Abstract
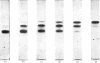
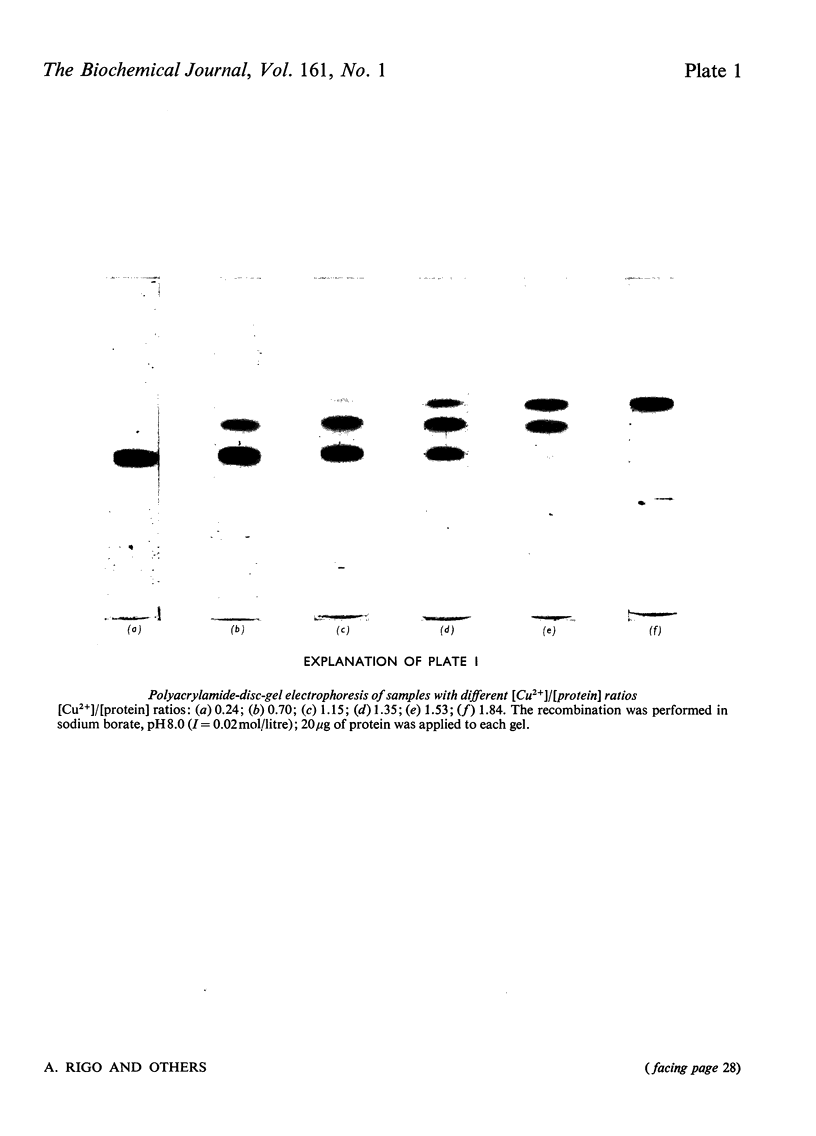
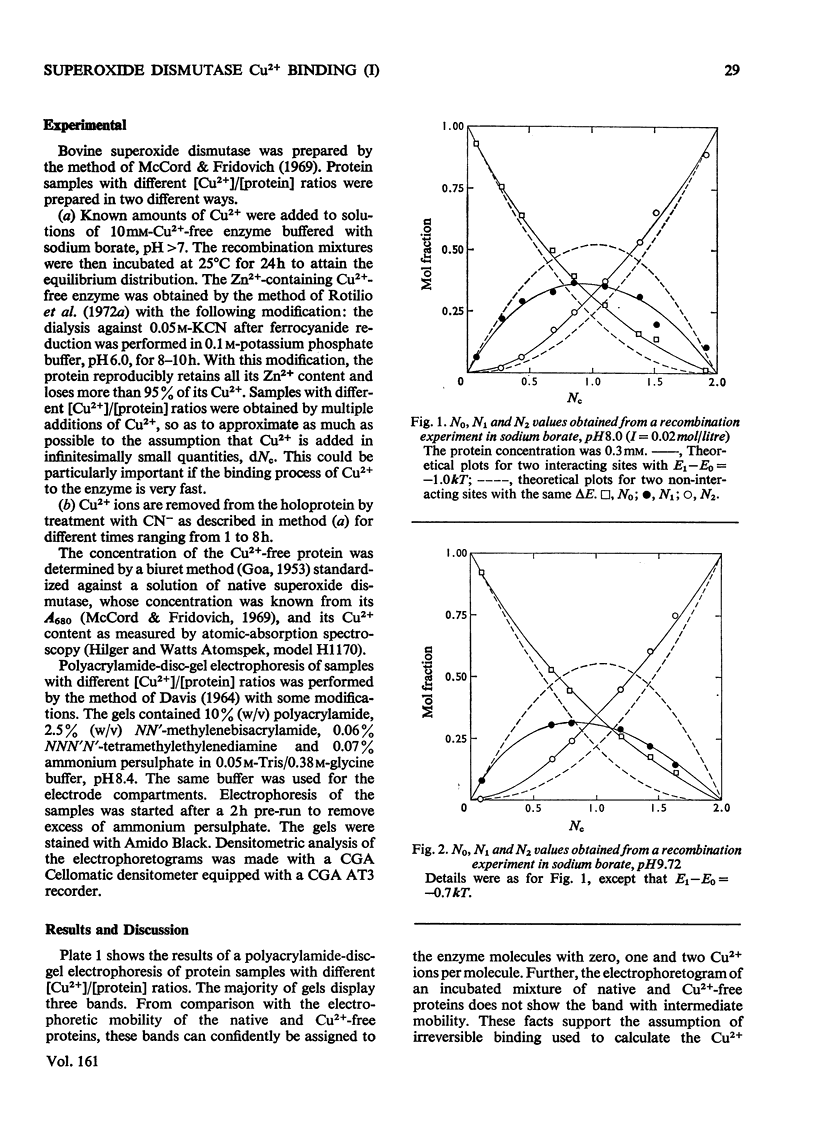
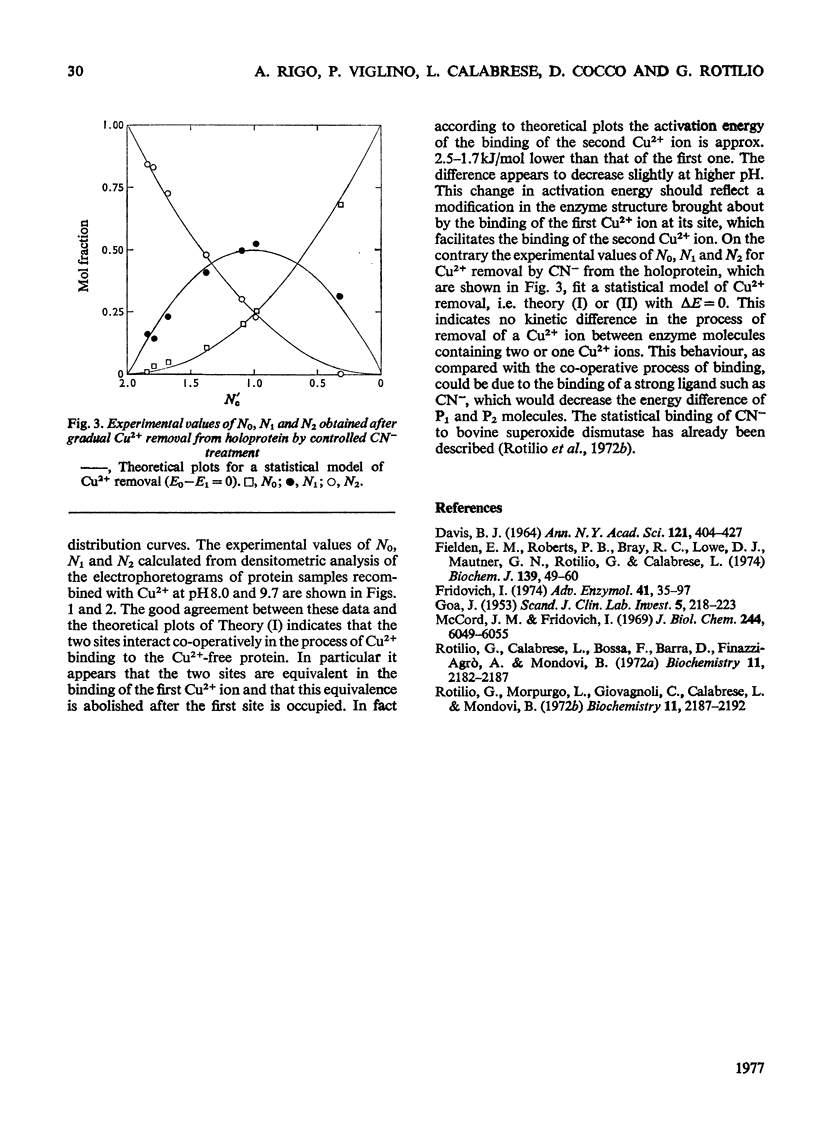
Samples of superoxide dismutase containing less than stoicheiometric amounts of Cu2+ were obtained by either partial re-addition of Cu2+ to the Cu2+-free protein or partial removal of Cu2+ by controlled CN-treatment. In these samples the distribution of the metal between the two identical sites on the two subunits was studied by quantitative gel electrophoresis and found to be statistical only in the process of copper removal by CN-. In the other case the distribution fits a model of co-operative interaction between the two sites, where the sites are equivalent for the binding of the first Cu2+ ion, but the occupation of the first site lowers the activation energy of the binding of the second Cu2+ ion. This indicates that binding of Cu2+ ion at its site on one subunit brings about conformational changes that facilitate Cu2+ binding on the other subunit. These results may relate to possible intersubunit interactions during the catalytic activity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Fielden E. M., Roberts P. B., Bray R. C., Lowe D. J., Mautner G. N., Rotilio G., Calabrese L. Mechanism of action of superoxide dismutase from pulse radiolysis and electron paramagnetic resonance. Evidence that only half the active sites function in catalysis. Biochem J. 1974 Apr;139(1):49–60. doi: 10.1042/bj1390049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):35–97. doi: 10.1002/9780470122860.ch2. [DOI] [PubMed] [Google Scholar]
- GOA J. A micro biuret method for protein determination; determination of total protein in cerebrospinal fluid. Scand J Clin Lab Invest. 1953;5(3):218–222. doi: 10.3109/00365515309094189. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Rotilio G., Calabrese L., Bossa F., Barra D., Agrò A. F., Mondovì B. Properties of the apoprotein and role of copper and zinc in protein conformation and enzyme activity of bovine superoxide dismutase. Biochemistry. 1972 May 23;11(11):2182–2187. doi: 10.1021/bi00761a027. [DOI] [PubMed] [Google Scholar]
- Rotilio G., Morpurgo L., Giovagnoli C., Calabrese L., Mondovì B. Studies of the metal sites of copper proteins. Symmetry of copper in bovine superoxide dismutase and its functional significance. Biochemistry. 1972 May 23;11(11):2187–2192. doi: 10.1021/bi00761a028. [DOI] [PubMed] [Google Scholar]