Abstract
Plant nucleotide-binding leucine-rich repeat immune receptor genes (NLRs) play an important role in plant defenses against pathogens, pathogenic nematodes, and piercing–sucking herbivores. However, little is known about their functions in plant defenses against chewing herbivores. Here, we identified a plasma membrane-localized coiled-coil-type NLR protein, OsPik-2-like, whose transcript levels were induced by the infestation of rice leaf folder (LF, Cnaphalocrocis medinalis) larvae, and by treatment with mechanical wounding. Knocking out OsPik-2-like in rice increased the LF-induced levels of jasmonic acid (JA) and jasmonoyl–isoleucine (JA-Ile), the activity of trypsin protease inhibitors (TrypPIs), and the basal levels of some flavonoids, which in turn decreased the performance of LF larvae. Moreover, knocking out OsPik-2-like reduced plant growth. These findings demonstrate that OsPik-2-like regulates the symbiosis between rice and LF by balancing plant growth and defense.
Keywords: rice, OsPik-2-like, Cnaphalocrocis medinalis, jasmonic acid, jasmonoyl–isoleucine, trypsin protease inhibitors
1. Introduction
Plants are constantly being challenged by a variety of stresses, including herbivores. As a consequence, plants have evolved complicated strategies to cope with herbivore infestation. When attacked by herbivores, plants employ two tiers of immune receptors—plasma membrane-localized pattern recognition receptors (PRRs) and intracellular nucleotide-binding domain leucine-rich repeat receptors (NLRs)—to initiate specific defense responses [1,2,3]. PRRs mainly recognize plant-derived damage-associated molecular patterns (DAMPs) or herbivore-associated molecular patterns (HAMPs, also called herbivore-associated elicitors) to activate pattern-triggered immunity (PTI) [2,4], whereas NLRs mainly perceive herbivore-secreted effectors to initiate effector-triggered immunity (ETI) [5,6]. After recognizing patterns/effectors, plants activate a series of defense responses, including calcium influx, the production of reactive oxygen species (ROS), the activation of mitogen-activated protein kinases (MAPKs), and signaling pathways mediated by phytohormones (such as jasmonic acid (JA), salicylic acid (SA), ethylene (ET), and abscisic acid (ABA)), and the accumulation of defense compounds. Acting together, these responses enhance the direct and indirect resistance of plants to herbivores [2,7].
Plant NLRs are usually composed of three conserved domains: a variable N-terminal domain; a central NB-ARC (nucleotide-binding adaptor shared by Apaf1, certain resistance genes, and a CED4) domain; and a C-terminal LRR (leucine-rich repeat) domain [8]. Based on their N-terminal domains, plant NLRs are divided into three classes, including Toll/interleukin-1 receptor/R protein (TIR)-type NLRs (TNLs); coiled-coil (CC)-type NLRs (CNLs); and resistance to powdery mildew 8 (RPW8)-type NLRs (RNLs) [8]. Numerous studies have revealed that NLRs usually regulate plant disease resistance by forming homomultimers or heteromultimers [8,9]. For example, PigmR (Pigm Resistant) and PigmS (Pigm Susceptible) are functional gene pairs identified at the rice Pigm locus for rice blast resistance. PigmR forms homologous dimers conferring broad-spectrum disease resistance to rice, but severely decreasing rice yields in the process; however, PigmS binds to PigmR to form heterodimers, thereby inhibiting PigmR-mediated broad-spectrum disease resistance and offsetting the negative effect of PigmR on rice yields [10]. In Arabidopsis and tobacco plants, ZAR1 (HopZ-activated resistance 1) interacts with multiple members of the receptor-like cytoplasmic kinase (RLCK) subfamily to form immune receptor complexes; these sense the pathogen-derived effectors and trigger immune responses [11,12,13,14]. For instance, the ZAR1 resistosome in Arabidopsis—a heterodimer composed of ZAR1, the resistance-related kinase 1 (RKS1), and the PBL2UMP (the acetylated form of PBL2 by the effector protein AvrAC of Xanthomonas campestris pv. campestris)—directly enters the lipid bilayer of the plasma membrane of cells, where it acts as a Ca2+ channel and promotes Ca2+ influx, thereby triggering ROS production and cell death [14].
Recently, NLRs have also been reported to play an important role in plant herbivore resistance [3]. For example, several NLR genes involved in plant resistance to aphids have been identified and cloned, such as Vat in Cucumis melo, Ra in Lactuca sativa, and AIN (Acyrthosiphon-induced necrosis) in Medicago truncatula [15,16,17,18]. Also, the resistance gene Mi1.2 in Lycopersicon peruvianum confers broad-spectrum resistance to root-knot nematodes, whitefly (Bemisia tabaci), and psyllid (Bactericerca cockerelli) on plants [19,20]. When Pieris brassicae egg deposition-induced hypersensitive response (HR)-like cell death was genetically mapped in the black mustard Brassica nigra, a cluster of TNL genes was identified as a potential source of R genes; these genes may regulate the ability of plants to kill herbivore eggs [21]. In rice, two CNL genes, Brown planthopper resistance 9 (Bph9) and Bph14, are well known to confer BPH resistance on rice: Bph9 regulates rice BPH resistance by affecting JA, SA, and ET-mediated signaling pathways, and Bph14 positively mediates the resistance of rice to BPH by promoting SA and ROS-mediated defenses [22,23,24,25]. Further studies proved that the CC and NB domains, as well as the full length of Bph14, interact with two WRKY proteins (OsWRKY46 and OsWRKY72); this interaction stabilizes the two WRKY proteins and enhances their transactivation activity, thereby increasing the expression of callose-biosynthesis genes and the deposition of calloses in the phloem of rice leaf sheaths [26]. Moreover, Bph14 interacts with the BPH-derived effector BISP (Bph14-interacting salivary protein) to activate ETI, which markedly increased rice resistance to BPH [27]. However, our understanding of the role of NLRs in plant defense against herbivores and their underlying mechanisms is limited.
Rice (Oryza sativa L.), one of the primary food crops in the world, is often seriously damaged in the field by multiple herbivores, including the rice leaf folder (LF, Cnaphalocrocis medinalis) [28]. LF larvae fold rice leaves longitudinally and feed on the green mesophyll tissues of these folded leaves, actions that reduce both the photosynthetic productivity and grain yield of rice plants [29]. It has been reported that the infestation of LF larvae activates defense-related signaling pathways mediated by JA, SA, ET, and H2O2 in rice; these signaling pathways jointly modulate the expression of defense genes and the accumulation of defense compounds, such as trypsin protease inhibitors (TrypPIs) and phenolamines [30,31]. Although a few NLRs have been reported to be involved in the resistance of rice to piercing–sucking herbivores, as stated above, whether NLRs participate in rice defense against chewing herbivores, such as LF, remains largely unknown.
In this study, we isolated a rice CNL gene OsPik-2-like (XM_015756755.2), which was induced by LF larval infestation [31], and investigated its function in interactions between rice and LF. By combining molecular tools, chemical analysis, and bioassays, we found that OsPik-2-like negatively regulates the biosynthesis of LF-induced JA and JA-Ile, the activity of induced TrypPIs, and the resistance of rice to LF, suggesting that NLRs participate in rice–LF interactions.
2. Results
2.1. Characterization of OsPik-2-like
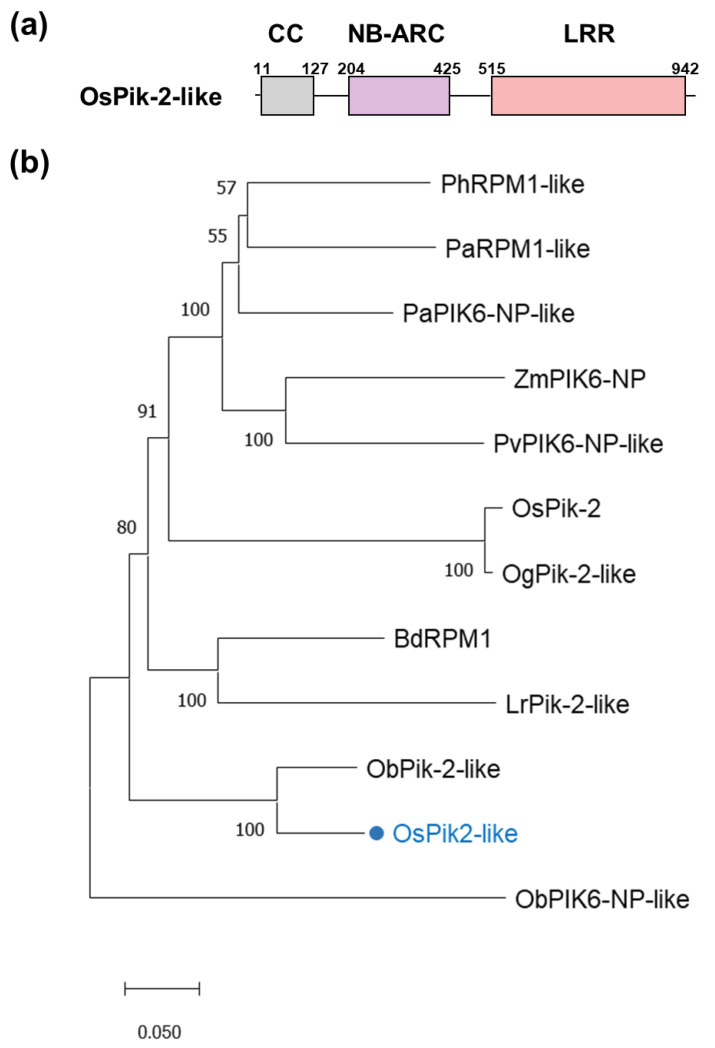
Using transcriptome data [31], we determined that a putative CNL gene was up-regulated by herbivore infestation. The full-length cDNA sequence obtained from a cDNA library of rice variety XS11 using the reverse-transcription polymerase chain reaction (RT-PCR) was identical to the sequence of a rice disease resistance gene OsPik-2-like (annotated in the Genbank database, https://www.ncbi.nlm.nih.gov/genbank/, under the ID: XM_015756755.2; accessed 30 August 2023). The gene includes an open-reading frame of 2,892 bp that encodes a protein of 963 amino acids with a predicted molecular weight of 109.03 kDa (Figure S1). The protein harbored three conserved domains: a CC domain, a NB-ARC domain, and a LRR domain (Figure 1a). OsPik-2-like shared high similarity with CNL proteins in other plants, including ObPik-2-like (88.30% identity) in Oryza brachyantha, BdRPM1 (72.28% identity) in Brachypodium distachyon, and PaPIK6-NP-like (71.06% identity) in Phragmites australis (Figure 1b). These results showed that OsPik-2-like belongs to the CNL protein family.
Figure 1.
Structure and phylogenetic relationship of OsPik-2-like. (a) Schematic structure of OsPik-2-like. (b) Sequence alignment of OsPik-2-like and its homologs. Species abbreviations are included before the protein names: Bd, Brachypodium distachyon; Lr, Lolium rigidum; Ob, Oryza brachyantha; Og, Oryza glaberrima; Os, Oryza sative; Pa, Phragmites australis; Ph, Panicum hallii; Pv, Panicum virgatum; and Zm, Zea mays. Plant species and accession numbers from the NCBI database are as follows: BdRPM1, XP_003576018.1; LrPik-2-like, XP_047085044.1; ObPIK6-NP-like, XP_040383595.1; ObPik-2-like, XP_006660848.1; OgPik-2-like, XP_052139461.1; OsPik-2, XP_015619167.2; OsPik-2-like, XP_015612241.1; PaPIK6-NP-like, XP_062198511.1; PaRPM1-like, XP_062193254.1; PhRPM1-like, XP_025803740.1; PvPIK6-NP-like, XP_039795950.1; ZmPIK6-NP, XP_008652943.2. The blue dot indicates OsPik-2-like. The scale bar represents 0.05 amino acid substitutions per site in the primary structure.
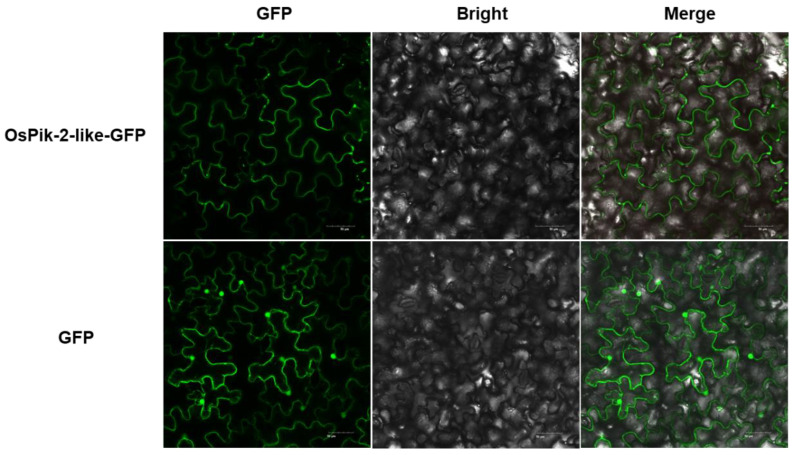
To elucidate the subcellular localization of OsPik-2-like, we generated the construct 35S::OsPik-2-like-EGFP (the enhanced green fluorescent protein was fused to OsPik-2-like), which was driven by a CaMV 35S promoter. Then, the construct was transiently expressed in Nicotiana benthamiana leaves using Agrobacterium tumefaciens-mediated transformation. As shown in Figure 2, compared with the wide distribution of the control EGFP, the OsPik-2-like-EGFP mainly localized to plasma membranes of tobacco leaf cells, indicating that OsPik-2-like was a plasma membrane (PM)-localized CNL protein.
Figure 2.
Subcellular localization of OsPik-2-like. Subcellular localization of the fused OsPik-2-like-GFP or GFP in N. benthamiana leaf cells. OsPik-2-like-GFP, green fluorescent protein (GFP) fluorescence from OsPik-2-like-GFP; Bright, bright field; Merged, the merged image of OsPik-2-like-GFP or GFP and Bright. Bar = 50 μm.
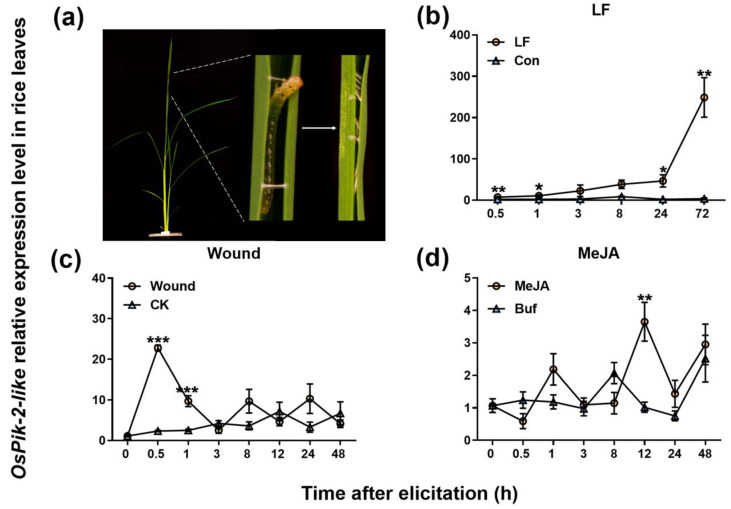
Quantitative real-time PCR (qRT-PCR) revealed that the constitutive transcript levels of OsPik-2-like in rice leaves were low. However, when rice plants (leaf blades) were infested by LF larvae (Figure 3a,b), the levels of OsPik-2-like transcripts in leaf blades were rapidly (starting 0.5 h after infestation) and lastingly up-regulated; the levels, which peaked at 72 h, were about 70-fold higher than the levels in control plants (Figure 3b; Table S1). Mechanical wounding also quickly (0.5 h after treatment) induced the expression of OsPik-2-like (levels, which peaked at 0.5 h, were about 10-fold higher than levels in control plants); however, the levels of OsPik-2-like transcripts rapidly declined to those of controls 3 h after treatment (Figure 3c; Table S1). MeJA treatment only slightly induced the expression of OsPik-2-like at 12 h after treatment (Figure 3d; Table S1). These results suggest that OsPik-2-like might be involved in LF-induced rice defense responses.
Figure 3.
Relative expression levels of OsPik-2-like in rice leaves after different treatments. (a) Phenotypes of rice plants that had been infested by one 3rd-instar LF larva. (b–d) Mean transcript levels (±SE, n = 3~6) of OsPik-2-like in rice leaves that were infested with one 3rd LF larva (LF) (b), or punctured by a fabric pattern wheel rolling (Wound) (c), or treated with methyl jasmonate (MeJA) by root absorption (d). Con, non-treated plants; Buf: plants treated with the same concentration of ethanol in the nutrient solution as MeJA treatment. Asterisks represent significant differences between the treatments and controls at each time point (* p < 0.05, ** p < 0.01, and *** p < 0.001, Student’s t-tests or t tests with Welch’s correction).
2.2. Knocking out OsPik-2-like in Rice
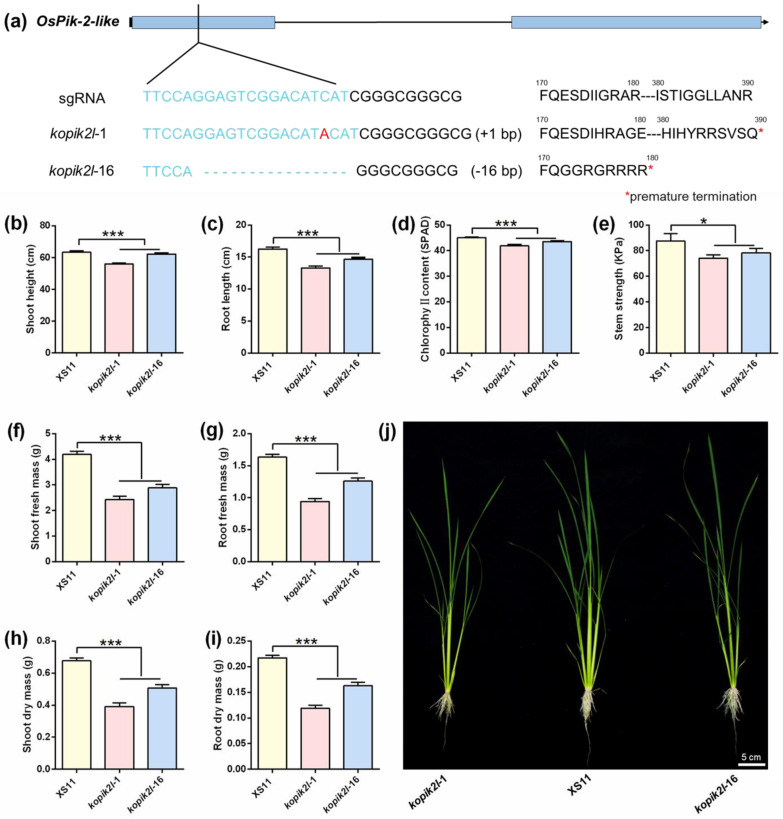
To explore the function of OsPik-2-like in interactions between rice and LF, we obtained two T-DNA-free homozygous rice lines with knocked-out OsPik-2-like in the variety XS11 using a CRISPR/Cas9-mediated gene-editing system (ko-pik2l: kopik2l-1 and kopik2l-16). As shown in Figure 4a, compared with the target sequence of OsPik-2-like gene in WT plants, the line kopik2l-1 displayed a single “A” insertion and the line kopik2l-16 showed a 16-bp deletion (Figure 4a). DNA sequencing analysis revealed no mutations of off-target sites (such mutations had been predicted by the CRISPR-P server, http://cbi.hzau.edu.cn/crispr/, accessed 25 June 2023), in ko-pik2l lines (Figure S2), suggesting that the CRISPR/Cas9-based OsPik-2-like-knockout was specific.
Figure 4.
OsPik-2-like gene-knockout rice lines and their growth phenotypes. (a) Sanger sequencing analysis of mutation patterns in the target site of OsPik-2-like gene in the wild-type (WT) and OsPik-2-like knockout rice lines. Blue boxes represent exons. The sgRNA sequence that specifically targets OsPik-2-like is indicated. The mutations, an ‘‘A’’ insertion in line kopik2l-1 and a 16 bp deletion in kopik2l-16, lead to premature translational termination of OsPik-2-like. (b–i) Mean shoot height (b), root length (c), chlorophyll content (d), stem strength (e), shoot fresh weight (f), root fresh weight (g), shoot dry weight (h), and root dry weight (i) (+SE, n = 20) of WT plants and ko-pik2l lines at 30 days old in the phytotron. Asterisks indicate significant differences in ko-pik2l lines compared with WT plants evaluated by Bayesian analysis of variance (* p < 0.05 and *** p < 0.001). (j) The phenotype of 30-day-old WT and ko-pik2l lines in the phytotron.
We investigated the effect of knocking out OsPik-2-like on plant growth. Knocking out OsPik-2-like reduced shoot height (by 11.75% in kopik2l-1 and 1.84% in kopik2l-16, compared with WT plants), root length (by 18.18% and 9.91%), chlorophyll content in leaves (by 7.15% and 3.47%), stem strength (by 15.34% and 10.7%), shoot fresh mass (by 42.24% and 31.21%) and shoot dry mass (by 42.17% and 25.14%), and root fresh mass (by 42.47% and 22.99%) and root dry mass (by 45.28% and 25.01%) of 30-day-old plants (Figure 4b–i; Table S2). These data suggest that OsPik-2-like positively regulated rice growth.
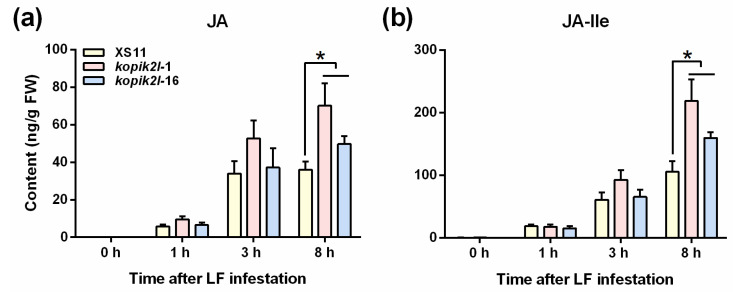
2.3. Knocking out OsPik-2-like-Enhances LF-Induced JA and JA-Ile Levels
The JA-mediated signaling pathway plays a central role in rice defenses against chewing herbivores [32]. Hence, we determined the levels of JA and JA-Ile in WT plants, as well as ko-pik2l lines before and after LF infestation. Consistent with previous studies [31,33], LF infestation significantly increased the contents of JA and JA-Ile in rice leaf blades in WT plants (Figure 5; Table S3). Knocking out OsPik-2-like did not influence the constitutive levels of JA and JA-Ile in rice leaves. However, it did enhance the induced levels of JA and JA-Ile, especially JA-Ile, in rice leaves 8 h after LF infestation: the LF-induced JA-Ile levels in the two ko-pik2l lines kopik2l-1 and kopik2l-16 were about 2- and 1.5-fold higher than those in WT plants (Figure 5; Table S3).
Figure 5.
Knocking out OsPik-2-like enhanced LF-induced JAs levels in rice leaves. Mean levels (+SE, n = 4~6) of JA (a) and JA-Ile (b) in WT plants and ko-pik2l lines that were individually infested by a 3rd-instar LF larva on the first fully expanded leaf at the indicated time points. Asterisks indicate significant differences in ko-pik2l lines compared with WT plants evaluated by Bayesian analysis of variance (* p < 0.05).
We also found that LF larval infestation (at 8 h) enhanced the content of 3-indoleacetic acid (IAA) in rice leaf blades. However, no difference was observed in IAA content between WT and ko-pik2l lines (Figure S3a; Tables S3 and S4). These results indicate that the JA signaling pathway might be involved in OsPik2-like-mediated rice defenses against LF.
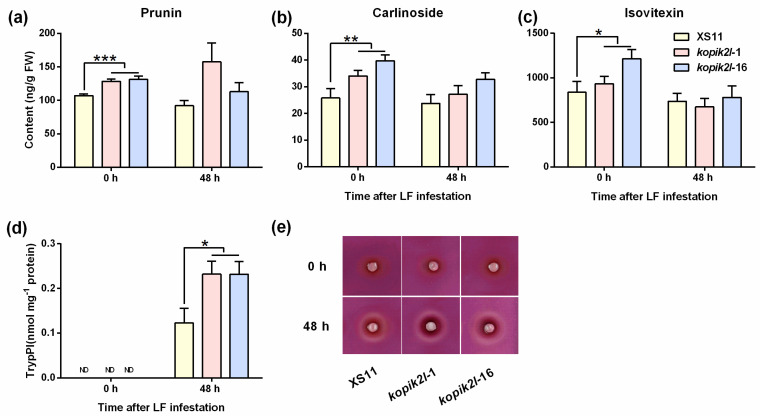
2.4. Knocking out OsPik-2-like Enhances the Level of Defense Compounds and Rice Resistance to LF
Plants employ multiple defense compounds to limit the population development of herbivores, such as flavonoids, phenolamides, and TrypPIs [32]. To evaluate whether OsPik-2-like regulates the production of defense compounds in rice plants, we examined the content of flavonoids and TrypPIs in WT plants and in ko-pik2l lines before and after LF infestation. Knocking out OsPik-2-like in rice constitutively increased the contents of three flavonoids—prunin, carlinoside, and isovitexin (Figure 6a–c; Table S5)—but decreased the contents of two flavonoids—astragalin and luteolin 7-O-glucoside (Figure S3b,c; Table S5). After LF larval infestation, knocking out OsPik-2-like also decreased the contents of five flavonoids: astragalin (by 49.83% in kopik2l-1 and 59.17% in kopik2l-16 compared to WT plants), luteolin 7-O-glucoside (by 49.89% and 59.76%), luteolin (by 65.71% and 72.89%), sakuranetin (by 100% and 100%), and isoquercitrin (by 32.61% and 28.04%) (Figure S3b–f; Table S5). Consistent with the results reported previously [31], the basal level of TrypPIs in rice leaves of WT plants was extremely low. However, when plants were infested by LF larvae, the TrypPI activity in leaves was markedly enhanced (Figure 6d,e; Table S5). Knocking out OsPik-2-like did not influence the basal activity of TrypPIs in plant leaf blades, but it did significantly enhance the LF-induced activities of TrypPIs in leaf blades (Figure 6d,e; Table S5).
Figure 6.
Flavonoid levels and TrypPI activity in the leaves of WT plants and ko-pik2l lines. Mean concentrations (+SE, n = 5~6) of prunin (a), carlinoside (b), isovitexin (c), and mean TrypPI activity (+SE, n = 7) (d) in WT plants and ko-pik2l lines that were individually infested by a 3rd-instar LF larva on the first fully expanded leaf for 0 h or 48 h. Asterisks indicate significant differences in ko-pik2l lines compared with WT plants evaluated by Bayesian analysis of variance (* p < 0.05, ** p < 0.01 and *** p < 0.001). (e) Representative pictures of TrypPI after different treatments measured by radial diffusion assay.
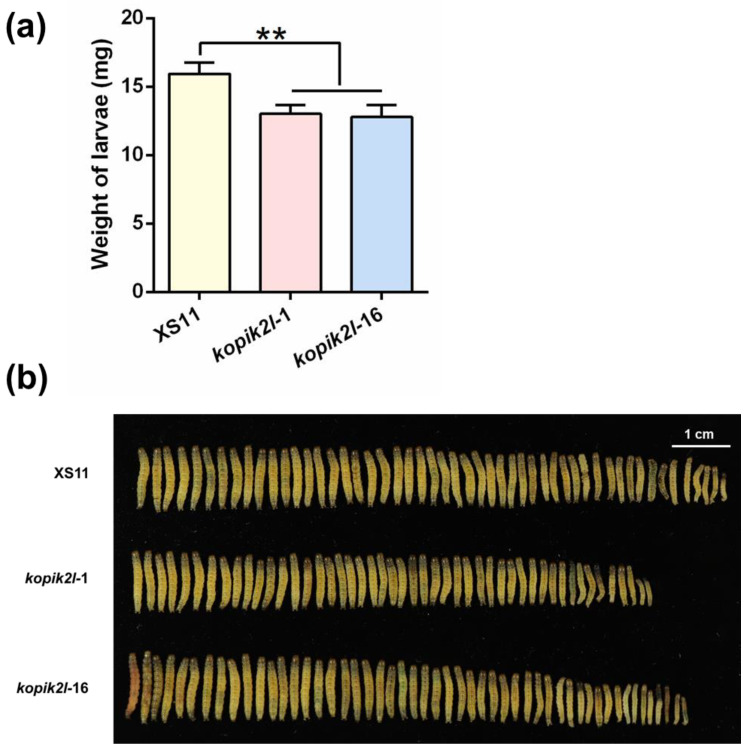
Given that knocking out OsPik-2-like in rice influenced the JA signaling pathway and the levels of defense compounds in plants, we asked whether knocking out OsPik-2-like affected rice defense against LF. As expected, LF larvae fed on ko-pik2l plants gained significantly less mass compared with those fed on WT plants: by day 11, the mass of LF larvae fed on the kopik2l-1 and kopik2l-16 plants decreased by about 18% and 20%, respectively, compared with the mass of larvae fed on WT plants (Figure 7; Table S6). These results indicate that OsPik-2-like negatively regulates rice defense against LF larvae.
Figure 7.
OsPik-2-like negatively regulates the resistance of rice to LF. (a) Mean larval mass (+SE, n = 48~51) of LF fed on WT plants and ko-pik2l lines for 11 days. (b) Phenotypes of LF larvae fed on different lines on day 11. Asterisks indicate significant differences in ko-pik2l lines compared with WT plants evaluated by Bayesian analysis of variance (** p < 0.01).
3. Discussion
Plant NLRs have widely been reported to act as resistance proteins that sense pathogen/herbivore-derived effectors, thereby triggering ETI. However, their role in plant defense responses to chewing herbivores remains poorly understood [15,16,17,18,19,20,21,22,23,24,25]. In this study, we revealed that a CNL protein, OsPik-2-like, plays an important role in LF larvae-induced rice defenses. First, OsPik-2-like localized to PM, and its transcript levels were significantly induced by LF larval infestation and mechanical wounding. Second, knocking out OsPik-2-like decreased the shoot mass, root mass leaf chlorophyll content, and stem strength of plants. Third, knocking out OsPik-2-like enhanced the LF-induced levels of JA and JA-Ile, as well as the activity of TrypPIs in rice, which in turn reduced the growth of LF larvae. Our findings demonstrate that OsPik-2-like positively regulates plant growth, but negatively mediates the resistance of rice plants to LF.
NLRs play an important role in regulating defense-related signaling pathways [3]. Two NLRs, Sw-5b and Sl5R-1, from Solanaceae, for instance, have been reported to activate the JA signaling pathway and then increase plant resistance to infection by tomato spotted wilt orthotospovirus (TSWV) [34,35]. In rice, Bph9 and Bph14, two NLRs, have been reported to negatively regulate the expression of genes related to JA biosynthesis, such as genes encoding allene oxide synthase 2 (OsAOS2) and lipoxygenase (OsLOX); moreover, the levels of JA and JA-Ile in plants carrying Bph9 or Bph14 were significantly lower than those in WT plants in response to BPH infestation [22,25]. Here, we observed that knocking out OsPik-2-like significantly increased the LF-elicited levels of JA and JA-Ile in rice leaf blades (Figure 5). Moreover, LF larval infestation up-regulated the transcript levels of OsPik-2-like, especially late in the process. These data suggest that, like Bph9 and Bph14, OsPik-2-like negatively modulated the biosynthesis of LF-induced JA and JA-Ile in rice. These results indicate that LF was able to suppress the JA-mediated rice defense by inducing the expression of OsPik-2-like. Further studies should elucidate the mechanism underlying the OsPik-2-like-mediated regulation of JA biosynthesis. It would also be interesting to know whether and how other defense-related signaling pathways are modulated by OsPik-2-like.
Why rice plants up-regulate the expression of OsPik-2-like at late stages of infestation by LF larvae, given that OsPik-2-like negatively regulated rice defense, remains a question. It may be that rice plants are trying to avoid the autotoxicity caused by excessive levels of defenses [36,37] and to restore growth. Given that OsPik-2-like suppressed the biosynthesis of LF-induced JA and JA-Ile, and that knocking out OsPik-2-like decreased plant growth (Figure 4 and Figure 5), the up-regulation of OsPik-2-like at the late stage of LF larval infestation caused plants to maintain appropriate levels of defenses. Moreover, it made plants regrow quickly. In rice, several such genes, such as OsMPK20-5 [38] and OsLRR2 [39], have been reported. Alternatively, it may be related to herbivore decoy strategies [40]. Thus far, many CNLs have been reported to regulate plant disease resistance [3]. In rice, two CNLs, Pik-1 and Pik-2, have been reported to enhance rice resistance to Magnaporthe oryzae (syn. Pyricularia oryzae) expressing the AvrPik effector [41,42,43]. Because many symbiotic microbe species live in herbivores [44], it may be that LF larvae regulate the expression of OsPik-2-like by secreting one of these microbes during their feeding, thereby suppressing the JA-mediated defense in rice. Further research should clarify this mechanism. Moreover, how OsPik-2-like influences rice growth, including which plant growth-related signaling pathways are regulated by OsPik-2-like, should also be elucidated.
The JA signaling pathway plays a central role in regulating the biosynthesis of multiple defense compounds [31,33]. For example, silencing or knocking out genes related to JA biosynthesis (such as herbivore-induced rice type 2 13-LOX gene, OsHI-LOX, and the allene oxide cyclase gene, OsAOC) or signaling (JA receptor gene, rice CORONATINE-INSENSITIVE1 (OsCOI1) and the core JA-responsive transcriptional factor OsMYC2) in rice reduces plant resistance to LF by impairing the activity of TrypPIs and the accumulation of some phenolamines in rice plants [31,33]. Therefore, the observed results—namely, that knocking out OsPik-2-like enhanced the activity of LF-induced TrypPIs in plants—were related, at least in part, to the increase in the levels of LF-induced JA and JA-Ile in plants. Interestingly, we observed that knocking out OsPik-2-like increased the basal levels of three flavonoids and decreased the basal levels of two flavonoids; moreover, it also decreased LF-induced levels of five flavonoids (Figure 6). Although some researchers have reported that the JA signaling pathway positively modulates the production of herbivore-induced flavonoids [45], these results indicate that the regulation of flavonoid biosynthesis was complex and that other signaling pathways were also involved in this process. It has been reported that signaling pathways mediated by brassinosteroids, SA, and ET also regulate the biosynthesis of flavonoids in plants [46,47,48]. Future research should elucidate which other signaling pathways regulated by OsPik-2-like mediate the biosynthesis of these compounds in rice.
TrypPIs and some flavonoids are important defense compounds against herbivores. TrypPIs, for example, have been reported to suppress the growth and development of chewing herbivores, including LF, by inhibiting the activity of digestive enzymes in their midgut [30,31,49]. Some flavonoids (including prunin, carlinoside, schaftoside, and its isomers, isoschaftoside and neoschaftoside) impede the feeding, survival, and development of rice planthoppers [50,51,52], although no flavonoids have been reported to affect the performance of LF larvae. Moreover, two isovitexin-derived compounds, isovitexin 2″-O-(6‴-(E)-feruloyl) glucoside and isovitexin-2′-O-β-[6-O-E-p-coumaroylglucopyranoside], decrease the probing responses of Nephotettix cincticepts [53] and the fecundity of Helicoverpa armigera, respectively [54]. Hence, the enhanced resistance of ko-pik2l plants to LF larvae is probably related to the increase in the levels of defense compounds, such as TrypPIs and some flavonoids. Future research should clarify which compounds are responsible for the performance of LF larvae on ko-pik2l plants.
In summary, our results demonstrate that the PM-localized rice CNL protein, OsPik-2-like, plays an important role in regulating the interaction between rice and LF larvae. When infested by LF larvae, plants perceive signals derived from the herbivore and then initiate defense-related signaling pathways, such as the JA signaling pathway. These changes enhance the expression of defense-related genes and the level of defense compounds, such as TrypPIs, in turn decreasing the performance of LF larvae. The up-regulation of OsPik-2-like in rice at late stages of LF larval infestation not only reduces plant defense by suppressing the JA signaling pathway, but also promotes plant regrowth. Both consequences may be beneficial for both plants and herbivores. Plants may avoid the autotoxicity associated with excessive defenses and also experience regrowth; herbivores may improve growth due to decreased plant defense. Our study provides an interesting example of how a single gene can act as a modulator for the symbiotic relationship between plants and herbivores, balancing plant growth and defense. From an application point of view, this study shows how specific genes can be edited in plants to control insect pests.
4. Materials and Methods
4.1. Plants and Insects
The japonica rice variety XiuShui 11 (XS11) was used as the wild type (WT) in this study. The gene-knockout rice lines of OsPik-2-like (ko-pik2l: kopik2l-1 and kopik2l-16) were generated and screened as below. Seeds of WT and ko-pik2l lines were pre-germinated in plastic culture dishes (diameter 90 mm, height 15 mm) in a climate incubator under the following conditions: 28 ± 2 °C, 60% relative humidity, and 14/10 h light/dark cycle. Ten days later, rice seedlings were transplanted to 25 L hydroponic boxes (length 51 cm, width 35 cm, and height 17 cm) brimming with rice nutrient solution [55] and cultivated in a phytotron (26 ± 2 °C, 14 h light phase, 60% relative humidity). Twenty-five-day-old rice plants were individually transferred into plastic pots (diameter 7 cm, height 9.5 cm) filled with 350 mL of nutrient solution. Plants were used for experiments 4 d after transplantation.
Colonies of LF were originally obtained from a rice paddy in Hangzhou, China, and subsequently reared with wheat seedlings in a climate chamber (26 ± 2 °C, 14 h light phase, and 65 ± 10% relative humidity) for more than 30 generations.
4.2. Plant Treatment
For LF treatments, a third-instar LF larva was starved for 2 h, then placed on the youngest fully expanded leaf of a plant. Untreated plants were used as controls. For mechanical wounding in rice leaves, the youngest fully expanded leaf was punctured by rolling a fabric pattern wheel over it [45]. Untreated plants were used as controls. For mechanical wounding in rice leaf sheaths, aerial parts 2 cm high from the roots of rice plants were individually punctured 200 times using an insect needle (length 40 mm and diameter 0.45 mm; Yuxiu, Taizhou, China). Untreated plants were used as controls. For treatment with methyl jasmonate (MeJA) (Sigma-Aldrich, Darmstadt, Germany), MeJA was dissolved in 1 mL of absolute ethyl alcohol and then added to the nutrient solution to make its concentration 100 μM, as stated in previous studies [56]. Plants cultivated in the nutrient solution with an equal volume of solvent were used as controls.
4.3. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
Total RNA was extracted from rice samples using FastPure® Universal Plant Total RNA Isolation Kit (Vazyme, Nanjing, China) following the manufacturer’s instructions. One microgram of total RNA was then reverse-transcribed by using HiScript® II Q RT SuperMix for qPCR (+gDNA wiper) (Vazyme, Nanjing, China) according to the manufacturer’s instructions. QRT-PCR assays were performed with Taq Pro Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China) on the CFX96 Real-Time PCR System (Bio-Rad, Richmond, CA, USA). With the OsACTIN (TIGR ID: LOC_Os03g50885) as an internal control, the relative expression levels of tested genes were analyzed by the −2ΔΔCt method [56]. All primers used in the qRT-PCR assays are listed in Table S7.
4.4. Isolating OsPik-2-like
Four micrograms of total RNA extracted from WT plants was reverse-transcribed to first-strand cDNAs using PrimeScript™ IV cDNA Synthesis Mix (TaKaRa, Dalian, China). Then, the opening read frame of OsPik-2-like (XM_015756755.2) was PCR-amplified from the cDNA library using specific primers OsPik-2-like-CDS-F (5′-TCTCCGCCATTTACACCCAC-3′) and OsPik-2-like-CDS-R (5′-TGCCAAAGAGGAACATCGGA-3′), and the KOD FX polymerase (TOYOBO, Shanghai, China) following the manufacturer’s instructions. PCR products were subsequently cloned into the pEASY®-Blunt Zero cloning vector (TransGen, Beijing, China) and sequenced. The primers used in this study are listed in Table S8.
4.5. Structure and Phylogenetic Analysis of OsPik-2-like
The conserved domains of the deduced OsPik-2-like protein were analyzed using CD-search (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi; accessed on 30 August 2023). The homologs of OsPik-2-like from different plant species were identified using the BLASTP plug-in in the NCBI online database (https://blast.ncbi.nlm.nih.gov/Blast.cgi; accessed on 24 June 2024). The amino acid sequences of OsPik-2-like and their homologs from other plant species were downloaded from the NCBI website. Then, multiple sequence alignment was carried out using ClustalW program in Molecular Evolutionary Genetics Analysis (MEGA, version 11.0.13) as described previously [56]. A neighbor-joining phylogenetic tree was constructed by MEGA with 1000 bootstrap replicates using the default parameters as described [56].
4.6. Subcellular Localization Assay
For subcellular localization, the full-length coding sequence of OsPik-2-like without the termination codon was inserted into the pCAMBIA1301-EGFP vector [39] using ClonExpress® II One Step Cloning Kit C112 (Vazyme, Nanjing, China), yielding a 35S::OsPik-2-like-EGFP construct. The constructed plasmid was then transformed into A. tumefaciens strain EHA105 by electroporation. A. tumefaciens—cultivated overnight and carrying the OsPik-2-like-EGFP construct—was injected into leaves of 4-week-old N. benthamiana plants as previously described [57]. Two days after infiltration, the EGFP fluorescence images were observed and recorded by Olympus FV3000 confocal laser scanning microscope (Olympus, Tokyo, Japan) at an excitation wavelength of 488 nm and an emission wavelength of 507 nm. The primers used in this study are listed in Table S8.
4.7. Generating and Characterizing OsPik-2-like Gene-Knockout Rice Plants
To generate OsPik-2-like gene-knockout rice lines, the target sequence (5′-TTCCAGGAGTCGGACATCAT-3′) was introduced into pLYsgRNAOsU6b to yield rice U6b promoter-driven single-guide RNA (sgRNA). The sgRNA expression cassette was then introduced into the plant CRISPR-Cas9 binary vector pYLCRISPR/Cas9Pubi-H [58]. The T-DNA was inserted into XS11 using A. tumefaciens (EHA105)-mediated transformation. Plants with mutations but lacking the T-DNA were screened by target DNA sequencing and hygromycin resistance gene identification using the method described in [39]. Two homozygous OsPik-2-like gene-knockout (ko-pik2l) lines, kopik2l-1 and kopik2l-16, were used for all experiments. Potential off-target sites were predicted by CRISPR-GE (http://skl.scau.edu.cn/home/, accessed 13 August 2023) and identified by target DNA sequencing using the specific primers listed in Table S9.
4.8. Measuring Plant Growth Parameters
Thirty-day-old WT plants and OsPik-2-like gene-knockout lines were used to measure plant growth parameters, which included root length, shoot height, root fresh mass, root dry mass, shoot fresh mass, shoot dry mass, stem strength, and chlorophyll content. To determine stem strength, the aerial parts (2 cm high from plant root) of rice plants were measured with a plant-stem-strength meter [59], YYD-1 (Top Cloud-Agri, Hangzhou, Zhehjiang, China). To determine chlorophyll content, the middle location of the youngest fully expanded leaves from each plant was measured with a chlorophyll meter [56], SPAD-502 Plus (Konica Minolta, Tokyo, Japan). Each experiment was replicated 20 times.
4.9. Herbivore Bioassays
To measure LF performance, freshly hatched LF neonates were allowed to feed on WT and ko-pik2l rice plants. The larval mass (to an accuracy of 0.1 mg) was individually measured 11 days later. Each treatment was replicated 48~51 times.
4.10. JA, JA-Ile, and IAA Analysis
For JA, JA-Ile, and IAA analysis, 30-day-old plants of the WT and ko-pik2l rice lines were randomly assigned to LF and control treatments as described above. The youngest fully expanded leaf of each plant was harvested at different time points (0, 1, 3, and 8 h after the start of treatment) and was ground in liquid nitrogen. JA, JA-Ile, and IAA were extracted from a sample of about 100 mg of ground rice with ethyl acetate containing labeled internal standards (D6-JA, D6-JA-Ile, and D5-IAA) and then quantified by high-performance liquid chromatography–mass spectrometry–mass spectrometry (HPLC-MS-MS) (Agilent Technologies, Santa Clara, CA, USA) using the method described in [60]. Each treatment was replicated 4~6 times.
4.11. Analysis of the Activity of TrypPIs
To analyze TrypPIs, 30-day-old plants of the WT and ko-pik2l rice lines were randomly assigned to LF and control treatments as described above. The youngest fully expanded leaf of each plant was harvested at 0 and 2 d after the start of treatment and was ground in liquid nitrogen. Samples containing about 100 mg of ground rice were homogenized with 300 μL of cooled extraction buffer (0.1 M Tris-HC1, pH 7.6, 5% polyvinylpolypyrrolidone (Sigma-Aldrich, Darmstadt, Germany), 2 mg/mL phenylthiourea (Sigma-Aldrich, Darmstadt, Germany), 5 mg/mL diethyldithiocarbamate (Sigma-Aldrich, Darmstadt, Germany), and 0.05 M Na2EDTA) as described previously [61]. TrypPI activity was measured by the radial diffusion method [62]. Each treatment was replicated 7 times.
4.12. Detecting Flavonoids
To detect flavonoids, 30-day-old plants of the WT and ko-pik2l rice lines were randomly assigned to LF and control treatments as described above. The youngest fully expanded leaf of each plant was harvested at 0 and 2 d after the start of treatment and was ground in liquid nitrogen. Flavonoids were extracted from samples of approximately 100 mg using 800 μL of 70% methanol, and then quantified by HPLC-MS-MS as previously described in [63]. The content of each compound was calculated using an external standard method [64]. Each treatment was replicated 5~6 times.
4.13. Data Analysis
Two-treatment data were analyzed using Student’s t-tests (equal SDs) or t tests with Welch’s correction (unequal SDs). For comparisons between data collected from WT plants and from two transgenic lines of OsPik-2-like (ko-pik2l), an a priori approach based on Bayesian analysis of variance was used. All statistical analyses were conducted with SPSS software version 26 (IBM Corp., Armonk, NY, USA).
Acknowledgments
We thank Yie Xu for technical assistance, and Genshen Xie for the assistance in LF rearing. We thank Emily Wheeler for editorial assistance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants13233275/s1, Figure S1: Sequences of nucleotides and deduced amino acids of OsPik-2-like; Figure S2: Sanger sequencing results of potential off-target sites; Figure S3: Other compounds in the leaves of WT plants and ko-pik2l lines; Table S1: Student’s t-test or t-test with Welch’s correction analysis with data from Figure 3; Table S2: Bayesian analysis of variance with data from Figure 4; Table S3: Bayesian analysis of variance with data from Figure 5; Table S4: Student’s t-test analysis with data from Figure S3; Table S5: Bayesian analysis of variance with data from Figure 6 and Figure S3; Table S6: Bayesian analysis of variance with data from Figure 7; Table S7: Primers used for real-time qPCR; Table S8: Primers used for OsPike-2-like cloning and subcellular localization assay; Table S9: Primers used for generation and characterization of transgenic plants.
Author Contributions
Conceptualization, Y.L.; methodology, Y.L., P.K., and C.Q.; validation, Y.L., P.K., and C.Q.; formal analysis, Y.L., P.K., and C.Q.; investigation, C.Q., X.J., and Y.Z.; resources, Y.L.; data curation, C.Q.; writing—original draft preparation, C.Q. and P.K.; writing—review and editing, Y.L.; supervision, Y.L.; project administration, Y.L.; funding acquisition, Y.L. and P.K. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was jointly supported by the Major Science and Technology project of Xinjiang (2023A0200904 to Y.L.), the earmarked fund for China Agriculture Research Systems (CARS-01-43 to Y.L.), and the National Natural Science Foundation of China (32202396 to P.K.).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Stahl E., Hilfiker O., Reymond P. Plant-arthropod interactions: Who is the winner? Plant J. 2018;93:703–728. doi: 10.1111/tpj.13773. [DOI] [PubMed] [Google Scholar]
- 2.Erb M., Reymond P. Molecular interactions between plants and insect herbivores. Annu. Rev. Plant Biol. 2019;70:527–557. doi: 10.1146/annurev-arplant-050718-095910. [DOI] [PubMed] [Google Scholar]
- 3.Ngou B., Ding P., Jones J. Thirty years of resistance: Zig-zag through the plant immune system. Plant Cell. 2022;34:1447–1478. doi: 10.1093/plcell/koac041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reymond P. Receptor kinases in plant responses to herbivory. Curr. Opin. Biotechnol. 2021;70:143–150. doi: 10.1016/j.copbio.2021.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Dodds P.N., Rathjen J.P. Plant immunity: Towards an integrated view of plant-pathogen interactions. Nat. Rev. Genet. 2010;11:539–548. doi: 10.1038/nrg2812. [DOI] [PubMed] [Google Scholar]
- 6.Jones J.D.G., Vance R.E., Dangl J.L. Intracellular innate immune surveillance devices in plants and animals. Science. 2016;354:aaf6395. doi: 10.1126/science.aaf6395. [DOI] [PubMed] [Google Scholar]
- 7.Snoeck S., Guayazán-Palacios N., Steinbrenner A.D. Molecular tug-of-war: Plant immune recognition of herbivory. Plant Cell. 2022;34:1497–1513. doi: 10.1093/plcell/koac009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duxbury Z., Wu C.H., Ding P. A comparative overview of the intracellular guardians of plants and animals: NLRs in innate immunity and beyond. Annu. Rev. Plant Biol. 2021;72:155–184. doi: 10.1146/annurev-arplant-080620-104948. [DOI] [PubMed] [Google Scholar]
- 9.Contreras M.P., Ludke D., Pai H., Toghani A., Kamoun S. NLR receptors in plant immunity: Making sense of the alphabet soup. EMBO Rep. 2023;24:e57495. doi: 10.15252/embr.202357495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng Y., Zhai K., Xie Z., Yang D., Zhu X., Liu J., Wang X., Qin P., Yang Y., Zhang G., et al. Epigenetic regulation of antagonistic receptors confers rice blast resistance with yield balance. Science. 2017;355:962–965. doi: 10.1126/science.aai8898. [DOI] [PubMed] [Google Scholar]
- 11.Seto D., Koulena N., Lo T., Menna A., Guttman D.S., Desveaux D. Expanded type III effector recognition by the ZAR1 NLR protein using ZED1-related kinases. Nat. Plants. 2017;3:17027. doi: 10.1038/nplants.2017.27. [DOI] [PubMed] [Google Scholar]
- 12.Schultink A., Qi T., Bally J., Staskawicz B. Using forward genetics in Nicotiana benthamiana to uncover the immune signaling pathway mediating recognition of the Xanthomonas perforans effector XopJ4. New Phytol. 2019;221:1001–1009. doi: 10.1111/nph.15411. [DOI] [PubMed] [Google Scholar]
- 13.Wang G., Roux B., Feng F., Guy E., Li L., Li N., Zhang X., Lautier M., Jardinaud M., Chabannes M., et al. The decoy substrate of a pathogen effector and a pseudokinase specify pathogen-induced modified-self recognition and immunity in plants. Cell Host Microbe. 2015;18:285–295. doi: 10.1016/j.chom.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Bi G., Su M., Li N., Liang Y., Dang S., Xu J., Hu M., Wang J., Zou M., Deng Y., et al. The ZAR1 resistosome is a calcium-permeable channel triggering plant immune signaling. Cell. 2021;184:3528–3541. doi: 10.1016/j.cell.2021.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Dogimont C., Chovelon V., Pauquet J., Boualem A., Bendahmane A. The Vat locus encodes for a CC-NBS-LRR protein that confers resistance to Aphis gossypii infestation and A. gossypii-mediated virus resistance. Plant J. 2014;80:993–1004. doi: 10.1111/tpj.12690. [DOI] [PubMed] [Google Scholar]
- 16.Klingler J.P., Nair R.M., Edwards O.R., Singh K.B. A single gene, AIN, in Medicago truncatula mediates a hypersensitive response to both bluegreen aphid and pea aphid, but confers resistance only to bluegreen aphid. J. Exp. Bot. 2009;60:4115–4127. doi: 10.1093/jxb/erp244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wroblewski T., Piskurewicz U., Tomczak A., Ochoa O., Michelmore R.W. Silencing of the major family of NBS-LRR-encoding genes in lettuce results in the loss of multiple resistance specificities. Plant J. 2007;51:803–818. doi: 10.1111/j.1365-313X.2007.03182.x. [DOI] [PubMed] [Google Scholar]
- 18.Casteel C.L., Walling L.L., Paine T.D. Behavior and biology of the tomato psyllid, Bactericerca cockerelli, in response to the Mi-1.2 gene. Entomol. Exp. Appl. 2006;121:67–72. doi: 10.1111/j.1570-8703.2006.00458.x. [DOI] [Google Scholar]
- 19.Nombela G., Williamson V.M., Muniz M. The root-knot nematode resistance gene Mi-1.2 of tomato is responsible for resistance against the whitefly Bemisia tabaci. Mol. Plant Microbe Interact. 2003;16:645–649. doi: 10.1094/MPMI.2003.16.7.645. [DOI] [PubMed] [Google Scholar]
- 20.Rossi M.U.O.C., Goggin F.L., Milligan S.B., Kaloshian I., Ullman D.E., Williamson V.M. The nematode resistance gene Mi of tomato confers resistance against the potato aphid. Proc. Natl. Acad. Sci. USA. 1998;95:9750–9754. doi: 10.1073/pnas.95.17.9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bassetti N., Caarls L., Bouwmeester K., Verbaarschot P., van Eijden E., Zwaan B.J., Bonnema G., Schranz M.E., Fatouros N.E. A butterfly egg-killing hypersensitive response in Brassica nigra is controlled by a single locus, PEK, containing a cluster of TIR-NBS-LRR receptor genes. Plant Cell Environ. 2024;47:1009–1022. doi: 10.1111/pce.14765. [DOI] [PubMed] [Google Scholar]
- 22.Du B., Zhang W., Liu B., Hu J., Wei Z., Shi Z., He R., Zhu L., Chen R., Han B., et al. Identification and characterization of Bph14, a gene conferring resistance to brown planthopper in rice. Proc. Natl. Acad. Sci. USA. 2009;106:22163–22168. doi: 10.1073/pnas.0912139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamura Y., Hattori M., Yoshioka H., Yoshioka M., Takahashi A., Wu J., Sentoku N., Yasui H. Map-based cloning and characterization of a brown planthopper resistance gene BPH26 from Oryza sativa L. ssp. indica cultivar ADR52. Sci. Rep. 2014;4:5872. doi: 10.1038/srep05872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ji H., Kim S., Kim Y., Suh J., Park H., Sreenivasulu N., Misra G., Kim S., Hechanova S.L., Kim H., et al. Map-based cloning and characterization of the BPH18 gene from wild rice conferring resistance to brown planthopper (BPH) insect pest. Sci. Rep. 2016;6:34376. doi: 10.1038/srep34376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Y., Huang J., Wang Z., Jing S., Wang Y., Ouyang Y., Cai B., Xin X., Liu X., Zhang C., et al. Allelic diversity in an NLR gene BPH9 enables rice to combat planthopper variation. Proc. Natl. Acad. Sci. USA. 2016;113:12850–12855. doi: 10.1073/pnas.1614862113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu L., Wu Y., Wu D., Rao W., Guo J., Ma Y., Wang Z., Shangguan X., Wang H., Xu C., et al. The coiled-coil and nucleotide binding domains of brown planthopper resistance14 function in signaling and resistance against planthopper in rice. Plant Cell. 2017;29:3157–3185. doi: 10.1105/tpc.17.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo J., Wang H., Guan W., Guo Q., Wang J., Yang J., Peng Y., Shan J., Gao M., Shi S., et al. A tripartite rheostat controls self-regulated host plant resistance to insects. Nature. 2023;618:799–807. doi: 10.1038/s41586-023-06197-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang L., Zhu Z., Ge L., Yang G., Wu J. Rice grain damage by combination and sequence infestations by the rice leaffolder, Cnaphalocrocis medinalis Guenee (Lepidoptera: Pyralidae), and the white-backed rice planthopper, Sogatella furcifera Horváth (Hemiptera: Delphacidae) J. Integr. Agric. 2014;13:2460–2470. doi: 10.1016/S2095-3119(14)60745-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Padmavathi C., Katti G., Padmakumari A.P., Voleti S.R., Subba Rao L.V. The effect of leaffolder Cnaphalocrocis medinalis (Guenee) [Lepidoptera: Pyralidae] injury on the plant physiology and yield loss in rice. J. Appl. Entomol. 2013;137:249–256. doi: 10.1111/j.1439-0418.2012.01741.x. [DOI] [Google Scholar]
- 30.Wang X. Master’s Thesis. Zhejiang University; Hang Zhou, China: 2006. Influence of Infestation by Herbivores with Different Feeding Habits or Treatment by β-Glucosidase on Levels of Main Defense-Related Signals in Rice Plants; p. 31. [Google Scholar]
- 31.Zhuang Y., Wang X., Llorca L.C., Lu J., Lou Y., Li R. Role of jasmonate signaling in rice resistance to the leaf folder Cnaphalocrocis medinalis. Plant Mol. Biol. 2022;109:627–637. doi: 10.1007/s11103-021-01208-x. [DOI] [PubMed] [Google Scholar]
- 32.Kuai P., Lou Y. Advances in molecular interactions between rice and insect herbivores. Crop Health. 2024;2:6. doi: 10.1007/s44297-024-00027-y. [DOI] [Google Scholar]
- 33.Ye M., Luo S.M., Xie J.F., Li Y.F., Xu T., Liu Y., Song Y.Y., Zhu-Salzman K., Zeng R.S. Silencing COI1 in rice increases susceptibility to chewing insects and impairs inducible defense. PLoS ONE. 2012;7:e36214. doi: 10.1371/journal.pone.0036214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Q., Tong C., Chen Z., Huang S., Zhao X., Hong H., Li J., Feng M., Wang H., Xu M., et al. NLRs derepress MED10b- and MED7-mediated repression of jasmonate-dependent transcription to activate immunity. Proc. Natl. Acad. Sci. USA. 2023;120:e1992741176. doi: 10.1073/pnas.2302226120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qi S., Shen Y., Wang X., Zhang S., Li Y., Islam M.M., Wang J., Zhao P., Zhan X., Zhang F., et al. A new NLR gene for resistance to tomato spotted wilt virus in tomato (Solanum lycopersicum) Theor. Appl. Genet. 2022;135:1493–1509. doi: 10.1007/s00122-022-04049-4. [DOI] [PubMed] [Google Scholar]
- 36.Gog L., Berenbaum M.R., DeLucia E.H., Zangerl A.R. Autotoxic effects of essential oils on photosynthesis in parsley, parsnip, and rough lemon. Chemoecology. 2005;15:115–119. doi: 10.1007/s00049-005-0294-8. [DOI] [Google Scholar]
- 37.He Z., Webster S., He S.Y. Growth-defense trade-offs in plants. Curr. Biol. 2022;32:R634–R639. doi: 10.1016/j.cub.2022.04.070. [DOI] [PubMed] [Google Scholar]
- 38.Li J., Liu X., Wang Q., Huangfu J., Schuman M.C., Lou Y. A group D MAPK protects plants from autotoxicity by suppressing herbivore-induced defense signaling. Plant Physiol. 2019;179:1386–1401. doi: 10.1104/pp.18.01411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuai P., Lin N., Ye M., Ye M., Chen L., Chen S., Zu H., Hu L., Gatehouse A.M.R., Lou Y. Identification and knockout of a herbivore susceptibility gene enhances planthopper resistance and increases rice yield. Nat. Food. 2024;5:846–859. doi: 10.1038/s43016-024-01044-4. [DOI] [PubMed] [Google Scholar]
- 40.Walling L.L. Avoiding effective defenses: Strategies employed by phloem-feeding insects. Plant Physiol. 2008;146:859–866. doi: 10.1104/pp.107.113142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanzaki H., Yoshida K., Saitoh H., Fujisaki K., Hirabuchi A., Alaux L., Fournier E., Tharreau D., Terauchi R. Arms race co-evolution of Magnaporthe oryzae AVR-Pik and rice Pik genes driven by their physical interactions. Plant J. 2012;72:894–907. doi: 10.1111/j.1365-313X.2012.05110.x. [DOI] [PubMed] [Google Scholar]
- 42.De la Concepcion J.C., Franceschetti M., Maqbool A., Saitoh H., Terauchi R., Kamoun S., Banfield M.J. Polymorphic residues in rice NLRs expand binding and response to effectors of the blast pathogen. Nat. Plants. 2018;4:576–585. doi: 10.1038/s41477-018-0194-x. [DOI] [PubMed] [Google Scholar]
- 43.Ashikawa I., Hayashi N., Yamane H., Kanamori H., Wu J., Matsumoto T., Ono K., Yano M. Two adjacent nucleotide-binding site-leucine-rich repeat class genes are required to confer Pikm-specific rice blast resistance. Genetics. 2008;180:2267–2276. doi: 10.1534/genetics.108.095034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pirttilä A.M., Brusila V., Koskimäki J.J., Wäli P.R., Ruotsalainen A.L., Mutanen M., Markkola A.M., Yount J. Exchange of microbiomes in plant-insect herbivore interactions. mBio. 2023;14:e321022. doi: 10.1128/mbio.03210-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu J., Wang X., Zu H., Zeng X., Baldwin I.T., Lou Y., Li R. Molecular dissection of rice phytohormone signaling involved in resistance to a piercing-sucking herbivore. New Phytol. 2021;230:1639–1652. doi: 10.1111/nph.17251. [DOI] [PubMed] [Google Scholar]
- 46.Chen H., Zhang Q., Lv W., Yu X., Zhang Z. Ethylene positively regulates Cd tolerance via reactive oxygen species scavenging and apoplastic transport barrier formation in rice. Environ. Pollut. 2022;302:119063. doi: 10.1016/j.envpol.2022.119063. [DOI] [PubMed] [Google Scholar]
- 47.Xu P., Wu T., Ali A., Wang J., Fang Y., Qiang R., Liu Y., Tian Y., Liu S., Zhang H., et al. Rice β-glucosidase 4 (Os1βGlu4) regulates the hull pigmentation via accumulation of salicylic acid. Int. J. Mol. Sci. 2022;23:10646. doi: 10.3390/ijms231810646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He Y., Zhao Y., Hu J., Wang L., Li L., Zhang X., Zhou Z., Chen L., Wang H., Wang J., et al. The OsBZR1-OsSPX1/2 module fine-tunes the growth-immunity trade-off in adaptation to phosphate availability in rice. Mol. Plant. 2024;17:258–276. doi: 10.1016/j.molp.2023.12.003. [DOI] [PubMed] [Google Scholar]
- 49.Kumar R., Bhutani S., Singh R., Chauhan R., Chowdhury V., Jain K., Rajinder K. Enhanced resistance against the rice leaffolder (Cnaphalocrocis medinalis) in transgenic rice plants containing the potato proteinase inhibitor II gene. Entomol. Gen. 2009;32:11–22. doi: 10.1127/entom.gen/32/2009/11. [DOI] [Google Scholar]
- 50.Stevenson P.C., Kimmins F.M., Grayer R.J., Raveendranath S. Schaftosides from rice phloem as feeding inhibitors and resistance factors to brown planthoppers, Nilaparvata lugens. Entomol. Exp. Appl. 1996;80:246–249. doi: 10.1111/j.1570-7458.1996.tb00928.x. [DOI] [Google Scholar]
- 51.Grayer R.J., Harborne J.B., Kimmins F.M., Stevenson P.C., Wijayagunasekera H.N.P. Phenolics in rice phloem sap as sucking deterrents to the brown planthopper, Nilaparvata lugens. Acta Hortic. 1994;381:691–694. doi: 10.17660/ActaHortic.1994.381.100. [DOI] [Google Scholar]
- 52.Sun K., Kuai P., Lu J., Lou Y. Exogenous application of paclobutrazol induces the resistance of rice to planthoppers. Acta Entomol. Sinica. 2024;67:21–30. [Google Scholar]
- 53.Zhan Z., Matsuo A., Kim C. Studies on probing stimulants for the green rice leafhopper, Nephotettix nigropictus (Stål) (Hemiptera: Cicadellidae), in the rice plant (Oryza sativa L.) J. Pestic. Sci. 2016;41:163–166. doi: 10.1584/jpestics.D16-052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Caasi-Lit M.T., Tanner G.J., Nayudu M., Whitecross M.I. Isovitexin-2′-O-β-[6-O-E-p-coumaroylglucopyranoside] from UV-B irradiated leaves of rice, Oryza sativa L. Inhibits fertility of Helicoverpa armigera. Photochem. Photobiol. 2007;83:1167–1173. doi: 10.1111/j.1751-1097.2007.00125.x. [DOI] [PubMed] [Google Scholar]
- 55.Yoshida S., Forno D.A., Cock J.H., Gomez K.A. Laboratory Manual for Physiological Studies of Rice. 3rd ed. International Rice Research Institute; Los Baños, Philippines: 1976. [Google Scholar]
- 56.Ye M., Kuai P., Chen S., Lin N., Ye M., Hu L., Lou Y. Silencing a simple extracellular leucine-rich repeat gene OsI-BAK1 enhances the resistance of rice to brown planthopper Nilaparvata lugens. Int. J. Mol. Sci. 2021;22:12182. doi: 10.3390/ijms222212182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun L., Andika I.B., Shen J., Yang D., Chen J. The P2 of Wheat yellow mosaic virus rearranges the endoplasmic reticulum and recruits other viral proteins into replication-associated inclusion bodies. Mol. Plant Pathol. 2014;15:466–478. doi: 10.1111/mpp.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma X., Zhang Q., Zhu Q., Liu W., Chen Y., Qiu R., Wang B., Yang Z., Li H., Lin Y., et al. A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol. Plant. 2015;8:1274–1284. doi: 10.1016/j.molp.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 59.Xie L. Ph.D. Thesis. Shandong Agricultural University; Tai’an, China: 2021. Genome-Wide Association Studies and Important Gene Mining of Maize Stalk Strength Related Traits; p. 14. [Google Scholar]
- 60.Lu J., Robert C.A.M., Riemann M., Cosme M., Mène-Saffrané L., Massana J., Stout M.J., Lou Y., Gershenzon J., Erb M. Induced jasmonate signaling leads to contrasting effects on root damage and herbivore performance. Plant Physiol. 2015;167:1100–1116. doi: 10.1104/pp.114.252700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jongsma M.A., Bakker P.L., Visser B., Stiekema W.J. Trypsin-inhibitor activity in mature tobacco and tomato plants is mainly induced locally in response to insect attack, wounding and virus-infection. Planta. 1994;195:29–35. doi: 10.1007/BF00206288. [DOI] [Google Scholar]
- 62.Van Dam N.M., Horn M., Mares M., Baldwin I.T. Ontogeny constrains systemic protease inhibitor response in Nicotiana attenuata. J. Chem. Ecol. 2001;27:547–568. doi: 10.1023/A:1010341022761. [DOI] [PubMed] [Google Scholar]
- 63.Caristi C., Bellocco E., Panzera V., Toscano G., Vadalà R., Leuzzi U. Flavonoids detection by HPLC-DAD-MS-MS in lemon juices from Sicilian cultivars. J. Agric. Food Chem. 2003;51:3528–3534. doi: 10.1021/jf0262357. [DOI] [PubMed] [Google Scholar]
- 64.Wang W.W., Zhou P.Y., Mo X.C., Hu L.F., Jin N., Chen X., Yu Z.X., Meng J.P., Erb M., Shang Z.C., et al. Induction of defense in cereals by 4-fluorophenoxyacetic acid suppresses insect pest populations and increases crop yields in the field. Proc. Natl. Acad. Sci. USA. 2020;117:12017–12028. doi: 10.1073/pnas.2003742117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.