Abstract
Laccase (LAC, EC 1.10.3.2) is integral to the formation of lignin synthesis, flavonoid production, and responses to both biotic and abiotic stresses. While recent studies have characterized numerous LAC gene families and their functions across various plants, information regarding LAC genes in woodland strawberry (Fragaria vesca) remains limited. In this study, we identified a total of 57 FvLAC genes in the Fragaria vesca genome, which were phylogenetically categorized into five distinct groups. Analysis of the gene structures revealed a uniformity in the exon–intron structure among the subgroups, while conserved motifs identified unique motifs specific to certain subgroups, suggesting functional variations. Chromosomal localization studies indicated that FvLACs are distributed across seven chromosomes, and collinearity analysis demonstrated that FvLACs exhibit collinearity within the species. Additionally, cis-acting element analysis suggested that FvLAC genes are involved in stress responses, hormone responses, light responses, and the growth and development of plants. qRT-PCR demonstrated that FvLACs responded to salt, drought, and hormone stresses, with the expression levels of FvLAC24, FvLAC32, and FvLAC51 continuously increasing under these stress conditions. Furthermore, transgenic yeast experiments revealed that FvLAC51 enhanced yeast tolerance to both salt and drought stresses, while FvLAC24 and FvLAC32 negatively regulated yeast tolerance under these same conditions. These findings provide a theoretical foundation for further investigation into the functions of FvLAC genes in woodland strawberry.
Keywords: Fragaria vesca, laccase, phylogenetic analysis, expression patterns, salt and drought stresses
1. Introduction
Laccase (LAC, EC 1.10.3.2) is a member of the multicopper oxidase family and contains a copper oxidase domain, which catalyzes the degradation of various aromatic or non-aromatic compounds (phenols, monolignols, lignins, anilines, thiols, and arylamines) [1]. In its catalytic mechanism, copper ions absorb electrons and reduce oxygen to water during the electron transfer process [2]. Laccases have been identified in bacteria, fungi, insects, and plants. Fungal laccases are capable of oxidizing and degrading phenolic lignin structural units and are involved in the recycling and utilization of natural lignin, making them widely used in the manufacturing industry [3]. In contrast, plant laccases primarily participate in defense-related processes, such as lignin synthesis in the cell wall and the polymerization of phenolic compounds; they also play crucial roles in plant growth and in conferring resistance to various stresses [4,5,6].
To date, laccase genes have been identified in various species, including Arabidopsis thaliana [7], citrus [8], pear [9], cotton [10], poplar [11], and rice [12]. Previous studies indicated that most plant laccases play a role in the oxidation of monolignols, which contributes to the formation of higher-order lignin structures. In Arabidopsis, AtLAC4 and AtLAC17 are involved in the constitutive lignification of floral stems. The silencing of AtLAC17 specifically affects the deposition of G lignin units in fibers, while the unit specificity of AtLAC4 remains less clear [7]. AtLAC5 is essential for the biosynthesis of seed-coat protective neolignans through its cooperation with the AtDP1 protein [13]. The absence of LAC8 and LAC5 double mutants results in a significant decrease in lignin content and a notable increase in saccharification yield without impacting plant integrity in Brachypodium distachyon [14]. In Populus deltoides, laccase (PdLAC2) is associated with the oxidation of phenolics and the conjugation of flavonoids that interact with lignin in the cell wall [15]. In cotton, the GhLAC15 gene enhances resistance to Verticillium wilt by increasing cell wall lignin content [16].
Laccases also play significant roles under abiotic stress conditions, including drought, salinity, heavy metal stress, and low temperature. In rice, the laccase OsLAC10 enhances the tolerance of Arabidopsis to copper (Cu) stress, potentially through lignification in the roots, which prevents excessive copper absorption [12]. Additionally, a putative laccase precursor gene, OsChI1, has been shown to increase the tolerance of transgenic Arabidopsis to drought and salinity stress [17]. Recently, the overexpression of PeLAC10 was demonstrated to elevate lignin content in transgenic Arabidopsis, thereby improving their adaptability to phenolic acid and drought stress [18]. Furthermore, PeuLAC2 enhances drought tolerance in Populus euphratica by improving water transport capacity [5]. In Poncirus trifoliata, silencing CsLAC18 significantly reduced the plant’s resistance to cold stress [19]. Overall, these studies indicate that laccases play a vital role in plant development and their responses to stress.
Strawberries are among the most popular fruits worldwide, known for their excellent taste and high content of sugars, vitamins, amino acids, and other essential nutrients [20]. Additionally, strawberry plants possess a large leaf area and shallow root distribution, and they are well recognized for their sensitivity to abiotic stresses [21]. The diploid woodland strawberry (Fragaria vesca, 2n = 2x = 14) has a relatively small genome (240 Mb) and a short growth cycle, which facilitates cultivation and reproduction. Furthermore, this species is increasingly regarded as an emerging model organism for research focused on the functions of specific genes and genomic analyses within the Rosaceae family [20]. In this study, we identified 57 members of the LAC gene family in woodland strawberry (Fragaria vesca). By analyzing the physical and chemical properties (position, protein length, molecular weight, isoelectric point, aliphatic index, grand average of hydropathicity, and subcellular localization) of these genes, as well as their protein structures and phylogenetic relationships, we clarified the evolutionary patterns of the FvLAC family members. Additionally, the analysis of the promoter sequence of FvLACs revealed the presence of 32 regulatory elements associated with stress responses, hormone responses, light response, and MYB-related and development-related processes. The treatments of 20 FvLACs in groups I to III with salt, drought, ABA, and MeJA demonstrated that all 20 genes exhibited varying degrees of response to these stressors. Experiments conducted on transgenic yeast indicated that the FvLAC51 gene enhances tolerance to salt and drought stresses, whereas FvLAC24 and FvLAC32 appear to reduce tolerance under similar stress conditions. Subcellular localization studies confirmed that FvLAC24, FvLAC32, and FvLAC51 are situated in the cell membrane, classifying them as membrane proteins. These findings enhance our understanding of FvLACs’ functions and aid in the identification of candidate genes for further exploration of salt and drought stress tolerance in strawberry plants.
2. Results
2.1. Identification of Laccase Genes from Fragaria vesca Genomes
A total of 57 members of the FvLAC gene family were identified in Fragaria vesca by using HMMER3.0 (http://hmmer.org/download.html, accessed on 3 July 2024) and SMART (http://smart.embl-heidelberg.de/, accessed on 25 May 2024), and these were designated from FvLAC1 to FvLAC57 based on their chromosomal positions. Detailed information of the FvLACs, including gene name, gene ID, position, protein length, molecular weight (MW), isoelectric point (pI), aliphatic index, grand average of hydropathicity (GRAVY), and subcellular localization is shown in Table 1. Among the 57 FvLAC proteins, their length ranged from 440 (FvLAC36) to 616 (FvLAC21) amino acids, with the MWs varying from 49.20 kDa (FvLAC36) to 69.3 kDa (FvLAC21). The isoelectric point (pI) varied from 4.52 (FvLAC44) to 9.78 (FvLAC40), with 54% classified as basic proteins and 46% as acidic proteins. Furthermore, the aliphatic index of the LAC family proteins ranged from 72.29 (FvLAC14) to 94.5 (FvLAC24), and the GRAVY values ranged from −0.369 (FvLAC29) to 0.05 (FvLAC38), indicating that 96.5% of the FvLAC gene members are hydrophilic proteins (the value of GRAVY < 0). Predicted subcellular localization results indicated that all FvLAC family proteins were located in the cell membrane. Table 1 provides detailed information about the physiochemical proprieties of each identified FvLAC protein.
Table 1.
Molecular characterization of FvLAC family genes and the corresponding proteins in Fragaria vesca.
| Gene Name | Gene ID | Position | Protein Length (aa) | MW (kDa) | pI (Isoelectric Point) |
Aliphatic Index | Grand Average of Hydropathicity (GRAVY) | Subcellular Localization |
|---|---|---|---|---|---|---|---|---|
| FvLAC1 | FvH4_1g13060.t1 | Fvb1: 7195820-7198114 | 566 | 62.59965 | 8.81 | 81.11 | −0.142 | Cell Membrane |
| FvLAC2 | FvH4_1g14980.t1 | Fvb1: 8412006-8415378 | 537 | 59.63128 | 9.49 | 89.11 | −0.084 | Cell Membrane |
| FvLAC3 | FvH4_1g14990.t1 | Fvb1: 8418523-8421631 | 539 | 60.25449 | 9.47 | 84.25 | −0.253 | Cell Membrane |
| FvLAC4 | FvH4_1g20010.t1 | Fvb1: 12113062-12129311 | 568 | 62.75757 | 4.92 | 77.75 | −0.182 | Cell Membrane |
| FvLAC5 | FvH4_1g21540.t1 | Fvb1: 13515806-13518950 | 588 | 65.9508 | 6.78 | 81.19 | −0.235 | Cell Membrane |
| FvLAC6 | FvH4_1g24380.t1 | Fvb1: 16242663-16247257 | 574 | 64.03903 | 8.02 | 81.69 | −0.224 | Cell Membrane |
| FvLAC7 | FvH4_2g08670.t1 | Fvb2: 7595223-7598610 | 562 | 62.08846 | 8.91 | 86.58 | −0.037 | Cell Membrane |
| FvLAC8 | FvH4_2g08840.t1 | Fvb2: 7714665-7717273 | 563 | 62.15149 | 9.07 | 85.22 | −0.075 | Cell Membrane |
| FvLAC9 | FvH4_2g12570.t1 | Fvb2: 11035380-11040775 | 526 | 57.81445 | 5.41 | 82.09 | −0.128 | Cell Membrane |
| FvLAC10 | FvH4_2g12590.t1 | Fvb2: 11047104-11051161 | 565 | 62.70119 | 6.18 | 79.84 | −0.144 | Cell Membrane |
| FvLAC11 | FvH4_2g12610.t1 | Fvb2: 11066186-11071363 | 567 | 62.35179 | 7.04 | 77.6 | −0.123 | Cell Membrane |
| FvLAC12 | FvH4_2g12620.t1 | Fvb2: 11082038-11085711 | 593 | 66.66999 | 4.71 | 73.31 | −0.199 | Cell Membrane |
| FvLAC13 | FvH4_2g12640.t1 | Fvb2: 11096982-11100699 | 597 | 67.32025 | 5.09 | 79.11 | −0.156 | Cell Membrane |
| FvLAC14 | FvH4_2g12820.t2 | Fvb2: 11263752-11267345 | 586 | 65.90307 | 4.78 | 72.29 | −0.282 | Cell Membrane |
| FvLAC15 | FvH4_2g12830.t1 | Fvb2: 11269702-11275196 | 615 | 69.20619 | 5.04 | 78.37 | −0.22 | Cell Membrane |
| FvLAC16 | FvH4_2g16000.t1 | Fvb2: 13973659-13977994 | 540 | 60.65865 | 9.29 | 81.89 | −0.296 | Cell Membrane |
| FvLAC17 | FvH4_2g17700.t1 | Fvb2: 15202774-15207062 | 588 | 65.01755 | 5.45 | 88.35 | −0.049 | Cell Membrane |
| FvLAC18 | FvH4_2g18990.t1 | Fvb2: 16155342-16159380 | 518 | 58.02463 | 5.13 | 82.16 | −0.141 | Cell Membrane |
| FvLAC19 | FvH4_2g21650.t1 | Fvb2: 17953843-17956181 | 586 | 64.92625 | 5.22 | 81.16 | −0.21 | Cell Membrane |
| FvLAC20 | FvH4_2g37780.t1 | Fvb2: 27427351-27432183 | 545 | 60.58551 | 9.2 | 86.9 | −0.219 | Cell Membrane |
| FvLAC21 | FvH4_2g40270.t1 | Fvb2: 28723626-28726549 | 616 | 69.37165 | 5.37 | 75.45 | −0.186 | Cell Membrane |
| FvLAC22 | FvH4_3g05920.t1 | Fvb3: 3441792-3444593 | 581 | 64.42843 | 9.33 | 92.62 | −0.018 | Cell Membrane |
| FvLAC23 | FvH4_3g14660.t1 | Fvb3: 9038326-9041336 | 579 | 65.03623 | 8.01 | 84.97 | −0.19 | Cell Membrane |
| FvLAC24 | FvH4_3g34100.t1 | Fvb3: 29509155-29511629 | 527 | 58.61884 | 9.34 | 94.5 | −0.038 | Cell Membrane |
| FvLAC25 | FvH4_3g34310.t1 | Fvb3: 29812823-29815653 | 581 | 64.54466 | 9.36 | 93.27 | −0.015 | Cell Membrane |
| FvLAC26 | FvH4_3g34340.t1 | Fvb3: 29823780-29826764 | 584 | 64.35617 | 9.33 | 89.76 | −0.044 | Cell Membrane |
| FvLAC27 | FvH4_3g38140.t1 | Fvb3: 32569772-32573695 | 561 | 61.86636 | 9.05 | 89.96 | −0.044 | Cell Membrane |
| FvLAC28 | FvH4_3g39750.t2 | Fvb3: 33703382-33706783 | 578 | 63.68979 | 8.72 | 83.2 | −0.106 | Cell Membrane |
| FvLAC29 | FvH4_3g46080.t1 | Fvb3: 38231205-38234498 | 552 | 61.78519 | 7.67 | 78.03 | −0.369 | Cell Membrane |
| FvLAC30 | FvH4_4g22740.t1 | Fvb4: 25453431-25457792 | 593 | 66.87838 | 5.33 | 80 | −0.21 | Cell Membrane |
| FvLAC31 | FvH4_5g00300.t1 | Fvb5: 211106-215572 | 590 | 65.88286 | 8.9 | 85.73 | −0.202 | Cell Membrane |
| FvLAC32 | FvH4_5g17150.t1 | Fvb5: 9786606-9789346 | 581 | 65.54888 | 8.6 | 76.82 | −0.278 | Cell Membrane |
| FvLAC33 | FvH4_5g29070.t2 | Fvb5: 20173849-20178790 | 578 | 63.94649 | 7.39 | 80.09 | −0.278 | Cell Membrane |
| FvLAC34 | FvH4_5g35760.t2 | Fvb5: 26184996-26188385 | 589 | 66.24063 | 5.07 | 75.59 | −0.237 | Cell Membrane |
| FvLAC35 | FvH4_5g36460.t1 | Fvb5: 26744115-26747456 | 590 | 66.75552 | 5.29 | 86.32 | −0.164 | Cell Membrane |
| FvLAC36 | FvH4_5g36541.t1 | Fvb5: 26803908-26806116 | 440 | 49.19912 | 4.84 | 76.86 | −0.243 | Cell Membrane |
| FvLAC37 | FvH4_6g11960.t1 | Fvb6: 7170306-7173452 | 561 | 61.07522 | 9.36 | 91.39 | −0.016 | Cell Membrane |
| FvLAC38 | FvH4_6g12410.t1 | Fvb6: 7488545-7491071 | 565 | 61.93886 | 6.78 | 91.45 | 0.05 | Cell Membrane |
| FvLAC39 | FvH4_6g12430.t1 | Fvb6: 7501544-7504444 | 565 | 61.79521 | 6.03 | 88.71 | 0.002 | Cell Membrane |
| FvLAC40 | FvH4_6g13390.t1 | Fvb6: 8140343-8143219 | 591 | 65.33345 | 9.78 | 81.76 | −0.143 | Cell Membrane |
| FvLAC41 | FvH4_6g13430.t1 | Fvb6: 8150815-8155584 | 585 | 64.61023 | 9.33 | 84.32 | −0.118 | Cell Membrane |
| FvLAC42 | FvH4_6g20700.t1 | Fvb6: 14273906-14275558 | 550 | 61.6282 | 8.34 | 81.55 | −0.319 | Cell Membrane |
| FvLAC43 | FvH4_6g20720.t1 | Fvb6: 14290925-14292572 | 466 | 51.90107 | 8.95 | 79.1 | −0.315 | Cell Membrane |
| FvLAC44 | FvH4_6g35560.t1 | Fvb6: 28057302-28057449 | 460 | 51.55499 | 4.52 | 83.65 | −0.175 | Cell Membrane |
| FvLAC45 | FvH4_6g39950.t1 | Fvb6: 31547912-31551090 | 592 | 66.35409 | 4.64 | 82.01 | −0.076 | Cell Membrane |
| FvLAC46 | FvH4_6g39970.t1 | Fvb6: 31557548-31561494 | 594 | 66.73349 | 4.95 | 76.3 | −0.178 | Cell Membrane |
| FvLAC47 | FvH4_6g39983.t1 | Fvb6: 31578149-31580632 | 595 | 66.64642 | 5.01 | 81.73 | −0.109 | Cell Membrane |
| FvLAC48 | FvH4_6g39990.t1 | Fvb6: 31584585-31587084 | 600 | 67.20496 | 4.8 | 82.2 | −0.134 | Cell Membrane |
| FvLAC49 | FvH4_6g42990.t2 | Fvb6: 33349236-33353616 | 542 | 60.26189 | 8.37 | 80.68 | −0.238 | Cell Membrane |
| FvLAC50 | FvH4_7g07750.t1 | Fvb7: 7643077-7645694 | 575 | 63.70139 | 9.06 | 76.45 | −0.285 | Cell Membrane |
| FvLAC51 | FvH4_7g07800.t1 | Fvb7: 7696581-7699286 | 574 | 63.57031 | 9.05 | 76.93 | −0.282 | Cell Membrane |
| FvLAC52 | FvH4_7g16960.t1 | Fvb7: 14530357-14533065 | 574 | 63.63589 | 8.23 | 82.46 | −0.087 | Cell Membrane |
| FvLAC53 | FvH4_7g18341.t1 | Fvb7: 15320904-15323455 | 564 | 63.01902 | 4.88 | 85.82 | −0.13 | Cell Membrane |
| FvLAC54 | FvH4_7g18350.t1 | Fvb7: 15330255-15333363 | 604 | 66.8756 | 6.05 | 85.03 | −0.169 | Cell Membrane |
| FvLAC55 | FvH4_7g23980.t1 | Fvb7: 18576510-18579531 | 560 | 61.70672 | 7.3 | 92.07 | −0.025 | Cell Membrane |
| FvLAC56 | FvH4_7g25330.t1 | Fvb7: 19368353-19371071 | 574 | 63.25964 | 8.23 | 77.09 | −0.254 | Cell Membrane |
| FvLAC57 | FvH4_7g27730.t1 | Fvb7: 20684142-20687949 | 594 | 66.40308 | 6.86 | 82.88 | −0.204 | Cell Membrane |
2.2. Phylogenetic, Gene Structure, and Conservation Motif Analysis of LAC Genes Among Fragaria vesca and Arabidopsis
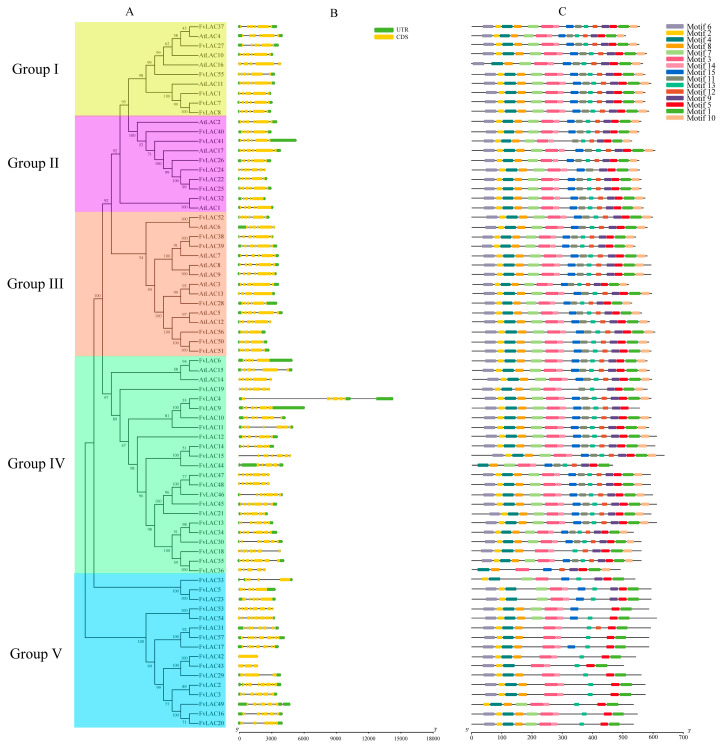
To gain insight into the phylogenetic relationships of the LAC gene families, a phylogenetic tree was constructed using the neighbor-joining (NJ) method among 17 Arabidopsis LACs and 57 FvLACs. Based on the analysis of Arabidopsis laccases, the laccases in Fragaria vesca were clustered into five distinct groups (Figure 1A), revealing an uneven distribution among these groups. Group Ⅰ included six FvLACs (FvLAC1, 7, 8, 27, 37, 55) and four AtLACs (AtLAC4, 6, 10, 11). In group Ⅱ, seven FvLACs (FvLAC22, 24, 25, 26, 32, 40, 41) were clustered alongside three AtLACs (AtLAC1, 2, 17). Group Ⅲ comprised eight AtLAC members (AtLAC3, 5, 6, 7, 8, 9, 12, 13) and seven FvLACs (FvLAC28, 38, 39, 50, 51, 52, 56). Group Ⅳ contained the largest number of LACs, consisting of 21 FvLACs (FvLAC4, 6, 9, 10, 11, 12, 13, 14, 15, 18, 19, 21, 30, 34, 35, 36, 44, 45, 46, 47, 48) and two AtLACs (AtLAC14, 15). In contrast, group Ⅴ encompassed 16 FvLACs (FvLAC2, 3, 5, 16, 17, 20, 23, 29, 33, 37, 42, 43, 49, 53, 54, 57) genes, with no AtLAC genes clustered with this group (Figure 1A).
Figure 1.
Phylogenetic relationships, gene structures, and conserved protein motifs of the LAC genes from Arabidopsis and F. vesca. (A) Phylogenetic tree of the LAC family. Various highlighted colors represent the different groups. (B) Exon–intron organizations of the LAC genes. Green boxes represent UTRs, yellow boxes represent exons, and black lines represent introns. (C) Conserved motif analysis of LACs within each group. Different color boxes represent different motifs.
The web server GSDS (Gene Structure Display Server) was utilized to analyze the gene structures of the FvLACs. As shown in Figure 1B, 40 FvLACs possessed two UTR regions, while 5 FvLAC genes (FvLAC2, 4, 33, 44, 49) contained three UTRs. Additionally, two FvLAC genes (FvLAC54, 55) included only the 3′UTR region, and ten FvLAC genes (FvLAC15, 18, 19, 24, 36, 42, 43, 47, 48, 53) lacked any UTR regions. Except for FvLAC42, the remaining 56 genes were broken genes, containing between three and nine exons in an uneven distribution. Furthermore, phylogenetic analysis revealed a similar exon–intron distribution pattern among genes with close evolutionary relationships, indicating that the gene structure of FvLACs corresponds to their evolutionary relationship. The conserved motif of all FvLAC proteins were constructed using the MEME (Multiple Em for Motif Elicitation) program, and a total of 15 conserved motifs (motif 1 to motif 15) were identified (Figure 1C). Apart from FvLAC9, all other FvLACs contained motif 1, while motif 3, motif 4, and motif 5 were widely distributed throughout the entire FvLAC family. Furthermore, the FvLAC proteins within the same group exhibited similar motif compositions, whereas genes from different groups displayed distinct compositions. In addition to group Ⅴ, most genes in groups Ⅰ, Ⅱ, and Ⅲ shared the same motifs, indicating a high degree of conservation in FvLAC gene family. In summary, the similarities in gene structures and conserved motif compositions within the same group forcefully supported the phylogenetic analysis for the group classifications.
2.3. Chromosomal Location, Genome Synteny, and Duplication of the FvLAC Genes
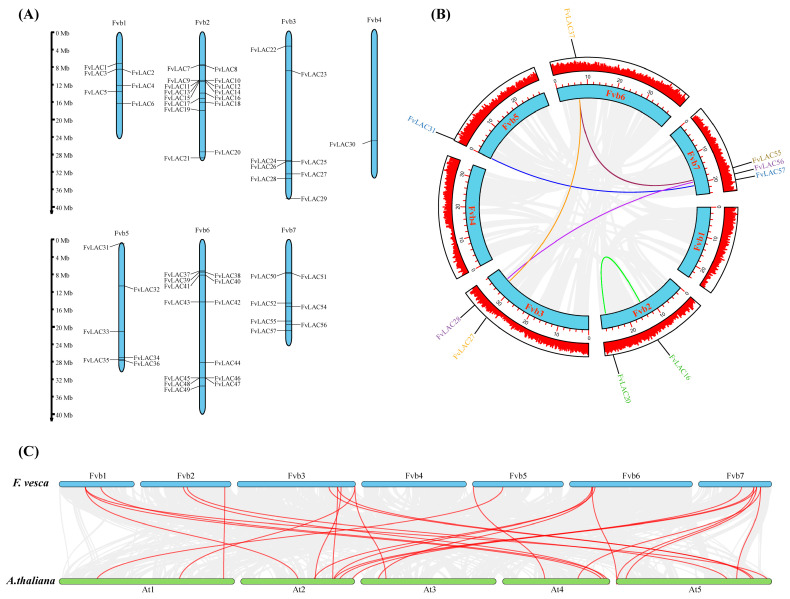
The chromosomal analysis revealed that 57 FvLAC genes were distributed across 1 to 7 chromosomes (Figure 2A). Among these genes, chromosomes 2 and 6 contained more than ten genes each, while six FvLAC genes were located on chromosomes 1 and 5. Eight FvLACs were distributed on chromosome 3, seven on chromosome 7, and chromosome 4 carried only one FvLAC gene. Additionally, some FvLAC gene members on chromosomes 1, 2, 3, and 6 were found to exist in the form of gene clusters (Figure 2A).
Figure 2.
Chromosome distribution and collinearity analysis of FvLACs. (A) Chromosomal localization of 57 FvLAC genes. The chromosome numbers are indicated at the top of each chromosome image, and the scale on the left is in mega-bases. (B) Collinearity relationship analysis of the FvLAC gene family. The colored lines indicate duplicated FvLAC gene pairs. (C) Synteny analysis of LACs between A. thaliana and F. vesca. Gray lines in the background indicate all the collinear blocks, and red lines highlight the syntenic LAC gene pairs.
To elucidate the underlying mechanisms associated with the expansion of FvLAC genes in the woodland strawberry, a collinear analysis was conducted to investigate potential gene duplication patterns using BLAST and MCScanX methods. Five pairs of duplicate genes (FvLAC16/FvLAC20, FvLAC27/FvLAC37, FvLAC28/FvLAC56, FvLAC31/FvLAC57, and FvLAC37/FvLAC55) and one triple (FvLAC27/FvLAC37/FvLAC55) were found in the woodland strawberry genome, with the majority of these genes concentrated on chromosome 3. This distribution may be attributed to tandem replication or whole-genome duplication (WGD) (Figure 2B). To further investigate the evolutionary mechanisms of FvLACs, a comparative syntenic map of F. vesca associated with A. thaliana was constructed. As illustrated in Figure 2C, seventeen FvLACs showed syntenic relationships with those in A. thaliana, predominantly located on chromosomes 3 and 7. These findings reflected the intraspecific collinearity observed in woodland strawberry plants.
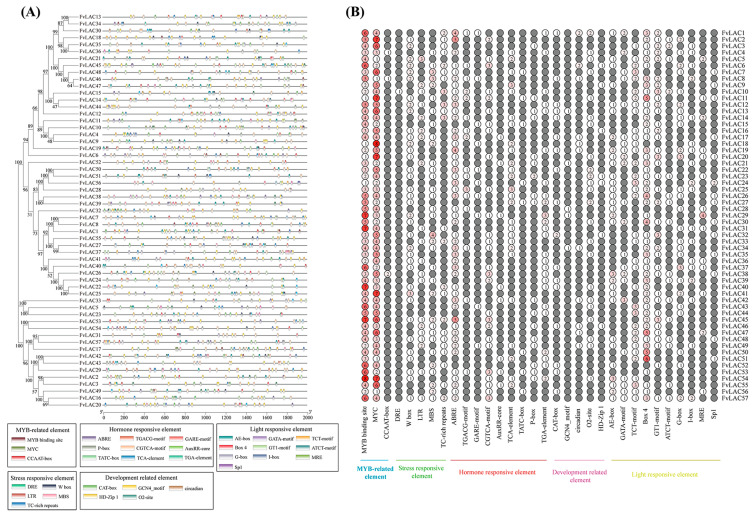
2.4. Promoter Cis-Elements of the FvLAC Gene Family
The analysis of promoter cis-elements can provide insights into the regulatory functions and stress response modes of genes [22]. To gain a deeper understanding of the functions of FvLAC genes in the woodland strawberry, we predicted and summarized cis-acting elements based on the 2000 bp upstream promoter sequences. A total of 32 cis-regulatory elements were identified and categorized into 5 functional groups, including MYB-related, stress-responsive, hormone-responsive, development-related, and light-responsive elements (Figure 3A,B, Table S1). All FvLAC genes contained MYB-related, hormone-responsive, and light-responsive elements. The hormone-responsive cis elements identified included abscisic acid response elements (ABREs, 48 members), MeJA response elements (TGACG-motif and CGTCA-motif, 16 and 27 members, respectively), gibberellin-responsive elements (GARE-motif, P-box, and TATC-box, 10, 17, and 8 members, respectively), and auxin-responsive elements (AuxRR-core and TGA element, 8 and 25 members, respectively), which were present in nearly all FvLAC genes. Except for FvLAC24, FvLAC43, and FvLAC44, all FvLACs contained at least one sequence of stress-responsive cis-acting elements. Regarding development-related elements, we identified CAT-box elements (associated with meristem expression), GCN4-motif elements (associated with endosperm-specific expression), O2-site (associated with corn protein metabolism), circadian elements, and HD-Zip 1. Notably, FvLAC2, FvLAC3, FvLAC41, FvLAC52, and FvLAC57 did not contain any of these five elements. Furthermore, the number of genes containing MYB/MYC binding site elements was the highest, followed by light-responsive (BOX 4) and abscisic acid response (ABRE) elements. Additionally, three genes (FvLAC1, FvLAC45, and FvLAC57) contained more than 30 elements, with FvLAC45 having the largest number (32) of cis-elements, while FvLAC36 and FvLAC56 contained the fewest (15). All FvLAC genes, except FvLAC44, possessed elements from more than three categories. These findings suggest that FvLAC genes may play significant roles in various developmental processes and stress responses.
Figure 3.
Identification of cis-acting regulatory elements in promoter region of FvLACs. (A) Cis-elements and their positions in the promoter region of the 57 FvLAC genes. Different colored boxes represent different cis-regulatory elements. (B) Number of each cis-acting element in the promoter region; the color intensity and numbers in the circles indicate the numbers of identified cis-acting elements.
2.5. Candidate miRNAs Targeted to Regulate FvLAC Family Genes
MicroRNA (miRNA) is a small, non-coding RNA molecule consisting of 20–24 nucleotides. It plays a crucial role in regulating gene expression at the post-transcriptional level by binding to target genes and inhibiting their activity [23]. Previous studies have demonstrated that LAC genes can undergo miRNA-mediated post-transcriptional regulation in Arabidopsis, rice, and poplar [24,25,26,27]. To investigate the potential role of miRNAs in regulating FvLAC family members in the woodland strawberry, a total of 57 FvLACs were analyzed for the presence of potential miRNA target sites. As shown in Table 2, miRNAs were predicted for 16 FvLAC genes, and most of them were identified as miR397 and miR857. FvLAC1, FvLAC32, and FvLAC55 might be regulated by miR397a only, while FvLAC7, FvLAC8, FvLAC27, FvLAC28, FvLAC37, FvLAC38, FvLAC39, and FvLAC41 might be regulated by both miR397a and miR397b. In addition, miR875 might target FvLAC3, FvLAC7, FvLAC23, FvLAC34, FvLAC41, and FvLAC52, with only FvLAC7 and FvLAC41 potentially co-regulated by miR397a, miR397b, and miR857. These results suggest that miR397a/b and miR875 may play significant regulatory roles in the post-transcriptional regulation of FvLACs, contributing to the diverse functions and complex expression regulation of FvLAC genes.
Table 2.
List of FvLACs with putative miRNA target sites in Fragaria vesca.
| Gene | Gene ID | Predicted miRNA Target Sites | miRNA Length | Expectation | Type of Inhibition |
|---|---|---|---|---|---|
| FvLAC1 | FvH4_1g13060.t1 | miR397a | 21 | 3.5 | Cleavage |
| FvLAC3 | FvH4_1g14990.t1 | miR857 | 21 | 4.5 | Cleavage |
| FvLAC7 | FvH4_2g08670.t1 | miR397a, miR397b | 21 | 1–2.5 | Cleavage |
| FvLAC7 | FvH4_2g08670.t1 | miR857 | 21 | 4.5 | Cleavage |
| FvLAC8 | FvH4_2g08840.t1 | miR397a, miR397b | 21 | 2–3.5 | Cleavage |
| FvLAC23 | FvH4_3g14660.t1 | miR857 | 21 | 5 | Translation |
| FvLAC26 | FvH4_3g34340.t1 | miR397b | 21 | 4 | Cleavage |
| FvLAC27 | FvH4_3g38140.t1 | miR397a, miR397b | 21 | 2–3.5 | Cleavage |
| FvLAC28 | FvH4_3g39750.t2 | miR397a, miR397b | 21 | 2.5–4 | Cleavage |
| FvLAC32 | FvH4_5g17150.t1 | miR397a | 21 | 3.5 | Cleavage |
| FvLAC34 | FvH4_5g35760.t2 | miR857 | 21 | 5 | Translation |
| FvLAC37 | FvH4_6g11960.t1 | miR397a, miR397b | 21 | 1–2.5 | Cleavage |
| FvLAC38 | FvH4_6g12410.t1 | miR397a, miR397b | 21 | 0–1.5 | Cleavage |
| FvLAC39 | FvH4_6g12430.t1 | miR397a, miR397b | 21 | 1.5–3 | Cleavage |
| FvLAC41 | FvH4_6g13430.t1 | miR397a, miR397b | 21 | 1.5–3 | Cleavage |
| FvLAC41 | FvH4_6g13430.t1 | miR857 | 21 | 4.5 | Cleavage |
| FvLAC52 | FvH4_7g16960.t1 | miR857 | 21 | 5 | Cleavage |
| FvLAC55 | FvH4_7g23980.t1 | miR397a | 21 | 4 | Cleavage |
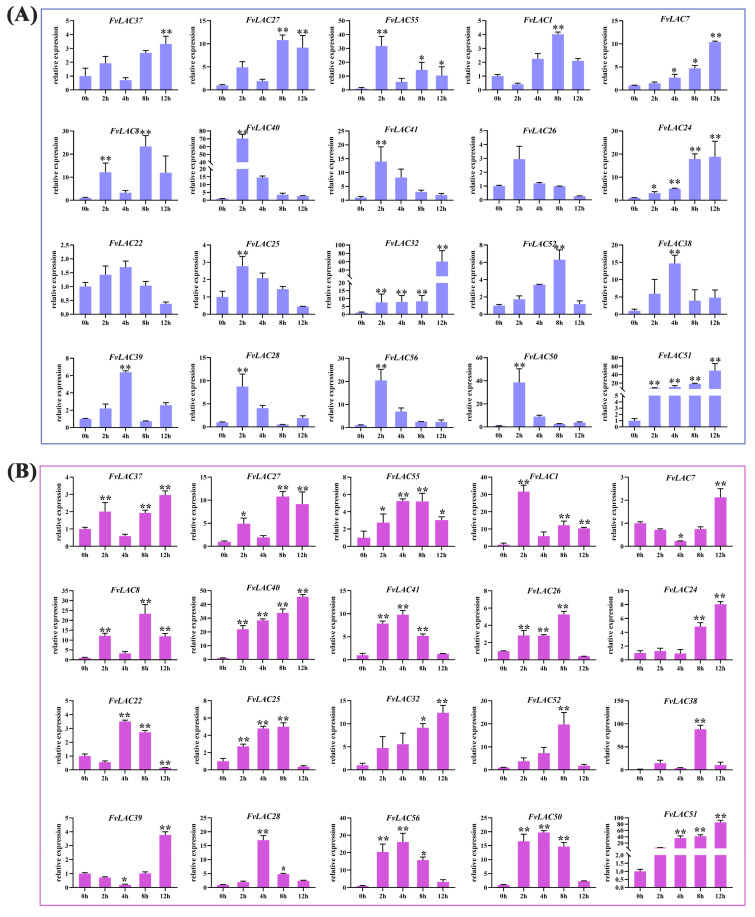
2.6. FvLAC Expression Responds to Salt and Drought Stresses
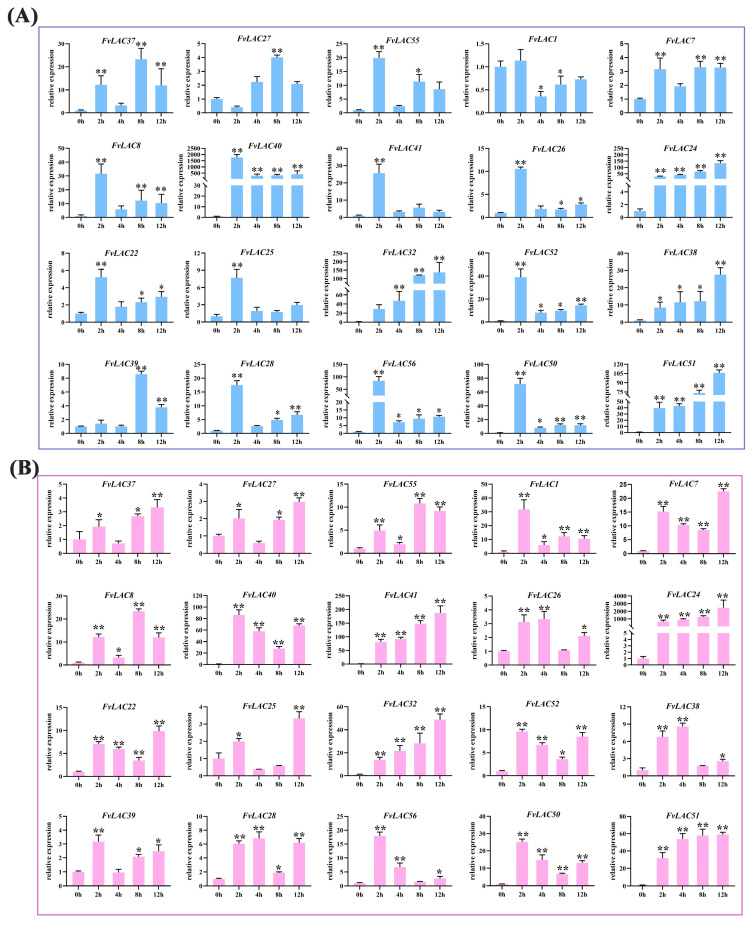
Salt and drought stresses are among the most prevalent abiotic stresses encountered by plants. To investigate the impact of these stresses on the FvLAC genes, we analyzed the relative expression of 20 FvLAC genes from groups I, II, and III in woodland strawberry subjected to salt and drought stresses using qRT-PCR. As illustrated in Figure 4A, under NaCl treatment, the expression levels of FvLAC7, FvLAC24, FvLAC32, and FvLAC51 exhibited a continuous increase over a 12 h period, while the expression levels of the remaining 16 FvLAC genes displayed a wave-like pattern. For instance, FvLAC40, FvLAC41, FvLAC26, FvLAC22, FvLAC25, and FvLAC52 demonstrated an initial increase followed by a decrease. Similarly, the expression trends of FvLAC37, FvLAC38, FvLAC39, FvLAC29, FvLAC28, and FvLAC56, along with the other 12 genes, were characterized by an initial upregulation followed by a decrease and a subsequent increase.
Figure 4.
Expression analysis of 20 FvLAC genes under salt and drought treatments by qRT-PCR. (A) The 20 FvLACs’ expression levels in seedlings under 300 mM NaCl treatments. (B) The 20 FvLACs’ expression levels in seedlings under 20% PEG-6000 treatments (Sangon Biotech, Shanghai, China). Error bars indicate standard deviations among three independent biological replications. FvActin was used as the internal control, 0 h was used as the mock, and the t-test was used to analyze three biological replicates of each sample. *: p-value < 0.05, **: p-value < 0.01.
In alignment with the results from NaCl treatment, the expression levels of FvLAC40, FvLAC24, FvLAC32, and FvLAC51 exhibited continuous increase after 12 h of PEG treatment. Additionally, seven FvLAC genes (FvLAC55, FvLAC41, FvLAC25, FvLAC52, FvLAC28, FvLAC56, FvLAC50) showed a trend of initial increase followed by a decrease, most with a peaking time at 4 h. FvLAC7 and FvLAC39 showed a trend of initial decrease followed by an increase, both reaching their lowest point at 4 h and their highest point at 12 h. The expression levels of the remaining seven FvLACs also displayed a wave pattern (Figure 4B). These results indicated that the 20 FvLAC genes respond differently to salt and drought stress over varying treatment times, which might contribute to their distinct biological functions under these stress conditions.
2.7. FvLACs Expression in Response to Hormone Treatments
The plant hormones abscisic acid (ABA) and methyl jasmonate (MeJA) play crucial roles in plant stress signaling pathways as well as in plant growth and development. To investigate whether the expression of FvLAC genes was induced by ABA and MeJA, we analyzed the expression patterns of these 20 genes in response to exogenous ABA and MeJA treatment using quantitative reverse transcription PCR (qRT-PCR). Under ABA treatment, the expression levels of 12 FvLAC genes peaked at 2 h before subsequently declining. These genes were FvLAC1, FvLAC7, FvLAC8, FvLAC40, FvLAC41, FvLAC26, FvLAC22, FvLAC25, FvLAC52, FvLAC28, FvLAC56, and FvLAC50. Notably, the expression levels of FvLAC24, FvLAC32, FvLAC28, and FvLAC51 consistently increased over time with the treatment, while the expression of the remaining four FvLAC genes (FvLAC37, FvLAC27, FvLAC55, and FvLAC39) exhibited a fluctuating pattern (Figure 5A). Specifically, the expression levels of FvLAC40 and FvLAC24 increased more than 100-fold following ABA treatment, whereas the expression of the other genes increased by several- to dozens-fold.
Figure 5.
Expression analysis of 20 FvLAC genes under ABA and MeJA treatments by qRT-PCR. (A) The 20 FvLACs’ expression levels in seedlings under 40 µmol/L ABA treatments. (B) The 20 FvLACs’ expression levels in seedlings under 40 µmol/L MeJA treatments. Error bars indicate standard deviations among three independent biological replications. FvActin was used as the internal control, 0 h was used as the mock, and t-test was used to analyze three biological replicates of each sample. *: p-value < 0.05, **: p-value < 0.01.
Under 12 h MeJA treatment, in addition to the continuous increase in the expression of FvLAC41, FvLAC24, FvLAC32, and FvLAC51, the expression of the remaining 16 genes displayed 2 distinct fluctuating patterns. For example, FvLAC37, FvLAC27, FvLAC7, FvLAC40, FvLAC26, FvLAC22, FvLAC25, FvLAC52, FvLAC38, FvLAC39, FvLAC28, FvLAC56, and FvLAC50 exhibited initial increases followed by decreases and subsequent increases (Figure 5B). Conversely, the expression of FvLAC55, FvLAC1, and FvLAC8 increased initially, then decreased, and then increased and decreased again. Among these, the expression of FvLAC24 increased by thousands of times, while the expression of the other genes increased by several- to tens-fold (Figure 5B).
2.8. The Tolerance of FvLACs to Salt and Drought Stresses in Yeast
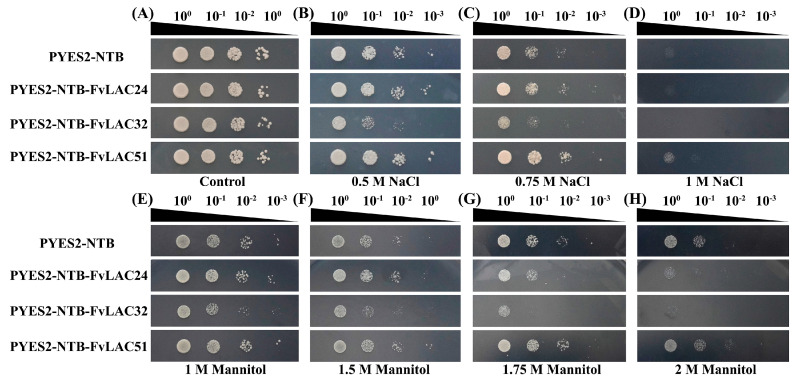
Through our analysis of FvLAC gene expression under conditions of salt, drought, and ABA and MeJA treatments, we observed a continuous increase in the expression levels of FvLAC24, FvLAC32, and FvLAC51 across all four treatments (Figure 4 and Figure 5). This observation prompted us to investigate the biological functions of these three genes further. The impact of FvLAC24, FvLAC32, and FvLAC51 on yeast growth and stress resistance was investigated in yeast strains harboring the FvLACs-overexpressing construct (PYES2-NTB-FvLAC24, PYES2-NTB-FvLAC32, and PYES2-NTB-FvLAC51). The results indicated that both the control (pYES2-NTB) and the three overexpression yeast strains exhibited typical growth characteristics on SG-Ura medium, suggesting that the enhanced expression of FvLACs in INVSc1 strains did not adversely affect their growth under normal conditions (Figure 6A). However, in media containing 0.5 M, 0.75 M, and 1 M NaCl, the FvLAC24 strain demonstrated a growth trend similar to that of the control, while the FvLAC32 strain exhibited significantly reduced salt tolerance, showing almost no growth after a 1000-fold dilution at 0.5 M NaCl and a 100-fold dilution at 0.75 M NaCl (Figure 6B,C). In contrast, the FvLAC51 strain displayed enhanced salt tolerance, while the other three strains perished without dilution under 1 M NaCl treatment. FvLAC51 remained weakly resistant to NaCl, indicating its potential to enhance salt tolerance in yeast strains (Figure 6D).
Figure 6.
The function analysis of FvLAC genes under salt and drought stresses in the yeast strain INVSC1. (A) The growth of control and FvLACs yeasts under unstress condition. (B–D) The function analysis of control, FvLAC24, FvLAC32, and FvLAC51 genes under different salt stresses in yeast strain INVSC1. (E–H) The function analysis of control, FvLAC24, FvLAC32, and FvLAC51 genes under different drought stresses in yeast strain INVSC1.
Similarly, FvLAC32 exhibited sensitivity to drought as mannitol concentration increased. In the presence of 1.75 M mannitol, the 10-fold diluted FvLAC32 strain perished, whereas the control, FvLAC24, and FvLAC51 strains continued to grow, with FvLAC51 demonstrating the strongest growth potential and insensitivity to drought stress (Figure 6E–H). The FvLAC24 gene exhibited higher sensitivity to both salt and drought stress in the presence of 1.75 M and 2 M mannitol compared with the control; these results suggest that FvLAC24 and FvLAC32 confer sensitivity to drought, while FvLAC51 appears to enhance tolerance to this stress (Figure 6E–H). Overall, these findings indicated that FvLAC32 may play a negative regulatory role under salt and drought stress, whereas FvLAC51 may play a positive regulatory role.
2.9. Subcellular Localization of FvLAC Genes
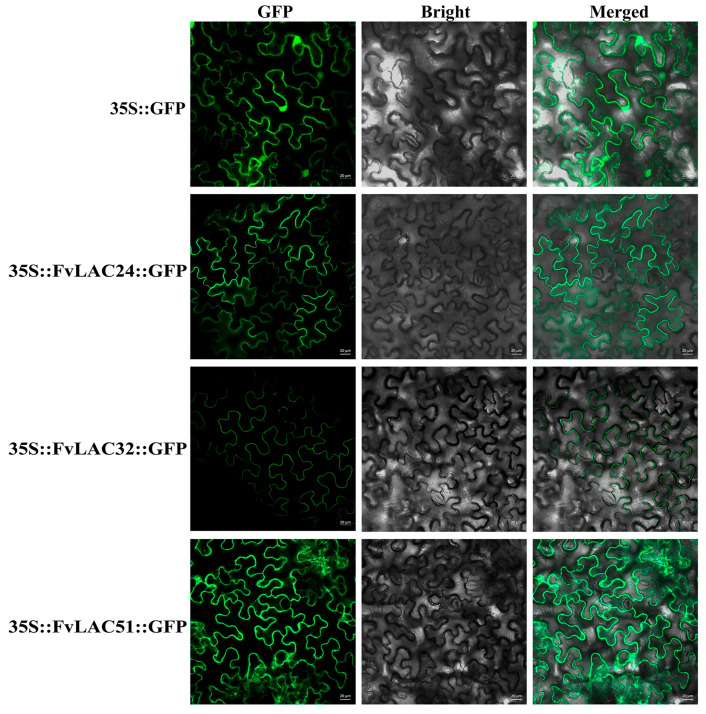
Subcellular localization provides crucial insights into a protein’s function. To further elucidate the function of FvLAC24, FvLAC32, and FvLAC51, we conducted a subcellular localization analysis. As illustrated in Figure 7, the green fluorescence of the empty vector (35S::GFP) was distributed on plasma membranes and in the nucleus, whereas the 35S::FvLAC24::GFP, 35S::FvLAC32::GFP, and 35S::FvLAC51::GFP fusion proteins were exclusively localized at the plasma membranes. This observation suggests that these three FvLAC proteins are likely localized at the plasma membranes. Furthermore, this result aligns with predictions made by the website and is consistent with findings regarding LAC protein subcellular localization in other species.
Figure 7.
The subcellular localization analysis of FvLAC24, FvLAC32, and FvLAC51 proteins. GFP driven by the CaMV35S promoter was used as a control. Bars = 20 µm.
3. Discussion
Plant laccase is a multifunctional oxidase primarily involved in lignin synthesis, flavonoid metabolism, wound repair, plant growth, and response to adverse stress-related processes [28]. Since the laccase gene was first cloned from Rhus vernicifera in 1995, numerous plant laccase genes have been cloned and reported. To date, we have identified 17 AtLACs in Arabidopsis [29], 29 laccases in Saccharum spontaneum L. [30], 24 CsLACs in Citrus sinensis [8], 45 BnLACs in Brassica napus [31], 44 GaLACs in Gossypium spp. [10], 27 SbLACs in Sorghum bicolor (L.) [32], and 54 EgrLACs in Eucalyptus grandis [33]. However, the laccases present in strawberry plants remain unknown. In this study, we identified the laccase gene family in the woodland strawberry using bioinformatics methods, resulting in the identification of a total of 57 family members. According to the evolutionary tree, the gene structures, conserved motifs, and physicochemical properties of FvLAC proteins within the same group exhibited remarkable similarities. These observations align closely with the presence of LACs in various other species [8,9] (Figure 1A).
The exon–intron structures and conserved motifs exhibited similar regularities. The FvLACs in groups Ⅰ to Ⅳ had 5 to 7 exons, which aligns with the exon–intron structure observed in Arabidopsis and soybeans. This similarity suggests that the FvLAC gene structure in groups I to IV has remained highly conserved throughout evolution (Figure 1B). Notably, the genes in group Ⅴ were found to be distantly related to Arabidopsis, with exon numbers varying from 1 to 9, highlighting a small degree of diversity in LAC gene structure that may ultimately contribute to variations in gene function (Figure 1B). A previous study indicated that divergences in coding regions, particularly those capable of altering gene function, could result from substitutions or modifications of amino acids that affect exon–intron structures [34]. This would cause the Arabidopsis and strawberry LAC genes with different functions to cluster into different groups. It has been reported that AtLAC4, AtLAC11, and AtLAC17 are necessary for lignin biosynthesis. Laccases of these two groups (Arabidopsis and strawberry) might be monolignol laccases that participate in lignin biosynthesis [7,35,36]. The genes encoding AtLAC5, AtLAC12, and AtLAC13 were found to respond to ABA signals; AtLAC6 was down-regulated when subjected to biological stress; and AtLAC8 affected the plants’ flowering time [29], therefore, it was speculated that group Ⅲ FvLAC members may be related to plant flowering time, abiotic stress, and the ABA signaling pathway.
Tandem repetition and fragment duplication are the primary mechanisms involved in the replication of gene families, contributing to the emergence and functional diversification of novel genes [37]. Our results indicate that most FvLAC genes are concentrated on chromosomes 3 and 7, comprising seven clusters of tandem repeat genes across four chromosomes and five pairs of duplicate genes on five chromosomes. This distribution may be attributed to tandem replication or whole-genome duplication (Figure 2A,B).
While many studies have characterized plant laccase genes, the detailed regulatory mechanisms remain poorly understood. Cis-regulatory elements can be bound by transcription factors (TFs) to regulate the gene expression at the transcriptional level. Our analysis identified 32 types of promoters, including 3 MYB-related elements, 5 stress-related elements, 9 hormone-related elements, 5 development-related elements, and 10 light-responsive elements. Notably, all FvLAC promoters contain MYB-related elements, integral components of MYB transcription factors (TFs). Previous studies have shown that these MYB TFs play a crucial role in secondary cell wall formation, plant growth, and stress response [38,39,40]. In Arabidopsis thaliana, AtMYB58/63 can activate the expression of AtLAC4 by interacting with AC elements in the promoter, enhancing lignin synthesis and regulating secondary cell walls’ formation [41]. ABRE elements generally exist in the promoters of ABA hormone-responsive genes and can be bound by AREB/ABF or bZIP TFs [42]. W-box elements primarily exist in the promoter regions of resistance genes related to the resistance to disease, drought, low temperature, and salt, and they regulate plant resistance by mediating hormone signal transduction pathways [43,44]. These results suggest that FvLACs are regulated by a variety of factors and functions in numerous physiological processes, including development, morphogenesis, and responses to biotic and abiotic stress.
In plants, miRNAs are essential for growth, development, and stress responses. The levels of LACs can be controlled by miRNAs through post-transcriptional regulation. Previous research has indicated that Csi-miR397 functions as a suppressor of LACs in citrus [45]. Additionally, strawberry miR397 can modulate the expression of LAC11a during the fruit-ripening process [46]. The miR397-LAC module also plays a role in the domestication of rice [12]. We found that only 16 LACs among the 57 LAC members can be regulated by miRNAs, specifically miR397a, miR397b, and miR857 (Table 2). Notably, these miRNAs also target and regulate the LAC genes in citrus, suggesting that the miRNAs targeting the FvLAC gene family are relatively conserved. In summary, whether through transcriptional regulation, post-transcriptional regulation, or post-translational modification, the in-depth research on these regulatory factors and their mechanisms will enhance our understanding of the FvLAC gene regulatory network involved in plant development and stress resistance.
Plant laccases are known to be associated with cytokinin degradation and the flavonoid process, both of which are essential for plant growth [47,48,49]. Furthermore, many studies have demonstrated that laccase genes are responsive to various types of stress. In Populus euphratica, the overexpression of PeuLAC2 c enhances drought tolerance by altering xylem structure and water transport [5]. Increased expression of the potential rice LAC family member OsChl1 in Arabidopsis significantly improves plant tolerance to drought and salt stresses [17]. Our qRT-PCR analysis revealed that the expression of the 20 selected FvLAC genes was significantly induced by salt, drought, and ABA and MeJA treatments, indicating that FvLACs play a role in these stress responses and providing novel targets for enhancing the stress resistance in the strawberry. Notably, three FvLAC genes exhibited continuously elevated expression levels over 12 h under both salt and drought stresses, prompting us to conduct functional validation of these genes in transgenic yeast. As illustrated in Figure 6, FvLAC24 and FvLAC32 were found to weaken the salt and drought tolerance of transgenic yeast, whereas FvLAC51 enhanced these tolerances (Figure 6A–H). These findings suggest that FvLAC24 and FvLAC32 may function as negative regulatory genes in response to salt and drought stress, while FvLAC51 acts as a positive regulatory gene under similar conditions. In terms of evolutionary relationships, FvLAC24 and FvLAC32 are classified within group Ⅱ, while FvLAC51 is categorized in group Ⅲ (Figure 1A); this clustering indicates that the functions of FvLACs within the same group are relatively conserved. Moreover, FvLAC32 displayed significantly greater sensitivity to salt and drought compared with FvLAC24, which may be attributed to its higher expression levels following stress treatment (Figure 4A,B). In conclusion, the findings of this research provide a systematic understanding of the FvLAC gene family in the woodland strawberry and enhance our comprehension of the roles of FvLAC genes in relation to salt and drought stress. However, the specific biological functions of FvLAC in response to salt and drought stresses in the woodland strawberry remain unclear, necessitating further exploration in future research.
4. Materials and Methods
4.1. Identification of LAC Family Genes in the Woodland Strawberry Genome
The whole genome sequence, coding sequence (CDS), and protein sequence of Fragaria vesca (v4.0.a2) were downloaded from the GDR database (https://www.rosaceae.org/search/genes (accessed on 21 July 2023)). The 17 AtLAC protein sequences in Arabidopsis were obtained from the TAIR database (https://www.arabidopsis.org/ (accessed on 21 July 2023)). Hidden Markov model (HMM) profiles (PF007731, PF00394, PF07732) of the LAC family were downloaded from the InterPro database (https://www.ebi.ac.uk/interpro/ (accessed on 21 July 2023)), and a local protein database was constructed for searches using Hmmer 3.0 with a predefined threshold of E < 1 × 10−3. Candidate proteins and domain verification were ultimately confirmed through the SMART website (http://smart.embl-heidelberg.de/ (accessed on 22 July 2023)). The ExPASy ProtParam tool (https://web.expasy.org/protparam/ (accessed on 22 July 2023)) was utilized to calculate protein length (number of amino acids [aa]), molecular weight (MW), isoelectric point (pI), aliphatic index, and grand averages of hydropathicity (GRAVY). The CELLO v.2.5 website (http://cello.life.nctu.edu.tw/ (accessed on 22 July 2023)) and Cell-PLoc 2.0 (http://www.csbio.sjtu.edu.cn/bioinf/Cell-PLoc-2 (accessed on 22 July 2023)) were used to speculate the subcellular localization of proteins.
4.2. Phylogenetic Tree, Multiple Sequence Alignments, and Gene Structure Analysis
The phylogenetic tree was constructed based on sequence data using the neighbor-joining method with 100 bootstrap replicates via MEGA-X software (https://www.megasoftware.net/) [50]. The Gene Structure Display Server 2.0 (http://gsds.gao-lab.org/ (accessed on 5 August 2023)) was employed to obtain the exon–intron structures of the FvLACs [51]. Conserved motifs were analyzed by using the online tool MEME (https://meme-suite.org/meme/ (accessed on 5 August 2023)), and both the gene structure and conserved motif map were generated through TBtools analysis and visualized using Adobe Illustrator 2020 software [52,53].
4.3. Chromosome Distribution and Collinearity Analysis of FvLACs
The chromosomal location information of the FvLAC genes was acquired from the gff3 annotation file of the woodland strawberry genome and was graphically visualized using TBtools [53]. To investigate the evolution of LAC genes, gene duplication and collinearity relationships were identified via MCScanX, and intergenomic synteny analysis was conducted between Arabidopsis thaliana and F. vesca [54]. The syntenic diagram was also generated using TBtools.
4.4. Promoter Cis-Acting Elements and Prediction of miRNA Targets in FvLACs
The 2000 bps upstream of the gene-coding sequence was extracted as the promoter region of FvLACs by the Gtf/gff3 Sequences Extractor option in TBtools [53]. Cis-acting element analyses were predicted through PlantCARE online tool (https://bioinformatics.psb.ugent.be/webtools/plantcare/html/ (accessed on 7 August 2023)), and the common functional elements were visually displayed via TBtools [22,53]. The online database psRNATarget server (https://www.zhaolab.org/psRNATarget/ (accessed on 7 August 2023)) was used to predict miRNAs with target sites in FvLAC genes, selecting those with an expectation number under 5 [55].
4.5. Plant Materials, Growth Conditions, Abiotic Stress Treatments, and Tissue Collection in F. vesca
The woodland strawberry (Ruegen) seeds used in this study were provided by Zhao Mizhen from the Jiangsu Academy of Agricultural Sciences. After germination, strawberry seedlings were transplanted into pots containing a mixture of soil and vermiculite (1:4) and grown in a phytotron under conditions of 16 h light /8 h dark at 22 °C, with light intensity of 12,000 lux and a relative humidity of 65%. Following a uniform growth period of 10 weeks, the seedlings were used for drought stress, salt stress, and hormone treatments concurrently. Specifically, 20% PEG 6000 and 300 mM NaCl solutions were employed to irrigate the roots to simulate drought and salt stresses, respectively [56,57]. In addition, 40 µmol/L ABA and 40 µmol/L MeJA solutions were sprayed on leaves to induce hormonal stresses. Samples consisting of the whole plant were collected at designated time points (0 h, 2 h, 4 h, 8 h, or 12 h) following stress and hormone treatments, with 0 h serving as the control. Each sample comprised three biological replicates, with each replicate including six plants. The collected samples were promptly wrapped in clean tinfoil, rapidly frozen in liquid nitrogen, and stored at −80 °C until further analysis.
4.6. RNA Extraction and Quantitative Real-Time PCR (RT-qPCR) Analysis
Total RNA from all samples was extracted using an RNAprep Pure Plant Plus Kit (Tiangen Biotech, Beijing, China) according to the manufacturer’s protocols. The quality and purity of RNA were evaluated by 1% agarose gels and a Nanodrop One microvolume UV-Vis spectrophotometer (Thermo Scientific, Waltham, MA, USA), with a 260/280 ratio ranging from 1.8 to 2.1 and deemed acceptable for reverse transcription. Total RNA was reverse transcribed into cDNA utilizing the EasyScript® One-Step gDNA Removal and cDNA Synthesis SuperMix Kit (Transgene, Beijing, China), and all cDNA samples were stored at −20 °C. Primers for candidate genes were designed using Primer Premier 5 software and NCBI-Primer Blast (https://www.ncbi.nlm.nih.gov/tools/primer-blast/ (accessed on 2 October 2023)), with FvActin and Fvtubulin serving as reference genes for RT-qPCR analysis (Table S1). The RT-qPCR reactions were conducted in a final volume of 15 μL f using PerfectStart® Green qPCR SuperMix (Transgene, Beijing, China), and the reaction program was executed in the ABI7500 thermal cycler (Applied Biosystems, Foster City, CA, USA). Relative expression levels were calculated using the 2−ΔΔCT method. The gene expression data are presented as mean ± SD and were analyzed for significant differences using ANOVA in GraphPad Prism 8 (NS: not significant; * p < 0.05; ** p < 0.01). Every experiment was conducted with three biological replicates, each of which was performed in triplicate.
4.7. Salt and Drought Stress Treatments of Transgenic Yeasts
The open reading frame (ORF) containing the termination codon of FvLAC24, FvLAC32, and FvLAC51 was amplified by PCR from the cDNA of the ‘Ruegen’ strawberry and subsequently cloned into the pYES2-NTB vector to create the fusion plasmids PYES2-NTB-FvLAC24, PYES2-NTB-FvLAC32, and PYES2-NTB-FvLAC51. The empty vector, along with the three fusion vectors, was transformed into the yeast strain INVSC1, which was then plated on SD-Ura medium and cultured at 29 °C for 3–4 days. Positive clones were dissolved in SD liquid medium and cultured until the OD600 reached 1.2 to 1.4. Following centrifugation, the cells were resuspended in SD-Ura liquid medium devoid of a carbon source and subjected to shaking culture for 3 h. After a second centrifugation, the cells were resuspended in SG-Ura containing 2% galactose and incubated for 8 to12 h. The cells were then centrifuged again, and the OD600 of the yeast cell suspension was adjusted to 0.45. Serial dilutions (100, 10−1, 10−2, 10−3) were performed, and the diluted samples were plated on SG-Ura agar plates containing varying concentrations of NaCl (0.5 M, 0.75 M, and 1 M) and mannitol (1 M, 1.5 M, 1.75 M, and 2 M) [58]. The plates were subsequently incubated at 29 °C for 3 days, and the growth of yeast colonies was observed and recorded [59].
4.8. Subcellar Localization Assays
The open reading frames (ORFs) of FvLAC24, FvLAC32, and FvLAC51, excluding the termination codon, were amplified via PCR from cDNA of ‘Ruegen’ strawberry and subsequently cloned into the pMDC43 vector to construct the fusion protein. The recombinant vectors, along with empty vector plasmids, were then transferred into Agrobacterium tumefaciens strain GV3101 and transiently expressed in tobacco (Nicotiana benthamiana) leaves. From 48 to 72 h after injection, the GFP signals were observed using a laser scanning confocal microscope (LSM 880, Zeiss, Germany), and the gene-specific clone primers are listed in Supplement Table S2.
5. Conclusions
In this study, we identified and analyzed a total of 57 FvLAC genes in the Fragaria vesca genome, focusing on their physical and chemical properties, phylogenetic relationships, gene structure, conserved motifs, chromosomal localization, gene duplications, and cis-acting elements. Furthermore, we assessed the expression levels from 20 FvLAC genes in groups I, II, and III in response to abiotic stress and hormone treatments, revealing that these genes are responsive to salt, drought, ABA, and MeJA stresses. Notably, the expression levels of FvLAC24, FvLAC32, and FvLAC51 consistently increased within 12 h following stress exposure. Transgenic yeast experiments demonstrated that FvLAC51 enhances yeast tolerance to salt and drought, while FvLAC24 and FvLAC51 negatively regulate yeast tolerance under these conditions. Subcellular localization experiments indicated that FvLAC24, FvLAC32, and FvLAC51 are situated within the cell membranes. These findings offer significant insights into the evolutionary processes, expansion, regulation, and expression patterns of FvLAC genes in the woodland strawberry, which provide valuable information for further investigation into the functions of FvLAC genes in the woodland strawberry.
Acknowledgments
We would like to thank the Strawberry Innovation team of the Fruit Tree Research Institute of Jiangsu Academy of Agricultural Sciences for generously providing strawberry seeds and all those who contributed to this article.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants13233366/s1, Table S1: Cis-elements analysis of FvLAC gene promoters; Table S2: The primers used in this study.
Author Contributions
Conceptualization, Q.S.; methodology, J.K., R.X., and K.Q.; software, X.L.; resources, L.L.; data curation, X.L. and D.L.; writing—original draft preparation, J.K. and J.Z.; writing—review and editing, J.K. and S.Z.; project administration, M.L.; funding acquisition, Q.S. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
All the accession numbers of the woodland strawberry (Fragaria vesca) FvLAC genes in this study can be found in Table 1.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by the Key Research and Development Program of Anhui Province (202104f06020004) and grants from the National Modern Agricultural Industrial Technology System Program (CARS-24-G-09).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Dwivedi U.N., Singh P., Pandey V.P., Kumar A. Structure–function relationship among bacterial, fungal and plant laccases. J. Mol. Catal. B Enzym. 2011;68:117–128. doi: 10.1016/j.molcatb.2010.11.002. [DOI] [Google Scholar]
- 2.Hoffmann N., Benske A., Betz H., Schuetz M., Samuels A.L. Laccases and Peroxidases Co-Localize in Lignified Secondary Cell Walls throughout Stem Development. Plant Physiol. 2020;184:806–822. doi: 10.1104/pp.20.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldrian P. Fungal laccases—Occurrence and properties. FEMS Microbiol. Rev. 2006;30:215–242. doi: 10.1111/j.1574-4976.2005.00010.x. [DOI] [PubMed] [Google Scholar]
- 4.Barros J., Serk H., Granlund I., Pesquet E. The cell biology of lignification in higher plants. Ann. Bot. 2015;115:1053–1074. doi: 10.1093/aob/mcv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niu Z., Li G., Hu H., Lv J., Zheng Q., Liu J., Wan D. A gene that underwent adaptive evolution, LAC2 (LACCASE), in Populus euphratica improves drought tolerance by improving water transport capacity. Hortic. Res. 2021;8:88. doi: 10.1038/s41438-021-00518-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin S., Fan C., Li X., Li Y., Hu J., Li C., Luo K. LACCASE14 is required for the deposition of guaiacyl lignin and affects cell wall digestibility in poplar. Biotechnol. Biofuels. 2020;13:197. doi: 10.1186/s13068-020-01843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berthet S., Demont-Caulet N., Pollet B., Bidzinski P., Cézard L., Le Bris P., Borrega N., Hervé J., Blondet E., Balzergue S., et al. Disruption of LACCASE4 and 17 Results in Tissue-Specific Alterations to Lignification of Arabidopsis thaliana Stems. Plant Cell. 2011;23:1124–1137. doi: 10.1105/tpc.110.082792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu X., Zhou Y., Wang B., Ding L., Wang Y., Luo L., Zhang Y., Kong W. Genome-wide identification and characterization of laccase gene family in Citrus sinensis. Gene. 2019;689:114–123. doi: 10.1016/j.gene.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 9.Cheng X., Li G., Ma C., Abdullah M., Zhang J., Zhao H., Jin Q., Cai Y., Lin Y. Comprehensive genome-wide analysis of the pear (Pyrus bretschneideri) laccase gene (PbLAC) family and functional identification of PbLAC1 involved in lignin biosynthesis. PLoS ONE. 2019;14:e0210892. doi: 10.1371/journal.pone.0210892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balasubramanian V.K., Rai K.M., Thu S.W., Hii M.M., Mendu V. Genome-wide identification of multifunctional laccase gene family in cotton (Gossypium spp.); expression and biochemical analysis during fiber development. Sci. Rep. 2016;6:34309. doi: 10.1038/srep34309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ranocha P., Chabannes M., Chamayou S., Danoun S.d., Jauneau A., Boudet A.-M., Goffner D. Laccase Down-Regulation Causes Alterations in Phenolic Metabolism and Cell Wall Structure in Poplar. Plant Physiol. 2002;129:145–155. doi: 10.1104/pp.010988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Q., Luo L., Wang X., Shen Z., Zheng L. Comprehensive Analysis of Rice Laccase Gene (OsLAC) Family and Ectopic Expression of OsLAC10 Enhances Tolerance to Copper Stress in Arabidopsis. Int. J. Mol. Sci. 2017;18:209. doi: 10.3390/ijms18020209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yonekura-Sakakibara K., Yamamura M., Matsuda F., Ono E., Nakabayashi R., Sugawara S., Mori T., Tobimatsu Y., Umezawa T., Saito K. Seed-coat protective neolignans are produced by the dirigent protein AtDP1 and the laccase AtLAC5 in Arabidopsis. Plant Cell. 2020;33:129–152. doi: 10.1093/plcell/koaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Bris P., Wang Y., Barbereau C., Antelme S., Cézard L., Legée F., D’Orlando A., Dalmais M., Bendahmane A., Schuetz M., et al. Inactivation of LACCASE8 and LACCASE5 genes in Brachypodium distachyon leads to severe decrease in lignin content and high increase in saccharification yield without impacting plant integrity. Biotechnol. Biofuels. 2019;12:181. doi: 10.1186/s13068-019-1525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bryan A.C., Jawdy S., Gunter L., Gjersing E., Sykes R., Hinchee M.A.W., Winkeler K.A., Collins C.M., Engle N., Tschaplinski T.J., et al. Knockdown of a laccase in Populus deltoides confers altered cell wall chemistry and increased sugar release. Plant Biotechnol. J. 2016;14:2010–2020. doi: 10.1111/pbi.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y., Wu L., Wang X., Chen B., Zhao J., Cui J., Li Z., Yang J., Wu L., Wu J., et al. The cotton laccase gene GhLAC15 enhances Verticillium wilt resistance via an increase in defence-induced lignification and lignin components in the cell walls of plants. Mol. Plant Pathol. 2018;20:309–322. doi: 10.1111/mpp.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho H.Y., Lee C., Hwang S.-G., Park Y.C., Lim H.L., Jang C.S. Overexpression of the OsChI1 gene, encoding a putative laccase precursor, increases tolerance to drought and salinity stress in transgenic Arabidopsis. Gene. 2014;552:98–105. doi: 10.1016/j.gene.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 18.Li L., Yang K., Wang S., Lou Y., Zhu C., Gao Z. Genome-wide analysis of laccase genes in moso bamboo highlights PeLAC10 involved in lignin biosynthesis and in response to abiotic stresses. Plant Cell Rep. 2020;39:751–763. doi: 10.1007/s00299-020-02528-w. [DOI] [PubMed] [Google Scholar]
- 19.Xu X., Zhang Y., Liang M., Kong W., Liu J. The Citrus Laccase Gene CsLAC18 Contributes to Cold Tolerance. Int. J. Mol. Sci. 2022;23:14509. doi: 10.3390/ijms232314509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L., Wang L., Zeng X., Chen R., Yang S., Pan S. Comparative transcriptome analysis reveals fruit discoloration mechanisms in postharvest strawberries in response to high ambient temperature. Food Chem. X. 2019;2:100025. doi: 10.1016/j.fochx.2019.100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H., Yue M., Jiang L., Liu Y., Zhang N., Liu X., Ye Y., Lin X., Zhang Y., Lin Y., et al. Genome-Wide Identification of Strawberry C2H2-ZFP C1-2i Subclass and the Potential Function of FaZAT10 in Abiotic Stress. Int. J. Mol. Sci. 2022;23:13079. doi: 10.3390/ijms232113079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lescot M., Déhais P., Thijs G., Marchal K., Moreau Y., Van de Peer Y., Rouzé P., Rombauts S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30:325–327. doi: 10.1093/nar/30.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu Y., Jia T., Chen X. The ‘how’ and ‘where’ of plant microRNAs. New Phytol. 2017;216:1002–1017. doi: 10.1111/nph.14834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang C.Y., Zhang S., Yu Y., Luo Y.C., Liu Q., Ju C., Zhang Y.C., Qu L.H., Lucas W.J., Wang X., et al. MiR397b regulates both lignin content and seed number in Arabidopsis via modulating a laccase involved in lignin biosynthesis. Plant Biotechnol. J. 2014;12:1132–1142. doi: 10.1111/pbi.12222. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Y., Lin S., Qiu Z., Cao D., Wen J., Deng X., Wang X., Lin J., Li X. MicroRNA857 Is Involved in the Regulation of Secondary Growth of Vascular Tissues in Arabidopsis. Plant Physiol. 2015;169:2539–2552. doi: 10.1104/pp.15.01011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y.-C., Yu Y., Wang C.-Y., Li Z.-Y., Liu Q., Xu J., Liao J.-Y., Wang X.-J., Qu L.-H., Chen F., et al. Overexpression of microRNA OsmiR397 improves rice yield by increasing grain size and promoting panicle branching. Nat. Biotechnol. 2013;31:848–852. doi: 10.1038/nbt.2646. [DOI] [PubMed] [Google Scholar]
- 27.Lu S., Li Q., Wei H., Chang M.-J., Tunlaya-Anukit S., Kim H., Liu J., Song J., Sun Y.-H., Yuan L., et al. Ptr-miR397a is a negative regulator of laccase genes affecting lignin content in Populus trichocarpa. Proc. Natl. Acad. Sci. USA. 2013;110:10848–10853. doi: 10.1073/pnas.1308936110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bai Y., Ali S., Liu S., Zhou J., Tang Y. Characterization of plant laccase genes and their functions. Gene. 2023;852:147060. doi: 10.1016/j.gene.2022.147060. [DOI] [PubMed] [Google Scholar]
- 29.Cai X., Davis E.J., Ballif J., Liang M., Bushman E., Haroldsen V., Torabinejad J., Wu Y. Mutant identification and characterization of the laccase gene family in Arabidopsis. J. Exp. Bot. 2006;57:2563–2569. doi: 10.1093/jxb/erl022. [DOI] [PubMed] [Google Scholar]
- 30.Zhang W., Lin J., Dong F., Ma Q., Wu S., Ma X., Fatima M., Jia H., Ming R. Genomic and Allelic Analyses of Laccase Genes in Sugarcane (Saccharum spontaneum L.) Trop. Plant Biol. 2019;12:219–229. doi: 10.1007/s12042-019-09239-x. [DOI] [Google Scholar]
- 31.Ping X., Wang T., Lin N., Di F., Li Y., Jian H., Wang H., Lu K., Li J., Xu X., et al. Genome-Wide Identification of the LAC Gene Family and Its Expression Analysis Under Stress in Brassica napus. Molecules. 2019;24:1985. doi: 10.3390/molecules24101985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J., Feng J., Jia W., Fan P., Bao H., Li S., Li Y. Genome-Wide Identification of Sorghum bicolor Laccases Reveals Potential Targets for Lignin Modification. Front. Plant Sci. 2017;8:714. doi: 10.3389/fpls.2017.00714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arcuri M.L.C., Fialho L.C., Vasconcellos Nunes-Laitz A., Fuchs-Ferraz M.C.P., Wolf I.R., Valente G.T., Marino C.L., Maia I.G. Genome-wide identification of multifunctional laccase gene family in Eucalyptus grandis: Potential targets for lignin engineering and stress tolerance. Trees. 2020;34:745–758. doi: 10.1007/s00468-020-01954-3. [DOI] [Google Scholar]
- 34.Xu G., Guo C., Shan H., Kong H. Divergence of duplicate genes in exon–intron structure. Proc. Natl. Acad. Sci. USA. 2012;109:1187–1192. doi: 10.1073/pnas.1109047109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao Q., Nakashima J., Chen F., Yin Y., Fu C., Yun J., Shao H., Wang X., Wang Z.-Y., Dixon R.A. LACCASE Is Necessary and Nonredundant with PEROXIDASE for Lignin Polymerization during Vascular Development in Arabidopsis. Plant Cell. 2013;25:3976–3987. doi: 10.1105/tpc.113.117770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu Y. LACCASE2 Negatively Regulates Lignin Deposition of Arabidopsis Roots. Plant Physiol. 2020;182:1190–1191. doi: 10.1104/pp.20.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Panchy N., Lehti-Shiu M., Shiu S.-H. Evolution of Gene Duplication in Plants. Plant Physiol. 2016;171:2294–2316. doi: 10.1104/pp.16.00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCarthy R.L., Zhong R., Fowler S., Lyskowski D., Piyasena H., Carleton K., Spicer C., Ye Z.-H. The Poplar MYB Transcription Factors, PtrMYB3 and PtrMYB20, are Involved in the Regulation of Secondary Wall Biosynthesis. Plant Cell Physiol. 2010;51:1084–1090. doi: 10.1093/pcp/pcq064. [DOI] [PubMed] [Google Scholar]
- 39.Kim S.H., Lam P.Y., Lee M.-H., Jeon H.S., Tobimatsu Y., Park O.K. The Arabidopsis R2R3 MYB Transcription Factor MYB15 Is a Key Regulator of Lignin Biosynthesis in Effector-Triggered Immunity. Front. Plant Sci. 2020;11:583153. doi: 10.3389/fpls.2020.583153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu Y., Liu H., Zhang N., Gao C., Qi L., Wang C. The BpMYB4 Transcription Factor From Betula platyphylla Contributes Toward Abiotic Stress Resistance and Secondary Cell Wall Biosynthesis. Front. Plant Sci. 2021;11:606062. doi: 10.3389/fpls.2020.606062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou J., Lee C., Zhong R., Ye Z.-H. MYB58 and MYB63 Are Transcriptional Activators of the Lignin Biosynthetic Pathway during Secondary Cell Wall Formation in Arabidopsis. Plant Cell. 2009;21:248–266. doi: 10.1105/tpc.108.063321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waadt R., Seller C.A., Hsu P.-K., Takahashi Y., Munemasa S., Schroeder J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022;23:680–694. doi: 10.1038/s41580-022-00479-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang J., Tian X., Mei E., He M., Gao J., Yu J., Xu M., Liu J., Song L., Li X., et al. WRKY53 negatively regulates rice cold tolerance at the booting stage by fine-tuning anther gibberellin levels. Plant Cell. 2022;34:4495–4515. doi: 10.1093/plcell/koac253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang L., Guo D., Zhao G., Wang J., Zhang S., Wang C., Guo X. Group IIc WRKY transcription factors regulate cotton resistance to Fusarium oxysporum by promoting GhMKK2-mediated flavonoid biosynthesis. New Phytol. 2022;236:249–265. doi: 10.1111/nph.18329. [DOI] [PubMed] [Google Scholar]
- 45.Huang J.H., Zhang L.Y., Lin X.J., Gao Y., Zhang J., Huang W.L., Zhao D., Ferrarezi R.S., Fan G.C., Chen L.S. CsiLAC4 modulates boron flow in Arabidopsis and Citrus via high-boron-dependent lignification of cell walls. New Phytol. 2022;233:1257–1273. doi: 10.1111/nph.17861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mittelsten Scheid O., Tang Y., Qu Z., Lei J., He R., Adelson D.L., Zhu Y., Yang Z., Wang D. The long noncoding RNA FRILAIR regulates strawberry fruit ripening by functioning as a noncanonical target mimic. PLoS Genet. 2021;17:e1009461. doi: 10.1371/journal.pgen.1009461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galuszka P., Frébortová J., Luhová L., Bilyeu K., English J., Frébort I. Tissue localization of cytokinin dehydrogenase in maize Possible involvement of quinone species generated from plant phenolics by other enzymatic systems in the catalytic reaction. Plant Cell Physiol. 2005;46:716–728. doi: 10.1093/pcp/pci074. [DOI] [PubMed] [Google Scholar]
- 48.Pourcel L., Routaboul J.-M., Kerhoas L., Caboche M., Lepiniec L.c., Debeaujon I. TRANSPARENT TESTA10 Encodes a Laccase-Like Enzyme Involved in Oxidative Polymerization of Flavonoids in Arabidopsis Seed Coat. Plant Cell. 2005;17:2966–2980. doi: 10.1105/tpc.105.035154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu Q., Xiao S., Guan Q., Tu L., Sheng F., Du X., Zhang X. The laccase gene GhLac1 modulates fiber initiation and elongation by coordinating jasmonic acid and flavonoid metabolism. Crop J. 2020;8:522–533. doi: 10.1016/j.cj.2019.11.006. [DOI] [Google Scholar]
- 50.Kumar S., Stecher G., Li M., Knyaz C., Tamura K., Battistuzzi F.U. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu B., Jin J., Guo A.-Y., Zhang H., Luo J., Gao G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics. 2015;31:1296–1297. doi: 10.1093/bioinformatics/btu817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bailey T.L., Johnson J., Grant C.E., Noble W.S. The MEME Suite. Nucleic Acids Res. 2015;43:W39–W49. doi: 10.1093/nar/gkv416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen C., Chen H., Zhang Y., Thomas H.R., Frank M.H., He Y., Xia R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant. 2020;13:1194–1202. doi: 10.1016/j.molp.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 54.Wang Y., Tang H., DeBarry J.D., Tan X., Li J., Wang X., Lee T.h., Jin H., Marler B., Guo H., et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40:e49. doi: 10.1093/nar/gkr1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dai X., Zhao P.X. pssRNAMiner: A plant short small RNA regulatory cascade analysis server. Nucleic Acids Res. 2008;36:W114–W118. doi: 10.1093/nar/gkn297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Z., Xie Q., Yan J., Chen J., Chen Q. Genome-Wide Identification and Characterization of the Abiotic-Stress-Responsive GRF Gene Family in Diploid Woodland Strawberry (Fragaria vesca) Plants. 2021;10:1916. doi: 10.3390/plants10091916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li W., Zhong J., Zhang L., Wang Y., Song P., Liu W., Li X., Han D. Overexpression of a Fragaria vesca MYB Transcription Factor Gene (FvMYB82) Increases Salt and Cold Tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2022;23:10538. doi: 10.3390/ijms231810538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guan C., Li W., Wang G., Yang R., Zhang J., Zhang J., Wu B., Gao R., Jia C. Transcriptomic analysis of ncRNAs and mRNAs interactions during drought stress in switchgrass. Plant Sci. 2024;339:111930. doi: 10.1016/j.plantsci.2023.111930. [DOI] [PubMed] [Google Scholar]
- 59.Jia T., Hou J., Iqbal M.Z., Zhang Y., Cheng B., Feng H., Li Z., Liu L., Zhou J., Feng G., et al. Overexpression of the white clover TrSAMDC1 gene enhanced salt and drought resistance in Arabidopsis thaliana. Plant Physiol. Biochem. 2021;165:147–160. doi: 10.1016/j.plaphy.2021.05.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the accession numbers of the woodland strawberry (Fragaria vesca) FvLAC genes in this study can be found in Table 1.