Abstract
The effect of ionic strength on the proteolysis by trypsin of the major membrane-penetrating protein (polypeptide 3) in the erythrocyte membrane was studied. Both the intracellular and extracellular regions of the protein are susceptible to trypsin proteolysis under hypo-osmotic conditions, whereas under iso-osmotic conditions the extracellular region of the protein is resistant to trypsin, and the intracellular region yields only two cleavage products with trypsin. Studies of the fragments obtained from polypeptide 3 by trypsin digestion under iso-osmotic conditions of 'ghosts' radioiodinated with lactoperoxidase confirmed our earlier conclusions that the polypeptide chain of polypeptide 3 traverses the membrane twice. Ionic-strength-dependent changes were also observed in the incorporation of iodine by lactoperoxidase into the individual extracellular tyrosine sites of the protein. These results show that polypeptide 3 undergoes ionic-strength-dependent changes in structure.
Full text
PDF
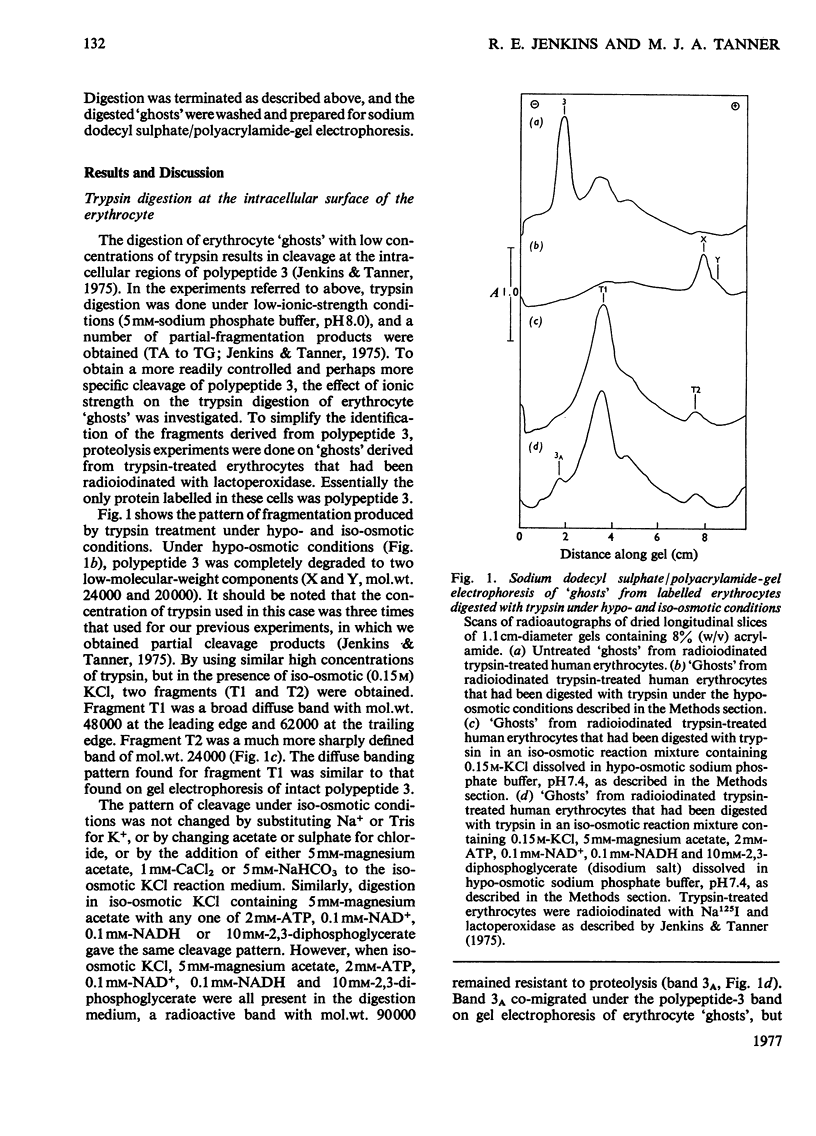
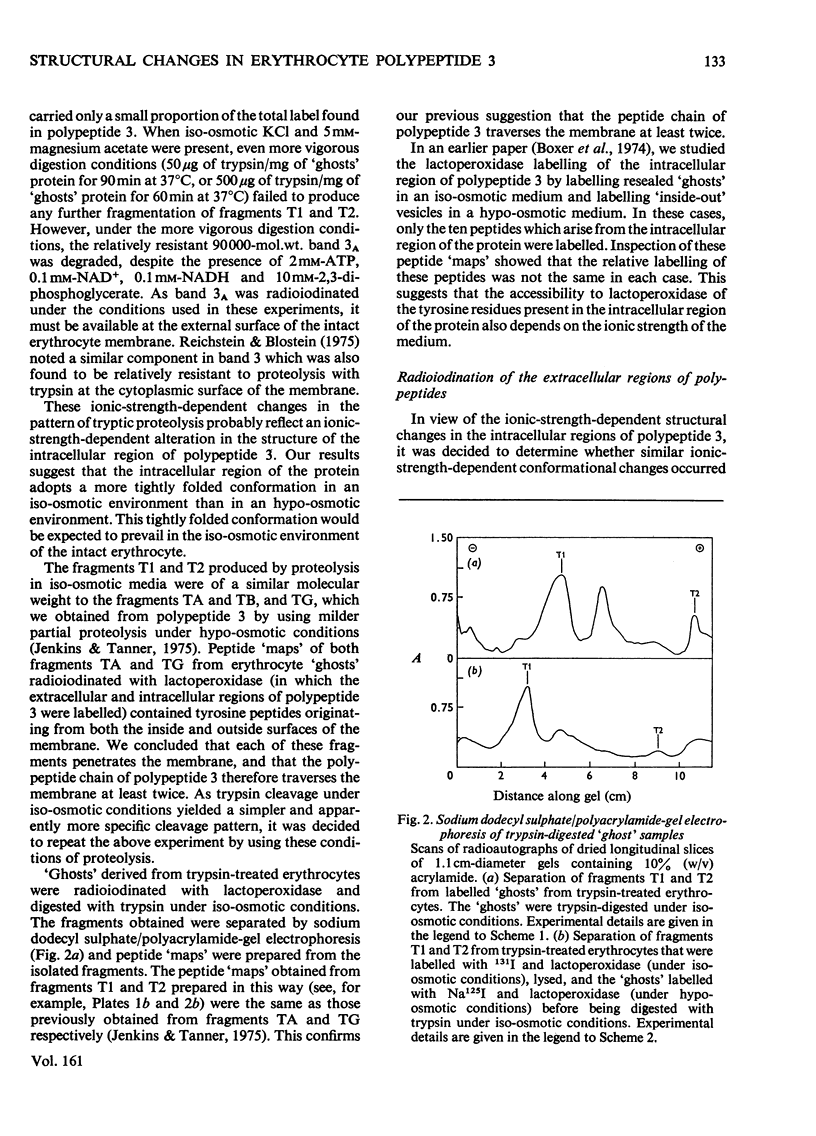



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender W. W., Garan H., Berg H. C. Proteins of the human erythrocyte membrane as modified by pronase. J Mol Biol. 1971 Jun 28;58(3):783–797. doi: 10.1016/0022-2836(71)90040-4. [DOI] [PubMed] [Google Scholar]
- Boxer D. H., Jenkins R. E., Tanner M. J. The organization of the major protein of the human erythrocyte membrane. Biochem J. 1974 Mar;137(3):531–534. doi: 10.1042/bj1370531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S. A major protein which spans the human erythrocyte membrane. J Mol Biol. 1971 Jul 28;59(2):351–357. doi: 10.1016/0022-2836(71)90055-6. [DOI] [PubMed] [Google Scholar]
- Cabantchik Z. I., Rothstein A. Membrane proteins related to anion permeability of human red blood cells. I. Localization of disulfonic stilbene binding sites in proteins involved in permeation. J Membr Biol. 1974;15(3):207–226. doi: 10.1007/BF01870088. [DOI] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. The enzymatic iodination of the red cell membrane. J Cell Biol. 1972 Nov;55(2):390–405. doi: 10.1083/jcb.55.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins R. E., Tanner J. A. The major human erythrocyte membrane protein. Evidence for an S-shaped structure which traverses the membrane twice and contains a duplicated set of sites. Biochem J. 1975 Jun;147(3):393–399. doi: 10.1042/bj1470393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins R. E., Tanner J. A. The structure of the major protein of the human erythrocyte membrane. Characterization of the intact protein and major fragments. Biochem J. 1977 Jan 1;161(1):139–147. doi: 10.1042/bj1610139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichstein E., Blostein R. Arrangement of human erythrocyte membrane proteins. J Biol Chem. 1975 Aug 25;250(16):6256–6263. [PubMed] [Google Scholar]
- Steck T. L., Fairbanks G., Wallach D. F. Disposition of the major proteins in the isolated erythrocyte membrane. Proteolytic dissection. Biochemistry. 1971 Jun 22;10(13):2617–2624. doi: 10.1021/bi00789a031. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Ramos B., Strapazon E. Proteolytic dissection of band 3, the predominant transmembrane polypeptide of the human erythrocyte membrane. Biochemistry. 1976 Mar 9;15(5):1153–1161. doi: 10.1021/bi00650a030. [DOI] [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki L., Fasold H., Schuhmann B., Passow H. Chemical modification of membrane proteins in relation to inhibition of anion exchange in human red blood cells. J Cell Physiol. 1975 Dec;86(3 Pt 1):471–494. doi: 10.1002/jcp.1040860305. [DOI] [PubMed] [Google Scholar]