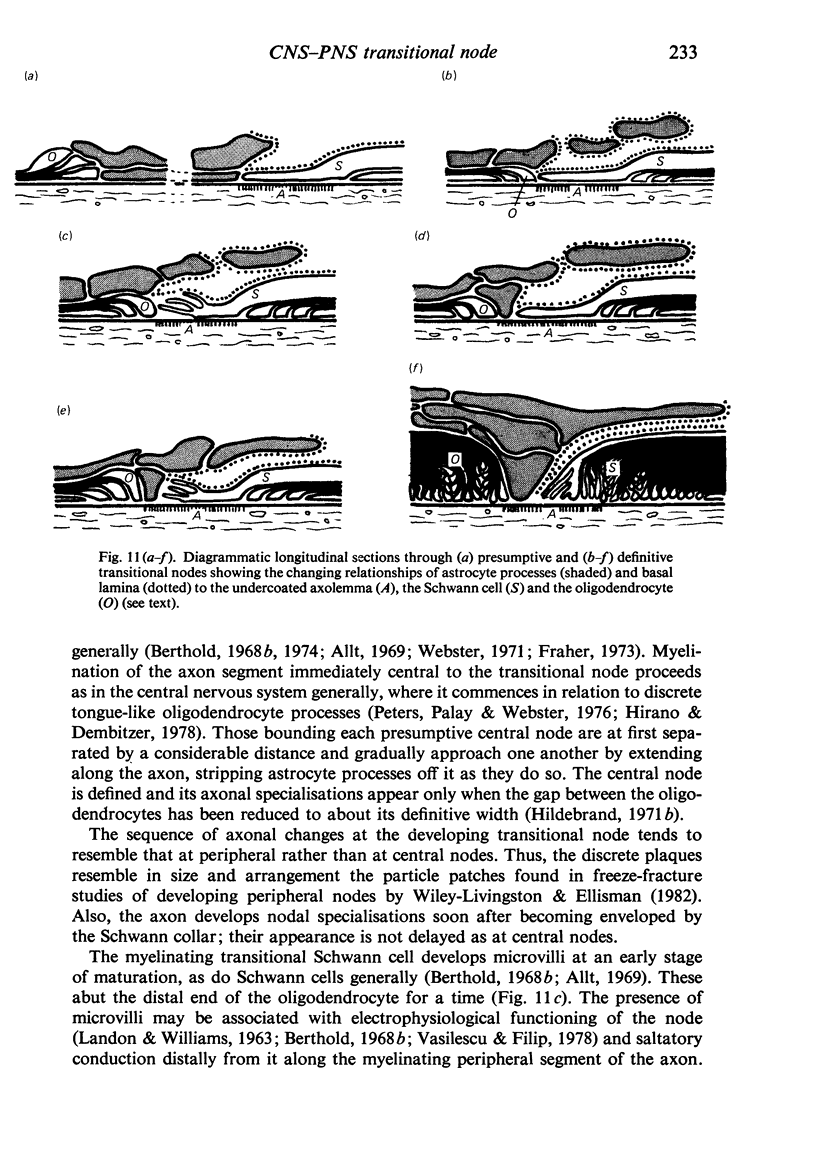
When the central-peripheral transitional node first appears it lies immediately distal to the astrocyte processes delineating the cord surface. Its initial location may be influenced indirectly by the astrocytes, since they determine the position of the transitional Schwann cell by preventing it from invading the central nervous system. The central end of the Schwann cell becomes specialised to form a narrow cytoplasmic collar which closely envelopes, and so may influence, the developing nodal axon segment. The earliest nodal specializations include subaxolemmal undercoating which first appears as discrete plaques. These soon fuse to form a complete layer. The transitional node is closely related to the Schwann cell collar throughout its maturation. However, the presumptive oligodendrocyte paranode lies a considerable distance central to the node for some time. The intervening axon segment is enveloped by astrocyte processes. Terminal pockets of the oligodendrocyte gradually extend distally along this segment until they reach the node. Here the distal end of the oligodendrocyte is at first apposed to the Schwann cell collar. With maturation, astrocyte processes extend into the node gap, intervening between the two. The Schwann collar gradually becomes retracted distally as it gives rise to microvilli which project into the node gap space. With maturation, the astrocyte processes form a progressively more complete barrier between the oligodendrocyte and the node gap space. As the myelin sheaths on either side of the transitional node become thicker, the angles through which their turns incline inwards towards the axon progressively increase. The node gap thus tends to become deeper and to be bounded by steeper walls. However, in small fibres and in a proportion of large fibres, this angle remains relatively small and the node gap is therefore relatively open. Axonal protrusions commonly arise from the nodal and the paranodal segments of the axon. With maturation they become more frequent at the latter. Small recurrent collateral axon branches arise at transitional nodes of large fibres in increasing numbers with maturation. They possess thin myelin sheaths. Most run centrally in the intramedullary bundle towards the anterior horn grey matter.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allt G. Ultrastructural features of the immature peripheral nerve. J Anat. 1969 Sep;105(Pt 2):283–293. [PMC free article] [PubMed] [Google Scholar]
- Berthold C. H. A comparative morphological study of the developing node-paranode region in lumbar spinal roots. I. Electron microscopy. Neurobiology. 1974;4(2):82–104. [PubMed] [Google Scholar]
- Berthold C. H., Carlstedt T. Observations on the morphology at the transition between the peripheral and the central nervous system in the cat. III. Myelinated fibres in S1 dorsal rootlets. Acta Physiol Scand Suppl. 1977;446:43–60. [PubMed] [Google Scholar]
- Berthold C. H., Rydmark M. Electron microscopic serial section analysis of nodes of Ranvier in lumbosacral spinal roots of the cat: ultrastructural organization of nodal compartments in fibres of different sizes. J Neurocytol. 1983 Jun;12(3):475–505. doi: 10.1007/BF01159386. [DOI] [PubMed] [Google Scholar]
- Berthold C. H. Ultrastructure of postnatally developing feline peripheral nodes of Ranvier. Acta Soc Med Ups. 1968;73(3-4):145–168. [PubMed] [Google Scholar]
- Blakemore W. F., Patterson R. C. Observations on the interactions of Schwann cells and astrocytes following X-irradiation of neonatal rat spinal cord. J Neurocytol. 1975 Oct;4(5):573–585. doi: 10.1007/BF01351538. [DOI] [PubMed] [Google Scholar]
- Blakemore W. F. Remyelination by Schwann cells of axons demyelinated by intraspinal injection of 6-aminonicotinamide in the rat. J Neurocytol. 1975 Dec;4(6):745–757. doi: 10.1007/BF01181634. [DOI] [PubMed] [Google Scholar]
- Bunge R. P. Glial cells and the central myelin sheath. Physiol Rev. 1968 Jan;48(1):197–251. doi: 10.1152/physrev.1968.48.1.197. [DOI] [PubMed] [Google Scholar]
- Conradi S. Observations on the ultrastructure of the axon hillock and initial axon segment of lumbosacral motoneurons in the cat. Acta Physiol Scand Suppl. 1969;332:65–84. [PubMed] [Google Scholar]
- Edwards A. V., Furness P. N., Helle K. B. Adrenal medullary responses to stimulation of the splanchnic nerve in the conscious calf. J Physiol. 1980 Nov;308:15–27. doi: 10.1113/jphysiol.1980.sp013458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraher J. P. A quantitative study of anterior root fibres during early myelination. II. Longitudinal variation in sheath thickness and axon circumference. J Anat. 1973 Sep;115(Pt 3):421–444. [PMC free article] [PubMed] [Google Scholar]
- Fraher J. P., Kaar G. F. The maturation of the ventral root-spinal cord transitional zone. Part 2. A quantitative ultrastructural study of the dynamics of its early development. J Neurol Sci. 1982 Jan;53(1):63–75. doi: 10.1016/0022-510x(82)90080-6. [DOI] [PubMed] [Google Scholar]
- Fraher J. P. Quantitative studies on the maturation of central and peripheral parts of individual ventral motoneuron axons. I. Myelin sheath and axon calibre. J Anat. 1978 Aug;126(Pt 3):509–533. [PMC free article] [PubMed] [Google Scholar]
- Fraher J. P. Quantitative studies on the maturation of central and peripheral parts of individual ventral motoneuron axons. II. Internodal length. J Anat. 1978 Sep;127(Pt 1):1–15. [PMC free article] [PubMed] [Google Scholar]
- Fraher J. P., Rossiter J. P. Cell clusters on fetal rat ventral roots: prenatal development. J Anat. 1983 Jan;136(Pt 1):111–128. [PMC free article] [PubMed] [Google Scholar]
- Fraher J. P., Rossiter J. P. Cell clusters on rat ventral roots: postnatal development. J Anat. 1983 Oct;137(Pt 3):555–571. [PMC free article] [PubMed] [Google Scholar]
- Fraher J. P. The growth and myelination of central and peripheral segments of ventral motoneurone axons. A quantitative ultrastructural study. Brain Res. 1976 Mar 26;105(2):193–211. doi: 10.1016/0006-8993(76)90421-2. [DOI] [PubMed] [Google Scholar]
- Fraher J. P. The maturation of the ventral root-spinal cord transitional zone. An ultrastructural study. J Neurol Sci. 1978 May;36(3):427–449. doi: 10.1016/0022-510x(78)90049-7. [DOI] [PubMed] [Google Scholar]
- Gilmore S. A., Sims T. J., Heard J. K. Autoradiographic and ultrastructural studies of areas of spinal cord occupied by Schwann cells and Schwann cell myelin. Brain Res. 1982 May 13;239(2):365–375. doi: 10.1016/0006-8993(82)90515-7. [DOI] [PubMed] [Google Scholar]
- Hildebrand C. Ultrastructural and light-microscopic studies of the developing feline spinal cord white matter. I. The nodes of Ranvier. Acta Physiol Scand Suppl. 1971;364:81–109. doi: 10.1111/j.1365-201x.1971.tb10979.x. [DOI] [PubMed] [Google Scholar]
- LANDON D. N., WILLIAMS P. L. ULTRASTRUCTURE OF THE NODE OF RANVIER. Nature. 1963 Aug 10;199:575–577. doi: 10.1038/199575a0. [DOI] [PubMed] [Google Scholar]
- Landon D. N., Langley O. K. The local chemical environment of nodes of Ranvier: a study of cation binding. J Anat. 1971 Apr;108(Pt 3):419–432. [PMC free article] [PubMed] [Google Scholar]
- METUZALS J. Ultrastructure of myelinated nerve fibers in the central nervous system of the frog. J Ultrastruct Res. 1963 Feb;8:30–47. doi: 10.1016/s0022-5320(63)80019-2. [DOI] [PubMed] [Google Scholar]
- Moll C., Meier C. The central-peripheral transition zone of cervical spinal nerve roots in Jimpy mutant and normal mice. Light- and electron-microscopic study. Acta Neuropathol. 1983;60(3-4):241–251. doi: 10.1007/BF00691872. [DOI] [PubMed] [Google Scholar]
- Nemecek S., Parízek J., Spacek J., Nemecková J. Histological, histochemical and ultrastructural appearance of the transitional zone of the cranial and spinal nerve roots. Folia Morphol (Praha) 1969;17(2):171–181. [PubMed] [Google Scholar]
- Phillips D. D., Hibbs R. G., Ellison J. P., Shapiro H. An electron microscopic study of central and peripheral nodes of Ranvier. J Anat. 1972 Feb;111(Pt 2):229–238. [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine C. S. Differences between the nodes of Ranvier of large and small diameter fibres in the P.N.S. J Neurocytol. 1982 Dec;11(6):935–947. doi: 10.1007/BF01148309. [DOI] [PubMed] [Google Scholar]
- Rosenstein J. M., Leure-DuPree A. E. Electron microscopic observations of nodes of Ranvier in the external cuneate nucleus. J Comp Neurol. 1976 Dec 15;170(4):461–483. doi: 10.1002/cne.901700406. [DOI] [PubMed] [Google Scholar]
- Saito K. Branchings at the central node of Ranvier, observed in the anterior horn and Clarke's nucleus of the cat. An electron microscopic study. Neuroscience. 1979;4(3):391–399. doi: 10.1016/0306-4522(79)90102-7. [DOI] [PubMed] [Google Scholar]
- Uhrík B., Stämpfli R. Ultrastructural observations on nodes of Ranvier from isolated single frog peripheral nerve fibres. Brain Res. 1981 Jun 29;215(1-2):93–101. doi: 10.1016/0006-8993(81)90493-5. [DOI] [PubMed] [Google Scholar]
- Vasilescu V., Filip D. A. The Ranvier node as a chemo-electric pulsatory unit: a study of its structure-functions relations. Physiologie. 1979 Apr-Jun;16(2):83–91. [PubMed] [Google Scholar]
- Waxman S. G., Foster R. E. Development of the axon membrane during differentiation of myelinated fibres in spinal nerve roots. Proc R Soc Lond B Biol Sci. 1980 Sep 26;209(1176):441–446. doi: 10.1098/rspb.1980.0105. [DOI] [PubMed] [Google Scholar]
- Weinberg E. L., Spencer P. S. Studies on the control of myelinogenesis. 3. Signalling of oligodendrocyte myelination by regenerating peripheral axons. Brain Res. 1979 Feb 23;162(2):273–279. doi: 10.1016/0006-8993(79)90289-0. [DOI] [PubMed] [Google Scholar]
- Wiley-Livingston C., Ellisman M. H. Development of axonal membrane specializations defines nodes of Ranvier and precedes Schwann cell myelin elaboration. Dev Biol. 1980 Oct;79(2):334–355. doi: 10.1016/0012-1606(80)90120-7. [DOI] [PubMed] [Google Scholar]