Abstract
An efficient and convenient strategy has been successfully developed for the preparation of novel hydroxylated alkaloid derivatives (also called fused multicyclic iminosugars) from p-toluenesulfonylated sugars through a Pictet–Spengler-type mechanism. This method is highly stereoselective, does not require metal catalysts, and capable of conducting gram level reactions (with a 53% yield). Some of such iminosugars had an intermediate antiproliferative effect on HCT116 tumor cells.
Keywords: hydroxylated alkaloids, fused multicyclic iminosugars, Pictet–Spengler reaction, antitumor activity
1. Introduction
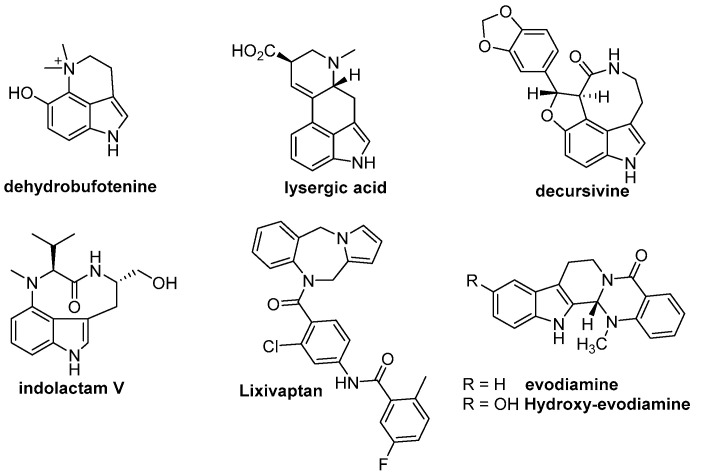
Indole or pyrrole, as the key structural skeleton, has been widely used in various therapeutic drugs, such as antitumor, antihypertensive, antiproliferative, antiviral, analgesic, anti-inflammatory, and antibacterial drugs, due to its alkaloid character. Currently, there are over a hundred indole or pyrrole drugs on the market [1,2]; the preparation [3,4] and modification of these [5,6,7,8] have been hot research topics in the field of organic synthesis. Fusing an additional ring at the 3, 4-positions of indole is a common strategy in the structural modification of indole, and well-known products of this process include dehydrobufotenine [9,10], lysergic acid [11,12], decursivine [13,14,15], and indolactam V [16,17,18] (Figure 1). Indolactam V not only can selectively activate protein kinase C (PKC) but can also induce the differentiation of human embryonic stem cells (ESCs) into pancreatic cells. Lixivaptan is a typical pyrrole alkaloid in the development of innovative non-peptide oxytocin and vasopressin small-molecule agonists and antagonists [19] (Figure 1). Additionally, fused indole alkaloids, such as evodiamine, have excellent antiproliferative activities in tumor cells, while the hydroxylated type, 10-hydroxyevodiamine [20], as a clinical chemotherapy drug, shows better antitumor activity than that of evodiamine, which suggests that the introduction of hydroxy groups into fused alkaloids can improve their biological activities, with the exception of their bioavailability.
Figure 1.
Alkaloids with good biological activities.
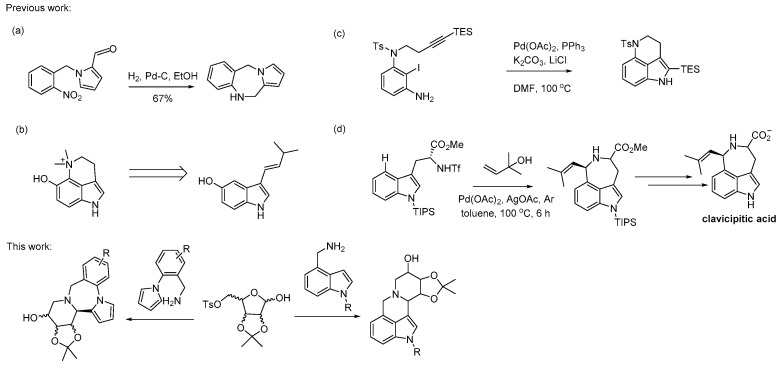
Due to the excellent biological activities of indole and pyrrole derivatives, many research groups have developed different synthetic methods for such alkaloids [21,22,23,24,25]. O’Brien’s research group successfully constructed a pyrrole-like alkaloid by utilizing a tandem hydrogenation–condensation–hydrogenation sequence [26] (Scheme 1a). Wang’s research group achieved efficient synthesis of toad alkaloid dehydrobufotenine in eight steps with an overall yield of 8% from 5-methoxyindole [27] (Scheme 1b). Jia’s research group conducted a lot of outstanding work in the synthesis of alkaloids, including the synthesis of 3,4-fused tricyclic indoles using intra-molecular Larock indole synthesis [28] (Scheme 1c). In addition, through C-H activation, Pd-catalyzed direct alkylation of the tryptophan derivatives at the C-4 position was undertaken to prepare 4-substituted tryptophan, which could be used for the synthesis of various sesquiterpenoid indole alkaloids [29,30] (Scheme 1d).
Scheme 1.
Different strategies for the synthesis of alkaloids. (a) O’Brien’ work [26]; (b) Wang’s work [27]. (c) Jia’s work [28]; (d) References [29,30].
However, the fusion of carbohydrates into indole or pyrrole alkaloids to construct new bioactive small molecules has rarely been reported. Considering the potent activity of hydroxylated alkaloids, herein, we would like to report the synthesis of novel indole- or pyrrole-type derivatives containing an iminosugar under metal-free catalytic conditions through a Pictet–Spengler-type reaction [31,32].
2. Results and Discussion
2.1. Optimization of the Reaction Conditions
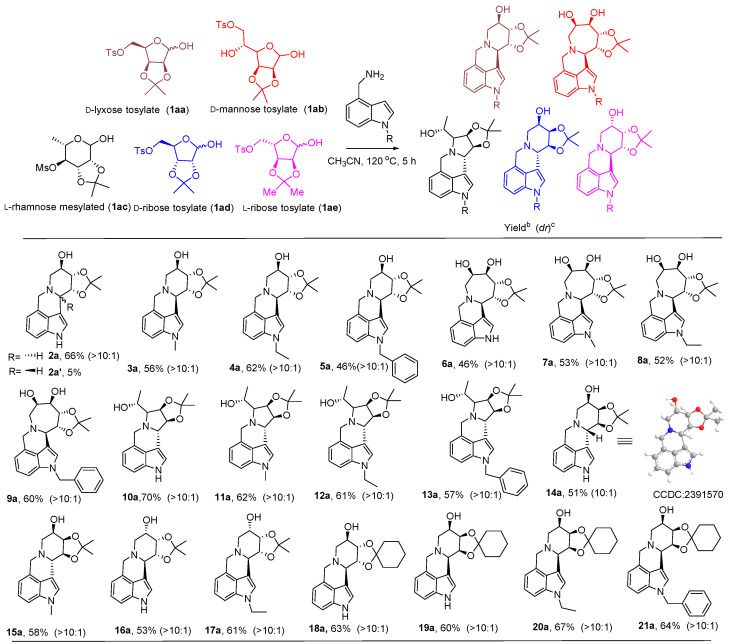
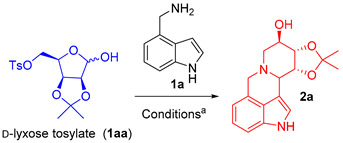
Recently, our group has been dedicated to the study of the synthesis of fused multicyclic iminosugars [33,34]. An effective approach has been developed for synthesizing hydroxylated alkaloid 2a (also called a fused multicyclic iminosugar) using 4-(aminomethyl)indole 1a and p-toluenesulfonylated sugars as the raw materials. This reaction can successfully synthesize various five membered, six membered, or seven membered iminosugar indole alkaloids by heating in an oil bath at 120 ° C in an air atmosphere without a metal catalyst.
d-lyxose tosylate (1aa) and 1H-indol-4-ylmethanamine (1a) were selected as the model substrates to optimize the conditions (Table 1). Firstly, compound 1a (1.5 equiv) was reacted with d-lyxose tosylate (1aa) in the presence of the metal catalysts Yb(OTf)3, Sc(OTf)3, and AlCl3 for 5 h, respectively, all of which were able to generate target product 2a, albeit at yields of 30–38% (entries 1–3). Subsequent attempts to conduct the reaction under metal-free reaction conditions, by employing TFA or 1N HCl as the acid catalysts, did not yield improvements (entries 4–5).
Table 1.
Optimization of reaction conditions for compound 2a.
| |||||
|---|---|---|---|---|---|
| Entry | 1a (equiv) | Catalyst (0.2 equiv) | Solvent (2.0 mL) | Temp (°C) | Yield b (%) |
| 1 | 1.5 | Yb(OTf)3 | CH3CN | 80 | 30 |
| 2 | 1.5 | Sc(OTf)3 | CH3CN | 80 | 35 |
| 3 | 1.5 | AlCl3 | CH3CN | 80 | 38 |
| 4 | 1.5 | CF3COOH | CH3CN | 80 | 37 |
| 5 | 1.5 | 1N HCl | CH3CN | 80 | 24 |
| 6 | 1.5 | - | CH3CN | 80 | 42 |
| 7 | 2.0 | - | CH3CN | 80 | 46 |
| 8 | 1.2 | - | CH3CN | 80 | 58 |
| 9 | 1.2 | - | CH3CN | 100 | 60 |
| 10 | 1.2 | - | CH3CN | 120 | 66 |
| 11 | 1.2 | - | Toluene | 120 | 53 |
| 12 | 1.2 | - | DMSO | 120 | 31 |
| 13 | 1.2 | - | THF | 120 | 43 |
| 14 c | 1.2 | - | CH3CN | 120 | 63 |
a Reaction conditions: d-lyxose tosylate (0.2 mmol, 1.0 equiv); reaction time: 5 h; oil bath heating; b isolated yield; c N2 atmosphere.
Without any catalyst, the expected product 2a could also be generated smoothly, and the yield slightly improved (entry 6). Varying the amount of 1a under 80 °C reaction conditions, it was found that 2a was achieved at a moderate yield of 58% when 1.2 equiv of 1a was used (entries 7–8). When the reaction was performed at increasing reaction temperatures, the yield gradually increased and reached the highest amount of 66% at 120 °C (entries 9–10). No more attempts were made above 120 °C. Finally, when toluene, DMSO, and THF were employed as different reaction solvents, the reactions produced target compound 2a at low yields (entries 11–13). In addition, the reaction could also be successfully carried out under the optimal conditions in a nitrogen atmosphere (entry 14).
2.2. Synthesis of the Iminosugar Alkaloids
Under the optimized reaction conditions, the substrate tolerance was studied, and a series of hydroxylated 3,4-position-fused indole alkaloids were prepared (Figure 2). By using different N-substituents, such as methyl, ethyl, benzyl, and different Ts-glycosides, specific products can be obtained. By reacting d-lyxose tosylate (1aa) or D-ribose tosylate (1ad) or L-ribose tosylate (1ae) with 3-methylaminoindole, products 2a–5a and 14a–17a were obtained at a moderate yield of 46–66%, respectively. The reaction of D-mannose tosylate (1ab) and indoleamine generated seven-membered-iminosugar-fused indole derivatives 6a–9a. When L-rhamnose mesylate (1ac) was used as the substrate, the reactions resulted in the formation of five-membered-iminosugar-fused indole alkaloids 10a–13a. Finally, the reaction was carried out replacing the isopropyl group with the cyclohexyl group on the sugar, which yielded products 18a–21a. NMR spectrum of 2a–21a is shown in the Figures S1–S42 in the Supplementary Materials. To sum up, a series of five-, six-, and seven-membered-iminosugar-fused indole alkaloids with high diastereomeric ratios (above 10:1) was successfully one-pot-synthesized. The single-crystal data for compound 14a (CCDC: 2391570, The single-crystal data Figure S90 in the Supplementary Materials) confirmed the structure.
Figure 2.
Synthesis of the iminosugar indole alkaloids. Reaction conditions: d/l-lyxose/ribose tosylate, d-mannose tosylate, or l-rhamnose sulfonate (0.2 mmol, 1.0 equiv), aminomethyl-indole (1.2 equiv), CH3CN (2.0 mL), air, 120 °C, 5 h, oil bath heating; b isolated yields; c determined using 1H NMR; (dr): the dr was determined by 1H NMR of the mixture.
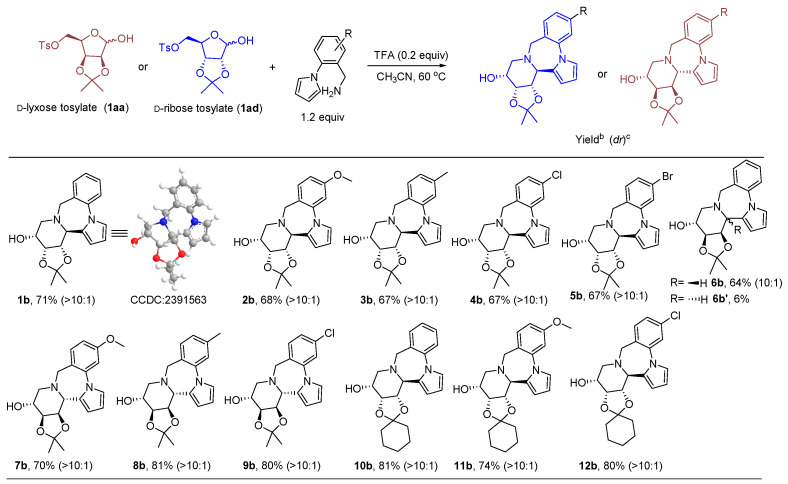
After optimization of the reaction conditions, it was found that under the catalysis of trifluoroacetic acid, reactions of D-ribose tosylate or d-lyxose tosylate and 2-(1-pyrrolyl)benzylamine at 60 °C generated six-membered iminosugar benzopiperazine pyrrole alkaloids 1b–9b. The single-crystal data for compound 1b confirmed the structure (CCDC: 2391563, The single-crystal data Figure S91 in the Supplementary Materials). The expected products were also successfully obtained when the sugar was protected with a cyclohexyl group, 10b–12b (Figure 3). NMR spectrum of 1b–12b is shown in the Figures S43–S68 in the Supplementary Materials.
Figure 3.
Synthesis of the iminosugar pyrrole benzodiazepine alkaloids. Reaction conditions: D-lyxose/ribose tosylate (0.2 mmol, 1.0 equiv), 2-(1-pyrrolyl)benzylamine (1.2 equiv), TFA (0.2 equiv), CH3CN (2.0 mL), air, 60 °C, 4 h, oil bath heating; b isolated yields. c determined using 1H NMR; (dr): the dr was determined by 1H NMR of the mixture.
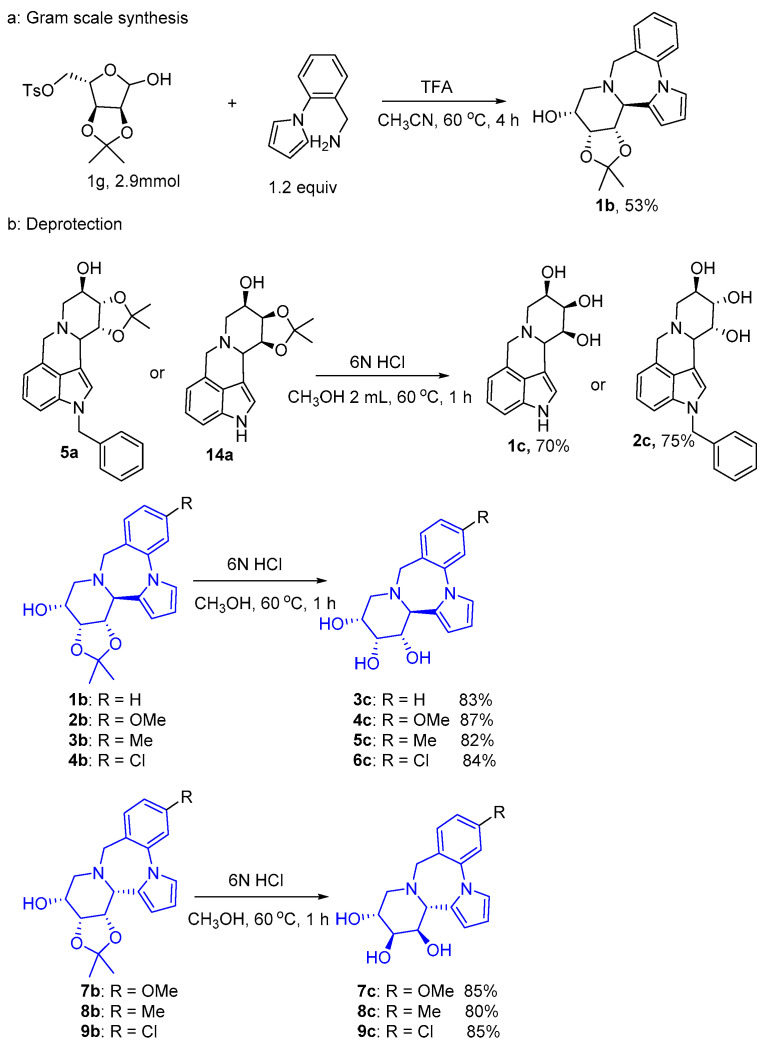
When the reaction was scaled up to the gram level, product 1b could be obtained at a 53% yield (Scheme 2a). Additionally, removing 2,3-O-isopropylidene in the presence of hydrochloric acid to generate corresponding products 1c–9c), respectively (Scheme 2b). NMR spectrum of 1c-9c is shown in the Figures S69–S86 in the Supplementary Materials.
Scheme 2.
Further transformations of the derivatives.
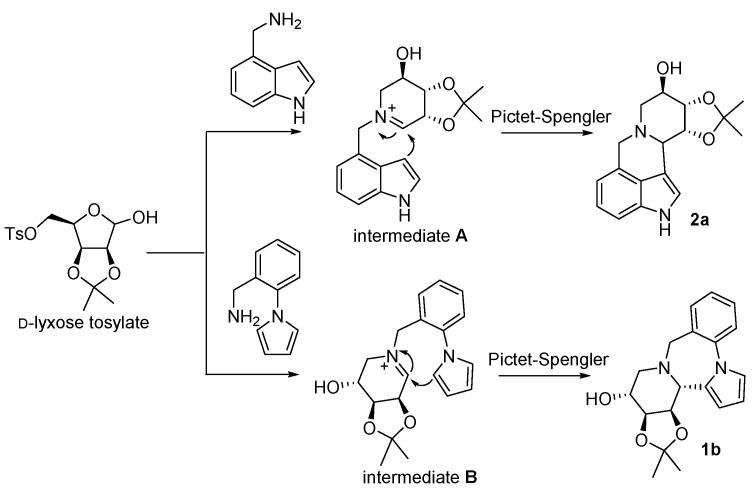
According to the above experimental results, a reasonable reaction mechanism is described (Scheme 3). Firstly, the reaction of d-lyxose tosylate and an amine yields a key intermediate iminium ion A or B [33,34]. Then, an intramolecular Pictet–Spengler-type [35] reaction will occur to form the final compound 2a or 2b, respectively, due to the high nucleophilic reactivities of indole or pyrrole.
Scheme 3.
A possible mechanism for the synthesis of the alkaloids.
Iminosugars 9a and 6c were preliminarily evaluated for their antitumor activities. The cytotoxicity of these compounds against HCT116 (human colon cancer cells) was examined using Cell Counting Kit-8 (CCK-8) assays after 48 h of drug treatment. The results showed that both compounds could inhibit the growth of the cells, and the inhibitory rates were above 45% at a concentration of 100 μM, which suggested such iminosugars had intermediate antiproliferative activities against HCT116 tumor cells.
3. Materials and Methods
3.1. General Information
The solvents were all analytical-grade, and the other reagents were purchased from Energy Chemical (Shanghai, China) and Bide Pharmatech Ltd (Shanghai, China). The 1H NMR spectra were measured on 600 MHz and 400 MHz Bruker AVANCE spectrometers (Fällanden, Switzerland). The 13C NMR spectra were recorded on Bruker 100 MHz spectrometers (Fällanden, Switzerland) with complete proton decoupling. Melting points were measured on glass slides on an SGW X-4 melting point apparatus (Shanghai Yidian Physical & Optical Instrument Co., Shanghai, China). Optical rotations were determined on an SGW-1 automatic polarimeter (Shanghai Yidian Physical & Optical Instrument Co., Shanghai, China). High-resolution mass spectrometry (HRMS) were conducted on an FTICR-MS (Ionspec 7.0T) mass spectrometer with electric spray ionization (ESI) (Bruker Daltonics, Billerica, MA, USA), manufactured by Ionspec Company in the United States. Thin-layer chromatography (TLC) was performed on pre-coated plates (GF254) with detection using UV light, and silica gel (200–300-mesh) was used for column chromatography (Qingdao Puke Spectrum Separation Material Co., Qingdao, China). X-ray diffraction data were gathered using a Bruker D8 VENTURE (Bruker, Bremen, Germany).
3.2. Extraction and Isolation
General experimental procedure: Ts-d-lyxose (69 mg, 0.2 mmol) and aminomethyl-indole (1.2 equiv) were added into a 20 mL flask, with 2.0 mL of CH3CN used as the solvent. Then, the solution was stirred at a temperature of 120 °C under an air atmosphere for 5 h. Upon completion, the mixture was cooled to room temperature, and the solvent was evaporated in vacuo. The crude product was purified using column chromatography (dichloromethane:methanol v/v = 30:1) to give 2a–5a as a pale white solid. Under similar conditions, different N-substituents 3-aminoindoles, Ts-d-mannose, Ms-l-rhamnose, Ts-d-ribose, and Ts-l-ribose, were used as the raw materials for the reaction, and the corresponding products were obtained, respectively (6a–17a).
Ts-d-ribose (69 mg, 0.2 mmol), 2-(1-pyrrolyl)benzylamine (1.2 equiv), and trifluoroacetic acid (0.2 equiv) were added into a 20 mL flask, with 2.0 mL of CH3CN used as the solvent. Then, the solution was stirred at a temperature of 60 °C under an air atmosphere for 4 h. Upon completion, the mixture was cooled to room temperature, and the solvent was evaporated in vacuo. The crude product was purified using column chromatography (petroleum ether/ethyl acetate v/v = 1:1) to give 1b–10b as a pale white solid.
Product 5a (30 mg, 0.07 mmol) or 14a and 6N HCl (5.0 equiv) were added into a 20 mL flask, with 2.0 mL of methanol used as the solvent. Then, the solution was stirred at a temperature of 60 °C under an air atmosphere for 2 h. Upon completion, the mixture was cooled to room temperature, and the solvent was evaporated in vacuo. The crude product was purified using silica gel column chromatography (dichloromethane:methanol v/v = 15:1) to give 1c or 2c as a pale white solid. Under similar conditions, compounds 1b–4b, 7b–9b were reacted with 1N HCl (5.0 equiv) to obtain compounds 3c–9c.
Ts-d-ribose (1 g, 2.9 mmol), 2-(1-pyrrylyl)benzylamine (1.2 equiv), and TFA (0.2 equiv) were added into a 50 mL flask, using 5.0 mL of CH3CN as the solvent. Then, the solution was stirred at a temperature of 60 °C under an air atmosphere for 4 h. Upon completion, the mixture was cooled to room temperature, and the solvent was evaporated in vacuo. The crude product was purified using silica gel column chromatography (petroleum ether/ethyl acetate v/v = 1:1) to obtain a 501 mg light yellow solid 1b with a yield of 53%.
3.3. Antitumor Activity
Cell Counting Kit-8 (CCK-8, APExBlO Technology LLC, Houston, TX, USA) assays were used to measure the cell viability. Cells were seeded into a 96-well plate at a density of 3000–5000 cells/well with 100 μL of complete culture medium. The tested compounds were added to the wells at different concentrations, and the plates were incubated at 37 °C for 48 h. Then, 10 μL of CCK-8 reagent was added to each well of the plate, which was kept in the dark for 2 h. The absorbance value at 450 nm was measured using a microplate reader to evaluate the cell viability. Calculate the survival rate according to the public notice (1) provided in the instruction manual
| survival rate = (ODtreated − ODblank)/(ODcontrol − ODblank) × 100% | (1) |
4. Conclusions
In summary, we have developed a novel method for the one-pot synthesis of iminosugar piperidone indole alkaloids and benzopiperazine pyrrole alkaloids, featuring simple operation and a stable structure which offers an efficient pathway for the construction of valuable alkaloid derivatives. Meanwhile, the absolute structure of the required product was confirmed using single-crystal X-ray diffraction.
Supplementary Materials
The Supporting Information is available free of charge at https://www.mdpi.com/article/10.3390/molecules29235709/s1, including the experimental methodologies, spectral analysis results, copies of the 1H and 13C NMR findings, as well as crystallographic information for the novel compounds. (PDF).
Author Contributions
Conceptualization, L.Z.; methodology, N.M., W.Y., L.F., S.X., L.W. and J.W.; software, L.Z.; writing—original draft preparation, J.W.; writing—review and editing, J.W.; supervision, H.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are contained within the article and Supplementary Materials.
Conflicts of Interest
Author Na Ma is employed by Asset Management Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Statement
This project was supported by the National Natural Science Foundation of China (NSFC) (21772031), the Natural Science Foundations of Hebei Province (B2019201398), the Project of Excellent Clinical Medicine of Hebei Province (ZF2024218), the Science and Technology Program of Baoding City (2372P004), Xingtai science and technology program (2022zz100), and the Graduate Innovation Funding Project of Hebei University (HBU2022bs015), Hebei Provincial Natural Science Foundation (B2024108004), Funded by Science Research Project of Hebei Education Department (QN2020512).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Humphrey G.R., Kuethe J.T. Practical Methodologies for the Synthesis of Indoles. Chem. Rev. 2006;106:2875–2911. doi: 10.1021/cr0505270. [DOI] [PubMed] [Google Scholar]
- 2.Aboul-Fadl T., Bin-Jubair F., Aboul-Wafa O. Schiff bases of indoline-2,3-dione (isatin) derivatives and nalidixic acid carbohydrazide, synthesis, antitubercular activity and pharmacophoric model building. Eur. J. Med. Chem. 2010;45:4578–4586. doi: 10.1016/j.ejmech.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 3.Cacchi S., Fabrizi G. Synthesis and Functionalization of Indoles Through Palladium-catalyzed Reactions. Chem. Rev. 2005;105:2873–2920. doi: 10.1021/cr040639b. [DOI] [PubMed] [Google Scholar]
- 4.Neto J.S.S., Zeni G. Recent advances in the synthesis of indoles from alkynes and nitrogen sources. Org. Chem. Front. 2020;7:155–210. doi: 10.1039/C9QO01315F. [DOI] [PubMed] [Google Scholar]
- 5.Wen J., Shi Z. From C4 to C7: Innovative Strategies for Site-Selective Functionalization of Indole C–H Bonds. Acc. Chem. Res. 2021;54:1723–1736. doi: 10.1021/acs.accounts.0c00888. [DOI] [PubMed] [Google Scholar]
- 6.Festa A.A., Voskressensky L.G., Van der Eycken E.V. Visible light-mediated chemistry of indoles and related heterocycles. Chem. Soc. Rev. 2019;48:4401–4423. doi: 10.1039/C8CS00790J. [DOI] [PubMed] [Google Scholar]
- 7.Liu S., Zhao F., Chen X., Deng G., Huang H. Aerobic Oxidative Functionalization of Indoles. Adv. Synth. Catal. 2020;362:3795–3823. doi: 10.1002/adsc.202000285. [DOI] [Google Scholar]
- 8.Huang G., Yin B. Recent Developments in Transition-Metal-Catalyzed Dearomative Cyclizations of Indoles as Dipolarophiles for the Construction of Indolines. Adv. Synth. Catal. 2018;361:405–425. doi: 10.1002/adsc.201800789. [DOI] [Google Scholar]
- 9.Peat A.J., Buchwald S.L. Novel Syntheses of Tetrahydropyrroloquinolines: Applications to Alkaloid Synthesis. J. Am. Chem. Soc. 1996;118:1028–1030. doi: 10.1021/ja953080t. [DOI] [Google Scholar]
- 10.Jackson B., Hester J. A Synthesis of 1,3,4,5-Tetrahydropyrrolo[4,3,2-de]quinolone. J. Org. Chem. 1964;29:1158–1160. [Google Scholar]
- 11.Liu Q., Jia Y. Total Synthesis of (+)-Lysergic Acid. Org. Lett. 2011;13:4810–4813. doi: 10.1021/ol2018467. [DOI] [PubMed] [Google Scholar]
- 12.Iwata A., Inuki S., Oishi S., Fujii N., Ohno H. Formal Total Synthesis of (+)-Lysergic Acid via Zinc(II)-Mediated Regioselective Ring-Opening Reduction of 2-Alkynyl-3-indolyloxirane. J. Org. Chem. 2011;76:5506–5512. doi: 10.1021/jo2008324. [DOI] [PubMed] [Google Scholar]
- 13.Qin H., Xu Z., Cui Y., Jia Y. Total Synthesis of (±)-Decursivine and (±)-Serotobenine: A Witkop Photocyclization/Elimination/O-Michael Addition Cascade Approach. Angew. Chem. Int. Ed. 2011;50:4447–4449. doi: 10.1002/anie.201100495. [DOI] [PubMed] [Google Scholar]
- 14.Hu W., Qin H., Cui Y., Jia Y. Total Synthesis of (+)- and (−)-Decursivine and (±)-Serotobenine through a Cascade Witkop Photocyclization/Elimination/Addition Sequence: Scope and Mechanistic Insights. Chem. Eur. J. 2013;19:3139–3147. doi: 10.1002/chem.201204137. [DOI] [PubMed] [Google Scholar]
- 15.Sun D., Zhao Q., Li C. Total Synthesis of (+)-Decursivine. Org. Lett. 2011;13:5302–5305. doi: 10.1021/ol2021669. [DOI] [PubMed] [Google Scholar]
- 16.Nicolaou K.C., Chen D.Y.K., Huang X., Ling T., Bella M., Snyder S.A. Chemistry and Biology of Diazonamide A: First Total Synthesis and Confirmation of the True Structure. J. Am. Chem. Soc. 2004;126:12888–12896. doi: 10.1021/ja040092i. [DOI] [PubMed] [Google Scholar]
- 17.Knowles R.R., Carpenter J., Blakey S.B., Kayano A., Mangion I.K., Sinz C.J., MacMil-lan D.W.C. Total synthesis of diazonamide. Chem. Sci. 2011;2:308–311. doi: 10.1039/C0SC00577K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mai C.K., Sammons M.F., Sammakia T. A Concise Formal Synthesis of Diazonamide A by the Stereoselective Construction of the C10 Quaternary Center. Angew. Chem. Int. Ed. 2010;49:2397–2400. doi: 10.1002/anie.200906318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mu S., Niu D., Liu Y., Zhang D.S., Liu D.K., Liu C.X. An Improved, Scalable and Impurity-Free Process for Lixivaptan. Heterocycl. Chem. 2015;52:1608–1613. doi: 10.1002/jhet.2176. [DOI] [Google Scholar]
- 20.Dong G.Q., Wang S.Z., Miao Z.Y., Yao J.Z., Zhang Y.Q., Guo Z.Z., Zhang W.N., Sheng C.Q. New tricks for an old natural product: Discovery of highly potent evodiamine derivatives as novel antitumor agents by systemic structure-activity relationship analysis and biological evaluations. J. Med. Chem. 2012;55:7593–7613. doi: 10.1021/jm300605m. [DOI] [PubMed] [Google Scholar]
- 21.Sure S.P., Narra R.R., Sundarababu B. One-Pot Synthesis of Structurally Diverse Iminosugar Based Hybrid Molecules. J. Org. Chem. 2018;83:9604–9618. doi: 10.1021/acs.joc.8b00748. [DOI] [PubMed] [Google Scholar]
- 22.Robaa D., Enzensperger C., Abul Azm S.E.D., El Khawass E.S., El Sayed O., Lehmann J. Dopamine Receptor Ligands. Part 18:1 Modification of the Structural Skeleton of Indolobenzazecine-Type Dopamine Receptor Antagonists. J. Med. Chem. 2010;53:2646–2650. doi: 10.1021/jm901291r. [DOI] [PubMed] [Google Scholar]
- 23.Ronald G., Visuanathar S., Selvaratnam S., Andrew W.B. Sequential and Cascade 1,3-Dipolar Cycloaddition-Palladium Catalysed Carbonylation-Cyclisation Reactions.Diastereospecific and Homochiral Processes. Tetrahedron. 1995;51:295–306. [Google Scholar]
- 24.Nitin T.P., Valmik S.S., Balasubramanian S. Relay Catalytic Branching Cascade: A Technique to Access Diverse Molecular Scaffolds. Angew. Chem. Int. Ed. 2013;52:2251–2255. doi: 10.1002/anie.201208738. [DOI] [PubMed] [Google Scholar]
- 25.Yusuke O., Hiroaki C., Shinya O., Nobutaka F., Hiroaki O. Concise Synthesis of Indole-Fused 1,4-Diazepines through Copper(I)-Catalyzed Domino Three-Component Coupling-Cyclization-N-Arylation under Microwave Irradiation. Org. Lett. 2008;10:3535–3538. doi: 10.1021/ol801383b. [DOI] [PubMed] [Google Scholar]
- 26.Eleni D., Richard H.J., Robin G.P., Gavin J.M., Matthew O.-B. Gas-liquid flow hydrogenation of nitroarenes: Efficient access to a pharmaceutically relevant pyrrolobenzo[1,4]diazepine scaffold. Tetrahedron. 2018;74:6795–6803. [Google Scholar]
- 27.Tian Z.Y., Liao A.C., Kang J., Gao Y.Y., Lu A.D., Wang Z.W., Wang Q.M. Toad Alkaloid for Pesticide Discovery: Dehydrobufotenine Derivatives as Novel Agents against Plant Virus and Fungi. J. Agric. Food Chem. 2021;69:9754–9763. doi: 10.1021/acs.jafc.1c03714. [DOI] [PubMed] [Google Scholar]
- 28.Yan G., Dong S., Jia Y.X. Intramolecular Larock Indole Synthesis for the Preparation of Tricyclic Indoles and Its Application in the Synthesis of Tetrahydropyrroloquinoline and Fargesine. Tetrahedron. 2014;70:5136–5141. [Google Scholar]
- 29.Liu Q., Li Q.J., Ma Y.F., Jia Y.X. Direct Olefination at the C-4 Position of Tryptophan via C–H Activation: Application to Biomimetic Synthesis of Clavicipitic Acid. Org. Lett. 2013;15:4528–4531. doi: 10.1021/ol4020877. [DOI] [PubMed] [Google Scholar]
- 30.Tahara Y., Ito M., Kanyiva K.S., Shibata T. Total Synthesis of cis-Clavicipitic Acid from Asparagine via Ir-Catalyzed C-H bond Activation as a Key Step. Chem.-Eur. J. 2015;21:11340–11343. doi: 10.1002/chem.201502300. [DOI] [PubMed] [Google Scholar]
- 31.Elisabeth E., Joerg H.S., Dennis W., Hans I., Bernd K., Wolfgang K. Asymmetric Biocatalytic Synthesis of 1-Aryltetrahydro-β-carbolines Enabled by “Substrate Walking”. Chem.-Eur. J. 2020;26:16281–16285. doi: 10.1002/chem.202004449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Surya K.S., Rumpa Sarkar U.S., Sayan D., Debabani G., Avantika H., Deepak C., Mrinal K.B. A direct entry to polycyclic quinoxaline derivatives via I2-DMSO mediated oxidative decarboxylation of α-amino acids and the subsequent Pictet–Spengler cyclization reaction. Org. Biomol. Chem. 2022;20:4650–4658. doi: 10.1039/d2ob00503d. [DOI] [PubMed] [Google Scholar]
- 33.Wu J.L., Su L.L., Jia T.G., Xu X.M., Cui Y.X., Wei C., Li X.L., Chen H. Efficient one-pot synthesis of the unexpected fused multicyclic iminosugars by an aza-Diels-Alder mechanism. Org. Chem. Front. 2022;9:6530–6534. doi: 10.1039/D2QO01284G. [DOI] [Google Scholar]
- 34.Xie S., Wu J.L., Zhou L.K., Wei C., Li X.L., Chen H. One-Pot Stereoselective synthesis of different fused multicyclic iminosugars based on the iminium-Ion intermediate. Chin. J. Chem. 2024;42:142–150. doi: 10.1002/cjoc.202300495. [DOI] [Google Scholar]
- 35.Ashish K.V., Ande C., Sateesh D., Yashwant D.V. Stereoselective synthesis of sugar-fused (or 1,2 annulated) isochromans and isochromanones by using Oxa-Pictet-Spengler reaction. Org. Biomol. Chem. 2018;16:8258–8262. doi: 10.1039/c8ob01698d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are contained within the article and Supplementary Materials.