Abstract
Due to their effectiveness at low doses and relative safety for non-target species, plant essential oils (EOs) are considered ideal alternatives to conventional pesticides for pest control. In this study, the chemical composition of Thymus serpyllum (T. serpyllum) EO was construed by Gas Chromatography-Mass Spectrometry (GC-MS), and its larvicidal and ovicidal activity against omnivorous pests Spodoptera litura (S. litura) was assessed. The effects of T. serpyllum EO on the activities of antioxidant detoxification enzymes were also measured. GC–MS analysis revealed that the main constituents of T. serpyllum EO were thymol (42.1%), p-cymene (22.4%), and γ-terpinene (18.6%). In the larvicidal toxicity experiment, the T. serpyllum EO demonstrated LC50 values of 0.606 and 0.664 mg/mL against the second- and third-instar larvae of S. litura, respectively, after 48 h exposure. Moreover, an EC50 value of 0.905 mg/mL was measured against S. litura eggs. In S. litura, T. serpyllum EO treatment reduced the enzymatic activity of ESTs and GST and, conversely, increased the enzymatic activity of AChE. Overall, this study demonstrated that T. serpyllum EO has the potential to be implemented as a novel eco-friendly insecticide against S. litura.
Keywords: essential oil, enzyme inhibition, agricultural pest control, chemical composition
1. Introduction
Spodoptera litura (Fabricius, 1775) (Lepidoptera: Noctuidae) is a typical destructive crop pest worldwide [1]. Over 300 plant species have been identified as its hosts, including cotton, maize, vegetables, rice, groundnut, and soybean, strongly impacting the agricultural industry [2,3,4]. S. litura (Spodoptera litura Fabricius, 1775) (Lepidoptera: Noctuidae) exhibits a high reproductive and developmental ability, resulting in five to six overlapping generations annually. S. litura larvae are characterized by their ability to feed on leaves, buds, fruits, and flowers. If not treated in time, it might cause severe crop losses or even destruction [5,6]. Globally, synthetic insecticides are often used to control S. litura. However, due to their extensive application and due to long-term interactions between pesticides and insects, S. litura has developed resistance to many conventional pesticides, including organochlorides, organophosphates, cyantraniliprole, pyrethroids, abamectin, avermectins, and indoxacarb [1,7,8,9]. To date, severe insecticide resistance has been observed in S. litura in numerous countries, including China, Puerto Rico, Mexico, India, Pakistan, and Thailand [1,3].
In recent years, considering the non-selectivity and persistence of chemical pesticides, plant essential oils (EOs) and their derivatives garnered a growing interest due to their effectiveness at low doses [10] and relative safety to non-target organisms [11]. They can be completely degraded in the environment, leaving no residues, and are regarded as a valuable resource for the development and formulation of environmentally friendly pesticides with low toxicity. Recently, extracts or EOs from various plants such as Crithmum maritimum L. [12], Couroupita guianensis (Aubl.) [13], Piper betle L. [14], Zanthoxylum armatum DC. [15], Inula racemosa (Asteraceae) [16], Zanthoxylum alatum Roxb. [17], Vernonia anthelmintica L. [18], Wedelia prostrata (Hook. et Arn.) Hemsl. [19], Acorus calamus L. [20], Alpinia galanga (Linn.) Willd, and Ocimum basilicum L. [21] have been assessed on S. litura to examine their toxicity and antifeedant potential. Essential oils and their major constituents can serve as safe alternatives for pest control. Mahajan et al. [20] reported that β-caryophyllene exhibited a satisfactory inhibitory activity on the growth and development of S. litura. Yooboon et al. [21] discovered that piperine and β-asarone showed acute toxicity against S. litura, with their combination demonstrating greater acute toxicity than the individual compounds. Additionally, thymol [22], pogostone [23], and pulegone [3] were found to be effective against S. litura.
Thyme L. plants belong to the Lamiaceae family and are perennial herbs or low shrubs. More than 250 species of this genus have been recorded worldwide, with wide distribution in Northern Africa, temperate regions of Europe, and Asia [24]. Essential oils have been extensively adopted and utilized by the pharmaceuticals sector and the food sector for their potential biological properties and activities [25]. Previous studies have shown that Thymus serpyllum (L.) (T. serpyllum) EO has valuable biological activities, including antibacterial [26], antimicrobial [27], and antifungal activities [28]. Regarding its insecticidal activity, T. serpyllum EO exhibited notable efficacy in the control of Frankliniella occidentalis (Pergande) [29], Acanthoscelides obtectus (Say) (A. obtectus) [30], Musca domestica L. (M. domestica) [24], Varroa destructor (V. destructor) (Anderson and Trueman) [31] and Reticulitermes dabieshanensis (R. dabieshanensis) according to Wang et Li [32]. The composition of T. serpyllum EO has been inadequately studied [24,32], and its toxicity against S. litura remains unknown.
This study aimed to elucidate the insecticidal efficacy and mechanism of action of T. serpyllum essential oil against S. litura. In the present research, we hypothesized that it has considerable activity against S. litura larvae and eggs and can inhibit its growth by modulating enzyme activities. We investigated the effects of T. serpyllum EO on S. litura second- and third-instar larvae. The objectives of our study were to (1) analyze the composition of T. serpyllum EO using GC-MS; (2) evaluate the larvicidal and ovicidal activity of T. serpyllum EO on S. litura; and (3) explore the impact of T. serpyllum EO on S. litura detoxification enzymes. The findings will facilitate the formulation of efficient and low-toxicity botanical insecticides for the management of S. litura.
2. Results
2.1. Chemical Composition of T. serpyllum EO
The major constituents of the T. serpyllum EO are presented in Table 1. The T. serpyllum EO comprised 13 primary chemical constituents, accounting for 99.2% of its composition. The most abundant constituent was thymol (42.1%). Furthermore, other constituents included p-Cymene (22.4%), γ-Terpinene (18.6%), Carvacrol (3.6%), β-Pinene (2.7%), Linalool (2.5%), α-Pinene (1.6%), α-Terpinene (1.5%), Camphene (1.3%), Limonene (1.2%), Camphor (0.8%), Terpin-4-ol (0.6%), and α-Terpineol (0.3%).
Table 1.
Components of Thymus serpillum by GC-MS.
| No | Compounds a | RI b | RI c | (%) |
|---|---|---|---|---|
| 1 | α-Pinene | 937 | 936 | 1.6 |
| 2 | Camphene | 954 | 954 | 1.3 |
| 3 | β-Pinene | 977 | 979 | 2.7 |
| 4 | α-Terpinene | 1018 | 1017 | 1.5 |
| 5 | p-Cymene | 1025 | 1025 | 22.4 |
| 6 | Limonene | 1030 | 1029 | 1.2 |
| 7 | γ-Terpinene | 1060 | 1060 | 18.6 |
| 8 | Linalool | 1099 | 1097 | 2.5 |
| 9 | Camphor | 1146 | 1134 | 0.8 |
| 10 | Terpin-4-ol | 1180 | 1177 | 0.6 |
| 11 | α-Terpineol | 1191 | 1186 | 0.3 |
| 12 | Thymol | 1292 | 1290 | 42.1 |
| 13 | Carvacrol | 1307 | 1299 | 3.6 |
a Components in order of elution from an HP-5 MS column. b RI, retention index computed on the HP-5MS column relative to C8–C28 n-alkanes. c Relative retention indices taken from Adams.
2.2. Larval Toxicity
The toxicity of T. serpyllum EO to S. litura second- and third-instar larvae was evaluated using the leaf-dipping approach (Table 2 and Table 3). T. serpyllum EO toxicity varied in a dose-dependent manner. As shown in Table 2, the LC50 and LC90 reached 1.632 and 4.463 mg/mL in second-instar larvae after 12 h of exposure. After 24 h, the LC50 and LC90 reached 1.033 and 3.294 mg/mL, after 36 h, 0.780 and 2.317 mg/mL; after 48 h, 0.606 and 1.749 mg/mL; and after 60 h, 0.444 and 1.313 mg/mL. Following 72 h of exposure, LC50 and LC90 were 0.300 and 0.959 mg/mL, respectively. The larval toxicity of T. serpyllum EO against S. litura 2nd-instar larvae showed an increased trend with increased concentration. At the same time, the effective concentration showed a decreasing trend with more prolonged treatment and exposure time.
Table 2.
Second-instar larvael activity of the Thymus serpillum EO.
| Time (h) | Concentration (mg/mL) |
Mortality (%) ± SD |
LC50 (mg/mL) (95%CL *) |
LC90 (mg/mL) (95%CL) |
χ2 |
|---|---|---|---|---|---|
| 12 | 0.25 | 3.3 ± 2.9 b | 1.632 (1.281–2.378) | 4.463 (2.886–11.051) | 15.980 |
| 0.5 | 5.0 ± 5.0 b | ||||
| 1.0 | 18.3 ± 5.8 b | ||||
| 2.0 | 66.7 ± 10.4 a | ||||
| F3,8 = 60.317, p < 0.0001 | |||||
| 24 | 0.25 | 11.7 ± 2.9 c | 1.033 (0.815–1.393) | 3.294 (2.186–7.114) | 15.789 |
| 0.5 | 16.7 ± 2.9 c | ||||
| 1.0 | 35.0 ± 5.0 b | ||||
| 2.0 | 83.3 ± 10.4 a | ||||
| F3,8 = 85.481, p < 0.0001 | |||||
| 36 | 0.25 | 15.0 ± 0.0 c | 0.780 (0.667–0.919) | 2.317 (1.794–3.384) | 10.300 |
| 0.5 | 23.3 ± 7.6 c | ||||
| 1.0 | 53.3 ± 7.6 b | ||||
| 2.0 | 93.3 ± 2.9 a | ||||
| F3,8 = 120.600, p < 0.0001 | |||||
| 48 | 0.25 | 20.0 ± 5.0 c | 0.606 (0.517–0.707) | 1.749 (1.386–2.460) | 10.254 |
| 0.5 | 35.0 ± 8.7 c | ||||
| 1.0 | 65.0 ± 5.0 b | ||||
| 2.0 | 98.3 ± 2.9 a | ||||
| F3,8 = 108.062, p < 0.0001 | |||||
| 60 | 0.25 | 31.7 ± 2.9 c | 0.444 (0.369–0.520) | 1.313 (1.049–1.835) | 8.827 |
| 0.5 | 46.7 ± 10.0 c | ||||
| 1.0 | 80.0 ± 5.0 b | ||||
| 2.0 | 100 ± 0.0 a | ||||
| F3,8 = 81.784, p < 0.0001 | |||||
| 72 | 0.25 | 46.7 ± 2.9 b | 0.300 (0.228–0.365) | 0.959 (0.767–1.357) | 9.744 |
| 0.5 | 65.0 ± 10.0 b | ||||
| 1.0 | 90.0 ± 10.0 a | ||||
| 2.0 | 100 ± 0.0 a | ||||
| F3,8 = 33.640, p < 0.0001 |
CL *: confidence limit which has been calculated with 95% confidence. The symbols “a”, “b” and “c” in the table denote levels of statistical significance, indicating meaningful differences between groups.
Table 3.
Third-instar Larval activity of the Thymus serpillum EO.
| Time (h) | Concentration (mg/mL) | Mortality (%) ± SD | LC50 (mg/mL) (95%CL) | LC90 (mg/mL) (95%CL) | χ2 |
|---|---|---|---|---|---|
| 12 | 0.25 | 1.7 ± 2.9 c | 1.725 (1.458–2.174) | 4.401 (3.205–7.486) | 13.964 |
| 0.5 | 5.0 ± 0.0 c | ||||
| 1.0 | 15.0 ± 5.0 b | ||||
| 2.0 | 63.3 ± 2.9 a | ||||
| F3,8 = 235.933, p < 0.0001 | |||||
| 0.25 | 11.7 ± 2.9 c | 1.159 (0.962–1.462) | 4.218 (2.918–7.646) | 9.307 | |
| 0.5 | 15.0 ± 5.0 c | ||||
| 1.0 | 35.0 ± 5.0 b | ||||
| 2.0 | 78.3 ± 2.9 a | ||||
| F3,8 = 169.333, p < 0.0001 | |||||
| 36 | 0.25 | 13.3 ± 2.9 d | 0.818 (0.696–0.972) | 2.554 (1.945–3.843) | 4.581 |
| 0.5 | 23.3 ± 2.9 c | ||||
| 1.0 | 55.0 ± 5.0 b | ||||
| 2.0 | 88.3 ± 2.9 a | ||||
| F3,8 = 276.000, p < 0.0001 | |||||
| 48 | 0.25 | 18.3 ± 2.9 d | 0.664 (0.560–0.786) | 2.166 (1.657–3.244) | 5.220 |
| 0.5 | 35.0 ± 5.0 c | ||||
| 1.0 | 60.0 ± 5.0 b | ||||
| 2.0 | 93.3 ± 2.9 a | ||||
| F3,8 = 191.667, p < 0.0001 | |||||
| 60 | 0.25 | 31.7 ± 2.9 c | 0.467 (0.389–0.547) | 1.398 (1.112–1.967) | 8.629 |
| 0.5 | 45.0 ± 5.0 c | ||||
| 1.0 | 76.7 ± 5.8 b | ||||
| 2.0 | 100 ± 0.0 a | ||||
| F3,8 = 142.500, p < 0.0001 | |||||
| 72 | 0.25 | 43.3 ± 10.4 c | 0.317 (0.245–0.382) | 0.994 (0.796–1.399) | 6.136 |
| 0.5 | 65.0 ± 5.0 b | ||||
| 1.0 | 88.3 ± 5.8 a | ||||
| 2.0 | 100 ± 0.0 a | ||||
| F3,8 = 45.667, p < 0.0001 |
The symbols “a”, “b” and “c” in the table denote levels of statistical significance, indicating meaningful differences between groups.
T. serpyllum EO demonstrated considerable efficacy against third-instar larvae of S. litura. As indicated in Table 3, the LC50 and LC90 values were 1.725 mg/mL and 4.401 mg/mL after 12 h of exposure. At a 0.25 mg/mL concentration, the mortality rate was 1.7 ± 2.9%, while it reached 63.3 ± 2.9% at 2.0 mg/mL. The toxicity of T. serpyllum EO to the third instar larvae increased with prolonged exposure. Mortality rates at each concentration rose progressively, with LC50 and LC90 values diminishing over time. After 72 h, they fell to 0.317 and 0.994 mg/mL, respectively. During this time point, the mortality rate reached 43.3 ± 10.4% at 0.25 mg/mL and increased to 100 ± 0.0% at 2.0 mg/mL. No mortality larvae mortality was observed in the control group treatment with acetone. One-way ANOVA analysis revealed a significant difference in mortality rates across the different EO concentrations (p < 0.0001). Tukey’s post hoc test also confirmed significant differences between the concentrations (p < 0.05). Logistic regression modelling indicated an EC50 value of 0.30 mg/mL at 72 h of exposure. These findings confirm that the toxicity of T. serpyllum EO to third-instar larvae of S. litura significantly increases with both exposure duration and concentration, while the effective concentration is reduced with increased exposure duration.
2.3. Ovicidal Activity
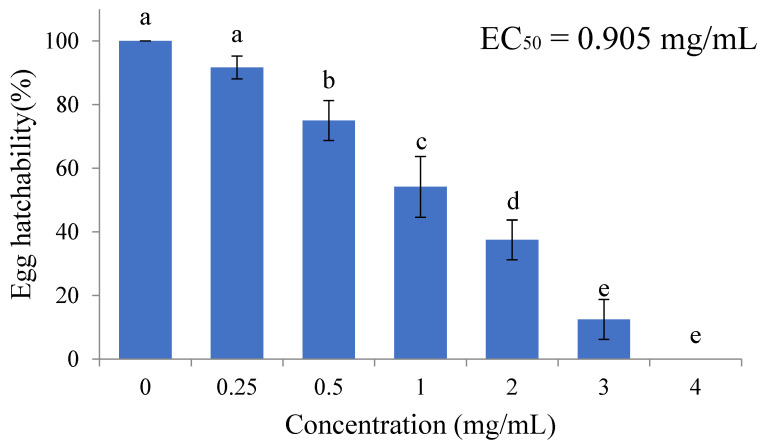
The ovicidal activity of T. serpyllum EO is shown in Figure 1. A dosage-dependent relationship was observed, with an EC50 = 0.905 mg/mL. The hatching rate in the 0, 0.25, 0.5, 1, 2, 3, and 4 mg/mL T. serpyllum EO treatment groups were 100 ± 0%, 91.7 ± 3.6%, 75.0 ± 6.3%, 54.2 ± 9.5%, 37.5 ± 6.3%, 12.5 ± 6.3%, and 0 ± 0%, respectively.
Figure 1.
Ovicidal activity of the Thymus serpillum on eggs of Spodoptera litura. Values are mean ± SD of 3 replicates, and significant differences are indicated by different letters (a–e) (ANOVA, Tukey’s HSD, p < 0.05).
2.4. Activity Against S. litura Cellular Detoxification Enzymes
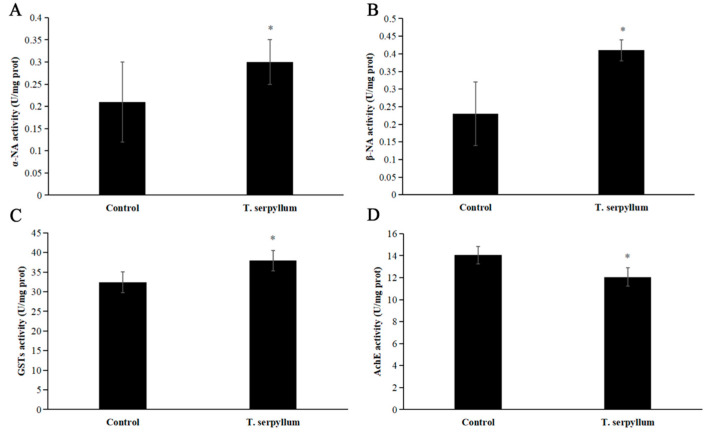
The potential role of T. serpyllum EO as an inhibitor of S. litura detoxifying enzymes involved in cellular detoxification was also investigated. CarE, GST, and AChE activities were measured, after 24 h of treatment with T. serpyllum (LC50 = 1.033 mg/mL). In the “Control group”, 10 μL of distilled water was used instead of 10 μL of the solution, and the rest of the reagents were kept unchanged. Among these three enzymes, AChE was significantly inhibited after treatment with T. serpyllum EO (Figure 2D). Compared with the control group, the α-NA activity, β-NA activity, and GST activities of S. litura were significantly increased by T. serpyllum EO (Figure 2A–C).
Figure 2.
The effects of Thymus serpillum EO on the activities of detoxifying enzymes of Spodoptera litura. (A) Esterases (α-NA) activity for 24 h of LC50 treatment; (B) esterases (β-NA) activity for 24 h of LC50 treatment; (C) GSTs activity for 24 h of LC50 treatment; (D) AChE activity for 24 h of LC50 treatment. p < 0.05 were illustrated by the asterisk according to an independent sample t-test.
3. Discussion
The main constituent of T. serpyllum EO was thymol (42.1%), which is in accordance with the findings of Xie et al., Hýbl et al., and Yang et al. [24,31,32].
S. litura is a predominant polyphagousan pest [3] and has developed resistance to many chemical pesticides, such as benzoate, emamectin, carbamates, and pyrethroids [33]. Plant essential oils can serve as an alternative to biopesticides for pest control, targeting species of agricultural importance [17].
According to the study findings, the LC50 of T. serpyllum EO against S. litura 2nd, and 3rd instar larvae after a 48 h exposure was 0.606 mg/mL and 0.664 mg/mL, respectively. These findings suggest that T. serpyllum EO exhibits substantial toxicity towards S. litura. GC-MS analysis identified the primary constituents of T. serpyllum EO, namely thymol, carvacrol, (S)-(+)-carvone, estragole, citral, linalool, (S)-(-)-limonene, and γ-terpinene, all known for their insecticidal properties against larvae of various pests [33]. A limonene analogue resulted in morphological and physiological alterations in Drosophila suzukii L3 larvae, contributing to elevated larval mortality. This aligns with the findings by Yang [34], which reported that T. serpyllum EO exhibited high toxicity to R. dabieshanensis. Additionally, Hýbl et al. highlighted the insecticidal properties of T. serpyllum EO on V. destructor (LC50 = 2.549 μL/L) [31]. Xie et al. demonstrated that this essential oil also has toxic effects on M. domestica (LC50 = 20.9 μL/L) [24]. Sertkaya (2021) documented an LC50 of 1.12 μL/L for T. serpyllum EO against A. obtectus, along with a 90.0% repellent efficacy against Frankliniella occidentalis at a 0.5% concentration [35]. Similarly, Lantana camara EO exhibited potent insecticidal activity against Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus larvae [36]. These findings align with prior research conducted by Yang et al. [32]. Similarly, T. serpyllum EO exhibited significant toxicity towards R. dabieshanensis. In addition, Hýbl et al. [31] demonstrated the insecticidal effect of T. serpyllum EO against Varroa destructor (LC50 = 2.549 μL/L). Xie et al. [24] showed T. serpyllum EO had toxic effects on M. domestica (LC50 = 20.9 μL/L). Research conducted by Sertkaya [30] indicated that the LC50 value of T. serpyllum EO against A. obtectus was 1.12 μL/L. Picard et al. [29] assessed the insecticidal ability of T. serpyllum EO, which showed a 90.0% repellent efficacy against F. occidentalis at 0.5%.
More recently, plant essential oils (EOs) have been implemented as a control agent against S. litura due to their effectiveness at low doses [10,11] and relative safety for non-target species [12]. Suresh et al. [10] reported that C. maritimum exhibited larvicidal activity against I-VI instar larvae of S. litura with LC50 values ranging from 102.1 to 237.0 μL/L. The larvicidal activity of a C. guianensis flower extract against S. litura third instar larvae (with an LC50 of 223 ppm) has been demonstrated by Ponsankar et al. [11]. The LC50 value of Piper betle L. EO against third instar S. litura larvae was 0.48%, as reported by Vasantha-Srinivasan et al. [12]. Similarly, Kaleeswaran et al. [13] reported that n-hexane (pericarp) Z. armatum pericarp extracts (0.209%), followed by Ethyl acetate (0.450%) and Methanol (0.654%) extracts, respectively, displayed larvicidal activity against third instar S. litura at 72 h. Benelli et al. [17] demonstrated that W. prostrata exhibited significant toxicity toward fourth instar larvae of S. litura (LC50 = 167.46 μL/mL). Strong toxicity of A. galanga (LD50 = 13.26 μg/larva) and O. basilicum (17.71 μg/larva) EO against S. litura has been reported by Ruttanaphan et al. [19].
The primary constituent of essential oils was identified as the determinant of their biological activity [24,31,34,35,36]. Thymol is one of the major volatile components of T. serpyllum EO. Ruttanaphan and Bullangpoti [3] demonstrated that thymol had significant toxicity against third instar larvae of S. litura after 24 and 48 h (LC50 = 5.610 μg/larva and 5.262 μg/larva). Koul et al. [22] reported a substantial insecticidal activity of thymol against S. litura, with LD50 of 28.5 μg/larva. Furthermore, thymol was determined to be toxic to various pest species (Table 4), including R. dabieshanensis [32,37,38]. As shown in Table 4, thymol has a broad-spectrum insecticide activity.
Table 4.
Insecticidal activity of Thymus serpyllum EO and its major component thymol.
| Oil/Compounds | Insect Species | LC50 or LD50 | References |
|---|---|---|---|
| Thymus serpyllum EO | Reticulitermes dabieshanensis | 0.092 μL/L | Yang et al. [32] |
| Varroa destructor | 2.549 μL/L | Hýbl et al. [31] | |
| Musca domestica | 20.9 μL/L | Xie et al. [24] | |
| Acanthoscelides obtectus | 1.12 μg/mL | Sertkaya [30] | |
| Frankliniella occidentalis | 0.5% | Picard et al. [29] | |
| thymol | Amblyomma sculptum; | 0.0156 mg/cm2 | da Silva Costa et al. [39] |
| Rhipicephalus sanguineus | 0.0041 mg/cm2 | ||
| Aedes aegypti | 0.1 mg/mL 100% mortality | Nascimento et al. [40] | |
| Reticulitermes dabieshanensis | 0.062 μL/L | Yang et al. [32] | |
| Tribolium castaneum; | 24.65 μg/adult; | Xie et al. [41] | |
| Lasioderma serricorne; | 9.9 μg/adult; | ||
| Liposcelis bostrychophila | 49.36 μg/adult | ||
| Solenopsis invicta | 0.98 μg/g, minimum repellent effective doses | Paudel et al. [42] | |
| Acromyrmex balzani | 2.23 µg/mg | Dantas et al. [43] | |
| Chilo suppressalis | 17.11 μg/larvae | Basij et al. [44] | |
| Plutella xylostella | 2.45 mg/mL | Zhao et al. [45] | |
| Sitophylus oryzae | 51.84%, repellency effects for 2% | Marsin and Muhamad [46] | |
| Tribolium confusum; | 0.3% | Amari et al. [47] | |
| Supella longipalpa | |||
| Sitophilus zeamais | 196 μmol/cm2 | Rodríguez et al. [48] | |
| Glyphodes pyloalis | 32.18 μg/larva | Goharrostami et al. [49] | |
| Spodoptera exigua | 9.54 μg/larva | Kumrungsee et al. [50] | |
| Spodoptera litura | 5.610 μg/larva | Ruttanaphan and Bullangpoti [3] | |
| Acanthoscelides obtectus | Females, 98.4 mg/kg; | Lazarević et al. [51] | |
| Males, 66.0 mg/kg | |||
| Plutella xylostella | 27.94 mg/L | da Camara et al. [52] | |
| Spodoptera exigua | 32.45 μg/larva | Pengsook et al. [53] | |
| Galleria mellonella | 0.5 mg/adult | Sohail et al. [54] | |
| Podisus nigrispinu; | 10.27 mg/g; | Lima et al. [55] | |
| Spodoptera frugiperda | 4.91 mg/g | ||
| Tuta absoluta | 7.72 μL/mL | Piri et al. [56] | |
| Mythimna separate ; | 6.67 μL/L; | Lu et al. [57] | |
| Myzus persicae ; | 5.58 μL/L; | ||
| Sitophilus zeamais ; | 59.20 μL/L; | ||
| Musca domestica ; | 1.66 μL/L; | ||
| Tetranychus cinnabarinus | 2.14 μL/L | ||
| Riptortus clavatus | 70.0% repellent activity at 2.83 μg/cm2 | Lee et al. [58] | |
| Culex pipiens | 49 mg/L | Youssefi et al. [59] | |
| Leishmania infantum | 7.22 μg/mL | Youssefi et al. [60] | |
| Musca domestica | 13 mg/L | Scalerandi et al. [61] | |
| Sitophilus zeamais | 84.06 μL/L | Oliveira et al. [62] | |
| Aedes albopictus | 12.9 mg/L | Giatropoulos et al. [63] | |
| Hyalomma lusitanicum | 100% at 5 mg/L | Navarro-Rocha et al. [64] | |
| Plutella xylostella | 0.00018 ppm | Webster et al. [65] | |
| Aedes aegypti | 35.71 ppm | de Mesquita et al. [66] | |
| Diaphania hyalinata | 2.99 μg/mg | Melo et al. [67] | |
| Blatta lateralis | 0.34 mg/nymph | Gaire et al. [68] | |
| Sitophilus zeamais | 17.08 μg/mg | Oliveira et al. [69] | |
| Cryptotermes brevis | 8.20 μg/mg | Santos et al. [70] | |
| Ixodes ricinus | 100% at 1% | Tabari et al. [71] | |
| Aedes aegypti | 11.1 μg/cm2 | Ali et al. [72] | |
| Blattella germanica | 100%, repellency effects for 10 μg/cm2 | Lee et al. [73] | |
| Sitophilus oryzae ; | 24.07 μg/cm2; | Kanda et al. [74] | |
| Tribolium castaneum; | 11.21 μg/cm2 | ||
| Rhyzopertha dominica; | 8.8 μg/cm2 | ||
| Aedes aegypti | 0.013 mg/cm2 | Rehman et al. [75] | |
| Aedes albopictus | 100%, larvicidal activity, 0.1 mg/mL | Seo et al. [76] | |
| Stegomyia aegypti | 68.05 μg/cm2 | Huang et al. [77] | |
| Plutella xylostella | 0.22 μg/larva | Kumrungsee et al. [78] | |
| Aedes aegypti | 13.9 ppm | Tabanca et al. [79] | |
| Spodoptera litura | 28.5 μg/larva | Koul et al. [22] | |
| Reticulitermes speratus | 0.65 mg/Petri dish | Sekine and Shibutani [80] | |
| Crithidia fasciculata; | 32.5 μg/mL; | Azeredo and Soares [81] | |
| Trypanosoma cruzi | 62 μg/mL | ||
| Aedes albopictus | 9 μL/L | Park et al. [82] | |
| Tenebrio molitor | 14.71 μL/L | Lima et al. [83] |
Previous studies have documented the toxicity of terpenoid complexes from essential oils to S. litura [3,18]. Mahajan et al. [18] determined the efficacy of β-caryophyllene, which resulted in a 13.33% adult emergence in S. litura at 3125 ppm. Similarly, β-asarone was toxic to S. litura larvae with LD50 values of 6.24 μg/larva [37]. Rotenone can be highly effective against third instar larvae of S. litura (LC50 = 5043 mg/L) [38]. According to Huang et al. [23], pogostone had significant larvicidal activity against S. litura, including oral toxicity (LC50 = 986.88 mg/L) and contact toxicity (LC50 = 1041.42 mg/L). Ruttanaphan and Bullangpoti [3] reported that pulegone exhibits high larvicidal activity against third instar larvae of S. litura after 24 h (LC50 = 8.348 μg/larva). Ruttanaphan et al. [21] discovered a remarkable insecticidal activity of linalool against S. litura, with an LD50 of 32.271 μg/larva and 49.742 μg/larva, respectively.
ESTs and GSTs are detoxification enzymes that play key roles in detoxifying botanical pesticides, and their activities are typically induced and up-regulated by exogenous compounds [84]. As shown in Figure 2, the ESTs and GSTs activities of S. litura were significantly elevated after exposure to an LC50 concentration of T. serpyllum EO. Yang et al. [32] reported that eight main components of EOs increased the activity of ESTs and GST of R. dabieshanensis; however, the activity of AChE was decreased. The EO from C. citratus, C. khasans, C. nardus and the main compound including citral, geraniol, and citronellal inhibited α-NA esterase activity and enhanced β-NA esterase activities [32]. The activities of ESTs and GST of R. dabieshanensis by treatment with M. citrate were significantly increased compared to the control [33]. This finding corresponded to studies by Yang et al. [32,84,85,86], which demonstrated the efficacy of T. serpyllum EO in enhancing the activity of detoxifying enzymes of treated insects.
Furthermore, AChE plays a significant role in the mechanism of action of essential oils (EOs) or relevant constituents, resulting in insecticidal effects. This study indicated that essential oil exerted a substantial inhibitory effect on acetylcholinesterase in vivo in S. litura. This is in agreement with findings by Jin et al. [34], who demonstrated that three Cymbopogon EOs and their primary components effectively inhibited the AChE activity of R. ffaviceps in vitro and in vivo. Additionally, Wu et al. [31] found that Mentha spp. EOs and their major constituents exhibited significant inhibitory activity against AChE both in vivo and in vitro. Previous studies have indicated that EOs and their major constituents can bind to the active site of AChE to inhibit AChE activity [34]. In addition, it has been demonstrated that most EOs can also exert toxic effects on insects by inhibiting cytochrome P450 enzymes (CYPs) [87], GABA receptors [88], octopus amine synapses [89], and tyramine receptors [90]. Therefore, EOs and their main constituents would lead to insect mortality by causing dysregulation in the nervous, antioxidant, and enzyme-based metabolic systems.
4. Materials and Methods
4.1. Insect Rearing
The eggs of S. litura were purchased from the Henan Jiyuan Baiyun Industry Company (Jiyuan, China). After the hatching, the larvae were raised individually on Chinese cabbage leaves. The laboratory temperature was maintained at 26 ± 2 °C, with 12:12 h (light/dark) cycles and RH of 75 ± 5%. The bioassay was conducted using eggs and healthy and uniform-sized 2nd and 3rd instar larvae.
4.2. Essential Oil
T. serpyllum EO was purchased from the online shop of Shanghai Zixin (Shanghai, China).
4.3. GC-MS Analysis
EOs of T. serpyllum were analyzed by an Agilent 7890B/5977A (Santa Clara, CA, USA) coupled with an HP-5 MS capillary column (30 m × 0.25 mm ID × 0.25 μm film thickness) (Santa Clara, CA, USA) and electron impact ionization (70 eV). Oven temperature increased at a rate of 10 °C min−1 from 50 °C (1 min) to 250 °C, subsequently kept at 250 °C for 2 min. Helium (99.999%) was applied as a carrier gas. Through GC-MS analysis of the essential oils, the peak areas of the major constituents were recorded in this study, and the relative percentage of each component was calculated by peak area normalization ():
where i is the individual compound; is the peak area per compound; and : is the sum of all compound peak areas corresponding to the total peak area.
The peaks of EOs of T. serpyllum were annotated by comparing the retention indices (RI) and mass spectra (NIST 11.0) and Wiley 275 library data with those of the literature.
4.4. Larvicidal Activity
Larvicidal bioassays were carried out using the modified leaf dipping method as described by Benelli et al. [17]. The T. serpyllum EO was evaluated in a series of concentration gradients (0, 0.25, 0.5, 1.0, and 2.0 mg/mL) diluted in the solvent Polysorbate 80 (P8010, Sigma-Aldrich, St. Louis, MO, USA). Then, Chinese cabbage leaves of uniform shape and size (3 cm × 3 cm) were immersed in the series of concentration gradients separately. After 10 s, the leaf discs were removed and dried, then placed in moisturized filter paper on plates. After that, each of the 20 larvae was placed onto Chinese cabbage leaves treated with different EO concentrations, respectively. The number of dead larvae was counted every 12 h and observed for 72 h. All experiments were performed in triplicate.
4.5. Ovicidal Bioassays
A total of 420 eggs were split into 7 groups (n = 60 eggs in each group). Each group was separately immersed in different concentrations (0, 0.25, 0.5, 1.0, and 2.0 mg/mL in Polysorbate 80) of T. serpyllum EO. Then, the number of eggs hatched in each group was counted. All experiments were performed in triplicate. Hatching rates were assessed at 120 h after the initiation of the treatment.
4.6. Enzyme Activities
4.6.1. Homogenate Preparation
The 2nd instar larvae were placed into the LC50 concentrations of T. serpyllum EO to evaluate the impact on detoxification enzymes, specifically esterases and acetylcholinesterase. Each group (20 larvae) was homogenized, and centrifuged at 12,000× g for 15 min at 4 °C. Then, the supernatants were stored at −80 °C for subsequent use. Each assay was repeated a minimum of three times.
4.6.2. Esterases (ESTs)
The EST assay was carried out based on the protocol of Piri et al. [56]. The EST was determined on two substrates, acetate-1-naphthyl ester (α-NA) (N1252, Sigma-Aldrich, St. Louis, MO, USA), and acetate-2-naphthyl ester (β-NA) (N1254, Sigma-Aldrich, St. Louis, MO, USA). The 20 µL substrate (α-NA or β-NA) (10 mM) and 50 µL Fast Blue RR Salt (1 mM) (F6250, Sigma-Aldrich, St. Louis, MO, USA) were mixed. Finally, 10 μL of the enzyme solution was supplemented into the mixture. In the control group, the 10 μL enzyme solution was replaced by 10 μL distilled water, and the rest of the reagents remained unchanged. After incubation at 27 °C for 5 min, the absorbance (OD value) was determined at 450 nm using a microplate reader (Synergy™ HTX, BioTek, Winooski, VT, USA). In addition, OD values were recorded every 1 min for 10 min. Each assay was repeated at least three times to ensure the reliability and reproducibility of the data. The EST activity was calculated using the following formula:
| EST activity (U/mg) = (ΔAtest − ΔAck) × Vtotal ÷ Vtest ÷ T |
ΔAtest corresponds to the absorbance change value of the treatment group measured for 10 min. ΔAck corresponds to the change in absorbance of the control group measured for 10 min. Vtotal represents the total volume of the reaction system in each well. Vtest represents the volume of the enzyme solution. T denotes the reaction time.
4.6.3. Glutathione S-Transferases (GSTs) Activity Assay
The enzymatic activity of GSTs was measured using the protocol employed by Piri et al. [56]. 20 μL CDNB (20 mM) and 510 μL enzyme solution were mixed. After incubation at 27 °C for 5 min, the absorbance was determined at 340 nm. Each assay was repeated a minimum of three times. The GST activity was calculated using the formula below:
| GST activity (U/mg) = (ΔOD340 × Vtotal) ÷ (ε × L) |
ΔOD340 corresponds to the GST-mediated absorbance change. Vtotal represents the total volume of the reaction system in each well. ε represents the molar extinction coefficient of GST (0.0096/(μmol·cm)), and L represents the optical range of the colourimetric cup (1 cm).
4.6.4. Acetylcholinesterase (AChE) Activity Assay
AChE (C4359, Sigma-Aldrich, St. Louis, MO, USA) activity was determined following the protocol of Ellman et al. [91]. 80 μL PBS solution (0.1 M, pH = 7.0), 50 μL ATCh (10 mM), and 50 μL 5,5-dithiobis-2-nitrobenzoic acid (DTNB) (D8130, Sigma-Aldrich, St. Louis, MO, USA) (10 mM) were mixed, respectively. After incubation at 27 °C for 5 min, 20 μL of enzyme solution was then added. Absorbance (OD) was measured at 405 nm using a microplate reader, and OD values were recorded at 1 min intervals for 30 min. Each assay was repeated at least three times to ensure the reliability and reproducibility of the data. The AChE activity was calculated using the following formula:
| AChE activity (U/mg) = (ΔOD405 × Vtotal) ÷ (ε × L) |
ΔOD405 corresponds to AChE-mediated absorbance change. Vtotal represents the total volume of the reaction system in each well. ε represents the molar extinction coefficient of AChE (0.0136/(μmol·cm)), and L represents the optical range of the colourimetric cup (1 cm).
4.7. Statistical Analysis
Larval mortality, hatching, and suppression rates were analyzed using nonparametric statistical methods. The Kruskal–Wallis test was implemented to evaluate overall differences among treatment groups (p < 0.05). Dunn’s post hoc test was used to identify specific groups with significant differences in pairwise comparisons. All statistical analyses were performed with SPSS v20.0 (SPSS Inc., Chicago, IL, USA). The LC50 and EC50 values were calculated by fitting dose–response curves with logistic regression models. Within-group differences were assessed using the independent samples Mann–Whitney U-test, denoting results with p < 0.05 with an asterisk (*).
5. Conclusions
In our study, thymol was the main constituent of T. serpyllum EO. Moreover, T. serpyllum EO was highly toxic to second- and third-instar larvae of S. litura. Moreover, it demonstrated significant inhibition of AChE activity, confirming that T. serpyllum EO can be developed and utilized control agent against S. litura. Before large-scale field application, the effects of T. serpyllum EO on non-target organisms must be determined. There is also a need to design slow-release formulations that can be used to extend the effectiveness of T. serpyllum EO. The findings disclosed in this study may facilitate the effective and environmentally friendly management of S. litura in the field.
Author Contributions
Conceptualization, J.Q., X.W. and L.W.; methodology, L.W.; validation, X.W. and J.Q.; formal analysis, S.J., S.W. and Z.X.; investigation, L.W., S.W. and Z.X.; data curation, L.W.; writing—original draft preparation, L.W. and S.J.; writing—review and editing, J.Q. and X.W.; supervision, J.Q. and X.W.; funding acquisition, J.Q., X.W. and L.W. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Our study was backed up by Jilin Province Science and Technology Major Projects (20230304002YY); Yantai University Doctoral Science Research Foundation (SM15B01, SM19B70 and SM19B28); “double-hundredaction” of Yantai (2320004-SM20RC02).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Tian L., Gao X., Zhang S., Zhang Y., Ma D., Cui J. Dynamic changes of transcriptome of fifth-instar Spodoptera litura larvae in response to insecticide. 3 Biotech. 2021;11:98. doi: 10.1007/s13205-021-02651-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liang L., Li J., Jin L., Yan K., Pan Y., Shang Q. Identification of inducible CYP3 and CYP4 genes associated with abamectin tolerance in the fat body and Malpighian tubules of Spodoptera litura. Pestic. Biochem. Physiol. 2024;198:105751. doi: 10.1016/j.pestbp.2023.105751. [DOI] [PubMed] [Google Scholar]
- 3.Ruttanaphan T., Bullangpoti V. The potential use of thymol and (R)-(+)-pulegone as detoxifying enzyme inhibitors against Spodoptera litura (Lepidoptera: Noctuidae) Phytoparasitica. 2022;50:913–920. doi: 10.1007/s12600-022-00989-1. [DOI] [Google Scholar]
- 4.Singh A., Kumar S., Yadav M., Kumari M., Singh I.K. Tailored midgut gene expression in Spodoptera litura (Lepidoptera: Noctuidae) feeding on Zea mays indicates a tug of war. Arthropod Plant Interact. 2024;18:547–567. doi: 10.1007/s11829-024-10048-7. [DOI] [Google Scholar]
- 5.Islam S.M.N., Chowdhury M.Z.H., Mim M.F., Momtaz M.B., Islam T. Biocontrol potential of native isolates of Beauveria bassiana against cotton leafworm Spodoptera litura (Fabricius) Sci. Rep. 2023;13:8331. doi: 10.1038/s41598-023-35415-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li L.L., Xu J.W., Yao W.C., Yang H.H., Dewer Y., Zhang F., Zhu X.Y., Zhang Y.N. Chemosensory genes in the head of Spodoptera litura larvae. Bull. Entomol. Res. 2021;111:454–463. doi: 10.1017/S0007485321000109. [DOI] [PubMed] [Google Scholar]
- 7.Che W.N., Li Y.Y., Zhang D.F., Qu C., Luo C., Wang R. Monitoring and characterization of field-evolved resistance to diamide insecticides in Spodoptera litura collected from eastern China. J. Appl. Entomol. 2024;148:253–260. doi: 10.1111/jen.13149. [DOI] [Google Scholar]
- 8.Tharamak S., Yooboon T., Pengsook A., Ratwatthananon A., Kumrungsee N., Bullangpoti V., Pluempanupat W. Synthesis of thymyl esters and their insecticidal activity against Spodoptera litura (Lepidoptera: Noctuidae) Pest Manag. Sci. 2020;76:928–935. doi: 10.1002/ps.5598. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z., Gao B., Qu C., Gong J., Li W., Luo C., Wang R. Resistance monitoring for six Insecticides in vegetable field-collected populations of Spodoptera litura from China. Horticulturae. 2022;8:255. doi: 10.3390/horticulturae8030255. [DOI] [Google Scholar]
- 10.Benelli G., Govindarajan M., Rajeswary M., Vaseeharan B., Alyahya S.A., Alharbi N.S., Kadaikunnan S., Khaled J.M., Maggi F. Insecticidal activity of camphene, zerumbone and α-humulene from Cheilocostus speciosus rhizome essential oil against the Old-World bollworm, Helicoverpa armigera. Ecotoxicol. Environ. Safe. 2018;148:781–786. doi: 10.1016/j.ecoenv.2017.11.044. [DOI] [PubMed] [Google Scholar]
- 11.Benelli G., Pavela R., Petrelli R., Cappellacci L., Bartolucci F., Canale A., Maggi F. Origanum syriacum subsp. syriacum: From an ingredient of Lebanese ‘manoushe’ to a source of effective and eco-friendly botanical insecticides. Ind. Crops Prod. 2019;134:26–32. doi: 10.1016/j.indcrop.2019.03.055. [DOI] [Google Scholar]
- 12.Suresh U., Murugan K., Panneerselvam C., Aziz A.T., Cianfaglione K., Wang L., Maggi F. Encapsulation of sea fennel (Crithmum maritimum) essential oil in nanoemulsion and SiO2 nanoparticles for treatment of the crop pest Spodoptera litura and the dengue vector Aedes aegypti. Ind. Crops Prod. 2020;158:113033. doi: 10.1016/j.indcrop.2020.113033. [DOI] [Google Scholar]
- 13.Ponsankar A., Vasantha-Srinivasan P., Senthil-Nathan S., Thanigaivel A., Edwin E.S., Selin-Rani S., Kalaivani K., Hunter W.B., Alessandro R.T., Abdel-Megeed A., et al. Target and non-target toxicity of botanical insecticide derived from Couroupita guianensis L. flower against generalist herbivore, Spodoptera litura Fab. and an earthworm, Eisenia foetida Savigny. Ecotoxicol. Environ. Safe. 2016;133:260–270. doi: 10.1016/j.ecoenv.2016.06.043. [DOI] [PubMed] [Google Scholar]
- 14.Vasantha-Srinivasan P., Senthil-Nathan S., Thanigaivel A., Edwin E.S., Ponsankar A., Selin-Rani S., Pradeepa V., Sakthi-Bhagavathy M., Kalaivani K., Hunter W.B., et al. Developmental response of Spodoptera litura Fab. to treatments of crude volatile oil from Piper betle L. and evaluation of toxicity to earthworm, Eudrilus eugeniae Kinb. Chemosphere. 2016;155:336–347. doi: 10.1016/j.chemosphere.2016.03.139. [DOI] [PubMed] [Google Scholar]
- 15.Kaleeswaran G., Firake D.M., Sanjukta R., Behere G.T., Ngachan S.V. Bamboo-Leaf Prickly Ash extract: A potential bio-pesticide against oriental leaf worm, Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) J. Environ. Manag. 2018;208:46–55. doi: 10.1016/j.jenvman.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 16.Kaur M., Saraf I., Kumar R., Singh I.P., Kaur S. Bioefficacy of Hexane Extract of Inula racemosa (Asteraceae) Against Spodoptera litura (Lepidoptera: Noctuidae) Gesunde Pflanz. 2019;71:165–174. doi: 10.1007/s10343-019-00462-w. [DOI] [Google Scholar]
- 17.Kumar A., Negi N., Haider S.Z., Negi D.S. Composition and efficacy of Zanthoxylum alatum essential oils and extracts against Spodoptera litura. Chem. Nat. Compd. 2014;50:920–923. doi: 10.1007/s10600-014-1118-2. [DOI] [Google Scholar]
- 18.Manimegalai T., Raguvaran K., Kalpana M., Maheswaran R. Facile synthesis of silver nanoparticles using Vernonia anthelmintica (L.) Willd. and their toxicity against Spodoptera litura (Fab.), Helicoverpa armigera (Hüb.), Aedes aegypti Linn. and Culex quinquefasciatus Say. J. Clust. Sci. 2022;33:2287–2303. doi: 10.1007/s10876-021-02151-z. [DOI] [Google Scholar]
- 19.Benelli G., Govindarajan M., AlSalhi M.S., Devanesan S., Maggi F. High toxicity of camphene and gamma-elemene from Wedelia prostrata essential oil against larvae of Spodoptera litura (Lepidoptera: Noctuidae) Environ. Sci. Pollut. Res. Int. 2018;25:10383–10391. doi: 10.1007/s11356-017-9490-7. [DOI] [PubMed] [Google Scholar]
- 20.Kumrungsee N., Wiwattanawanichakun P., Phankaen P., Saiyaitong C., Koul O., Nobsathian S., Bullangpoti V., Dunkhunthod B. Phenolic secondary metabolites from Acorus calamus (Acorales: Acoraceae) rhizomes: The feeding deterrents for Spodoptera litura (Lepidoptera: Noctuidae) J. Econ. Entomol. 2023;116:1613–1620. doi: 10.1093/jee/toad135. [DOI] [PubMed] [Google Scholar]
- 21.Ruttanaphan T., Pluempanupat W., Aungsirisawat C., Boonyarit P., Goff G.L., Bullangpoti V. Effect of plant essential oils and their major constituents on cypermethrin tolerance associated detoxification enzyme activities in Spodoptera litura (Lepidoptera: Noctuidae) J. Econ. Entomol. 2019;112:2167–2176. doi: 10.1093/jee/toz126. [DOI] [PubMed] [Google Scholar]
- 22.Koul O., Singh R., Kaur B., Kanda D. Comparative study on the behavioral response and acute toxicity of some essential oil compounds and their binary mixtures to larvae of Helicoverpa armigera, Spodoptera litura and Chilo partellus. Ind. Crops Prod. 2013;49:428–436. doi: 10.1016/j.indcrop.2013.05.032. [DOI] [Google Scholar]
- 23.Huang S.H., Xian J.D., Kong S.Z., Li Y.C., Xie J.H., Lin J., Chen J.N., Wang H.F., Su Z.R. Insecticidal activity of pogostone against Spodoptera litura and Spodoptera exigua (Lepidoptera: Noctuidae) Pest Manag. Sci. 2014;70:510–516. doi: 10.1002/ps.3635. [DOI] [PubMed] [Google Scholar]
- 24.Xie Y., Jin H., Yang X., Gu Q., Zhang D. Toxicity of the essential oil from Thymus serpyllum and thymol to larvae and pupae of the housefly Musca domestica L. (Diptera: Muscidae) Environ. Sci. Pollut. Res. Int. 2020;27:35330–35340. doi: 10.1007/s11356-020-09633-z. [DOI] [PubMed] [Google Scholar]
- 25.Dong Y., Wei Z., Yang R., Zhang Y., Sun M., Bai H., Mo M., Yao C., Li H., Shi L. Chemical compositions of essential oil extracted from eight thyme species and potential biological functions. Plants. 2023;12:4164. doi: 10.3390/plants12244164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ouedrhiri W., Balouiri M., Bouhdid S., Moja S., Chahdi F.O., Taleb M., Greche H. Mixture design of Origanum compactum, Origanum majorana and Thymus serpyllum essential oils: Optimization of their antibacterial effect. Ind. Crops Prod. 2016;89:1–9. doi: 10.1016/j.indcrop.2016.04.049. [DOI] [Google Scholar]
- 27.Verma R.S., Padalia R.C., Saikia D., Chauhan A., Krishna V., Sundaresan V. Chemical composition and antimicrobial activity of the essential oils isolated from the Herbage and Aqueous Distillates of two Thymus Species. J. Essent. Oil Bear. Plants. 2016;19:936–943. doi: 10.1080/0972060X.2014.935071. [DOI] [Google Scholar]
- 28.Mugnaini L., Nardoni S., Pistelli L., Leonardi M., Giuliotti L., Benvenuti M.N., Pisseri F., Mancianti F. A herbal antifungal formulation of Thymus serpillum, Origanum vulgare and Rosmarinus officinalis for treating ovine dermatophytosis due to Trichophyton mentagrophytes. Mycoses. 2013;56:333–337. doi: 10.1111/myc.12034. [DOI] [PubMed] [Google Scholar]
- 29.Picard I., Hollingsworth R.G., Salmieri S., Lacroix M. Repellency of essential oils to Frankliniella occidentalis (Thysanoptera: Thripidae) as affected by type of oil and polymer release. J. Econ. Entomol. 2012;105:1238–1247. doi: 10.1603/EC11292. [DOI] [PubMed] [Google Scholar]
- 30.Sertkaya E. Fumigant toxicity of the essential oils from medicinal plants against Bean weevil, Acanthoscelides obtectus (Say) (Coleoptera: Bruchidae) Asian J. Chem. 2013;25:553–555. doi: 10.14233/ajchem.2013.13581. [DOI] [Google Scholar]
- 31.Hýbl M., Bohatá A., Rádsetoulalová I., Kopecký M., Hoštičková I., Vaníčková A., Mráz P. Evaluating the efficacy of 30 different essential oils against Varroa destructor and Honey Bee Workers (Apis mellifera) Insects. 2021;12:1045. doi: 10.3390/insects12111045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang X., Jin C., Wu Z., Han H., Zhang Z., Xie Y., Zhang D. Toxicity and physiological effects of nine Lamiaceae essential oils and their major compounds on Reticulitermes dabieshanensis. Molecules. 2023;28:2007. doi: 10.3390/molecules28052007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rathod N.B., Kulawik P., Ozogul F., Regenstein J.M., Ozogul Y. Biological activity of plant-based carvacrol and thymol and their impact on human health and food quality. Trends Food Sci. Technol. 2021;116:733–748. doi: 10.1016/j.tifs.2021.08.023. [DOI] [Google Scholar]
- 34.De Souza M.T., de Souza M.T., Bernardi D., de Melo D.J., Zarbin P.H.G., Zawadneak M.A.C. Insecticidal and oviposition deterrent effects of essential oils of Baccharis spp. and histological assessment against Drosophila suzukii (Diptera: Drosophilidae) Sci. Rep. 2021;11:3944. doi: 10.1038/s41598-021-83557-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ebadollahi A., Jalali Sendi J., Ziaee M., Krutmuang P. Acaricidal, insecticidal, and nematicidal efficiency of essential oils isolated from the Satureja genus. Int. J. Environ. Res. Public Health. 2021;18:6050. doi: 10.3390/ijerph18116050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sonter S., Dwivedi M.K., Mishra S., Singh P., Kumar R., Park S., Jeon B.-H., Singh P.K. In vitro larvicidal efficacy of Lantana camara essential oil and its nanoemulsion and enzyme inhibition kinetics against Anopheles culicifacies. Sci. Rep. 2024;14:16325. doi: 10.1038/s41598-024-67148-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yooboon T., Bullangpoti V., Kainoh Y. Contact toxicity and antifeedant activity of binary mixtures of piperine and β-asarone against the crop pests, Spodoptera litura and Mythimna separata (Lepidoptera: Noctuidae) Int. J. Pest Manag. 2021;69:81–88. doi: 10.1080/09670874.2020.1853275. [DOI] [Google Scholar]
- 38.Li Z., Huang R., Li W., Cheng D., Mao R., Zhang Z. Addition of cinnamon oil improves toxicity of rotenone to Spodoptera litura (Lepidoptera: Noctuidae) Larvae. Fla. Entomol. 2017;100:515–521. doi: 10.1653/024.100.0304. [DOI] [Google Scholar]
- 39.da Silva Costa J.R., do Vale T.L., da Silva G.F., da Silva N.C.S., da Silva Lima A., Costa-Junior L.M., Luz H.R. Encapsulation of carvacrol and thymol with yeast cell wall and its repellent activity against Amblyomma sculptum and Rhipicephalus sanguineus (Sensu Lato) Exp. Appl. Acarol. 2024;92:555–565. doi: 10.1007/s10493-023-00896-y. [DOI] [PubMed] [Google Scholar]
- 40.Nascimento G., Oliveira L., Rique H., Leite R., Nunes F. Repellence and insecticidal activity mediated by necrosis in Aedes aegypti mosquitoes exposed to thymol. Arq. Bras. Med. Vet. E Zootec. 2024;76:77–83. doi: 10.1590/1678-4162-13027. [DOI] [Google Scholar]
- 41.Xie Q.H., Tian L., Li B.Y., Yu J.N., Zheng Y., Du S.S., Borjigidai A. Bioactivities of thymol and p-cymene from the essential oil of Adenosma buchneroides against three stored-product insects. Environ. Sci. Pollut. Res. Int. 2023;30:110841–110850. doi: 10.1007/s11356-023-30068-9. [DOI] [PubMed] [Google Scholar]
- 42.Paudel P., Shah F.M., Guddeti D.K., Ali A., Chen J., Khan I.A., Li X.-C. Repellency of Carvacrol, Thymol, and their acetates against imported fire ants. Insects. 2023;14:790. doi: 10.3390/insects14100790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dantas J.O., Cavalcanti S.C.H., Araújo A.P.A., Silva J.E., Brito T.B., Andrade V.S., Pinheiro H.S.S., Tavares S.R.S.A., Blank A.F., Bacci L. Formicidal potential of Thymol derivatives: Adverse effects on the survival and behavior of Acromyrmex balzani. Agriculture. 2023;13:1410. doi: 10.3390/agriculture13071410. [DOI] [Google Scholar]
- 44.Basij M., Sahebzadeh N., Shahriari M., Panahandeh S. Insecticidal potential of Ajwain essential oil and its major components against Chilo suppressalis Walker. J. Plant Dis. Protect. 2023;130:735–745. doi: 10.1007/s41348-023-00762-4. [DOI] [Google Scholar]
- 45.Zhao M., Tao Z., Wang L., Wang T., Wang C., Li S., Huang S., Wei Y., Jiang T., Li P. Structural modification of (3E)-4,8-dimethyl-1,3,7-nontriene enhances its ability to kill Plutella xylostella insect pests. Pest Manag. Sci. 2023;79:3280–3289. doi: 10.1002/ps.7508. [DOI] [PubMed] [Google Scholar]
- 46.Marsin A.M., Muhamad I.I. Effectiveness of insect-repellent food packaging film incorporating thymol against rice weevil, Sitophylus oryzae. Curr. Sci. 2023;125:551. [Google Scholar]
- 47.Amari R., Guesmi F., Saidi I., Bouzenna H., Khaled I., Ali M.B., Hedfi A., Alghamdi A.S., Hfaiedh N., Allagui M.S., et al. Insecticidal and antimicrobial potential of the volatile oils of the aerial parts of Teucrium ramosissimum and Thymus hirtus subsp. algeriensis growing in the south-west of Tunisia. J. Essent. Oil Bear. Plants. 2022;25:1185–1207. doi: 10.1080/0972060X.2022.2150008. [DOI] [Google Scholar]
- 48.Rodríguez A., Beato M., Usseglio V.L., Camina J., Zygadlo J.A., Dambolena J.S., Zunino M.P. Phenolic compounds as controllers of Sitophilus zeamais: A look at the structure-activity relationship. J. Stored Prod. Res. 2022;99:102038. doi: 10.1016/j.jspr.2022.102038. [DOI] [Google Scholar]
- 49.Goharrostami M., Sendi J.J., Hosseini R., Allah Mahmoodi N.O. Effect of thyme essential oil and its two components on toxicity and some physiological parameters in mulberry pyralid Glyphodes pyloalis Walker. Pestic. Biochem. Physiol. 2022;188:105220. doi: 10.1016/j.pestbp.2022.105220. [DOI] [PubMed] [Google Scholar]
- 50.Kumrungsee N., Dunkhunthod B., Manoruang W., Koul O., Pluempanupat W., Kainoh Y., Yooboon T., Piyasaengthong N., Bullangpoti V., Nobsathian S. Synergistic interaction of thymol with Piper ribesioides (Piperales: Piperaceae) extracts and isolated active compounds for enhanced insecticidal activity against Spodoptera exigua (Lepidoptera: Noctuidae) Chem. Biol. Technol. Agric. 2022;9:38. doi: 10.1186/s40538-022-00306-2. [DOI] [Google Scholar]
- 51.Lazarevic J., Jevremovic S., Kostic I., Vuleta A., Manitasevic Jovanovic S., Kostic M., Seslija Jovanovic D. Assessment of sex-specific toxicity and physiological responses to thymol in a common bean pest Acanthoscelides obtectus Say. Front. Physiol. 2022;13:842314. doi: 10.3389/fphys.2022.842314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cmara C.D.D., Doboszewski B., Melo J.D.D., Nazarenko A., Santos R.D., Moraes M. Novel Insecticides from alkylated and acylated derivatives of thymol and eugenol for the control of Plutella xylostella (Lepidoptera: Plutellidae) J. Braz. Chem. Soc. 2022;33:196–204. [Google Scholar]
- 53.Pengsook A., Tharamak S., Keosaeng K., Koul O., Bullangpoti V., Kumrungsee N., Pluempanupat W. Insecticidal and growth inhibitory effects of some thymol derivatives on the beet armyworm, Spodoptera exigua (Lepidoptera: Noctuidae) and their impact on detoxification enzymes. Pest Manag. Sci. 2022;78:684–691. doi: 10.1002/ps.6678. [DOI] [PubMed] [Google Scholar]
- 54.Sohail M., Aqueel M.A., Dai P., Ellis J.D. The larvicidal and adulticidal effects of selected plant essential oil constituents on greater wax moths. J. Econ. Entomol. 2020;114:397–402. doi: 10.1093/jee/toaa249. [DOI] [PubMed] [Google Scholar]
- 55.Lima A.P.S., Santana E.D.R., Santos A.C.C., Silva J.E., Ribeiro G.T., Pinheiro A.M., Santos Í.T.B.F., Blank A.F., Araújo A.P.A., Bacci L. Insecticide activity of botanical compounds against Spodoptera frugiperda and selectivity to the predatory bug Podisus nigrispinus. Crop Prot. 2020;136:105230. doi: 10.1016/j.cropro.2020.105230. [DOI] [Google Scholar]
- 56.Piri A., Sahebzadeh N., Zibaee A., Sendi J.J., Shamakhi L., Shahriari M. Toxicity and physiological effects of ajwain (Carum copticum, Apiaceae) essential oil and its major constituents against Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) Chemosphere. 2020;256:127103. doi: 10.1016/j.chemosphere.2020.127103. [DOI] [PubMed] [Google Scholar]
- 57.Lu X., Weng H., Li C., He J., Zhang X., Ma Z. Efficacy of essential oil from Mosla chinensis Maxim. cv. Jiangxiangru and its three main components against insect pests. Ind. Crops Prod. 2020;147:112237. doi: 10.1016/j.indcrop.2020.112237. [DOI] [Google Scholar]
- 58.Lee S.C., Seo S.M., Huh M.J., Kwon J.H., Nam I., Park J.H., Park I.K. Behavioral and electrophysiological effects of Ajowan (Trachyspermum ammi Sprague) (Apiales: Apiaceae) essential oil and its constituents on nymphal and adult bean bugs, Riptortus clavatus (Thunberg) (Hemiptera: Alydidae) Insects. 2020;11:104. doi: 10.3390/insects11020104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Youssefi M.R., Tabari M.A., Esfandiari A., Kazemi S., Moghadamnia A.A., Sut S., Dall’Acqua S., Benelli G., Maggi F. efficacy of two monoterpenoids, carvacrol and thymol, and their combinations against eggs and larvae of the west nile vector culex pipiens. Molecules. 2019;24:1867. doi: 10.3390/molecules24101867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Youssefi M.R., Moghaddas E., Tabari M.A., Moghadamnia A.A., Hosseini S.M., Farash B.R.H., Ebrahimi M.A., Mousavi N.N., Fata A., Maggi F., et al. In vitro and in vivo effectiveness of carvacrol, thymol and linalool against Leishmania infantum. Molecules. 2019;24:2072. doi: 10.3390/molecules24112072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scalerandi E., Flores G.A., Palacio M., Defagó M.T., Carpinella M.C., Valladares G., Bertoni A., Palacios S.M. Understanding synergistic toxicity of terpenes as insecticides: Contribution of metabolic detoxification in Musca domestica. Front. Plant Sci. 2018;9:1579. doi: 10.3389/fpls.2018.01579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oliveira A.P., Santos A.A., Santana A.S., Lima A.P.S., Melo C.R., Santana E.D.R., Sampaio T.S., Blank A.F., Araújo A.P.A., Cristaldo P.F., et al. Essential oil of Lippia sidoides and its major compound thymol: Toxicity and walking response of populations of Sitophilus zeamais (Coleoptera: Curculionidae) Crop Prot. 2018;112:33–38. doi: 10.1016/j.cropro.2018.05.011. [DOI] [Google Scholar]
- 63.Giatropoulos A., Kimbaris A., Michaelakis A., Papachristos D.P., Polissiou M.G., Emmanouel N. Chemical composition and assessment of larvicidal and repellent capacity of 14 Lamiaceae essential oils against Aedes albopictus. Parasitol. Res. 2018;117:1953–1964. doi: 10.1007/s00436-018-5892-9. [DOI] [PubMed] [Google Scholar]
- 64.Navarro-Rocha J., Barrero A.F., Burillo J., Olmeda A.S., González-Coloma A. Valorization of essential oils from two populations (wild and commercial) of Geranium macrorrhizum L. Ind. Crops Prod. 2018;116:41–45. doi: 10.1016/j.indcrop.2018.02.046. [DOI] [Google Scholar]
- 65.Webster A.E., Manning P., Sproule J.M., Faraone N., Cutler G.C. Insecticidal and synergistic activity of two monoterpenes against diamondback moth (Lepidoptera: Plutellidae) Can. Entomol. 2018;150:258–264. doi: 10.4039/tce.2017.63. [DOI] [Google Scholar]
- 66.Mesquita B.M.D., Nascimento P.G.D., Souza L.G., Farias I.F.D., Silva R.A.D., Lemos T.L.D., Monte F.J.Q., Oliveira I.R., Trevisan M.T.S., da Silva H.C., et al. Synthesis, larvicidal and acetylcholinerase inhibitory activities of carvacrol/thymol and derivatives. Quim. Nova. 2018;41:412–416. doi: 10.21577/0100-4042.20170189. [DOI] [Google Scholar]
- 67.Melo C.R., Picanço M.C., Santos A.A., Santos I.B., Pimentel M.F., Santos A.C.C., Blank A.F., Araújo A.P.A., Cristaldo P.F., Bacci L. Toxicity of essential oils of Lippia gracilis chemotypes and their major compounds on Diaphania hyalinata and non-target species. Crop Prot. 2018;104:47–51. doi: 10.1016/j.cropro.2017.10.013. [DOI] [Google Scholar]
- 68.Gaire S., O’Connell M., Holguin F.O., Amatya A., Bundy S., Romero A. Insecticidal properties of essential oils and some of their constituents on the Turkestan Cockroach (Blattodea: Blattidae) J. Econ. Entomol. 2017;110:584–592. doi: 10.1093/jee/tox035. [DOI] [PubMed] [Google Scholar]
- 69.Oliveira A.P., Santana A.S., Santana E.D.R., Lima A.P.S., Faro R.R.N., Nunes R.S., Lima A.D., Blank A.F., Araújo A.P.A., Cristaldo P.F., et al. Nanoformulation prototype of the essential oil of Lippia sidoides and thymol to population management of Sitophilus zeamais (Coleoptera: Curculionidae) Ind. Crops Prod. 2017;107:198–205. doi: 10.1016/j.indcrop.2017.05.046. [DOI] [Google Scholar]
- 70.Santos A.A., de Oliveira B.M.S., Melo C.R., Lima A.P.S., Santana E.D.R., Blank A.F., Picanco M.C., Araujo A.P.A., Cristaldo P.F., Bacci L. Sub-lethal effects of essential oil of Lippia sidoides on drywood termite Cryptotermes brevis (Blattodea: Termitoidea) Ecotoxicol. Environ. Safe. 2017;145:436–441. doi: 10.1016/j.ecoenv.2017.07.057. [DOI] [PubMed] [Google Scholar]
- 71.Tabari M.A., Youssefi M.R., Maggi F., Benelli G. Toxic and repellent activity of selected monoterpenoids (thymol, carvacrol and linalool) against the castor bean tick, Ixodes ricinus (Acari: Ixodidae) Vet. Parasitol. 2017;245:86–91. doi: 10.1016/j.vetpar.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 72.Ali A., Cantrell C.L., Khan I.A. A New In Vitro Bioassay system for the discovery and quantitative evaluation of mosquito repellents. J. Med. Entomol. 2017;54:1328–1336. doi: 10.1093/jme/tjx100. [DOI] [PubMed] [Google Scholar]
- 73.Lee H.R., Kim G.H., Choi W.S., Park I.K. Repellent activity of apiaceae plant essential oils and their constituents against adult German cockroaches. J. Econ. Entomol. 2016;110:552–557. doi: 10.1093/jee/tow290. [DOI] [PubMed] [Google Scholar]
- 74.Kanda D., Kaur S., Koul O. A comparative study of monoterpenoids and phenylpropanoids from essential oils against stored grain insects: Acute toxins or feeding deterrents. J. Pest Sci. 2017;90:531–545. doi: 10.1007/s10340-016-0800-5. [DOI] [Google Scholar]
- 75.Rehman J.U., Tabanca N., Khan I.A. A Novel in vitro bioassay to explore the repellent effects of compounds against Mosquito Aedes aegypti (Diptera: Culicidae) J. Med. Entomol. 2016;53:157–165. doi: 10.1093/jme/tjv182. [DOI] [PubMed] [Google Scholar]
- 76.Seo S.-M., Jung C.-S., Kang J., Lee H.-R., Kim S.-W., Hyun J., Park I.-K. Larvicidal and Acetylcholinesterase inhibitory activities of apiaceae plant essential oils and their constituents against Aedes albopictus and formulation development. J. Agric. Food Chem. 2015;63:9977–9986. doi: 10.1021/acs.jafc.5b03586. [DOI] [PubMed] [Google Scholar]
- 77.Huang T.H., Tien N.Y., Luo Y.P. An in vitro bioassay for the quantitative evaluation of mosquito repellents against Stegomyia aegypti (Aedes aegypti) mosquitoes using a novel cocktail meal. Med. Vet. Entomol. 2015;29:238–244. doi: 10.1111/mve.12114. [DOI] [PubMed] [Google Scholar]
- 78.Kumrungsee N., Pluempanupat W., Koul O., Bullangpoti V. Toxicity of essential oil compounds against diamondback moth, Plutella xylostella, and their impact on detoxification enzyme activities. J. Pest. Sci. 2014;87:721–729. doi: 10.1007/s10340-014-0602-6. [DOI] [Google Scholar]
- 79.Tabanca N., Bernier U.R., Ali A., Wang M., Demirci B., Blythe E.K., Khan S.I., Baser K.H.C., Khan I.A. Bioassay-Guided Investigation of two Monarda essential oils as repellents of yellow fever Mosquito Aedes aegypti. J. Agric. Food Chem. 2013;61:8573–8580. doi: 10.1021/jf402182h. [DOI] [PubMed] [Google Scholar]
- 80.Sekine N., Shibutani S. Chemical structures of p-menthane monoterpenes with special reference to their effect on seed germination and termite mortality. J. Wood Sci. 2013;59:229–237. doi: 10.1007/s10086-013-1327-5. [DOI] [Google Scholar]
- 81.Azeredo C.M.O., Soares M.J. Combination of the essential oil constituents citral, eugenol and thymol enhance their inhibitory effect on Crithidia fasciculata and Trypanosoma cruzi growth. Rev. Bras. Farmacogn. 2013;23:762–768. doi: 10.1590/S0102-695X2013000500007. [DOI] [Google Scholar]
- 82.Park Y.U., Koo H.N., Kim G.H. Chemical composition, larvicidal action, and adult repellency of Thymus magnus against Aedes albopictus. J. Am. Mosq. Control Assoc. 2012;28:192–198. doi: 10.2987/12-6250R.1. [DOI] [PubMed] [Google Scholar]
- 83.Lima R.K., Cardoso M.G., Moraes J.C., Carvalho S.M., Rodrigues V.G., Guimarães L.G.L. Chemical composition and fumigant effect of essential oil of Lippia sidoides Cham. and monoterpenes against Tenebrio molitor (L.) (Coleoptera: Tenebrionidae) Ciênc. E Agrotecno. 2011;35:664–671. doi: 10.1590/S1413-70542011000400004. [DOI] [Google Scholar]
- 84.Jin C., Wu Z., Chen Y., Gong X., Yang S., Zhang Z., Zhang D., Xie Y. Insights into the toxicity, behavioral responses, biochemical activity, and molecular docking of three Cymbopogon essential oils and their major constituents on Reticulitermes flaviceps. Ind. Crops Prod. 2024;214:118563. doi: 10.1016/j.indcrop.2024.118563. [DOI] [Google Scholar]
- 85.Wu Z., Jin C., Chen Y., Yang S., Yang X., Zhang D., Xie Y. Mentha spp. essential oils: A potential toxic fumigant with inhibition of acetylcholinesterase activity on Reticulitermes dabieshanensis. Plants. 2023;12:4034. doi: 10.3390/plants12234034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang X., Han H., Li B., Zhang D., Zhang Z., Xie Y. Fumigant toxicity and physiological effects of spearmint (Mentha spicata, Lamiaceae) essential oil and its major constituents against Reticulitermes dabieshanensis. Ind. Crops Prod. 2021;171:113894. doi: 10.1016/j.indcrop.2021.113894. [DOI] [Google Scholar]
- 87.Pavela R. Acute, synergistic and antagonistic effects of some aromatic compounds on the Spodoptera littoralis Boisd. (Lep., Noctuidae) larvae. Ind. Crops Prod. 2014;60:247–258. doi: 10.1016/j.indcrop.2014.06.030. [DOI] [Google Scholar]
- 88.Pavela R. Acute toxicity and synergistic and antagonistic effects of the aromatic compounds of some essential oils against Culex quinquefasciatus Say larvae. Parasitol. Res. 2015;114:3835–3853. doi: 10.1007/s00436-015-4614-9. [DOI] [PubMed] [Google Scholar]
- 89.Arokiyaraj C., Bhattacharyya K., Reddy S.G.E. Toxicity and synergistic activity of compounds from essential oils and their effect on detoxification enzymes against Planococcus lilacinus. Front. Plant Sci. 2022;13:1016737. doi: 10.3389/fpls.2022.1016737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pavela R., Maggi F., Petrelli R., Cappellacci L., Buccioni M., Palmieri A., Canale A., Benelli G. Outstanding insecticidal activity and sublethal effects of Carlina acaulis root essential oil on the housefly, Musca domestica, with insights on its toxicity on human cells. Food Chem. Toxicol. 2020;136:111037. doi: 10.1016/j.fct.2019.111037. [DOI] [PubMed] [Google Scholar]
- 91.Ellman G.L., Courtney K.D., Andres V., Jr., Feather-Stone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.