Abstract
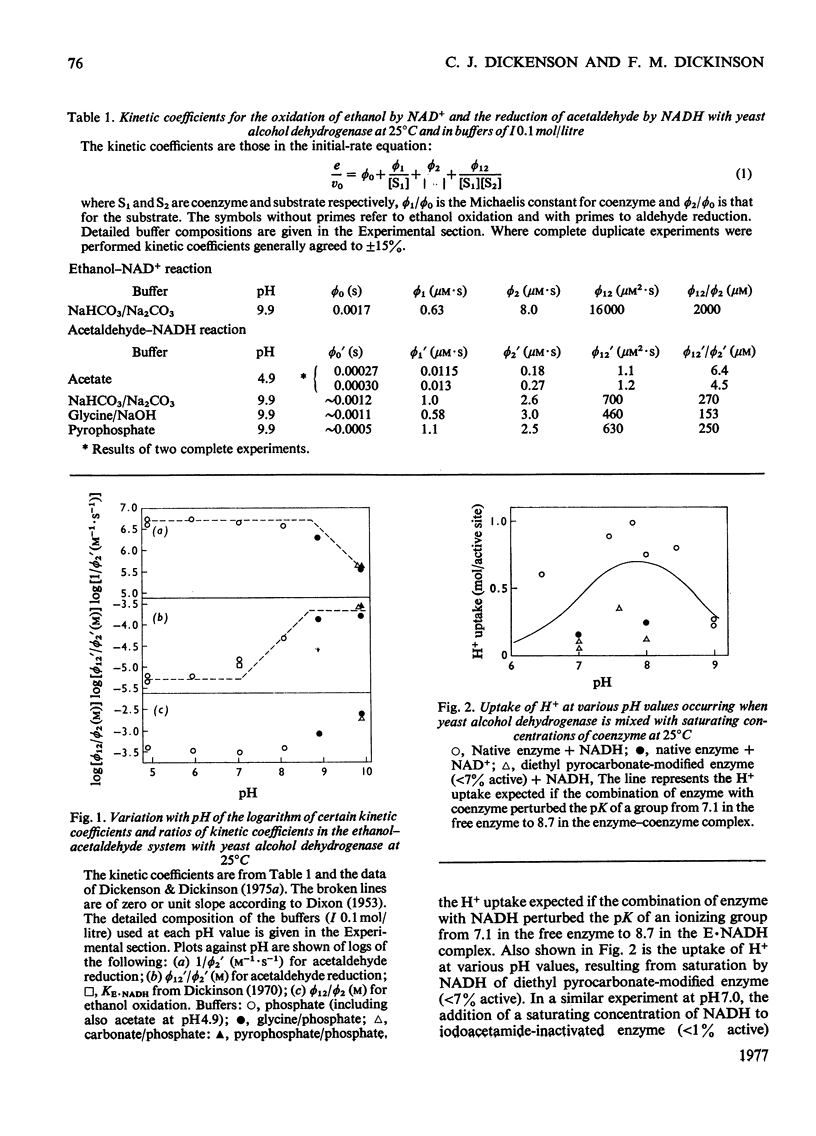
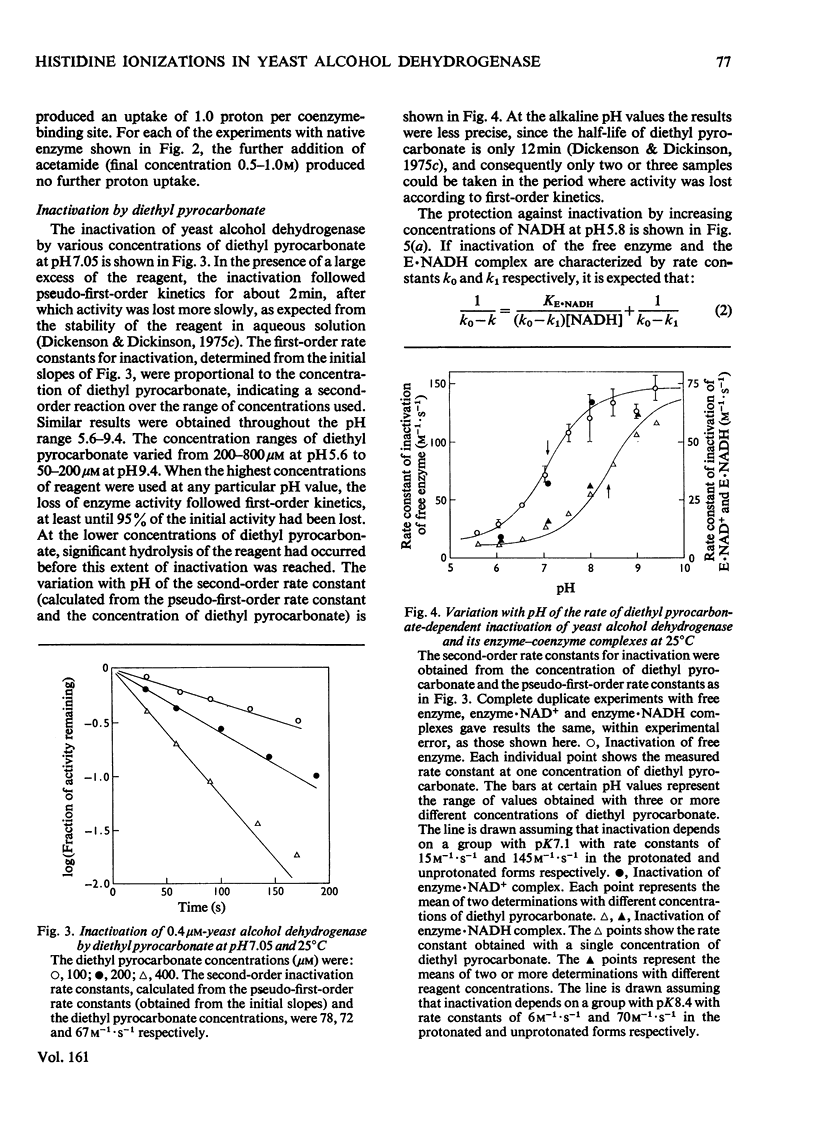
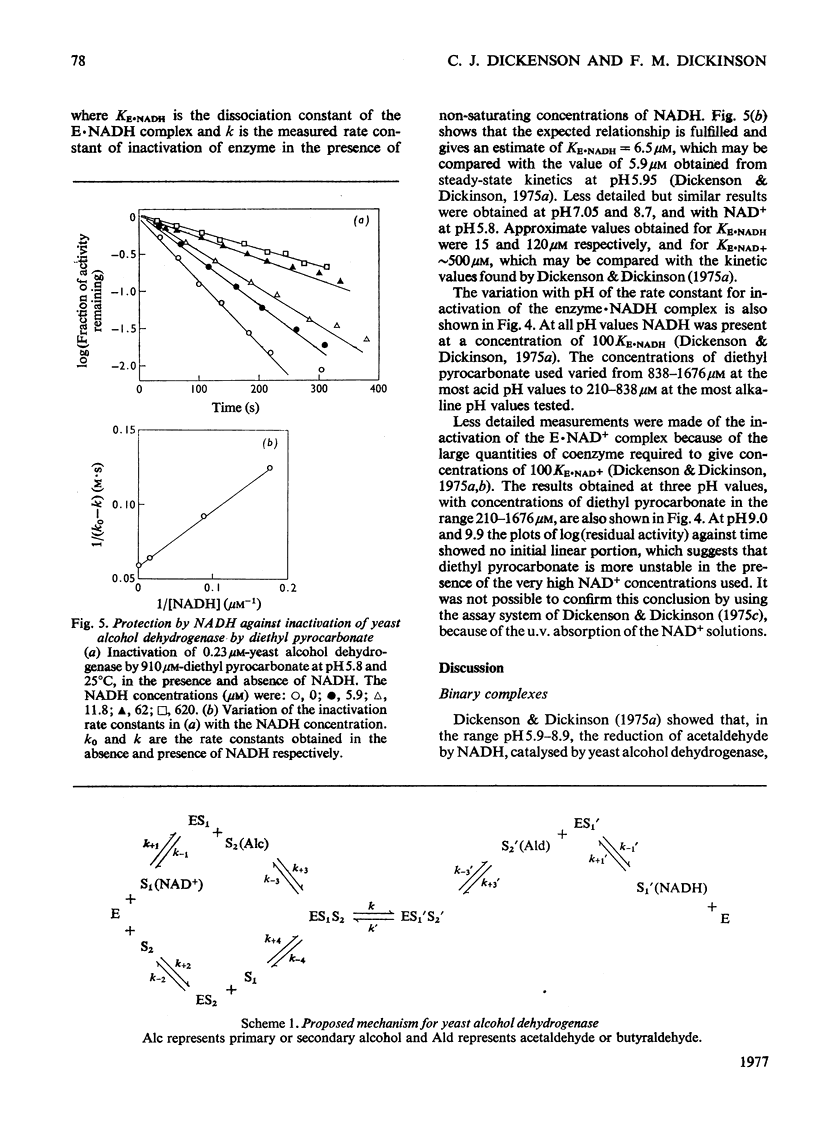
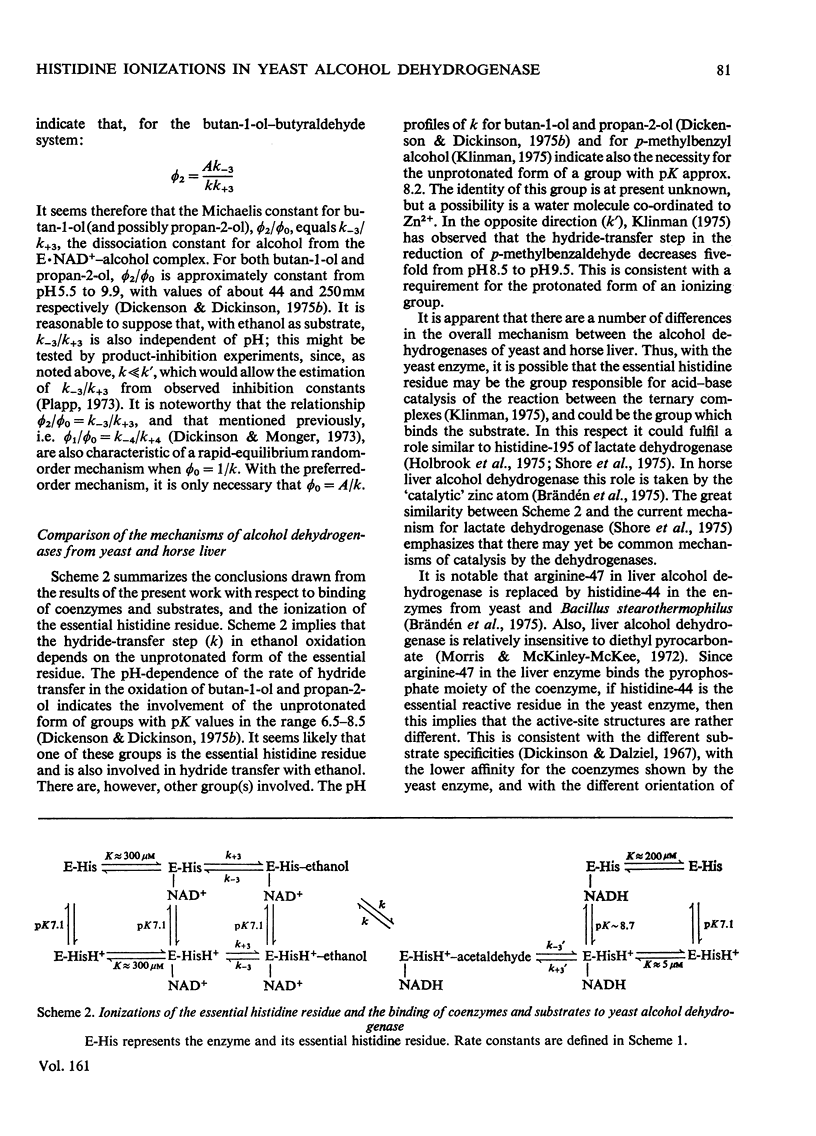
1. Initial-rate studies of the reduction of acetaldehyde by NADH, catalysed by yeast alcohol dehydrogenase, were performed at pH 4.9 and 9.9, in various buffers, at 25 degrees C. The results are discussed in terms of the mechanism previously proposed for the pH range 5.9-8.9 [Dickenson & Dickinson (1975) Biochem. J. 147, 303-311]. 2. Acetaldehyde forms a u.v.-absorbing complex with glycine. This was shown not to affect the results of kinetic experiments under the conditions used in this and earlier work. 3. The variation with pH of the dissociation constant for the enzyme-NADH complex, calculated from the initial-rate data, indicates that the enzyme possesses a group with pK7.1 in the free enzyme and pK8.7 in the complex. 4. The pH-dependences of the second-order rate constants for inactivation of the enzyme by diethyl pyrocarbonate were determined for the free enzymes (pK7.1), the enzyme-NAD+ complex (pK approx. 7.1) and the enzyme-NADH complex (pK approx. 8.4). The essential histidine residue may therefore be the group involved in formation and dissociation of the enzyme-NADH complex. 5. Estimates of the rate constant for reaction of acetaldehyde with the enzyme-NADH complex indicate that acetaldehyde may combine only when the essential histidine residue is protonated. The dissociation constants for butan-1-ol and propan-2-ol, calculated on the basis of earlier kinetic data, are, however, independent of pH. 6. The results obtained are discussed in relation to the role of the essential histidine residue in the mechanism of formation of binary and ternary complexes of the enzyme with its coenzymes and substrates.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DALZIEL K. KINETIC STUDIES OF LIVER ALCOHOL DEHYDROGENASE AND PH EFFECTS WITH COENZYME PREPARATIONS OF HIGH PURITY. J Biol Chem. 1963 Aug;238:2850–2858. [PubMed] [Google Scholar]
- DALZIEL K. The purification of nicotinamide adenine dinucleotide and kinetic effects of nucleotide impurities. J Biol Chem. 1963 Apr;238:1538–1543. [PubMed] [Google Scholar]
- DIXON M. The effect of pH on the affinities of enzymes for substrates and inhibitors. Biochem J. 1953 Aug;55(1):161–170. doi: 10.1042/bj0550161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson C. J., Dickinson F. M. A study of the oxidation of butan-1-ol and propan-2-ol by nicotinamide-adenine dinucleotide catalysed by yeast alcohol dehydrogenase. Biochem J. 1975 Jun;147(3):541–547. doi: 10.1042/bj1470541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson C. J., Dickinson F. M. A study of the pH- and temperature-dependence of the reactions of yeast alcohol dehydrogenase with ethanol, acetaldehyde and butyraldehyde as substrates. Biochem J. 1975 May;147(2):303–311. doi: 10.1042/bj1470303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson C. J., Dickinson F. M. The role of an essential histidine residue of yeast alcohol dehydrogenase. Eur J Biochem. 1975 Apr 1;52(3):595–603. doi: 10.1111/j.1432-1033.1975.tb04031.x. [DOI] [PubMed] [Google Scholar]
- Dickinson F. M., Dalziel K. The specificities and configurations of ternary complexes of yeast and liver alcohol dehydrogenases. Biochem J. 1967 Jul;104(1):165–172. doi: 10.1042/bj1040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson F. M., Monger G. P. A study of the kinetics and mechanism of yeast alcohol dehydrogenase with a variety of substrates. Biochem J. 1973 Feb;131(2):261–270. doi: 10.1042/bj1310261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson F. M. Role of the essential thiol groups of yeast alcohol dehydrogenase. Biochem J. 1972 Jan;126(1):133–138. doi: 10.1042/bj1260133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson F. M. The binding of dihydronicotinamide--adenine dinucleotide and pyridine-3-aldehyde--adenine dinucleotide by yeast alcohol dehydrogenase. Biochem J. 1970 Dec;120(4):821–830. doi: 10.1042/bj1200821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson M. Measurements of the concentration of active sites in preparations of yeast alcohol dehydrogenase. Eur J Biochem. 1974 Jan 3;41(1):31–36. doi: 10.1111/j.1432-1033.1974.tb03240.x. [DOI] [PubMed] [Google Scholar]
- Holbrook J. J., Ingram V. A. Ionic properties of an essential histidine residue in pig heart lactate dehydrogenase. Biochem J. 1973 Apr;131(4):729–738. doi: 10.1042/bj1310729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinman J. P. Acid-base catalysis in the yeast alcohol dehydrogenase reaction. J Biol Chem. 1975 Apr 10;250(7):2569–2573. [PubMed] [Google Scholar]
- Klinman J. P. The mechanism of enzyme-catalyzed reduced nicotinamide adenine dinucleotide-dependent reductions. Substituent and isotope effects in the yeast alcohol dehydrogenase reaction. J Biol Chem. 1972 Dec 25;247(24):7977–7987. [PubMed] [Google Scholar]
- Lee G. D., van Etten R. L. Evidence of an essential histidine residue in rabbit liver aryl sulfatase A. Arch Biochem Biophys. 1975 Dec;171(2):424–434. doi: 10.1016/0003-9861(75)90051-x. [DOI] [PubMed] [Google Scholar]
- Leskovac V., Pavkov-Pericin D. Evidence for a histidine and a cysteine residue in the substrate-binding site of yeast alcohol dehydrogenase. Biochem J. 1975 Mar;145(3):581–590. doi: 10.1042/bj1450581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUELLER-HILL B., WALLENFELS K. MECHANISMUS DER WASSERSTOFFUEBERTRAGUNG MIT PYRIDINNUCLEOTIDEN. XXV. IST ACETALDEHYD ODER SEIN HYDRAT SUBSTRAT DER ALKOHOLDEHYDROGENASE? Biochem Z. 1964 Mar 12;339:349–351. [PubMed] [Google Scholar]
- Morris D. L., McKinley-McKee J. S. The histidines in liver alcohol dehydrogenase. Chemical modification with diethylpyrocarbonate. Eur J Biochem. 1972 Sep 25;29(3):515–520. doi: 10.1111/j.1432-1033.1972.tb02016.x. [DOI] [PubMed] [Google Scholar]
- Plapp B. V. On calculation of rate and dissociation constants from kinetic constants for the Ordered Bi Bi mechanism of liver alcohol dehydrogenase. Arch Biochem Biophys. 1973 May;156(1):112–114. doi: 10.1016/0003-9861(73)90347-0. [DOI] [PubMed] [Google Scholar]
- SILVERSTEIN E., BOYER P. D. EQUILIBRIUM REACTION RATES AND THE MECHANISMS OF LIVER AND YEAST ALCOHOL DEHYDROGENASE. J Biol Chem. 1964 Nov;239:3908–3914. [PubMed] [Google Scholar]
- Twu J. S., Chin C. C., Wold F. Studies on the active-site sulfhydryyl groups of yeast alcohol dehydrogenase. Biochemistry. 1973 Jul 17;12(15):2856–2862. doi: 10.1021/bi00739a013. [DOI] [PubMed] [Google Scholar]
- Twu J. S., Wold F. Butyl isocyanate, an active-site-specific reagent for yeast alcohol dehydrogenase. Biochemistry. 1973 Jan 30;12(3):381–386. doi: 10.1021/bi00727a003. [DOI] [PubMed] [Google Scholar]
- Whitehead E. P., Rabin B. R. The thiol groups of yeast alcohol dehydrogenase. Biochem J. 1964 Mar;90(3):532–539. doi: 10.1042/bj0900532. [DOI] [PMC free article] [PubMed] [Google Scholar]


