Abstract
Background
To reduce the clinical burden of COVID-19, healthcare providers, and policymakers need a clear understanding of the illness severity during epidemic waves. This study aimed to identify the clinical severity of patients with COVID-19 during different stages of an epidemic wave (pre-peak, peak, post-peak) in four provinces in Iran.
Methods
We conducted a secondary analysis of the data on COVID-19 patients admitted to hospitals (25,382 cases), which were recorded in the Medical Care Monitoring Center. Data included adult patients (≥18 years) who were hospitalized due to COVID-19 infection, confirmed by a positive SARS-CoV-2 RT-PCR test. No exclusion criteria were applied. A pairwise comparison method was used to evaluate clinical severity. Then, based on univariable and multivariable linear regression models, the severity scores of patients were compared during various stages of an epidemic wave.
Results
The findings showed that the level of severity of the disease was higher during and after the peak in the total population. The means (SD) of severity scores were 0.16 (0.25), 0.18 (0.26), and 0.19 (0.26) before, during and after the peak, respectively. Besides, age and the underlying disease had a positive and significant relationship with disease severity.
Conclusion
During the middle and late phases of the COVID-19 epidemic wave, hospitals are seeing patients with more severe illnesses than in the early stages. Enhancing hospital preparedness is essential to avert excess deaths and critical cases. Moreover, it is important to maintain ongoing monitoring of clinical symptoms during the recovery phase to support individual patients, guide public health policy, and enhance scientific understanding of epidemic recovery processes.
Keywords: COVID-19, Epidemics, Clinical Severity, Hospitalization
↑What is “already known” in this topic:
Healthcare providers and policymakers require a clear understanding of the COVID-19 severity during epidemic waves to reduce the clinical burden.
→What this article adds:
This study provides insights into the clinical severity of hospitalized COVID-19 patients across different stages of an epidemic wave (before, during, and after the peak), highlighting the significance of ongoing monitoring during the recovery phase to ensure timely interventions.
Introduction
Despite more than four years since COVID-19 broke out, epidemic waves can continue to spread due to its propagation nature. Epidemiological data from hospitals serve as a key source of disease monitoring and surveillance (1, 2).
Based on the literature, numerous studies have been conducted on the clinical characteristics or severity of hospitalized patients during the COVID-19 pandemic and have compared these characteristics in subsequent waves (3-5). As the COVID-19 pandemic unfolds with multiple waves (6), there is a lack of evidence regarding whether the clinical severity of patients may vary during specific phases of an epidemic wave, particularly before, during, and after the peak time. Knowing a patient’s clinical severity at various phases of an epidemic will help to better understand the current situation and formulate best practices for controlling infectious disease outbreaks (7-9).
The number of patients referred to medical centers or hospitalizations may change for different reasons, for instance, patient characteristics, changes in interventions, and policies like variations in the capacity of laboratory tests or therapeutic methods (10, 11). We can define two distinct situations for investigating epidemic phases: 1. Each wave consists of three phases pre-peak (p1), during the peak (p2), and post-peak (p3). 2. The intervals between epidemic waves are referred to as the bp phases. Typically, in the p2 phase, it is expected that the number of patients who require hospitalization and severe cases will increase. According to this, healthcare systems can be overwhelmed, leading to worse outcomes. During this phase, most efforts are focused on severe COVID-19 patients to save lives (12).
Moreover, sometimes people assume that the downward trend and passage of the peak signal the end of danger and the return to the normal situation, while each wave, depending on its magnitude, may have a long-term detrimental effect on one's health (13, 14).
In resource-constrained countries, where healthcare systems are more susceptible to pandemics (15), consistent monitoring of each wave is crucial for reducing the clinical burden of diseases. In this study, for the first time, we aimed to assess the clinical severity of COVID-19 hospitalized patients during different phases of the epidemic wave including pre-peak, peak, and post-peak in four provinces in Iran.
Methods
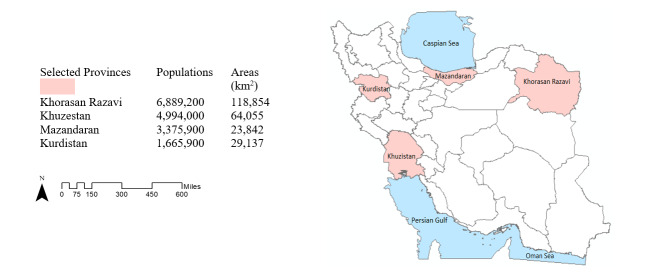
We conducted a secondary analysis of hospitalizations for laboratory-confirmed COVID-19 based on medical records in the “COVID-19 inpatient Dashboard” at the Medical Care Monitoring Center (MCMC), a national 24/7 center established to monitor healthcare service delivery. During the first nine months of the epidemic (from 19 February 2020 to 20 November 2020), four provinces, Mazandaran, Khuzestan, Kurdistan, and Khorasan Razavi, were selected from different geographical locations (north, south, west and east) using a convenience sampling strategy. These provinces were also considered in terms of their risk level for infection, using data gathered in late May and June 2020, when there were more investigations and diagnostic tests following the start of the epidemic in the country. Khuzestan and Kurdistan were identified as high-risk areas, while Razavi Khorasan and Mazandaran were considered lower risk at that time (16). The selected provinces are shown in Figure 1. During this period, the dominant strain of COVID-19 infection in the country was the Wuhan variant (Beta) and intervention measures including vaccination or specific drug treatments such as Remdesivir (one of the most widely used antiviral medications), were not available.
Figure 1.
Geographical location map of selected provinces. Population size and area of provinces are shown on the left side (Map was created using ArcGIS 10.7 (ESRI, 2018)).
The recorded data included patient age of at least 18 years, admitted to the hospital for COVID-19, and SARS-CoV-2 genome positive by reverse transcription–-polymerase chain reaction (RT-PCR) test. All patients were included during the first 24 hours after hospitalization, and the disease symptoms were recorded based on the information obtained from them during this time. No exclusion criteria were applied.
First, 7-day moving average trends of hospitalized cases were plotted for each province. After conducting a visual normality check, the practical threshold for identifying three phases of an epidemic wave (p1, p2, and p3 phases) was set at the geometric mean plus one standard deviation. Regarding this, during the phase p2, the number of cases was equal to or larger than the threshold, and fewer cases were considered as phases before and after the peak (p1 starts from a plateau in the disease trend and ends when the number of cases reaches the peak threshold, while p3 starts from the peak threshold and ends when the number of cases reaches another plateau). The minimum time interval for each phase was at least 14 days (period of the diseases on average). During 9 months, each province experienced two or three epidemic waves. From each province, the largest complete wave with three phases was selected for analysis.
Based on the literature (17-19) and the opinion of seven clinical experts, seven variables related to disease severity including respiratory distress, oxygen saturation (93% and less), deteriorated consciousness, hospitalization in an intensive care unit (ICU), intubation, referral status (the patient arrived by ambulance or not) and death, were selected. Then, variables were weighed using the Paired Comparison Method (20) and relative weight was assigned to each criterion based on their importance (scores were between 0.1 and 10). Finally, the disease severity score was calculated based on the total frequency of criteria by taking into account the specific weight of each criterion. The calculation of the Disease Severity Score is shown as follows:
Disease Severity Score = Σ (Frequency of Criterion i × Weight of Criterion i).
Where i represent each of the seven variables related to disease severity, and the weights are assigned based on their importance using the Paired Comparison Method.
Illness severity was compared in three phases in total and provincial levels by ANOVA and Chi-square tests. Furthermore, univariable linear regression models were used to investigate the relationship between disease severity and different wave phases. The multivariable linear regression model was performed to evaluate the severity of the disease in different phases of the epidemic wave, assuming the constant effect of other related variables (which had a P-value less than 0.2 in the univariable model).
Covariates: The underlying variables were age, sex, and history of at least one underlying disease (diabetes, immunodeficiency disorders, nervous system diseases, liver, renal and cardiovascular diseases). Common symptoms of the disease were fever, cough and muscle pain. Since the variable 'duration of hospitalization' is likely influenced by both the exposure variables and the outcome (disease severity) and is considered a 'collider' variable, it was not included in the modeling process.
Model selection: The stepwise backward Wald method with a lock term of epidemic phases was used to refine the regression model. This method is particularly useful for selecting the most relevant variables and enhancing the model's predictive accuracy.
Analyses were performed using STATA 14 software (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP.), and the P-value less than 5% was considered statistically significant.
Results
The data of 25382 hospitalized patients with COVID-19 from Khorasan Razavi (7741 people), Khuzestan (10285 people), Kurdistan (1598 people), and Mazandaran (5758 people) were analyzed. Characteristics of hospitalized COVID-19 patients in the largest epidemic wave in four provinces are shown in Table 1.
Table 1. The background characteristics of confirmed COVID-19 hospitalized patients in the largest epidemic wave in four provinces.
| Variable | Khorasan Razavi | Khuzestan | Kurdistan | Mazandaran | Total population |
|---|---|---|---|---|---|
| Age Mean (SD) | 58.9 (20.01) | 55.06 (18.17) | 56.60 (18.80) | 54.91 (17.67) | 56.04 (18.73) |
| Sex Frequency (%) | 4059 (52.44) | 5500 (53.48) | 794 (49.69) | 2702 (46.93) | 13055 (51.43) |
| Underlying Disease Frequency (%) | 2449 (31.64) | 2517 (24.47) | 353 (22.09) | 2037 (35.38) | 7356 (28.98) |
| Duration of Hospitalization Median (IQR) | 5 (3-9) | 5 (2-8) | 4 (2-7) | 6 (4-8) | 5 (3-8) |
The mean and standard deviation of disease severity scores before (p1), during (p2), and after (p3) the peak in four provinces were as follows: Khorasan Razavi province 0.22 (0.28), 0.23 (0.27), and 0.20 (0.25); Khuzestan province 0.13 (0.23), 0.19 (0.27), and 0.24 (0.30); Kurdistan province 0.13 (0.21), 0.20 (0.25), and 0.18 (0.22); Mazandaran province 0.08 (0.17), 0.12 (0.21), and 0.12 (0.21). Additionally, the mean scores of the total target population in the four provinces in three periods (p1, o2, and p3) were 0.16 (0.25), 0.18 (0.26), and 0.19 (0.26), respectively.
Among hospitalized patients in four provinces, the proportion of deaths, ICU admissions, decreased oxygen levels, and respiratory distress in phases p2 and p3 was significantly higher than in phase p1 (P < 0.001) (Table 2).
Table 2. The variables related to the severe disease in different phases of epidemic waves in four provinces.
| Characteristics | Epidemic phases | Khorasan Razavi | P | Khuzestan | P | Kurdistan | P | Mazand aran | P | Total population |
|---|---|---|---|---|---|---|---|---|---|---|
| Death No. (%) | <0.001 | <0.001 | 0.012 | <0.001 | ||||||
| Before the peak | 245 (22.58) | 146 (14.11) | 58 (14.79) | 24 (8.42) | 473 (16.91) | |||||
| During the peak | 1582 (27.11) | 1904 (22.11) | 220 (21.86) | 646 (12.70) | 4352(21.19) | |||||
| After the peak | 185 (22.50) | 173 (27.03) | 39 (19.50) | 44 (11.28) | 441 (2149) | |||||
| Hospitalization in ICU / CCU No. (%) | 0.031 | <0.001 | 0.064 | 0.737 | ||||||
| Before the peak | 151 (13.91) | 119 (11.50) | 31 (7.90) | 34 (11.92) | 335 (11.98) | |||||
| During the peak | 935 (16.02) | 1227 (14.24) | 123 (12.22) | 684 (13.45) | 2969 (14.45) | |||||
| After the peak | 151 (18.36) | 180 (28.12) | 24 (12.00) | 54 (13.84) | 409 (19.93) | |||||
| Before the peak | 151 (13.91) | 119 (11.50) | 31 (7.90) | 34 (11.92) | 335 (11.98) | |||||
| During the peak | 935 (16.02) | 1227 (14.24) | 123 (12.22) | 684 (13.45) | 2969 (14.45) | |||||
| After the peak | 151 (18.36) | 180 (28.12) | 24 (12.00) | 54 (13.84) | 409 (19.93) | |||||
| Intubation No. (%) | <0.001 | 0.027 | 0.024 | <0.001 | ||||||
| Before the peak | 152 (14.00) | 97 (9.38) | 18 (4.59) | 12 (4.21) | 279 (9.97) | |||||
| During the peak | 595 (10.19) | 985 (11.43) | 87 (8.64) | 236 (4.64) | 1903 (9.26) | |||||
| After thepeak | 63 (7.66) | 87 (13.59) | 12 (6.00) | 35 (8.97) | 197 (9.60) | |||||
| Loss of consciousness No. (%) | <0.001 | <0.001 | 0.034 | 0.095 | ||||||
| Before the peak | 126 (11.61) | 20 (1.93) | 8 (2.04) | 3 (1.05) | 157 (5.61) | |||||
| During the peak | 482(8.26) | 449 (5.21) | 43 (4.27) | 149 (2.93) | 1123 (5.46) | |||||
| After the peak | 68 (8.27) | 68 (10.62) | 3 (1.50) | 15 (3.82) | 154 (7.50) | |||||
| Oxygen saturation (<93%) No. (%) | <0.001 | 0.015 | <0.001 | <0.001 | ||||||
| Before the peak | 629 (57.97) | 287 (27.75) | 174 (44.38) | 38 (13.33) | 1128
(40.34) |
|||||
| During the peak | 3976 (68.15) | 2752 (31.95) | 727 (72.26) | 1290 (25.37) | 8745 (42.58) | |||||
| After the peak | 520 (63.26) | 213 (33.28) | 148 (74.00) | 105 (26.92) | 986 (48.05) | |||||
| Respiratory Distress No. (%) | <0.001 | <0.001 | <0.001 | <0.001 | ||||||
| Before the peak | 675 (62.21) | 339 (32.78) | 166 (42.34) | 86 (30.17) | 1266(45.27) | |||||
| During the peak | 4029 (69.06) | 4650 (54.00) | 635 (63.12) | 2297 (45.18) | 11611 (56.54) | |||||
| After the peak | 511 (62.16) | 358 (55.93) | 121 (60.50) | 152 (38.97) | 1142 (55.65) | |||||
| Use of ambulance service No. (%) | 0.072 | <0.001 | 0.062 | <0.001 | ||||||
| Before the peak | 323 (29.76) | 228 (22.05) | 23 (5.86) | 21 (7.36) | 595 (21.28) | |||||
| During the peak | 1685 (28.88) | 1321 (15.34) | 138 (13.71) | 310 (6.09) | 3454 (16.82) | |||||
| After the peak | 246 (29.92) | 135 (21.09) | 16 (8.00) | 35 (8.97) | 432 (21.05) |
The most important criteria associated with COVID-19 severity in the weighting process were death (0.44), intubation (0.15), ICU admission (0.14), deteriorated consciousness (0.12), low oxygen saturation (0.05), respiratory distress (0.05) and referral status (0.02), respectively.
Both univariable and multivariable linear regressions indicated that the frequency of illness severity was higher during phases p2 and p3 in the four provinces when comparing different phases of the epidemic wave. In the univariable model, the assessments of the COVID-19 severity in three phases at provincial levels revealed that in Khuzestan, Kurdistan, and Mazandaran, the severity of the disease was higher during p2 and p3, but in the Khorasan Razavi, although this average was higher during the p2 phase, it was not statistically significant (Table 3).
Table 3. Univariable linear regression model comparing severe disease in different phases of epidemic waves in four provinces.
| Disease severity core/population | Variable* | Coef. | t statistics | P>|t| (Univariable model) |
|---|---|---|---|---|
| Khorasan Razavi | ||||
| During the peak | 0.01 | 1.48 | 0.140 | |
| After the peak | -0.02 | -1.60 | 0.109 | |
| Khuzestan | ||||
| During the peak | 0.06 | 6.44 | <0.001 | |
| After the peak | 0.11 | 8.15 | <0.001 | |
| Kurdistan | ||||
| During the peak | 0.07 | 5.05 | <0.001 | |
| After the peak | 0.05 | 2.52 | 0.011 | |
| Mazandaran | ||||
| During the peak | 0.04 | 2.94 | 0.003 | |
| After the peak | 0.04 | 2.30 | 0.020 | |
| Total population | ||||
| During the peak | 0.02 | 4.70 | <0.001 | |
| After the peak | 0.03 | 4.76 | <0.001 |
*"Before the peak" is the reference category.
Furthermore, in the multivariable model, age and history of underlying disease had a significant positive relationship with disease severity (P < 0.001), and this relationship with the cough as a general symptom of the disease was significantly negative (P < 0.001) (Table 4).
Table 4. Multivariable and univariate linear regression model comparing severe disease status in the total population.
| Disease severity score/total population | Variable* | Coef. | t statistics | CI | P>|t| (Multivariable models) | P>|t| (Univariable model) |
|---|---|---|---|---|---|---|
| During the peak | 0.01 | 4.36 | 0.006, 0.02 | <0.001 | <0.001 | |
| After the peak | 0.01 | 3.00 | 0.004, 0.02 | 0.003 | <0.001 | |
| Total population | Age | 0.0007 | 15.96 | 0.0006, 0.0008 | <0.001 | <0.001 |
| Underlying diseases | 0.02 | 9.68 | 0.01, 0.02 | <0.001 | <0.001 | |
| Cough | -0.005 | -3.54 | -0.009, - 0.003 | <0.001 | <0.001 |
*"Before the peak" is the reference category.
Discussion
We investigated the illness severity in hospitalized COVID-19 patients in four provinces to explore the question of whether these characteristics differ during different phases of an epidemic wave.
The univariable and multivariable regression models showed that the COVID-19 severity index increased during phases p2 and p3 among all patients. At the provincial level, COVID-19 severity was higher during p2 and p3 in three provinces. Targeted health planning, considering local situations, could make the current system more effective, equitable, and accessible to the population.
Variations of clinical features during a wave's time intervals may be influenced by various factors, including demographic data, disease transmission, patient referral patterns, local legislation, and service delivery. As expected, the patients with worse health conditions are more likely to be hospitalized during the p2 due to the high load and burden of the disease, as well as the higher proportion of critically ill patients. On the other hand, there are some hypotheses on the severity of the disease after the peak, including the high burden of the disease during the peak, delay in severe consequences of illness like death and hospitalization in the ICU, more cases of long-term COVID-19 and the underestimation of disease risk by people in the phase p3 (10, 21). All mentioned factors can affect the recovery rates after the peak. Besides, disease severity and mortality rate indifferent phases can be in terms of changes in the patient characteristics such as age and history of underlying diseases. Demographic changes in the Iranian population, which are ageing the country, could create more pressure on disease management during emergencies and pandemics (22). In a study in Israel, the results showed that COVID-19 mortality rates were significantly associated with heavy patient load (23).
In the current study, although the clinical severity scores of COVID-19 have slightly increased during the phases, and the differences were minimal, serious complications such as death are still important even in small numbers. Additionally, no evidence was found regarding the Minimum Clinically Important Difference (MCID) criterion for COVID-19- patients in the literature (24).
Therefore, strengthening preparedness through the targeted interventions, i.e., allocating sufficient resources to the clinical sector, and improving access to medicines, vaccines, and health products is required during the phases p2 and p3 to prevent excess mortality and serious illness (12, 25).
The findings of this study also represented that severe diseases had a positive and significant relationship with variables, such as age and history of the underlying disease. These results are consistent with findings from other studies (17, 18, 26). Some hypotheses suggest that a decrease in mortality may be due to a reduced hospital load and improved handling of difficult illnesses by healthcare teams over time (27).
Clinical information on a large population of hospitalized patients during different phases of an epidemic wave can give us a comprehensive view of the overall variations in clinical severity on a national scale. However, various aspects, such as the characteristics of the target population, health infrastructures, and policy interventions, should be considered when interpreting the results.
Conclusion
In conclusion, hospitals in the middle and end of the epidemic wave are facing patients with more severe diseases than in the initial phase of the disease. Particularly, a higher proportion of severe COVID-19 cases in phase p3 is a cause for concern, as it can be attributed to factors such as limited hospital resources and delayed referral of patients for hospitalization. To prevent excess COVID-19 mortality and critical conditions, it is necessary to enhance the readiness of the hospitals until the end of the peak. In addition, to prepare the medical system in times of crisis, health policymakers need to take the necessary precautions to declare the end of the state of emergency and exit the epidemic phase.
Ethical Considerations
The study was reviewed and approved by the Research Ethics Committee at the Pasteur Institute of Iran (Ethics approval code: IR.PII.REC.1399.081).
Conflict of Interests
Dr MMG had been the former director of the Iranian Center for Communicable Diseases at Ministry of Health during the time of the study. All other authors declare that they have no competing interests. All other authors declare that they have no competing interests.
Funding
This paper is part of a PhD thesis at the Pasteur Institute of Iran, which funded this study.
Acknowledgment
We acknowledge the contributions of the Pasteur Institute of Iran, and the Ministry of Health for conducting the research. We thank all specialists and clinical experts who participated in classifying, scoring and interpreting clinical variables, and also thank Dr Mohammad Karamouzian for his valuable comments on data interpretation.
Authors’ Contributions
EM, AAH were the project managers and coordinated the study and data acquisition. EM, AAH and LM contributed to the conceptualization, methodology and design of the study. LM and SE contributed to the data curation. LM conducted the statistical analysis. LM wrote the first draft of the manuscript. EM, AAH, LM, SGH, MP, LH, MMG and AH contributed to the analysis and interpretation of the findings. All authors critically appraised the manuscript and approved the final version.
Cite this article as : Mounesan L, Gharibzadeh S, Parsaeian M, Gouya MM, Eybpoosh S, Hosseini A, Haghjou L, Haghdoost AA, Mostafavi E. Differences in the Clinical Severity Scores of Hospitalized COVID-19 Patients across Different Phases of an Epidemic Wave in Iran: a Secondary Analysis. Med J Islam Repub Iran. 2024 (13 Aug);38:93. https://doi.org/10.47176/mjiri.38.93
References
- 1.Wise J. Covid-19: WHO declares end of global health emergency. BMJ. 2023 May 9:1041. doi: 10.1136/bmj.p1041. [DOI] [PubMed]
- 2.Nikolay B, Salje H, Sturm-Ramirez K, Azziz-Baumgartner E, Homaira N, Ahmed M. et al. Evaluating hospital-based surveillance for outbreak detection in Bangladesh: analysis of healthcare utilization data. PLoS Med. 2017;14(1):e1002218. doi: 10.1371/journal.pmed.1002218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saito S, Asai Y, Matsunaga N, Hayakawa K, Terada M, Ohtsu H. et al. First and second COVID-19 waves in Japan: a comparison of disease severity and characteristics. J Infect. 2021;82(4):84–123. doi: 10.1016/j.jinf.2020.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eid RA, Attia AM, Hassan M, Shaker MA, Kamal MA. Demographic, clinical, and laboratory characteristics of patients with COVID-19 during the second and third waves of the pandemic in Egypt. J Infect Prev. 2021;14(10):1358. doi: 10.1016/j.jiph.2021.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valladares-Garrido MJ, Failoc-Rojas VE, Soto-Becerra P, Zeña-Ñañez S, Torres-Roman JS, Fernández-Mogollón JL. et al. Clinical-epidemiologic variation in patients treated in the first and second wave of COVID-19 in Lambayeque, Peru: A cluster analysis. J Infect Dis. 2022;123:212. doi: 10.1016/j.ijid.2022.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cacciapaglia G, Cot C, Sannino F. Multiwave pandemic dynamics explained: How to tame the next wave of infectious diseases. Sci Rep. 2021;11(1):6638. doi: 10.1038/s41598-021-85875-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Souza, Buss LF, Candido DdS, Carrera J-P, Li S, Zarebski AE. et al. Epidemiological and clinical characteristics of the COVID-19 epidemic in Brazil. Nat Hum Behav. 2020;4(8):856. doi: 10.1038/s41562-020-0928-4. [DOI] [PubMed] [Google Scholar]
- 8.Schoch-Spana M. An epidemic recovery framework to jump-start analysis, planning, and action on a neglected aspect of global health security. Clin Infect Dis. 2020;71(9):2516. doi: 10.1093/cid/ciaa486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horney J, Dwyer C, Aminto M, Berke P, Smith G. Developing indicators to measure post‐disaster community recovery in the United States. Disasters. 2017;41(1):124. doi: 10.1111/disa.12190. [DOI] [PubMed] [Google Scholar]
- 10.Charnley GE, Kelman I, Gaythorpe KA, Murray KA. Traits and risk factors of post-disaster infectious disease outbreaks: a systematic review. Sci Rep. 2021;11(1):5616. doi: 10.1038/s41598-021-85146-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Presanis AM, Kunzmann K, Grosso FM, Jackson CH, Corbella A, Grasselli G. et al. Risk factors associated with severe hospital burden of COVID-19 disease in Regione Lombardia: a cohort study. BMC Infect Dis. 2021;21:1–16. doi: 10.1186/s12879-021-06750-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tuech JJ, Gangloff A, Di Fiore, Benyoucef A, Michel P, Schwarz L. The day after tomorrow: how should we address health system organization to treat cancer patients after the peak of the COVID-19 epidemic. Oncology. 2020;98(12):827. doi: 10.1159/000509650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang R-p, Jiang Y-g, Zhao G-m, Guo X-q, Michael E. ‘Outbreak gold standard’selection to provide optimized threshold for infectious diseases early-alert based on China infectious disease automated-alert and response system. Curr Med Sci. 2017;37:833. doi: 10.1007/s11596-017-1814-9. [DOI] [PubMed] [Google Scholar]
- 14. Johnson J. COVID-19 Alert-Level System Indicators, Triggers and Thresholds 2021. 2021. Available from: https://preventepidemics.org/wp-content/uploads/2020/05/Annex-2_Example-of-an-alert-level-system_US_FINAL.pdf.
- 15.Madhav N, Oppenheim B, Gallivan M, Mulembakani P, Rubin E, Wolfe N. Disease control priorities: improving health and reducing poverty. 3rd edition. 2017 Nov 27. Pandemics: risks, impacts, and mitigation. [Google Scholar]
- 16.Doosti-Irani A, Haghdoost AA, Najafi F, Eybpoosh S, Moradi G, Amiri FB. et al. How can the epidemic curve of COVID-19 in Iran be interpreted. J Health Sci Res. 2020;20(3):e00491. doi: 10.34172/jrhs.2020.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia PDW, Fumeaux T, Guerci P, Heuberger DM, Montomoli J, Roche-Campo F. et al. Prognostic factors associated with mortality risk and disease progression in 639 critically ill patients with COVID-19 in Europe: Initial report of the international RISC-19-ICU prospective observational cohort. EClinicalMedicine. 2020;25:100449. doi: 10.1016/j.eclinm.2020.100449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez-Nava G, Yanez-Bello MA, Trelles-Garcia DP, Chung CW, Friedman HJ, Hines DW. Performance of the quick COVID-19 severity index and the Brescia-COVID respiratory severity scale in hospitalized patients with COVID-19 in a community hospital setting. Int J Infect Dis. 2021;102:571. doi: 10.1016/j.ijid.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haimovich AD, Ravindra NG, Stoytchev S, Young HP, Wilson FP, Van Dijk. et al. Development and validation of the quick COVID-19 severity index: a prognostic tool for early clinical decompensation. Ann Emerg Med. 2020;76(4):442. doi: 10.1016/j.annemergmed.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saaty TL. RWS publications;; 1994. Fundamentals of decision making and priority theory with the analytic hierarchy process. [Google Scholar]
- 21.Du L, Pang Y. A novel data-driven methodology for influenza outbreak detection and prediction. Scientific reports. 2021;11(1):13275. doi: 10.1038/s41598-021-92484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davari M, Haycox A, Walley T. Health care challenges in Iran. Iran J Public Health. 2005;34(Supple 1):30. [Google Scholar]
- 23.Rossman H, Meir T, Somer J, Shilo S, Gutman R, Ben Arie. et al. Hospital load and increased COVID-19 related mortality in Israel. Nat Commun. 2021;12(1):1904. doi: 10.1038/s41467-021-22214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shabat S, Marmor A, Shiri S, Tsenter J, Meiner Z, Schwartz I. Correlations between disease severity and rehabilitation outcomes in patients recovering from COVID-19 infection. J Rehabil Med. 2023;55:jrm00344. doi: 10.2340/jrm.v54.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gouya MM, Seif-Farahi K, Hemmati P. An overview of Iran's actions in response to the COVID-19 pandemic and in building health system resilience. Front Public Health. 2023;11:1073259. doi: 10.3389/fpubh.2023.1073259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allameh SF, Nemati S, Ghalehtaki R, Mohammadnejad E, Aghili SM, Khajavirad N. et al. Clinical characteristics and outcomes of 905 COVID-19 patients admitted to Imam Khomeini hospital complex in the capital city of Tehran, Iran. Arch Iran Med. 2020;23(11):766–775. doi: 10.34172/aim.2020.102. [DOI] [PubMed] [Google Scholar]
- 27.Roth GA, Emmons-Bell S, Alger HM, Bradley SM, Das SR, De Lemos. et al. Trends in patient characteristics and COVID-19 in-hospital mortality in the United States during the COVID-19 pandemic. JAMA Netw Open. 2021;4(5):e218828. doi: 10.1001/jamanetworkopen.2021.8828. [DOI] [PMC free article] [PubMed] [Google Scholar]