Abstract
Lagerstroemia thorelli (L. thorelli) is a member of the Lythraceae family and has not been previously researched. Thus, this study aimed to investigate its unexplored potential and identify novel therapeutic prospects. This research evaluated antioxidant, antidiabetic, and cytotoxic potentials along with compound characterization of the ethanolic leaf extract of L. thorelli. The antioxidant potential was assessed using 1, 1‐diphenyl‐2‐picrylhydrazyl (DPPH) free radical and hydrogen peroxide (H2O2) scavenging assays, total antioxidant capacity (TAC), total phenolic content (TPC), total flavonoid content (TFC) determination, antidiabetic property was assessed using α‐amylase inhibition, and the cytotoxic effect was examined on HeLa and Vero cells using MTT colorimetric assay. Chemical characterization was performed using gas chromatography‐mass spectrometry (GC‐MS). The findings demonstrated strong antioxidant, strong antidiabetic, and moderate cytotoxic activities. Comprehensive phytochemical analysis revealed its abundance in flavonoids, phenols/phenolics, tannins, glycosides, steroids, resin, etc. GC‐MS analysis of the L. thorelli extract identified 80 important compounds including cis‐11‐eicosenamide, beta‐D‐glucopyranoside, methyl‐, alpha‐D‐glucopyranoside, methyl‐, phthalic acid, gamma‐sitosterol, phytol, silicic acid, squalene, butanoic acid, cyclobarbital, etc. which are well‐documented for their antioxidant, antidiabetic, and anticancer effects. Thus, it can be inferred that L. thorelli could hold new promises in treating diseases like diabetes and free radical‐induced conditions, including neurodegenerative diseases.
Keywords: Lagerstroemia thorelli, Antioxidant, Antidiabetic, Cytotoxicity, Phytochemicals, GC-MS
1. Introduction
Throughout history, plants used for medicinal purposes have played a vital role in healthcare and research worldwide, proving their effectiveness, and generating a market worth over $100 billion. [1] Up to 80 % of the world's population, as per the World Health Organization (WHO) reports, relies on traditional medicines, including herbal remedies, for primary care.[ 2 , 3 ] Researchers are increasingly turning to medicinal plants as they serve as an excellent source of bioactive compounds, with approximately 40 % of pharmaceuticals on the market today coming from natural sources. [4] Scientists explore the beneficial phytochemicals present in medicinal plants like anthraquinones, glycosides, cardiac terpenoids, phenolic compounds, saponins, alkaloids, tannins, etc., through phytochemical analysis to investigate their therapeutic potential, thereby contributing to the advancement of novel therapeutics. [5]
Reactive oxygen species (ROS) are essential in tissue homeostasis and cell signaling, where it is critical to maintain the delicate harmony between generating and eliminating ROS. [6] The occurrence of any disruption in the state of balance due to excess or a lack of ROS results in the development of diseases. Consequently, modulating strategies become necessary to restore equilibrium and effective disease treatment. [6] Several studies have found a correlation between ROS and the development of carcinogenesis, mutations, and cellular transformation, primarily attributed to DNA, lipid, and protein damage.[ 7 , 8 , 9 , 10 ] Previous studies have documented that synthetic antioxidants like butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA) have potential carcinogenic and hepatotoxic effects. [11] Therefore, exploring naturally occurring antioxidants from medicinal plants is imperative. Antioxidants present in medicinal plants, such as flavonoids, tannins, and phenolic acids, play a crucial role in neutralizing ROS due to their potential antioxidant properties. [12] Thus, they offer a potentially effective approach for alleviating oxidative stress's deleterious effects.
Cervical cancer stands as the fourth most common malignancy among women worldwide, accounting for 342,000 deaths and 604,000 new cases in 2020. [13] Alarmingly, about 90 % of these fatalities and new cases were reported from countries with low‐ and middle‐incomes, underscoring its status as the third most prevalent malignancy type and the fourth most common cause of death from cancer in women.[ 13 , 14 ] While effective, the primary chemotherapy, cisplatin, has notable side effects and resistance issues, limiting its clinical use.[ 15 , 16 ] This has driven the pursuit of novel chemotherapeutic agents to enhance cervical cancer treatment, with a focus on reducing patient exposure to side effects through combination therapies. Recognizing these challenges, efforts are being made to explore the use of natural products derived from medicinal plants to reduce side effects and improve treatment outcomes.
The global incidence of type 2 diabetes has substantially increased during the previous three decades, impacting approximately 422 million individuals. [17] By the year 2025, it has been estimated that there will be 1.5 million annual deaths attributed to diabetes and obesity. [17] Therefore, affordable treatment is urgently needed to address this growing concern. Studies have identified several medicinal plants with antidiabetic properties due to their high content of phenol/phenolic compounds, alkaloids, terpenoids, flavonoids, and glycosides.[ 18 , 19 ] Quercetin from onions and resveratrol from grapevines are two examples of these compounds, which have been reported to enhance carbohydrate metabolism and improve insulin secretion. [20] Garlic, turmeric, and rosella flowers have been identified as potential remedies for diabetes. [21] Thus, researchers are increasingly exploring medicinal plants to uncover bioactive compounds for affordable diabetes treatments.
An unexplored species with no prior study, Lagerstroemia thorelli, is a perfect candidate for in‐depth phytochemical analysis and bioactivity study. L. thorelli belongs to the Lythraceae family, falls under the Lagerstroemia genus, and is locally identified as “Jarul” in Bangladesh.[ 22 , 23 ] In this study, we aimed to analyze the antioxidant, cytotoxic, and antidiabetic properties of L. thorelli plant extract. It is important to note that no compounds have been identified from this plant as of today. Therefore, we conducted gas chromatography‐mass spectrometry (GC‐MS)‐based phytochemical characterization to identify the individual compounds responsible for contributing to the screened activities.
2. Methodology
2.1. Plant Collection and Authentication
The leaves of L. thorelli were collected from Nabiganj Upazila, Habiganj (24.3750°N 91.4167°E.), Bangladesh, in October 2022 and were identified and authenticated by experts at the National Herbarium Bangladesh (NHB), Mirpur, Dhaka (Accession number DACB‐87494).
2.2. Preparation of Extract
The cleaned and sun‐dried leaves of L. thorelli were ground into a coarse powder, yielding an approximate mass of 306 g. For extraction, the powder was soaked in 1 L ethanol for 2–3 days at a temperature between 22–25 °C (room temperature), with intermittent agitation according to the method described by Phrompittayarat et al. with a slight modification. [24] After filtration, filtrate was concentrated in a water bath at 50–55 °C. To prevent cross‐contamination, the concentrated extract was transferred to a petri dish and subjected to drying under laminar airflow. A total of 17.21 g extract was produced from 306 g of dry powder and the % yield of extract was 5.62 % w/w. Finally, the extract was stored at 4 °C in the refrigerator.
2.3. Preliminary Phytochemical Screening
Preliminary phytochemical analysis standard protocols were followed with slight modifications to identify the chemical components of the examined extract, including carbohydrates, flavonoids, glycosides, alkaloids, tannins, etc. (Table 1).
Table 1.
Preliminary phytochemical screening of L. thorelli leaf extract.
|
Name of the test |
Class of compounds |
Presence (+)/Absence (−) |
|---|---|---|
|
Potassium dichromate test, Lead acetate test, and Ferric chloride test |
Tannins |
+++ |
|
Zinc ribbon test, Lead acetate test, and Alkaline reagent test |
Flavonoids |
+++ |
|
Ferric Chloride test, Lead acetate test, and Iodine test |
Phenol/ Phenolic compound |
+++ |
|
Acetic anhydride test and Turbidity test |
Resins |
++ |
|
Hager's test, Mayer's test, and Wagner's test |
Alkaloids |
– |
|
Molisch's test and Fehling's test |
Carbohydrates |
++ |
|
Libermann Burchard's test and Acetic anhydride test |
Phytosterols |
– |
|
Borntrager's Test and Modified Borntrager's test |
Glycosides |
++ |
|
Froth test and Olive oil test |
Saponins |
– |
|
Libermann‐Burchard's test and Salkowski's test |
Steroids |
++ |
Note: Any phytochemical group is indicated by a “+” when it is present and by a “−” when it is not. Key to bioavailability: (++) ve indicates medium intensity, (+++) ve strong intensity, (+) ve weak intensity, and (−) ve indicates absence.
2.4. Gas Chromatography‐Mass Spectrometry (GC‐MS) Employed Phytochemical Characterization of L. Thorelli Leaf Extract
GC‐MS analysis of L. thorelli leaf extract was analyzed using GCMS‐TQ8040 instrument (Shimadzu, Japan) with high sensitivity and high performance according to the method described here. A DB‐5 ms non‐polar column (internal diameter 30×0.25 mm and film thickness 0.25 μm was used. Initially, the column temperatures were kept at 50 °C for 1 minute, then gradually increased to 300 °C for 20 minutes. A volume of 0.5 μL of the extract was injected for analysis. Helium was used as the carrier gas with a flow rate set at 1 mL/min in the spitless mode. The split ratio was 5, and the temperatures of the sample injector and detector were maintained at 250 and 230 °C, respectively. Electrons having an energy of about +0.50 kV were used in electron ionization mass spectrometry. Furthermore, 40 minutes were spent recording mass spectra in the 50 m/z to 600 m/z region.
2.5. Quantification and Identification of Individual Phytoconstituents of L. Thorelli Leaf Extract
Identification and quantification of individual phytoconstituents of the L. thorelli leaf extract were done by comparing retention indices and mass spectra fragmentation patterns of each compound reference sample available in the Wiley database and National Institute of Standards & Technology (NIST) libraries. [25] Furthermore, the relative proportion of each component was determined by quantifying and relating its % peak area to the overall peak areas.
2.6. Assessment of In‐Vitro Antioxidant Activity Using DPPH, H2O2, TPC, TFC, and TAC Methods
Numerous comprehensive techniques, including the DPPH free radical scavenging test, H2O2 scavenging assay, total antioxidant capacity assessment, and determination of the total flavonoid and phenol/phenolic content, were used to assess the antioxidant properties of the extract.
2.7. Evaluation of DPPH Free Radical Scavenging Activity in L Thorelli Leaf Extract
The widely employed technique of free radical scavenging with DPPH as mentioned by Rahman et al., [26] was used to assess the free radical scavenging activity. It is measured through the discoloration of the DPPH solution following adding extract solution at varying concentrations (50–500 μg/mL). Each test tube contained a mixture of 3 mL of ethanol and 0.2 % DPPH, to which 1 mL of sample and the standard solution were added, followed by a 30‐minute incubation in the dark. Absorbance was measured at 517 nm using a UV‐visible spectrophotometer. This analysis used ethanol as the blank and ascorbic acid as the standard. The percentage of DPPH inhibition (% I) was calculated and plotted against extract concentration to determine IC50 values. The DPPH free radical's percent inhibition (% I) was determined using the following equation, % Inhibition=(1‐A/Ao)×100, where Ao stands for the blank's absorbance (1 mL ethanol+3 mL DPPH solution), and A denotes the sample's or the standard's absorbance. [27]
2.8. H2O2 Scavenging Activity
The H2O2 scavenging activity was assessed by following the procedure outlined by Nabavi et al. [28] This method involved measuring the absorbance at 230 nm using a 40 mM H2O2 solution in a 50 mM phosphate buffer with a pH of 7.4. Following that, a volume of 2 mL of H2O2 was combined with a volume of 1 mL of the sample extract. After a 10‐minute incubation time, the absorbance was compared to a blank solution containing only a phosphate buffer. The H2O2 scavenging activity was calculated using this formula, H2O2 scavenge (%)=[(A230 Control –A230 Sample )/A230 Control ]×100, where, A represents the absorbance, A230 Control is denoted as the absorbance at 230 nm for the control solution and A230 Sample is the absorbance at 230 nm for the sample solution.
2.9. Assessment of Total Phenolic Content (TPC)
A slightly modified version of the method narrated by Ainsworth and Gillespie (2007) was used to determine the total phenolic content (TPC). [29] The assessment of total phenol/phenolic content utilizes Folin‐Ciocalteu's reagent as an oxidative agent and gallic acid solution as the standard as stated by Lamuela‐Raventós et al. [30] 2 mL of Na2CO3 solution and 0.5 mL of gallic acid standard solutions at different concentrations (25 to 100 μg/mL) were added to test tubes. 0.5 mL of sample solutions were transferred to the test tubes containing Na2CO3 solution. Absorbance was measured at 760 nm wavelength after incubation for 20 minutes. A standard curve was generated employing gallic acid as a standard reference to determine the total phenolic content of the test sample. The TPC content of the extract was quantified in terms of gallic acid equivalents utilizing this equation: C=(c×V)÷m, where, C represents the TPC content, expressed as gallic acid equivalent (GAE), measured in milligrams of gallic acid per gram of dried plant extract, c denotes the gallic acid concentration calculated from the calibration curve (mg/mL), V is the volume of the sample solution in milliliters, and m is the weight of the sample in grams.
2.10. Assessment of Total Flavonoid Content (TFC)
A slightly modified approach derived from the methodology mentioned by Haida and Hakiman was used to assess the TFC of the plant extract. [31] Five different concentrations (100 to 1200 μg/mL) of standard and sample solutions were taken to which 3 mL of ethanol was combined, followed by the addition of 200 μL of 10 % AlCl3 solution and 200 μL of 1 M CH3COOK solution. After the mixtures were allowed to rest for 30 minutes following dilution with 5.6 mL of distilled water, the absorbance was measured at 415 nm, with ethanol used as the blank and quercetin as the standard. The TFC was quantified using this formula, C=(c×V)/m, where C represents the TFC, given as quercetin equivalent (QE), in milligrams of quercetin per gram of dried plant extract, c denotes the concentration of quercetin calculated from the calibration curve (mg/mL), V is the volume of the sample in milliliters and m is the weight of the sample in grams.
2.11. Assessment of Total Antioxidant Capacity (TAC)
The TAC of L. thorelli leaf extract was determined using the previously described method by Pisoschi and Negulescu. [32] 300 μL of the sample and standard solution were added to each test tube, containing 2 mL of the reagent solution mixture (0.6 M sulfuric acid, 0.028 M sodium phosphate, and 0.004 M ammonium). The test tubes were incubated in a water bath at 95 °C for 90 minutes. The absorbance was recorded at 765 nm using a U‐2910 UV‐Vis spectrometer. The TAC of the extract was quantified in terms of ascorbic acid equivalent (AAE) using this formula, A=(c×V)÷m, where A represents the ascorbic acid equivalent measured in milligrams of ascorbic acid per gram of dry plant extract, c is the concentration of ascorbic acid (mg/mL), V is the volume of the sample in (mL), and m is the weight of the sample in grams.
2.12. Evaluation of In‐Vitro Antidiabetic Activity
Kifle and Enyew, 2020, outlined the 3,5‐dinitrosalicylic acid (DNSA) process used in the α‐amylase inhibition assay. [33] DNSA reduces the yellow‐colored DNS through a redox reaction, yielding a red color. Various concentrations ranging from 125–1000 μg/mL of plant extract and standard solutions were prepared and used to assess this activity. Glimepiride was used as a standard and the absorbance of standard and sample solutions was measured using a UV‐Vis spectrophotometer (U‐2910, Hitachi High Technologies, USA). The formula, % α‐amylase inhibition=(Ao–A1)÷Ao×100 was used to calculate the percentage inhibition of α‐amylase where Ao represents the absorbance of the control and A1 is the absorbance of the sample/standard.
2.13. MTT Assay
2.13.1. Cell Culture
The cytotoxicity screening of L. thorelli leaf extract was conducted using a human cervical cancer cell line (HeLa, ATCC CCL‐2) and a normal cell line, healthy kidney cells from monkeys (VERO, ATCC CCL‐81). The cell lines were procured from ATCC, based in Manassas, VA 20108, USA. The cell lines were placed in Advanced DMEM, supplemented with 10 % inactivated NBCS and 5 mM L‐glutamine, and incubated at 37 °C in a humidified environment with 5 % CO2. [12]
2.13.2. The MTT Colorimetric Assay
To determine the cytotoxicity of L. thorelli leaf extract, the MTT colorimetric assay was described and validated by Akter et al. and Uddin et al.[ 34 , 35 ] Cells were placed at a specific density of 1.0×104–2.0×104 cells/well in 96‐well plates and then incubated, allowing them to adhere for 24 hours before being treated with different concentrations (1.0–2.5 mg/mL) of L. thorelli extract for 48 hours. Subsequently, the cells were washed, incubated with MTT solution for 2 hours, lysed with dimethyl sulfoxide (DMSO), and absorbance was measured at 560 nm after 45 minutes using a microplate reader (Wallac 1420 Multilabel counter, PerkinElmer). 2 % DMSO and cycloheximide functioned as the negative and positive control, respectively. The cytotoxicity was determined with the equation, % of cytotoxic activity=100–(Absorbance of the test sample/ Absorbance of the negative control) * 100.
2.14. Statistical Analysis
Experiments for assessing antioxidant potential (DPPH, H2O2, TPC, TFC, and TAC) were repeated and conducted three times to increase accuracy and precision. The MTT colorimetric assay was carried out in triplicates while antidiabetic activity was performed in duplicates. All statistical analyses, as well as the graphs, were done using MS Excel (2013), with all results presented as mean±SD.
3. Results
3.1. Preliminary Phytochemical Screening of L. Thorelli Leaf Extract
The symbol (+) indicates the presence of phytochemicals in one experiment, (++) in two experiments, (+++) in three experiments, and absence is denoted by (−) (Table 1). The presence of flavonoids, glycosides, phenolic compounds, carbohydrates, tannins, steroids, and resins presence was confirmed by phytochemical screening of L. thorelli leaf extract (Table 1). However, the extract was devoid of saponins, phytosterols, and alkaloids.
3.2. GC‐MS Employed Identification and Quantification of Individual Phytoconstituents of L. Thorelli Leaf Extract
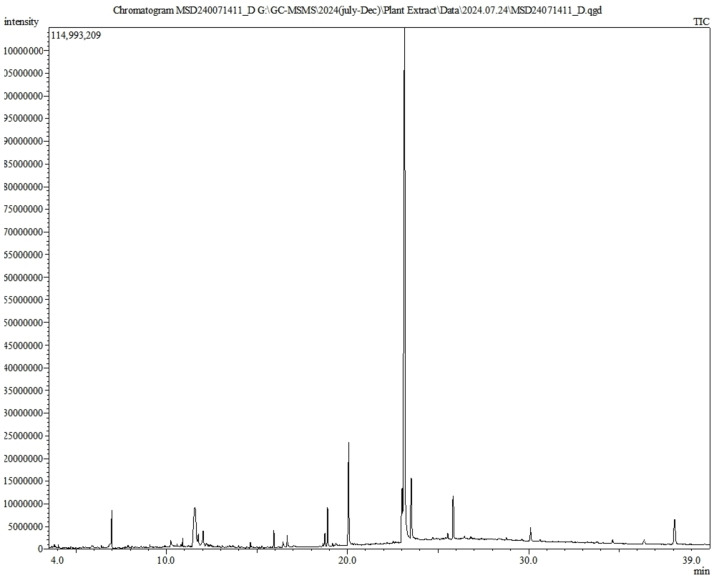
GC‐MS analysis of individual constituents of L. thorelli leaf extract led to the identification of 80 compounds belonging to different phytochemical classes (Figure 1, Table 2). Based on the % area, the major compounds detected among them were cis‐11‐eicosenamide (49.25 %), hexadecanamide (6.53 %), octadecanamide (5.03 %), beta‐D‐glucopyranoside, methyl‐ (4.16 %), alpha‐D‐glucopyranoside, methyl‐ (4.04 %), 6,9‐octadecadienoic acid, methyl ester (4.03 %), phthalic acid, di(2‐propylpentyl) ester (2.8 %), gamma‐sitosterol (2.8 %), phytol (2.22 %), silicic acid (1.916), alpha‐methyl‐l‐sorboside (1.74 %), methyl (methyl 2,4‐di‐O‐acetyI‐3‐O‐methyl‐alpha‐D galactoside uronate (1 %) and squalene (0.87 %). The compounds of L. thorelli leaf extract identified through GC‐MS analysis exhibit a diverse range of bioactivities that have been studied previously, as summarized in Table 3. These bioactivities include potential antidiabetic, antioxidant, anticancer, antimicrobial, anti‐inflammatory, antifungal, neuroprotective, cardioprotective, antihyperlipidemic properties, and many others.
Figure 1.
GC‐MS chromatogram of L. thorelli leaf extract.
Table 2.
Phytoconstituents of L. thorelli identified and quantified by GC‐MS.
|
Sl. No. |
R. Time |
Area % |
Compound Name |
Molecular Formula |
Chemical Class |
|---|---|---|---|---|---|
|
1 |
3.525 |
0.14 |
1‐Gala‐l‐ido‐octose |
C8H16O8 |
Sugars |
|
2 |
3.665 |
0.12 |
Succinic acid, 3‐methoxybenzyl nonyl ester |
C20H28O4 |
Esters |
|
3 |
3.765 |
0.11 |
Furfural |
C5H4O2 |
Aldehydes |
|
4 |
3.823 |
0.13 |
3,3‐Dimethoxy‐2‐butanone |
C8H12O4 |
Ketones |
|
5 |
3.862 |
0.06 |
Silane, methyldiethoxymethoxy‐ |
C6H16O3Si |
Organosilanes |
|
6 |
3.895 |
0.05 |
1,3‐Dioxolane‐4‐methanol, 2‐ethyl‐ |
C6H12O3 |
Dioxolanes |
|
7 |
4.046 |
0.08 |
1,1,3,3‐Tetramethyl‐3‐(1‐methylpropoxy) disiloxan‐l‐ol |
C8H22O3Si2 |
Siloxanes |
|
8 |
4.737 |
0.06 |
6‐Oxa‐bicyclo [3. I .0] hexan‐3‐one |
C5H6O2 |
Bicyclic ketones |
|
9 |
5.891 |
0.13 |
Arsenous acid, tris(trimethylsilyl) ester |
C9H27AsO3Si3 |
Organoarsenic compounds |
|
10 |
5.961 |
0.08 |
D‐Limonene |
C1OH16 |
Monoterpenes |
|
11 |
6.095 |
0.05 |
Pantolactone |
C6H10O3 |
Lactones |
|
12 |
6.413 |
0.07 |
7‐Octen‐2‐ol, 2,6‐dimethyl‐ |
C10H20O |
Alcohols |
|
13 |
7.885 |
0.1 |
Alpha‐Terpineol |
C10H18O |
Terpenes |
|
14 |
8.868 |
0.05 |
Acetic acid, l,7,7‐trimethyl‐bicyclo [2.2] hept‐2‐yl ester |
C12H20O2 |
Esters |
|
15 |
9.096 |
0.11 |
2‐Methoxy‐4‐vinylphenol |
C9H10O2 |
Phenols |
|
16 |
9.936 |
0.07 |
2‐(4′‐Methoxyphenyl)‐2‐(2′‐methoxyphenyl) propane |
C17H20O2 |
Alkyl propane |
|
17 |
10.242 |
0.62 |
2‐(Isobutoxymethyl)oxiranehexadecanoic acid |
C7H14O2 |
Oxiranes |
|
18 |
10.475 |
0.1 |
Dodecane, 2,6,11‐trimethyl‐ |
C15H32 |
Alkanes |
|
19 |
10.54 |
0.06 |
Tetradecane, 3‐methyl‐ |
C5H32 |
Alkanes |
|
20 |
10.589 |
0.12 |
1 ‐Undecanol |
C11H24O |
Alcohols |
|
21 |
10.695 |
0.07 |
Octadecane, 3‐ethyl‐5‐(2‐ethylbutyl)‐ |
C26H54 |
Alkanes |
|
22 |
10.805 |
0.12 |
Beta‐D‐Glucopyranose, 1,6‐anhydro‐ |
C6H10O |
Sugars |
|
23 |
11.224 |
0.06 |
Pentadecane, 7‐methyl‐ |
C16H34 |
Alkanes |
|
24 |
11.55 |
4.04 |
Alpha‐D‐Glucopyranoside, methyl |
C7H14O6 |
Glycosides |
|
25 |
11.588 |
4.16 |
Beta‐D‐GIucopyranoside, methyl |
C7H14O6 |
Glycosides |
|
26 |
11.756 |
1 |
Methyl (methyl 2,4‐di‐O‐acetyl‐3‐O‐methyl‐alpha‐D‐galactoside uronate |
– |
Glycosides |
|
27 |
11.895 |
0.2 |
Silane, [[(3beta,5alpha,11beta,20 R)‐pregnane‐3,11,20,21‐ tetrayl]tetrakis(oxy)]tetrakis[trimethyl |
C33H68O4Si4 |
Organosilanes |
|
28 |
12.026 |
1.74 |
Alpha‐Methyl‐l‐sorboside |
C7H14O6 |
Sugar alcohols |
|
29 |
12.2 |
0.45 |
9‐methylheptadecane |
C18H38 |
Alkanes |
|
30 |
12.345 |
0.12 |
Tridecane, 4‐cyclohexyl‐ |
C19H38 |
Alkanes |
|
31 |
12.434 |
0.2 |
1‐Ethynyl‐3,5‐dimethyIadamantane |
C14H20 |
Adamantanes |
|
32 |
12.501 |
0.1 |
Octadecane, 3‐methyl‐ |
C19H40 |
Alkanes |
|
33 |
12.596 |
0.04 |
1‐Tetradecanol |
C14H30O |
Alcohols |
|
34 |
12.836 |
0.07 |
Heptadecane |
C17H36 |
Alkanes |
|
35 |
13.084 |
0.11 |
Ethanol, 2‐(dodecyloxy)‐ |
C14H30O2 |
Alkyl ethers |
|
36 |
13.369 |
0.07 |
Eicosane |
C20H42 |
Alkanes |
|
37 |
13.472 |
0.15 |
(E)‐4‐(3‐Hydroxyprop‐1‐en‐1‐yl)‐2‐methoxyphenol |
C10H12O3 |
Phenolic compounds |
|
38 |
13.56 |
0.06 |
Heptadecane, 4‐methyl‐ |
C18H38 |
Alkane |
|
39 |
13.675 |
0.15 |
Dodecanamide |
C12H25NO |
Fatty amide of Lauric acid |
|
40 |
13.994 |
0.1 |
Loliolide |
C11H16O3 |
Benzofuran |
|
41 |
14.635 |
0.28 |
Neophytadiene |
C20H38 |
Diterpenes |
|
42 |
14.718 |
0.12 |
1 ‐(2‐Propen‐1‐yloxy) dodecane |
C15H28O |
Alkyl ether |
|
43 |
15.262 |
0.13 |
3,7,11,15‐Tetramethyl‐2‐hexadecen‐1‐ol |
C20H40O |
Phytol |
|
44 |
15.586 |
0.05 |
Isopropyl tetradecyl ether |
C17H36O |
Alkyl ether |
|
45 |
15.642 |
0.02 |
Butanoic acid |
C10H16F3NO3 |
Fatty acid |
|
46 |
15.933 |
0.87 |
Hexadecanoic acid, methyl ester |
C17H34O2 |
Fatty acid ester |
|
47 |
16.435 |
0.51 |
n‐Hexadecanoic acid |
C16H32O2 |
Fatty acid |
|
48 |
16.666 |
0.85 |
Tetradecanamide |
C16H34O3 |
Fatty amide of myristic acid |
|
49 |
17.035 |
0.12 |
Diethylene glycol monododecyl ether |
C19H34O2 |
Fatty acid ester |
|
50 |
18.646 |
0.19 |
9,12‐Octadecadienoic acid, methyl ester |
C19H34O2 |
Fatty acid ester |
|
51 |
18.75 |
0.79 |
8,11,14‐Docosatrienoic acid, methyl ester |
C23H40O2 |
Fatty acid ester |
|
52 |
18.901 |
2.22 |
Phytol |
C20H40O |
Diterpene alcohol |
|
53 |
19.172 |
0.17 |
Methyl stearate |
C19H38O2 |
Fatty acid methyl ester |
|
54 |
19.357 |
0.19 |
7‐Hexadecenal, (Z)‐ |
C16H30O |
Fatty aldehyde |
|
55 |
20.061 |
6.53 |
Hexadecanamide |
C16H33NO |
Fatty amide |
|
56 |
20.275 |
0.13 |
Heptasiloxane, hexadecamethyl‐ |
C16H48O6Si7 |
Fatty acid methyl ester |
|
57 |
20.715 |
1.92 |
Silicic acid |
C10H28O4Si3 |
Silicon oxoacid |
|
58 |
22.525 |
0.18 |
Methyl myristoleate |
C15H28O2 |
Fatty acid methyl ester |
|
59 |
23.008 |
4.03 |
6,9–0ctadecadienoic acid, methyl ester |
C19H34O2 |
Fatty acid ester |
|
60 |
23.155 |
49.25 |
cis‐11‐Eicosenamide |
C20H39NO |
Fatty amide |
|
61 |
23.517 |
5.03 |
Octadecanamide |
C18H37NO |
Fatty amide of stearic acid |
|
62 |
23.821 |
0.09 |
2‐Methylhexacosane |
C27H56 |
Alkane |
|
63 |
24.5.11 |
0.05 |
Cyclobarbital |
C12H16N2O3 |
Barbiturates |
|
64 |
24.895 |
0.1 |
4‐Hydroxy‐4‐methyIhex‐5‐enoic acid, tert.‐butyl ester |
C11H20O3 |
Fatty acid ester |
|
65 |
25.019 |
0.04 |
2,2‐DimethyI‐6‐methylene‐1‐[3,5‐dihydroxy‐1‐pentenyllcyclohexan‐1‐ perhydrol |
C14H24O4 |
– |
|
66 |
25.065 |
0.05 |
Bis(3,7‐dimethyIoct‐6‐enyI) phthalate |
C28H42O4 |
Phthalic acid ester |
|
67 |
25.312 |
0.03 |
Pregn‐4‐ene‐3,11,20‐trione |
C32H58N2O6Si3 |
Pregnane derivative |
|
68 |
25.435 |
0.19 |
11‐Methyltricosane |
C24H50 |
Alkane |
|
69 |
25.539 |
0.49 |
Hexadecanoic acid, 2‐hydroxy‐l ‐(hydroxymethyl)ethyl ester |
C19H38O4 |
Fatty acid ethyl ester |
|
70 |
25.693 |
0.07 |
Undec‐10‐ynoic acid, octadecyl ester |
C29H54O2 |
Fatty acid |
|
71 |
25.834 |
2.84 |
Phthalic acid, di(2‐propyIpentyl) ester |
C26H26O4 |
Phthalate ester |
|
72 |
26.72 |
0.04 |
Nonadecyl heptafluorobutyrate |
C23H39F7O2 |
Fatty acid ester |
|
73 |
26.85 |
0.12 |
18,19‐Secoyohimban‐19‐oic acid, 16,17,20,21‐tetradehydro‐16(hydromethyl)‐, methyl ester, (15. beta.,16 E) ‐ |
C21H28N2O2 |
Secoyohimban alkaloid |
|
74 |
26.984 |
0.14 |
Tetrapentacontane, 1,54‐dibromo‐ |
C54H108Br2 |
Dibromoalkane |
|
75 |
30.116 |
0.87 |
Squalene |
C30H50 |
Triterpene |
|
76 |
33.797 |
0.12 |
2‐ButenaI,2‐methyl‐4‐(2,6,6‐trimethyl‐1‐cyclohexen‐1‐yl)‐ |
C14H22O |
Aldehyde |
|
77 |
34.351 |
0.14 |
4‐Pyridinecarbaldehyde 4‐methyl‐3‐thiosemicarbazone |
C8H10N4S |
Organo thiocompound |
|
78 |
34.643 |
0.24 |
Vitamin E |
C29H50O2 |
Tocopherol |
|
79 |
36.38 |
0.44 |
Campesterol |
C28H48O |
Phytosterol |
|
80 |
38.066 |
2.84 |
Gamma‐Sitosterol |
C29H50O |
Phytosterol |
Table 3.
Bioactivity of the identified phytoconstituents studied previously.
|
Sl. No. |
Compound Name |
Bioactivity |
References |
|---|---|---|---|
|
1 |
1‐Gala‐l‐ido‐octose |
Memory drug production to prevent dementia |
[36] |
|
2 |
Succinic acid, 3‐methoxybenzyl nonyl ester |
Potent antioxidant |
[37] |
|
3 |
Furfural |
Antiviral, antioxidant, anti‐tumor, anti‐histaminic, and fungicides |
[38] |
|
4 |
3,3‐Dimethoxy‐2‐butanone |
– |
– |
|
5 |
Silane, methyldiethoxymethoxy‐ |
– |
– |
|
6 |
1,3‐Dioxolane‐4‐methanol, 2‐ethyl‐ |
Potential antibacterial and antifungal activity |
[39] |
|
7 |
1,1,3,3‐Tetramethyl‐3‐(1‐methylpropoxy) disiloxan‐l ‐ol |
– |
– |
|
8 |
6‐Oxa‐bicyclo [3. I .0] hexan‐3‐one |
– |
– |
|
9 |
Arsenous acid, tris(trimethylsilyl) ester |
Anti‐rheumatic activity and treat skin infections |
[40] |
|
10 |
D‐Limonene |
Antimicrobial |
[41] |
|
11 |
Pantolactone |
– |
– |
|
12 |
7–0cten‐2‐ol, 2,6‐dimethyl‐ |
– |
– |
|
13 |
Alpha‐Terpineol |
Antioxidant, anti‐inflammatory, antimicrobial, anticancer, analgesic, gastroprotective, cardioprotective, neuroprotective, and antidiarrheal |
[42] |
|
14 |
Acetic acid, l,7,7‐trimethyl‐bicyclo [2.2]hept‐2‐yl ester |
– |
– |
|
15 |
2‐Methoxy‐4‐vinylphenol |
Anti‐bacterial |
[43] |
|
16 |
2‐(4′‐Methoxyphenyl)‐2‐(2′‐methoxyphenyl) propane |
– |
– |
|
17 |
2‐(Isobutoxymethyl)oxirane |
– |
– |
|
18 |
Dodecane, 2,6,11‐trimethyl‐ |
Antimicrobial |
[44] |
|
19 |
Tetradecane, 3‐methyl‐ |
Potential antimicrobial activity |
[45] |
|
20 |
1 ‐Undecanol |
– |
– |
|
21 |
Octadecane, 3‐ethyl‐5‐(2‐ethylbutyl)‐ |
– |
– |
|
22 |
Beta‐D‐Glucopyranose, 1,6‐anhydro‐ |
– |
– |
|
23 |
Pentadecane, 7‐methyl‐ |
Antioxidant and antimicrobial |
[46] |
|
24 |
Alpha‐D‐Glucopyranoside, methyl |
Antimicrobial |
[47] |
|
25 |
Beta‐D‐GIucopyranoside, methyl |
– |
– |
|
26 |
Methyl (methyl 2,4‐di‐O acetyI‐3‐O‐methyl‐alpha‐ D‐galactoside uronate |
Antimicrobial |
[47] |
|
27 |
Silane, [[(3.beta,5.alpha, 11.beta 20 R)‐ prenane3,11,20,21tetrayl)[tetrakis(oxy)[tetrakis trimethyl‐ |
– |
– |
|
28 |
Alpha‐Methyl‐l‐sorboside |
Antioxidant |
[48] |
|
29 |
9‐methylheptadecane |
– |
– |
|
30 |
Tridecane, 4‐cyclohexyl‐ |
– |
– |
|
31 |
1‐Ethynyl‐3,5‐dimethyIadamantane |
Treat Alzheimer's disease |
[49] |
|
32 |
Octadecane, 3‐methyl‐ |
– |
– |
|
33 |
1‐Tetradecanol |
– |
– |
|
34 |
Heptadecane |
Antifungal and antimicrobial |
[50] |
|
35 |
Ethanol, 2‐(dodecyloxy)‐ |
Local anesthetic and sclerosing agent |
[51] |
|
36 |
Eicosane |
Antifungal activity |
[52] |
|
37 |
(E)‐4‐(3‐Hydroxyprop‐1‐en‐1‐yl)‐2‐methoxyphenol |
Antifungal activity |
[53] |
|
38 |
Heptadecane, 4‐methyl‐ |
Antioxidant and antimicrobial |
[46] |
|
39 |
Dodecanamide |
Anti‐inflammatory and antimicrobial |
[54,55] |
|
40 |
Loliolide |
Neuroprotective, antiapoptotic, anti‐inflammatory, antiaging, antidiabetic, antioxidant, antidiarrheal, and anthelmintic |
[56–59] |
|
41 |
Neophytadiene |
anti‐inflammatory, anti‐microbial, antioxidant, antipyretic, and anticonvulsant |
[60,61] |
|
42 |
1 ‐(2‐Propen‐1‐yloxy) dodecane |
– |
– |
|
43 |
3,7,11,15‐Tetramethyl‐2‐hexadecen‐1 ‐ol |
Anti‐inflammatory, antibacterial, antioxidant, antitumor, and potent antidiabetic (Inhibit alpha‐amylase and alpha‐glucosidase) |
[62,63] |
|
44 |
Isopropyl tetradecyl ether |
– |
– |
|
45 |
Butanoic acid |
Antithyroid, vasoconstrictor, anticancer, anti‐inflammatory, gut protective, potential antidiabetic, treat mental health problem (schizophrenia) |
[64,65] |
|
46 |
Hexadecanoic acid, methyl ester |
Antioxidant, anticancer, antidiabetic, nephroprotective, anti‐inflammatory, and antibacterial |
[66–70] |
|
47 |
n‐Hexadecanoic acid |
Anti‐inflammatory antimicrobial, antioxidant, antiatherosclerotic antiandrogenic anticancer Antitumor Hypocholesterolemic |
[71–73] |
|
48 |
Tetradecanamide |
– |
– |
|
49 |
Diethylene glycol monododecyl ether |
– |
– |
|
50 |
9,12‐Octadecadienoic acid, methyl ester |
Antioxidant, antimicrobial, and anti‐inflammatory |
[74] |
|
51 |
8,11,14‐Docosatrienoic acid, methyl ester |
– |
– |
|
52 |
Phytol |
Antioxidant, antidiabetic, anticancer, anti‐inflammatory, diuretic, antitumor, genotoxic, chemoprotective, antimicrobial, antiprotozoal, histamine release inhibitor, and antimicrobial |
[75,76] |
|
53 |
Methyl stearate |
Anti‐inflammatory, antidiarrheal, cytotoxic, antiproliferative, and antioxidant |
[77–79] |
|
54 |
7‐Hexadecenal, (Z)‐ |
Antiviral, and antimicrobial |
[80,81] |
|
55 |
Hexadecanamide |
Anti‐inflammatory, anticancer, antitumor, antimicrobial, antioxidant, antiatherosclerotic antiandrogenic, and hypocholesterolemia |
[82] |
|
56 |
Silicic acid |
Skin disorder, bone health, atherosclerosis, Alzheimer's disease, and immune system enhancement |
[83] |
|
57 |
Heptasiloxane, hexadecamethyl‐ |
Antioxidant, antibacterial, anticancer, and antifungal |
[84] |
|
58 |
Methyl myristoleate |
– |
– |
|
59 |
6,9–0ctadecadienoic acid, methyl ester |
– |
– |
|
60 |
cis‐11‐Eicosenamide |
Antimicrobial |
[85] |
|
61 |
Octadecanamide |
Hypolipidemic |
[86,87] |
|
62 |
2‐Methylhexacosane |
Anticancer and antidiabetic (Alpha‐glucosidase inhibitor) |
[88] |
|
63 |
Cyclobarbital |
Anesthetic general, testosterone 17beta‐dehydrogenase (NADP+) inhibitor, neurotransmitter antagonist, anticonvulsant, skeletal muscle relaxant, and antiproliferative |
[89] |
|
64 |
4‐Hydroxy‐4‐methyIhex‐5‐enoic acid, tert.‐butyl ester |
Antimicrobial, anticancer, and Antioxidant |
[88,90] |
|
65 |
2,2‐DimethyI‐6‐methylene‐1‐[3,5‐dihydroxy‐1‐ pentenyllcyclohexan‐1‐ perhydrol |
– |
– |
|
66 |
Bis(3,7‐dimethyIoct‐6‐enyI) phthalate |
– |
– |
|
67 |
Pregn‐4‐ene‐3,11,20‐trione |
Hormone biosynthesis (aldosterone) |
[91] |
|
68 |
11‐Methyltricosane |
Antioxidant |
[73] |
|
69 |
Hexadecanoic acid, 2‐hydroxy‐l ‐(hydroxymethyl)ethyl ester |
Hemolytic, antioxidant hypocholesterolemia, pesticidal, and nematicidal |
[73,92] |
|
70 |
Undec‐10‐ynoic acid, octadecyl ester |
Inhibitor of cytochrome P450 4 A1, antioxidant, antifungal, and wound healing activity |
[93] |
|
71 |
Phthalic acid, di(2‐propyIpentyl) ester |
Antimicrobial and anticancer |
[94,95] |
|
72 |
Nonadecyl heptafluorobutyrate |
– |
– |
|
73 |
18,19‐Secoyohimban‐19‐oic acid, 16,17,20,21‐ tetradehydro‐16(hydromethyl)‐, methyl ester, (15. beta.,16 E) ‐ |
– |
– |
|
74 |
Tetrapentacontane, 1,54‐dibromo‐ |
Hypolipidemic and antioxidant |
[73] |
|
75 |
Squalene |
Antioxidant, antitumor, and antidiabetic |
[96,97] |
|
76 |
2‐ButenaI,2‐methyl‐4‐(2,6,6‐trimethyl‐1‐ cyclohexen‐1‐yl)‐ |
– |
– |
|
77 |
4‐Pyridinecarbaldehyde 4‐methyl‐3‐ thiosemicarbazone |
Anticancer, metal‐chelating, and anti‐prolative |
[98] |
|
78 |
Vitamin E |
Antioxidant, anticancer, antidiabetic, immunomodulator, and anticoagulant |
[99,100] |
|
79 |
Campesterol |
Antidiabetic, anticancer, and antitumor |
[101,102] |
|
80 |
Gamma‐Sitosterol |
Antidiabetic (Increase insulin secretion and inhibit glucogenesis) |
[73,103] |
3.3. DPPH Free Radical Scavenging Activity of L. Thorelli Extract (LTE)
The antioxidant capacity of LTE was evaluated using DPPH free radical scavenging assay at various concentrations ranging from 500 μg/mL to 31.25 μg/mL, with ascorbic acid (AA) as the standard acid (Table 4). The findings showed an increase in the percentage of inhibition with the increasing concentration of L. thorelli extract and the standard ascorbic acid, suggesting a concentration‐dependent antioxidant activity. At lower concentrations (between 31.25 μg/mL to 125 μg/mL), the antioxidant activity of the LTE was almost identical to that of ascorbic acid, the standard. The percentage inhibition of LTE showed a significant increase as the concentration rose to 500 μg/mL, compared to the value observed at 31.25 μg/mL. The half‐maximal inhibitory concentration (IC50) for LTE was 83.05 μg/mL, while for AA it was 47.55 μg/mL, indicating that LTE requires a comparatively greater concentration to inhibit at the same level as ascorbic acid.
Table 4.
DPPH free radical scavenging activity of L. thorelli extract (LTE).
|
Conc. (μg/mL) |
% of Inhibition by Ascorbic acid (AA) (Mean±SD) |
% of Inhibition by LTE (Mean±SD) |
IC50 (μg/mL) |
|---|---|---|---|
|
500 |
96.62±1.022 |
88.44±2.041 |
LTE=83.05 AA=47.55 |
|
250 |
93.90±0.892 |
78.49±1.205 |
|
|
125 |
90.21±0.621 |
68.21±0.854 |
|
|
62.5 |
75.28±0754 |
66.29±0.251 |
|
|
31.25 |
57.95±0.551 |
59.06±1.015 |
The data was presented as the mean value of duplicate of the triplicate experiments with standard deviation.
3.4. Hydrogen Peroxide (H2O2) Scavenging Activity of L. Thorelli Leaf Extract
The hydrogen peroxide (H2O2) scavenging activity of the LTE is displayed in Table 5. The antioxidant activity of the LTE was assessed at various doses (31.25 to 500 μg/mL), using ascorbic acid (AA) as the standard. An increasing percentage saw a concentration‐dependent antioxidant effect of inhibition of the sample extract at higher concentrations. The sample extract of LTE showed a strong inhibition of 120.07 % at the maximum concentration of 500 μg/mL, whereas the standard, AA, showed a little higher inhibition of 142.967 %. The LTE's antioxidant activity consistently closely resembled that of AA at lower doses (between 62.5 to 250 μg/mL), indicating similar efficacy in scavenging free radicals at these concentrations. The sample's efficacy was similar to the standard, as evidenced by the close IC50 values of 2.84 for AA (standard) and 2.22 for the LTE (sample).
Table 5.
Hydrogen peroxide (H2O2) scavenging activity of L. thorelli extract (LTE).
|
Sample Concentration (μg/mL) |
% of inhibition by AA (Mean±SD) |
% of inhibition by LTE (Mean±SD) |
IC50 value (μg/mL) |
|---|---|---|---|
|
500 |
142.967±0.019 |
120.07±0.032 |
LTE=2.22 AA=2.84 |
|
250 |
85.82±0.019 |
88.96±0.032 |
|
|
125 |
74.57±0.018 |
78.82±0.070 |
|
|
62.5 |
60.59±0.078 |
69.2±0.077 |
|
|
31.25 |
33.03±0.019 |
61.9±0.102 |
The data was presented as the mean value of duplicate of the triplicate experiments with standard deviation.
3.5. Total Phenolic Content (TPC), Total Flavonoid Content (TFC), and Total Antioxidant Capacity (TAC) of L. Thorelli Leaf Extract
The TPC, TFC, and TAC of L. thorelli leaf extract were assessed. The data obtained as the average of three duplicate experiments are summarized in Table 6.
Table 6.
Total phenolic content, total flavonoid content, and total antioxidant capacity of L. thorelli leaf extract.
|
Concentration (μg/mL) |
TPC present in sample (mg) of Gallic acid per gram of dried extract (Mean±SD) |
TFC present in Sample (mg) of quercetin per gram of dried extract (Mean±SD) |
TAC present (mg) of ascorbic acid per gram of dry extract in the sample |
|---|---|---|---|
|
1200 |
750±4.138 |
893±0.221 |
97.916±0.003 |
|
800 |
650±1.617 |
752±0.127 |
50.583±0.002 |
|
400 |
470±0.087 |
575±0.144 |
22.916±0.014 |
|
200 |
210±0.399 |
273±0.142 |
13.08±0.003 |
|
100 |
157±0.076 |
125±0.175 |
5.916±0.009 |
The data was presented as the mean value of duplicate of the triplicate experiments with standard deviation.
The total phenolic content (TPC) of the LTE at concentrations ranging from 100–1200 μg/mL was determined through absorbance measurements of the standard gallic acid and the sample extract (Table 6). The TPC present in the sample was expressed in milligrams of gallic acid equivalent per gram of dried extract. At the maximum concentration of 1200 μg/mL, the absorbance of the L. thorelli leaf extract sample's estimated TPC was 750±4.138 mg/g of dry extract. The data pattern suggested that the TPC in the sample reduced as the extract concentration decreased.
The total flavonoid content (TFC), calculated through absorbance, given in mg of quercetin equivalent per gram of dry extract, is shown in Table 6 for LTE at various doses ranging from 100 to 1200 μg/mL. The trend indicated TFC of the leaf extract of L. thorelli varied with concentration. The TFC falls consistently (from 893±0.221 to 125±0.175) as the concentration of the L. thorelli (sample) extract dropped (from 1200 to 100), implying a decline in flavonoid content as concentration was lowered.
The total antioxidant capacity (TAC) of the extract at different concentrations (100–1200 սg/mL) is shown in Table 6. TAC values were expressed in mg of ascorbic acid equivalent per gram of dry extract in the sample. As the concentration increased from 100–1200 μg/mL, the TAC in the sample increased from 5.916±0.009 to 97.916±0.003 mg, showing an increase in TAC as the concentration increased.
Overall, the data trend implied a concentration‐dependent variation, the higher the extract concentration, the greater the phenolic and flavonoid content, as well as enhanced antioxidant activity.
3.6. Percentage Inhibition of α‐Amylase by Standard (glimepiride) and Sample (LT)
The % inhibition of α‐amylase by the standard (glimepiride) and sample (LT) at different concentrations (125–1000 μg/mL) is given in Table 7. The data suggested that the α‐amylase activity was effectively inhibited by L. thorelli in a concentration‐dependent manner and increased inhibition was observed with increasing concentrations of L. thorelli. It is of note that the inhibitory action of L. thorelli (sample) was close to that of glimepiride (standard), highlighting its potential as an antidiabetic medication.
Table 7.
Percentage inhibition of α‐amylase by standard (glimepiride) and sample (LT).
|
Conc. of Standard (μg/mL) |
% α‐amylase inhibition of glimepiride (mean±SD) |
% α‐amylase inhibition of LT (mean±SD) |
|---|---|---|
|
125 |
84.14±6.109 % |
80.1±10.324 % |
|
250 |
88.49±2.333 % |
84.7±6.951 % |
|
500 |
90.98±2.496 % |
88.5±6.088 % |
|
1000 |
95.36±3.345 % |
93.9±3.429 % |
The data was presented as the mean value of duplicate experiments with standard deviation.
3.7. Cytotoxic Activity of L. Thorelli Leaf Extract Against Cervical Cancer Cells (HeLa)
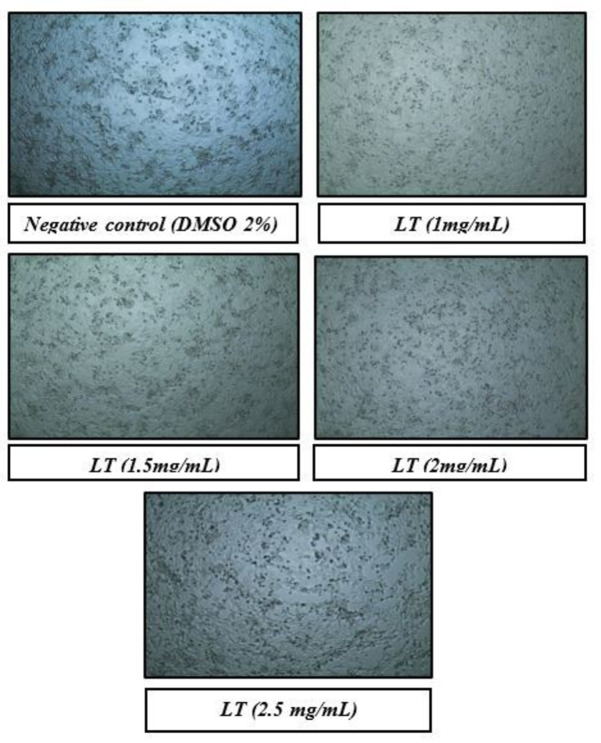
The percentage inhibition of L. thorelli leaf extract was assessed at different concentrations from 1–2.5 mg/mL against healthy monkey kidney cells (Vero) and cervical cancer cells (HeLa). L. thorelli leaf extract was found to possess concentration‐dependent anticancer activity. DMSO 2 % concentration was used as the negative control, showing no impact on cell growth (Figure 1). The inhibition of cell growth of HeLa cells was less at lower concentrations (1 mg/mL, 1.5 mg/mL, and 2 mg/mL) as seen in Figure 2 and Table 8. At maximum concentration, (2.5 mg/mL) of sample (LTE) extract, a notable 75 % inhibition of HeLa cells was recorded while Vero cells exhibited only 38.66 % inhibition, indicating greater cytotoxic potential of L. thorelli at higher concentrations. Furthermore, the IC50 for HeLa cells (IC50 2.36 mg/mL), was nearly half of that for Vero cells (IC50 4.66 mg/mL), illustrating a relatively stronger cytotoxic effect on Hela, cervical cancer cells compared to Vero, healthy monkey kidney cells which indicated less toxicity of L. thorelli extract to normal healthy cells (Figure 2 and Table 8).
Figure 2.
Hella cell survival at all the concentrations of the L. thorelli leaf extract.
Table 8.
Cytotoxic activity of L. thorelli leaf extract against cervical cancer cells (HeLa) and Vero cells.
|
Sample concentration |
% of cell growth inhibition (Vero) Mean±SD |
% of cell growth inhibition (HeLa) Mean±SD |
IC50 (mg/mL) |
|---|---|---|---|
|
2 % DMSO (Negative control) |
0 |
0 |
2.36 mg/mL (HeLa) 4.66 (Vero) |
|
1 mg/mL |
1.22±2.031 |
11.08±3.016 |
|
|
1.5 mg/mL |
3.76±1.640 |
19.8±2.841 |
|
|
2 mg/mL |
5.13±1.241 |
22.66±1.642 |
|
|
2.5 mg/mL |
38.66±3.015 |
75±3.954 |
The data was presented as the mean value of triplicate experiments with standard deviation.
4. Discussions
Different species of the Lagerstroemia genus have been scientifically investigated to determine their biological and therapeutic activities.[ 104 , 105 , 106 , 107 , 108 , 109 ] Among them, the most studied species was Lagerstroemia speciosa (L. spceiosa) and its antioxidant, hypoglycemic, antibacterial, anti‐inflammatory, antidiarrheal, antiobesity, antiviral, antimicrobial, etc. properties have been identified and reported.[ 104 , 108 ] In addition to that, different phytochemicals were isolated and identified from this species. [108] For example, corosolic acid, ursolic acid, amyl alcohol, lageracetal, gallic acid, 4‐hydroxybenzoic acid, beta‐sitosterol, ellagic acid, 3,3,4‐tri‐O‐methyl ellagic acid, 3‐O‐methyl‐3,4‐methylenedioxy ellagic acid, asiatic acid, alphitolic acid, 3,31–di‐O‐methyl ellagic acid, 3,4,3,4‐tetra‐O‐methyl flavellagic acid, 31, 41‐di‐O‐methyl‐3, 4‐methylenedioxy flavellagic acid, 3‐O‐methyl ellagic acid, alanine, alpha amino butyric acid, isoleucine, 6,7‐dihydroxy ellagitannin 7, methionine, neolignan, and coumarin have been identified and characterized from L. speciosa. From Lagerostromia floribunda a wide range of phytochemicals such as ursolic acid, 23‐hydroxy ursolic acid, alphitolic acid, sesamin, dihydro‐β‐cyclopyrethrosin, β‐sitosterol, betulinic acid, Clauslactone‐K, linguee resinol, etc., have been identified.[ 104 , 108 ]
L. thorelli belongs to the Lythraceae family and has remained scientifically unexplored. This study is the first to investigate its antioxidant, antidiabetic, and cytotoxic effects against cervical cancer cells (HeLa) using ethanolic leaf extract. Additionally, we are reporting some compounds that were identified for the first time from the leaf extract of this plant using GC‐MS followed by a preliminary phytochemical analysis of the extract.
Preliminary phytochemical analysis of ethanol leaf extract of L. thorelli revealed the presence of flavonoids, phenols/phenolic compounds, steroids, tannins, glycosides, resins, and carbohydrates. [110] However, alkaloids, phytosterols, and saponins were absent in the extract (Table 1). Phytochemicals have been reported to possess great antioxidant potential and provide beneficial effects on human health. Among phytoconstituents mentioned as health benefits providers are primarily flavonoids, iso‐flavonoids, phytosterols, phytoestrogens, anthocyanidins, terpenoids, etc. [110] Conversely, in a previous study, preliminary phytochemical analysis of ethanol and aqueous extracts of other species L. speciosa, identified the presence of steroids, phenolic compounds, alkaloids, flavonoids, glycosides, terpenoids, saponins, α‐amino acids, carbohydrates, starch, organic acids, reducing sugars and tannins in the samples, while cyanogenic glycosides were not detected. [104]
GC‐MS analysis of the ethanol extract of L. thorelli characterized the individual phytochemicals present in the leaf of this plant. GC‐MS analysis enabled the identification of a significant number of important compounds (80 compounds) with diverse chemical natures, including furfural, D‐limonene, alpha‐terpineol, loliolide, neophytadiene, butanoic acid, silicic acid, β‐D‐glucopyranoside, 4‐pyridinecarbaldehyde 4‐methyl‐3‐thiosemicarbazone, cyclobarbital, pregn‐4‐ene‐3,11,20‐trione, phytol, squalene, vitamin E, campesterol, gamma‐sitosterol, etc. and all of them possess distinct pharmacological properties (Table 3). It is noteworthy to mention that butanoic acid has been reported to possess antidiabetic, anticancer, anti‐inflammatory, antithyroid, gut‐protective properties and β‐D‐glucopyranoside has antioxidant, antidiabetic, anticancer, anti‐inflammatory, and analgesic activities.[ 65 , 111 , 112 ] Moreover, anticancer and antiproliferative effects were reported for alpha‐terpineol, phytol, hexadecanoic acid, methyl ester, n‐hexadecanoic acid, hexadecanamide, vitamin E, campesterol, squalene, cyclobarbital, 4‐Pyridinecarbaldehyde 4‐methyl‐3‐thiosemicarbazone, etc.[ 89 , 98 , 113 ] Silicic acid has the potential to treat skin disorders, atherosclerosis, Alzheimer's disease, enhance the immune system, and maintain bone health. [83] Pregn‐4‐ene‐3,11,20‐trione plays a vital role in aldosterone biosynthesis. [87] In addition to that, a significant number of compounds have been reported previously for their antioxidant activity including furfural, alpha‐terpineol, loliolide, neophytadiene, n‐hexadecanoic acid, hexadecanamide, phytol, squalene, vitamin E, etc. (Table 3). The findings of many studies have demonstrated the potent antidiabetic effect of multiple compounds namely 3,7,11,15‐Tetramethyl‐2‐hexadecen‐1‐ol, butanoic acid, phytol, 2‐methylhexacosane, squalene, vitamin E, campesterol, and gamma‐sitosterol (Table 3).
A literature search on the selected plant divulged no investigations had been carried out on L. thorelli to determine its biological and therapeutic activities. Most importantly, previous studies with other species of Lagerstroemia disclosed their strong antioxidant and antidiabetic properties and moderate cytotoxic activity which formed the basis of this study.[ 104 , 108 ] It is worth mentioning that this is the first study of assessing antioxidant, antidiabetic, and cytotoxic activity of L. thorelli species.
In the current study antioxidant activity was evaluated using five different comprehensive methods: DPPH free radical scavenging test, H2O2 scavenging assay, total antioxidant capacity assessment, and determination of the total phenolic and flavonoid content. Concentration‐dependent strong DPPH free radical scavenging potential of L. thorelli was identified in the current study where the highest DPPH free radical scavenging activity was 88.44±2.041 % at the highest concentration of 500 μg/mL and the IC50 value was 83.05 μg/mL whereas IC50 value of standard ascorbic acid was 47.55 μg/mL. In a previous study, L. speciosa methanol flower extract showed excellent DPPH free radical scavenging activity with an IC50 value of 41.51 μg/mL and the effect was concentration‐dependent. [114] Since the species, plant part, and solvent were different, the antioxidant effect also seemed to be different between the previous study and our study. In another study conducted by Mousa et al. where L. speciosa ethanol leaf extract exhibited 97.71 % DPPH free radical scavenging at the concentration of 100 μg/mL with an ED50 value 10.21±1.33 μg/mL and ED50 value of the standard, ascorbic acid was 1.83±1.41 μg/mL and the antioxidant effect was also concentration‐dependent. [115] In the present study, L. thorelli extract exhibited strong H2O2 scavenging activity with an IC50 value of 2.22 μg/mL, which was lower than the IC50 of standard ascorbic acid (IC50=2.84 μg/mL), and the effect was concentration‐driven. Pareek et al. assessed the H2O2 scavenging activity of L. speciosa hydro‐alcoholic leaf extract and found a very strong H2O2 scavenging effect with an IC50 of 28.00±0.16 μg/mL, which was much lower than ascorbic acid (IC50 =187.33±3.45 μg/mL). [116]
TFC, TPC, and TAC measurement assays with L. thorelli leaf extract displayed a higher quantity of flavonoids (893±0.221 mg quercetin equivalent/g of dry extract) and phenolics (750±4.138 mg gallic acid equivalent/g of dry extract) in the extract at the concentration of 1200 μg/mL, and 97.916±0.003 mg of ascorbic acid equivalent/g of dry extract was the TAC. The findings indicate that the extract is rich in flavonoids and phenolics. A previous study with L. speciosa methanol flower extract also showed that the extract contains a remarkable quantity of phenolics (418.0 mg/g) and flavonoids (50.8 mg/g). [114] A comparison of our study findings with the previous results evident that the leaf extract contained much higher flavonoids and phenolics than the flower extract. Flavonoids are powerful exogenous antioxidants, and they reduce free radicals to generate less reactive oxygen species, and the strong antioxidant potential of flavonoids is associated with their molecular structure, particularly the number and location of hydroxyl groups present in it, resonance, and conjugation effect. [117] Another investigation reported the total phenolic content of aqueous extract of L. speciosa where the TPC was found to be 72.3±0.293 mg gallic acid equivalent/100 mg of dry extract and the phenolic compounds have been reported as powerful natural antioxidants due to their hydroxyl groups, which contribute to free radical scavenging and their ability to donate hydrogen. [107] The overall strong antioxidant effect of the L. thorelli leaf extract was attributed to the presence of a significant number of compounds in this extract particularly‐ furfural, alpha‐terpineol, loliolide, silicic acid, neophytadiene, n‐hexadecanoic acid, hexadecanamide, phytol, squalene, vitamin E, etc (Table 3).
The α‐amylase inhibition assay was conducted to assess the antidiabetic property of L. thorelli ethanol leaf extract, and this is the first report of the antidiabetic effect of this plant. Carbohydrate metabolism has been reported to increase postprandial glucose levels, and one of the approaches to lowering postprandial glucose levels is the inhibition of carbohydrate digestive enzyme activity. [118] α‐amylase is the key enzyme that causes the breakdown of polysaccharides into glucose by catalyzing the hydrolysis of α‐1,4‐glucan linkages existing in starch, maltodextrins, and related carbohydrates.[ 119 , 120 ] Inhibition of this enzyme activity can prevent the conversion of polysaccharides into glucose molecules and thus, can control the glucose level in the body. [119] Rigorous control of postprandial glucose levels by inhibiting α‐amylase activity is crucial in preventing, developing, and treating diabetes. Alpha‐amylase inhibitors inhibit the digestibility and absorption of carbohydrates in the gastrointestinal tract, and thus this enzyme can act as a carbohydrate blocker. [119] This research confirmed that L. thorelli methanol leaf extract possessed strong α‐amylase inhibition activity (93.9±3.429 %) which was very close to the standard drug, glimepiride (95.36±3.345 %). Our result was in congruence with the finding of a previous study where another species called L. speciosa (methanol extract) exhibited very high α‐amylase inhibition activity which was 90.82±2.70 %. However, ethyl acetate or hexane extract of L. speciosa showed low inhibition with values of 54.42±2.36 % and 58.50±11.19 %, respectively. [121] The effect of solvent plays a crucial role as polar protic solvents like methanol, ethanol, etc. have higher extraction yield compared to non‐polar aprotic solvents like ethyl acetate and hexane. [122] Thus, polar protic solvents helped to extract more phytochemicals belonging to diverse classes which showed increased inhibition compared to extract of other solvents. Therefore, the use of ethanol (polar protic solvent) in the experiment ensured a higher yield of extract, causing a strong inhibition of α‐amylase by L. thorelli, making it a potent contributor as an antidiabetic agent. Natural compounds that act as α‐amylase inhibitors include flavonoids, alkaloids, terpenes, iminosugars, and thiosugars. [123] Preliminary phytochemical analysis identified the presence of flavonoids, polyphenols, and carbohydrates that may be responsible for the strong α‐amylase inhibition by LTE. GC‐MS analysis of LTE identified 3,7,11,15‐Tetramethyl‐2‐hexadecen‐1‐ol, butanoic acid, phytol, 2‐methylhexacosane, squalene, vitamin E, campesterol, gamma‐sitosterol, butanoic acid and methyl beta‐D‐glucopyranoside as its individual compounds, and most importantly, these compounds have been reported to possess antidiabetic effects (Table 3). [65] Therefore, the strong α‐amylase inhibition by LTE may be attributed to these compounds. The mechanism of antidiabetic action of 2‐methylhexacosane involved inhibition of alpha‐glucosidase and inhibition of both alpha‐amylase and alpha‐glucosidase resulting in potent hypoglycemic effect of 3,7,11,15‐tetramethyl‐2‐hexadecen‐1‐ol.[ 88 , 124 ] Antidiabetic action of gamma‐sitosterol was attributed to increased insulin secretion and inhibition of gluconeogenesis. [103]
Cervical cancer is one of the significant global public health burdens and the leading cause of morbidity and mortality in women, including in Bangladesh. [125] Since the treatment cost and drug resistance remain a concern in cancer treatment discovery and development of novel drugs are of utmost importance. Plant‐based medicines have huge potential as chemotherapeutics and some plant‐derived drugs serve as mainstream therapy for different cancers, for example, paclitaxel for breast cancer, vinblastine, and vincristine for leukemia, and flavopiridol for colorectal cancer. [126] Since no cytotoxic/anticancer effect studies were conducted previously on L. thorelli species the current study attempted to screen the anticancer potential of L. thorelli against cervical cancer cells (HeLa). The findings of the MTT test against this cell line demonstrated moderate cytotoxicity with an IC50 value of 2.36 mg/mL and 75 % cell growth inhibition was measured at the highest concentration of 2.5 mg/mL. Compared to HeLa cells this extract demonstrated lower cytotoxicity, 38.66 % cell growth inhibition (IC50: 4.66 mg/mL) at the highest concentration of 2.5 mg/mL against healthy monkey kidney cells, Vero. Cytotoxic potentials of ethanolic L. speciosa leaf extract induced G1‐phase of cell cycle arrest and apoptosis in human hepatocellular carcinoma, HepG2 cells. [127] MTT assay of aqueous ethanolic extract of L. speciosa against human lung adenocarcinoma cells (A549) showed a decrease in cell viability of 50.92±0.5 % with an IC50 value of 841.23 μg/mL at the concentration of 1 mg/mL. [115]
5. Conclusions
This study reported bioactivities and characterization of compounds of L. thorelli that have remained unexplored. It primarily focused on the investigations into antioxidant, antidiabetic, and cytotoxic effects of L. thorelli, along with the characterization of its compounds, offering a novel perspective on possible pharmacological interventions. The results demonstrated strong antioxidant, strong antidiabetic, and moderate cytotoxic effects of L. thorelli. Preliminary phytochemical analysis revealed the presence of different phytochemical classes like flavonoids, phenols/phenolics, tannins, resins, and glycosides which are known to possess important health benefits with strong antioxidant, antidiabetic, and cytotoxic properties. Furthermore, GC‐MS analysis of the extract identified 80 compounds and most importantly many of them have been reported previously for their potent antioxidant, antidiabetic, and anticancer effects. Therefore, these compounds may be responsible for the bioactivities detected in this study. The findings from this study indicate that L. thorelli could be a promising starting point for developing new medications targeting diabetes and free‐radical‐induced diseases.
Funding
This research did not receive any financial support.
Declaration of Competing Interests
The authors declare that they have no known competing interests.
Author Contributions
Conceptualization: RA, SHN; Methodology and Experiments: RA, SHN, SH, EST, IKL, AHT, MMRMM, FAR, SS; Writing‐Original draft: RA, LMF; Writing‐review and editing: RA, SH, FAR. All authors revised the manuscript and approved the final submitted version.
Conflict of Interests
The authors declare no conflict of interest.
6.
Akter R., Maknun Fariha L., Halder S., Sharmin S., Sabet Taki E., Kabir Lihu I., Hamja Tipu A., Rubaiyat Muntasir Meem M. M., Alam Ripa F., Sharmin S., Chem. Biodiversity 2024, 21, e202400999. 10.1002/cbdv.202400999
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Sofowora A., Ogunbodede E., Onayade A., Afr. J. Tradit. Complement Altern. Med. 2013, 10, 210–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rajkumar G., Panambara P. A. H. R., Sanmugarajah V., Borneo J. Pharm. 2022, 5, 93–103. [Google Scholar]
- 3. Badraoui R., Siddiqui A. J., Bardakci F., Ben-Nasr H., Ethnobotany and Ethnopharmacology of Medicinal and Aromatic Plants, CRC Press, Boca Raton, 2023, 115–128. [Google Scholar]
- 4.WHO, “Traditional medicine has a long history of contributing to conventional medicine and continues to hold promise,” can be found under https://www.who.int/news-room/feature-stories/detail/traditional-medicine-has-a-long-history-of-contributing-to-conventional-medicine-and-continues-to-hold-promise, 2023.
- 5. Agidew M. G., Bull. Natl. Res. Cent. 2022, 46, 87. [Google Scholar]
- 6. Zhou Z., Ni K., Deng H., Chen X., Adv. Drug. Deliv. Rev. 2020, 158, 73–90. [DOI] [PubMed] [Google Scholar]
- 7. Badraoui R., Zouhaier S., Bouayed A. N., Hakim A., Moncef F., Tarek R., Pestic. Biochem. Physiol. 2007, 88, 149–155. [Google Scholar]
- 8. Lobo V., Patil A., Phatak A., Chandra N., Pharmacogn. Rev. 2010, 4, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rahmouni F., Saoudi M., Amri N., El-Feki A., Rebai T., Badraoui R., Arch. Physio.l Biochem. 2018, 124, 1–9. [DOI] [PubMed] [Google Scholar]
- 10. Schieber M., Chandel N. S., Curr. Biol. 2014, 24, R453–R462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krishnaiah D., Sarbatly R., Nithyanandam R., Food Bioprod. Process. 2011, 89, 217–233. [Google Scholar]
- 12. Akter R., Khan S. S., Kabir Md. T., Halder S., Saudi J. Biol. Sci. 2022, 29, 103287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO, “Cervical Cancer,” can be found under https://www.who.int/news-room/fact-sheets/detail/cervical-cancer, 2022.
- 14. Siegel R. L., Miller K. D., Jemal A., CA. Cancer. J. Clin. 2017, 67, 7–30. [DOI] [PubMed] [Google Scholar]
- 15. Florea A.-M., Büsselberg D., Cancers. (Basel) 2011, 3, 1351–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Silva V. A. O., Alves A. L. V., Rosa M. N., Silva L. R. V., Melendez M. E., Cury F. P., Gomes I. N. F., Tansini A., Longato G. B., Martinho O., Oliveira B. G., Pinto F. E., Romão W., Ribeiro R. I. M. A., Reis R. M., Invest. New. Drugs 2019, 37, 602–615. [DOI] [PubMed] [Google Scholar]
- 17.WHO, “Diabetes,” can be found under https://www.who.int/health-topics/diabetes#tab=tab_1, 2023.
- 18. Fabio G., Romanucci V., Marino C., Pisanti A., Zarrelli A., Curr. Pharm. Biotechnol 2015, 16, 506–516. [DOI] [PubMed] [Google Scholar]
- 19. Kooti W., Farokhipour M., Asadzadeh Z., Ashtary-Larky D., Asadi-Samani M., Electron Physician 2016, 8, 1832–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Atanasov A. G., Waltenberger B., Pferschy-Wenzig E.-M., Linder T., Wawrosch C., Uhrin P., Temml V., Wang L., Schwaiger S., Heiss E. H., Rollinger J. M., Schuster D., Breuss J. M., Bochkov V., Mihovilovic M. D., Kopp B., Bauer R., Dirsch V. M., Stuppner H., Biotechnol Adv. 2015, 33, 1582–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Salleh N. H., Zulkipli I. N., Mohd Yasin H., Ja'afar F., Ahmad N., Wan Ahmad W. A. N., Ahmad S. R., Evidence-Based Complementary Altern. Med. 2021, 2021, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.“Bangladesh Forest Department,” can be found under http://bfis.bforest.gov.bd/nef/index.php/data/dataSpecies/50, 2023.
- 23.“International Plant Names Index and World Checklist of Vascular Plants,” can be found under https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:553625–1, 2023.
- 24.W. Phrompittayarat, W. Putalun, K. Jetiyanon, K. Ingkaninan, Comparison of Various Extraction Method of Bacopa Monnieri, 2007.
- 25. ALILOU H., AKSSIRA M., Saudi J. Biol. Sci. 2021, 28, 6756–6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rahman Md. M., Islam Md. B., Biswas M., Khurshid Alam A. H. M., BMC Res. Notes 2015, 8, 621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Salar R. K., Sharma P., Purewal S. S., TANG [HUMANITAS MEDICINE] 2015, 5, 14.1–14.6. [Google Scholar]
- 28. Nabavi S. M., Ebrahimzadeh M. A., Nabavi S. F., Hamidinia A., Bekhradnia A. R., Pharmacologyonline 2008, 2, 560–567. [Google Scholar]
- 29. Ainsworth E. A., Gillespie K. M., Nat. Protoc. 2007, 2, 875–877. [DOI] [PubMed] [Google Scholar]
- 30. Lamuela-Raventós R. M., Measurement of Antioxidant Activity & Capacity, John Wiley & Sons, Ltd, Chichester, UK, 2017, 107–115. [Google Scholar]
- 31. Haida Z., Hakiman M., Food Sci. Nutr. 2019, 7, 1555–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pisoschi A. M., Negulescu G. P., Biochem. Anal. Biochem. 2012, 01, DOI 10.4172/2161-1009.1000106. [DOI] [Google Scholar]
- 33. Kifle Z. D., Enyew E. F., J Evid Based Integr. Med. 2020, 25, 2515690X2093582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Akter R., Uddin S. J., Grice I. D., Tiralongo E., J. Nat. Med. 2014, 68, 246–252. [DOI] [PubMed] [Google Scholar]
- 35. Uddin S. J., Shilpi J. A., Alam S. M. S., Alamgir M., Rahman M. T., Sarker S. D., J. Ethnopharmacol 2005, 101, 139–143. [DOI] [PubMed] [Google Scholar]
- 36. Krishnappa S., Karthik Y., Pratap G. K., Shantaram M., Umarajashekhar A., Soumya J., Bhatt B., Sayed S. M., Alhelaify S. S., Aharthy O. M., Mushtaq M., 3 Biotech 2024, 14, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Karthik Y., Ishwara Kalyani M., Krishnappa S., Devappa R., Anjali Goud C., Ramakrishna K., Wani M. A., Alkafafy M., Hussen Abduljabbar M., Alswat A. S., Sayed S. M., Mushtaq M., Front. Microbiol. 2023, 14, DOI 10.3389/fmicb.2023.1096826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Donlawson C., Nweneka D. O., Orie K. J., Okah R., Am. J. Analyt. Chem. 2020, 11, 280–288. [Google Scholar]
- 39. Küçük H. B., Yusufoğlu A., Mataracı E., Döşler S., Molecules 2011, 16, 6806–6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Manikandan G., Pandiselvi P., Sobana N., Murugan M., Int. Res. J. Pharm. 2019, 10, 135–140. [Google Scholar]
- 41. Lin H., Li Z., Sun Y., Zhang Y., Wang S., Zhang Q., Cai T., Xiang W., Zeng C., Tang J., Appl. Sci. 2024, 14, 4605. [Google Scholar]
- 42. Chen Y., Zhang L.-L., Wang W., Wang G., J. Essent. Oil Res. 2023, 35, 274–288. [Google Scholar]
- 43. Rubab M., Chelliah R., Saravanakumar K., Barathikannan K., Wei S., Kim J.-R., Yoo D., Wang M.-H., Oh D.-H., Foods 2020, 9, 568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Harikrishnan S., Parivallal M., Alsalhi M. S., Sudarshan S., Jayaraman N., Devanesan S., Rajasekar A., Jayalakshmi S., J. Infect. Public. Health. 2021, 14, 1903–1910. [DOI] [PubMed] [Google Scholar]
- 45. Faridha Begum I., Mohankumar R., Jeevan M., Ramani K., Indian J Microbiol 2016, 56, 426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ashraf I., Zubair M., Rizwan K., Rasool N., Jamil M., Khan S. A., Tareen R. B., Ahmad V. U., Mahmood A., Riaz M., Zia-Ul-Haq M., Jaafar H. Z., Chem. Cent. J. 2018, 12, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Islam S., Hosen M. A., Ahmad S., ul Qamar M. T., Dey S., Hasan I., Fujii Y., Ozeki Y., Kawsar S. M. A., J. Mol. Struct. 2022, 1260, 132761. [Google Scholar]
- 48. Qadir A., Aqil M., Ali A., Farhan, Ahmad J., Ahmad S., Arif M., Khan N., Turk. J. Chem. 2020, 44, 352–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Robinson D. M., Keating G. M., Drugs 2006, 66, 1515–1534. [DOI] [PubMed] [Google Scholar]
- 50. Ahmed S. N., Kalaivani P., Amudha P., Usharani B., Res. J. Pharm. Technol. 2021, 14, 6511–6515. [Google Scholar]
- 51.“Ethanol-2-dodecyloxy | Drug Information, Uses, Side Effects, Chemistry,” can be found under https://www.pharmacompass.com/chemistry-chemical-name/ethanol-2-dodecyloxy, n.d.
- 52. Bhat M. P., Kumar R. S., Chakraborty B., Nagaraja S. K., Gireesh Babu K., Nayaka S., Environ. Res. 2024, 251, 118666. [DOI] [PubMed] [Google Scholar]
- 53.“Coniferyl Alcohol | Antifungal | Endogenous Metabolite,” can be found under https://www.targetmol.com/compound/Coniferyl%20Alcohol-, n.d.
- 54.“N,N-Bis(2-hydroxyethyl)dodecanamide,” can be found under https://www.biosynth.com/p/FB144319/120-40-1-nn-bis2-hydroxyethyldodecanamide#:~:text=N%2CN%2DBis(2%2Dhydroxyethyl)dodecanamide%20also,action%20of%20angiotensin%20II%20receptors, n.d.
- 55. Baig T. A., Haniffa H. M., Siddiqui H., Shah S. F., Jabeen A., Inflammopharmacology 2023, 31, 3303–3316. [DOI] [PubMed] [Google Scholar]
- 56. Silva J., Alves C., Martins A., Susano P., Simões M., Guedes M., Rehfeldt S., Pinteus S., Gaspar H., Rodrigues A., Goettert M. I., Alfonso A., Pedrosa R., Int. J. Mol. Sci. 2021, 22, DOI 10.3390/ijms22041888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Park S. H., Kim D. S., Kim S., Lorz L. R., Choi E., Lim H. Y., Hossain M. A., Jang S., Choi Y. I., Park K. J., Yoon K., Kim J.-H., Cho J. Y., Int. J. Mol. Sci. 2019, 20, 651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hunyadi A., Veres K., Danko B., Kele Z., Weber E., Hetenyi A., Zupko I., Hsieh T., Phytother. Res. 2013, 27, 847–851. [DOI] [PubMed] [Google Scholar]
- 59. Han E.-J., Fernando I. P. S., Kim H.-S., Lee D.-S., Kim A., Je J.-G., Seo M.-J., Jee Y.-H., Jeon Y.-J., Kim S.-Y., Ahn G., Antioxidants (Basel) 2021, 10, 856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bhardwaj M., Sali V. K., Mani S., Vasanthi H. R., Inflammation. 2020, 43, 937–950. [DOI] [PubMed] [Google Scholar]
- 61. Gonzalez-Rivera M. L., Barragan-Galvez J. C., Gasca-Martínez D., Hidalgo-Figueroa S., Isiordia-Espinoza M., Alonso-Castro A. J., Molecules 2023, 28, DOI 10.3390/molecules28083457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Palaniyappan S., Sridhar A., Kari Z. A., Téllez-Isaías G., Ramasamy T., Mar Drugs 2023, 21, 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yin G., Zeng H., He M., Wang M., Int. J. Mol. Sci. 2009, 10, 4330–4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gerunova L. K., Gerunov T. V., P'yanova L. G., Lavrenov A. V., Sedanova A. V., Delyagina M. S., Fedorov Y. N., Kornienko N. V., Kryuchek Y. O., Tarasenko A. A., J. Vet. Sci. 2024, 25, e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mayorga-Ramos A., Barba-Ostria C., Simancas-Racines D., Guamán L. P., Front. Nutr. 2022, 9, 1067647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cagnini C. Z., Dias A. B., Vilas Boas M. R., Batista F. P. R., Faria M. G. I., Glamočlija J., Soković M., Tešević V., de S. Ferreira E., Colauto N. B., Linde G. A., Gazim Z. C., Nat. Prod. Res. 2022, 36, 4781–4787. [DOI] [PubMed] [Google Scholar]
- 67. Hasan Md. M., Tasmin Most. S., El-Shehawi A. M., Elseehy M. M., Reza Md. A., Haque A., BMC Complement. Med. Ther. 2021, 21, 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Aparna V., Dileep K. V., Mandal P. K., Karthe P., Sadasivan C., Haridas M., Chem. Biol. Drug Des. 2012, 80, 434–439. [DOI] [PubMed] [Google Scholar]
- 69. Mahmood R., Kayani W. K., Ahmed T., Malik F., Hussain S., Ashfaq M., Ali H., Rubnawaz S., Green B. D., Calderwood D., Kenny O., Rivera G. A., Mirza B., Rasheed F., BMC Complement Med. Ther. 2020, 20, 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rizvi S. N. R., Afzal S., Khan K.-U.-R., Aati H. Y., Rao H., Ghalloo B. A., Shahzad M. N., Khan D. A., Esatbeyoglu T., Korma S. A., Molecules 2023, 28, DOI 10.3390/molecules28093847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Aparna V., Dileep K. V., Mandal P. K., Karthe P., Sadasivan C., Haridas M., Chem. Biol. Drug Des. 2012, 80, 434–439. [DOI] [PubMed] [Google Scholar]
- 72. Nabi M., Tabassum N., Ganai B. A., Front Plant Sci 2022, 13, DOI 10.3389/fpls.2022.937946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Saha S., Nisa F. Y., Majid M., Rafi Md. K. J., Siddique T. A., Sultana F., Khan Md. A. N., Abu Ahmed A. M., Rahman M. A., Gholamreza A., J. Funct. Foods. 2024, 115, 106103. [Google Scholar]
- 74. Taiyeb M., Hartati H., Arwansyah A., Dahlia, Muis Abd., Mu'nisa A., Arif A. R., Salleh L. M., Inform. Med. Unlocked 2024, 47, 101517. [Google Scholar]
- 75. Baishya T., Das P., Ashraf G. J., Dua T. K., Paul P., Nandi G., Jajo H., Dutta A., Kumar A., Bhattacharya M., Sahu R., Kuwait J. Sci. 2024, 51, 100229. [Google Scholar]
- 76. Islam Md. T., de Alencar M. V. O. B., da Conceição Machado K., da Conceição Machado K., de Carvalho Melo-Cavalcante A. A., de Sousa D. P., de Freitas R. M., Chem. Biol. Interact 2015, 240, 60–73. [DOI] [PubMed] [Google Scholar]
- 77. Khatun Mst. S., Mia N., Al Bashera M., Murad M. A., Zahan R., Parvin S., Akhtar Most. A., J. Ethnopharmacol 2024, 320, 117366. [DOI] [PubMed] [Google Scholar]
- 78. Baishya T., Das P., Ashraf G. J., Dua T. K., Paul P., Nandi G., Jajo H., Dutta A., Kumar A., Bhattacharya M., Sahu R., Kuwait J. Sci. 2024, 51, 100229. [Google Scholar]
- 79. Javeed A., Ahmed M., Sajid A. R., Sikandar A., Aslam M., ul Hassan T., Samiullah, Nazir Z., Ji M., Li C., Molecules 2022, 27, 2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Joseph D. D., Veerasamy K., Siva Singaram S., Asian J. Pharm. Clin. Res. 2016, 10, 364. [Google Scholar]
- 81. Hossen S. M. M., Eva T. A., Karim M. S., Mamurat H., Rahat M. H. H., Nipun T. S., Biochem. Biophys. Rep. 2024, 37, 101648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.“National Center for Biotechnology Information (2024). PubChem Compound Summary for CID 69421, Hexadecanamide.,” can be found under ational Center for Biotechnology Information (2024). PubChem Compound Summary for CID 69421, Hexadecanamide., n.d.
- 83. Jurkić L. M., Cepanec I., Pavelić S. K., Pavelić K., Nutr. Metab. (Lond) 2013, 10, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sohail N., Farhat H., Qureshi S. A., Ullah I., Ali M. S., Ann. Pharm. Fr. 2024, DOI 10.1016/j.pharma.2024.06.001. [DOI] [PubMed] [Google Scholar]
- 85. Tlou M., Ndou B., Mabona N., Khwathisi A., Ateba C., Madala N., Serepa-Dlamini M. H., Front. Microbiol. 2024, 15, 1383854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cheng M.-C., Ker Y.-B., Yu T.-H., Lin L.-Y., Peng R. Y., Peng C.-H., J Agric. Food Chem. 2010, 58, 1502–1508. [DOI] [PubMed] [Google Scholar]
- 87.J. W. Honour, Encyclopedia of Endocrine Diseases, Elsevier, London, Uk, 2019, 531–539.
- 88. Ali A., Ali A., Husain Warsi M., Ahmad W., Tahir A., Saudi J. Biol. Sci. 2021, 28, 4575–4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Maher T., Kabbashi N. A., Mirghani M. E. S., Alam M. Z., Daddiouaissa D., Abdulhafiz F., Reduan M. F. H., Omran J. I., Abdul Razab M. K. A., Mohammed A., Antioxidants (Basel) 2021, 10, 1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Asong J. A., Amoo S. O., McGaw L. J., Nkadimeng S. M., Aremu A. O., Otang-Mbeng W., Plants (Basel) 2019, 8, 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.“National Center for Biotechnology Information (2024). PubChem Compound Summary for CID 5839, Aldosterone,” can be found under National Center for Biotechnology Information (2024). PubChem Compound Summary for CID 5839, Aldosterone, n.d.
- 92. Khan I. H., Javaid A., J. Anim. Plant Sci. 2022, 32, 609–614. [Google Scholar]
- 93. Borkotoky S., Borah V. V., Int. J. Pharm. Sci. Res. 2023, 14, 4489–4508. [Google Scholar]
- 94. Alghamdi A., Alshehri W., Sajer B., Ashkan M., Ashy R., Gashgari R., Hakmi H., J. Chem. 2023, 2023, 1–15. [Google Scholar]
- 95. Ukwubile C. A., Ikpefan E. O., Malgwi T. S., Bababe A. B., Odugu J. A., Angyu A. N., Otalu O., Bingari M. S., Nettey H. I., Sci. Afr. 2020, 8, e00421. [Google Scholar]
- 96. Huang Z.-R., Lin Y.-K., Fang J.-Y., Molecules 2009, 14, 540–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kim S.-K., Karadeniz F., Adv. Food Nutr. Res. 2012, 65, 223–233. [DOI] [PubMed] [Google Scholar]
- 98. Bai X.-G., Zheng Y., Qi J., Front. Pharmacol. 2022, 13, 1018951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Brigelius-Flohé R., Nutr. Res. Rev. 2006, 19, 174–186. [DOI] [PubMed] [Google Scholar]
- 100. Asbaghi O., Nazarian B., Yousefi M., Anjom-Shoae J., Rasekhi H., Sadeghi O., Nutr. J. 2023, 22, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Prasad M., Jayaraman S., Eladl M. A., El-Sherbiny M., Abdelrahman M. A. E., Veeraraghavan V. P., Vengadassalapathy S., Umapathy V. R., Jaffer Hussain S. F., Krishnamoorthy K., Sekar D., Palanisamy C. P., Mohan S. K., Rajagopal P., Molecules 2022, 27, 1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Shahzad N., Khan W., MD S., Ali A., Saluja S. S., Sharma S., Al-Allaf F. A., Abduljaleel Z., Ibrahim I. A. A., Abdel-Wahab A. F., Afify M. A., Al-Ghamdi S. S., Biomed. Pharmacother. 2017, 88, 786–794. [DOI] [PubMed] [Google Scholar]
- 103. Tripathi N., Kumar S., Singh R., Singh C. J., Singh P., Varshney V. K., Orient. J. Chem. 2013, 29, 705–707. [Google Scholar]
- 104. Koduru R. L., Babu P. S., Varma I. V., Kalyani G. G., Nirmala P., Int J. Pharm. Sci. Res. 2017, 8, 4540–4545. [Google Scholar]
- 105.P. P. Myint, M. T. Soe, H. H. Hlaing, “A study of phytoconstituents, α-glucosidase inhibitory effect and antioxidant activity of Lagerstroemia speciosa L. Leaf and Fruit,” 2017.
- 106. Nurcahyanti A. D. R., Liliana M., Surja S. S., J. Appl. Pharm. Sci. 2023, DOI 10.7324/JAPS.2023.152976. [DOI] [Google Scholar]
- 107. Saumya S. M., Basha P. M., Indian J. Exp. Biol. 2011, 49, 125–31. [PubMed] [Google Scholar]
- 108.M. S. Sikarwar, L. C. Chung, L. W. Ting, L. C. Chee, S. Fuloria, K. Balaji, J. Appl. Pharm. Sci. 2016, 185–190.
- 109. Unno T., Sakane I., Masumizu T., Kohno M., Kakuda T., Biosci. Biotechnol. Biochem. 1997, 61, 1772–1774. [DOI] [PubMed] [Google Scholar]
- 110.M. Thakur, K. Singh, R. Khedkar, Functional and Preservative Properties of Phytochemicals, Elsevier, Varanasi, India, 2020, 341–361.
- 111. Borycka-Kiciak K., Banasiewicz T., Rydzewska G., Gastroenterol. Rev. 2017, 2, 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Li X., Fan X., Yuan X., Pang L., Hu S., Wang Y., Huang X., Song X., Front. Psychiatry 2021, 12, DOI 10.3389/fpsyt.2021.724664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.S. Britha, S. Rajesh, R. Renuka, V. P. Santhanakrishnan, R. Gnanam, “Phytochemical analysis and bioactivity prediction of compounds in methanolic extract of Curculigo orchioides Gaertn. Journal of Pharmacognosy and Phytochemistry,” 2017.
- 114. Bin Rahman F., Ahmed S., Noor P., Rahman M. Md. M., Huq S. M. A., Akib Md. T. E., Shohael A. M., Biochem. Biophys. Rep. 2020, 24, 100805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Mousa A. M., El-Sammad N. M., Abdel-Halim A. H., Anwar N., Khalil W. K. B., Nawwar M., Hashim A. N., Elsayed E. A., Hassan S. K., Biomolecules 2019, 9, 871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Pareek A., Suthar M., Rathore G. S., Bansal V., Kumawat T., Pharmacogn. J. 2010, 2, 357–360. [Google Scholar]
- 117. Ullah A., Munir S., Badshah S. L., Khan N., Ghani L., Poulson B. G., Emwas A.-H., Jaremko M., Molecules 2020, 25, 5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Truscheit E., Frommer W., Junge B., Müller L., Schmidt D. D., Wingender W., Angew. Chem., Int. Ed. Engl. 2010, 20, 744–761. [Google Scholar]
- 119. Gong L., Feng D., Wang T., Ren Y., Liu Y., Wang J., Food. Sci. Nutr. 2020, 8, 6320–6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Soussi A., Gargouri M., Magné C., Ben-Nasr H., Kausar M. A., Siddiqui A. J., Saeed M., Snoussi M., Adnan M., El-Feki A., Chappard D., Badraoui R., Chem. Biol. Interact. 2022, 368, 110230. [DOI] [PubMed] [Google Scholar]
- 121. Ratnadewi A. A. I., Wahyudi L. D., Rochman J., Susilowati, Nugraha A. S., Siswoyo T. A., Arabian J. Chem. 2020, 13, 1831–1836. [Google Scholar]
- 122. El Mannoubi I., J. Umm Al-Qura Univ. for Appl. Sci. 2023, 9, 176–184. [Google Scholar]
- 123. Ghani U., Eur. J. Med. Chem. 2015, 103, 133–162. [DOI] [PubMed] [Google Scholar]
- 124. Abdel-Karim O. H., Abo-Shady A. M., Ismail G. A., Gheda S. F., Int J Environ. Health Res. 2022, 32, 1447–1468. [DOI] [PubMed] [Google Scholar]
- 125. Kma L., Asian Pac. J. Cancer Prev. 2013, 14, 3429–3436. [DOI] [PubMed] [Google Scholar]
- 126.D. Uğur, H. Güneş, F. Güneş, R. Mammadov, Turk. J. Pharm. Sci. 2017, 14, 222–230. [DOI] [PMC free article] [PubMed]
- 127. Rohit Singh T., Ezhilarasan D., Nutr. Cancer 2020, 72, 146–156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.