Abstract
The benzenoid ester, methylbenzoate is one of the most abundant scent compounds detected in the majority of snapdragon (Antirrhinum majus) varieties. It is produced in upper and lower lobes of petals by enzymatic methylation of benzoic acid in the reaction catalyzed by S-adenosyl-l-methionine:benzoic acid carboxyl methyltransferase (BAMT). To identify the location of methylbenzoate biosynthesis, we conducted an extensive immunolocalization study by light and electron microscopy at cellular and subcellular levels using antibodies against BAMT protein. BAMT was immunolocalized predominantly in the conical cells of the inner epidermal layer and, to a much lesser extent, in the cells of the outer epidermis of snapdragon flower petal lobes. It was also located in the inner epidermis of the corolla tube with little BAMT protein detected in the outer epidermis and in the yellow hairs within the tube on the bee's way to the nectar. These results strongly suggest that scent biosynthetic genes are expressed almost exclusively in the epidermal cells of floral organs. Immunogold labeling studies reveal that BAMT is a cytosolic enzyme, suggesting cytosolic location of methylbenzoate biosynthesis. The concentration of scent production on flower surfaces that face the pollinators during landing may increase pollination efficiency and also help to minimize the biosynthetic cost of advertising for pollinators.
Flowers of many plant species attract pollinators by producing complex mixtures of volatile compounds that give each species their unique, characteristic fragrances. Among the various floral stimuli, odors play a prominent role in the location and selection of flowers by insects, particularly moths, which search and visit flowers at night (Dobson, 1994). Although little is known about how insects respond to individual components found within floral scents, it is clear that they are capable of distinguishing complex floral scent mixtures and that discriminatory visitation based upon floral scent has important implications for plant reproductive success. Therefore, floral scent can be considered to perform a vital function in the plant life cycle.
Floral fragrances belong to a broad category of secondary metabolites and are dominated by terpenoids (monoterpenes and sesquiterpenes), phenylpropanoid, and benzenoid compounds (Knudsen et al., 1993). Despite the importance of floral scent compounds in plant biology, floral scent research has, until recently, remained largely descriptive. A total of 700 floral scent compounds have been described from more than 60 plant families (Knudsen et al., 1993), yet most of the biochemical pathways and the enzymes involved in the biosynthesis of these compounds have not been elucidated.
Clarkia breweri, an annual native to California, was the first model system in which the isolation of enzymes and genes responsible for the formation of flower scent volatiles was accomplished (Dudareva and Pichersky, 2000). It was shown that C. breweri flowers produce scent compounds de novo within the tissues from which they are emitted and that their emission levels, corresponding enzyme activities, and corresponding mRNA levels are all spatially and temporally correlated. A positive correlation between amounts of emitted scent compounds, corresponding enzyme activity, and mRNA indicates that activity levels of enzymes involved in scent production and, indirectly, scent emission are regulated mainly by the transcription of corresponding genes at the site of emission (Dudareva et al., 1999; for review, see Dudareva and Pichersky, 2000).
We recently have begun investigations into the biogenesis of scent production in snapdragon (Antirrhinum majus) flowers and have isolated and characterized S-adenosyl-l-Met:benzoic acid carboxyl methytransferase (BAMT), the final enzyme in the biosynthesis of the volatile ester, methylbenzoate (Dudareva et al., 2000; Murfitt et al., 2000). We showed that common regulatory mechanisms are involved in floral scent production in different plant species. Similar to C. breweri, the activities of scent biosynthetic enzymes in snapdragon are regulated by the transcription of corresponding genes in the flower (Dudareva et al., 2000). In addition, the earlier steps in the biochemical pathway are also involved in the regulation of volatile production and emission by controlling the substrate supply required for the reaction (Dudareva et al., 2000).
One unique aspect of scent production is that small organic molecules, produced by the plant cells and are usually water-insoluble, have to move to the exterior of the plant for evaporation. It is not known, however, if these compounds are synthesized in cells at the plant surface or whether they are transported from internal cells. The first and only available information came from in situ RNA hybridization experiments in C. breweri, demonstrating that the biosynthesis of volatile compounds is restricted to specific tissues at the site of emission. It has been shown that linalool synthase and S-adenosyl-l-Met:(iso) eugenol O-methyltransferase genes are expressed uniformly and almost exclusively in cells of the epidermal layers of petals and other parts of C. breweri flowers, positions from which the compounds can readily escape into the atmosphere (Dudareva et al., 1996; Dudareva and Pichersky, 2000). However, to the best of our knowledge, there is limited information regarding the subcellular localization of biosynthesis of scent compounds.
To gain more insight into the significant aspects of floral scent production, we conducted an extensive light and electron microscopic immunolocalization study at cellular and subcellular levels using antibodies against BAMT protein. Our results show that, within snapdragon petals, BAMT is predominantly localized in the conical cells of the inner epidermal layer and, to a much lesser extent, in outer epidermal cells that line the exterior lobe surface. In addition, immunogold labeling studies reveal that BAMT is a cytosolic enzyme, suggesting cytosolic location of methylbenzoate biosynthesis.
RESULTS
Immunolocalization of BAMT in Conical Cells of the Petal Epidermis
S-Adenosyl-l-Met:benzoic acid carboxyl methyltransferase (BAMT) catalyzes the transfer of the methyl group of S-adenosyl-l-Met to the carboxyl group of benzoic acid to make the volatile ester, methylbenzoate, one of the most abundant scent compounds of snapdragon (Dudareva et al., 2000; Murfitt et al., 2000). Methylbenzoate production in snapdragon flowers is mostly restricted to the upper and lower lobes (Fig. 1A) where the majority of total BAMT activity and BAMT transcripts were found (Dudareva et al., 2000). Production of methylbenzoate is regulated by benzoic acid quantity and the amount of BAMT protein, which in turn is regulated at the transcriptional level. To determine the cellular localization of methylbenzoate production, we investigated the tissue distribution of the BAMT protein by immunolocalization at the light microscope level.
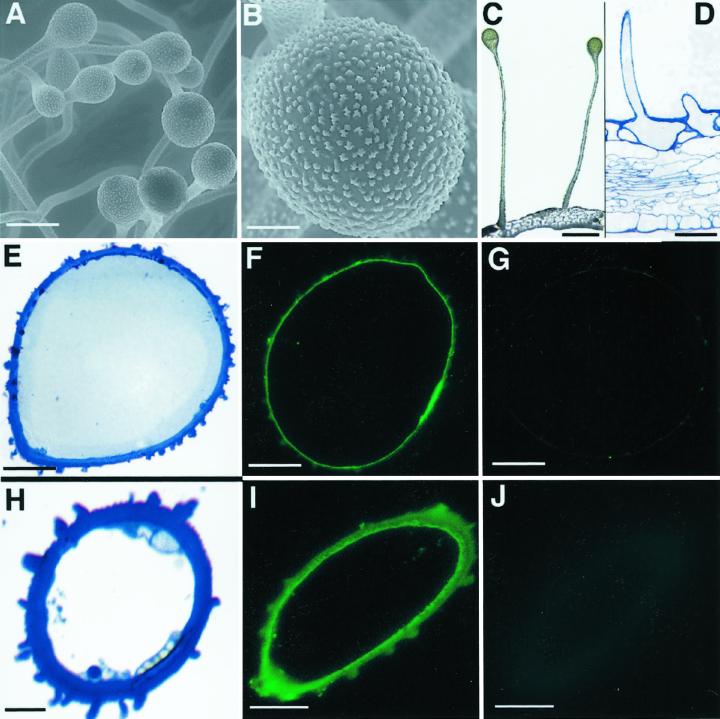
Figure 1.
Immunofluorescence localization of BAMT in snapdragon flowers. A, Antirrhinum flower. B, Environmental scanning electron micrograph of conical cells from the inner epidermis of the lower petal lobe. C, Transverse section of 7-d-old snapdragon lower petal lobe. D, Transverse section of 7-d-old snapdragon lower petal lobe treated with anti-BAMT antibodies and visualized by fluorescent FITC-conjugated secondary antibodies. E and F, Control sections corresponding to D. E, Transverse section of 7-d-old snapdragon lower petal lobe treated with preimmune serum and visualized by fluorescent FITC-conjugated secondary antibodies. F, Transverse section of 1-d-old snapdragon lower petal lobe treated with anti-BAMT antibodies and visualized by fluorescent FITC-conjugated secondary antibodies. Nonspecific signal is due to starch granules. G, Transverse section of corolla tube of 7-d-old snapdragon flower. H, Transverse section of corolla tube of 7-d-old snapdragon flower treated with anti-BAMT antibodies and visualized by fluorescent FITC-conjugated secondary antibodies. I, Control section corresponding to H. Transverse section of corolla tube of 7-d-old snapdragon flower treated with preimmune serum and visualized by fluorescent FITC-conjugated secondary antibodies. Vascular tissues in H and I show nonspecific labeling. vt, Vascular tissue. Scale bars = 20 μm (B) and 50 μm (C–I).
Immunofluorescent BAMT localization was performed on the lower lobes of 7-d-old snapdragon flowers, a stage of flower development with the highest amount of BAMT protein as determined by protein blot analysis (Dudareva et al., 2000). The polyclonal antibodies previously prepared against the snapdragon BAMT recognized both denatured BAMT protein (as determined by immunoblotting; see Dudareva et al., 2000), as well as the native protein (determined by immunoprecipitation of enzymatic activity; data not shown). Cross sections of lower petal lobes were incubated with primary antibodies, which were visualized using secondary antibodies coupled to fluorescein isothiocyanate (FITC). The two epidermal layers of the petal are referred to here as the inner layer, lining the throat of the tube and contiguous surface of the lobes, and the outer layer, lining the exterior surface of the tube and lobes (Fig. 1A). Within the lobes, the cells of the inner (adaxial) epidermis have a conical shape that is pentagonal at the base in contrast to the normal flattened (lenticular) shape of the outer (abaxial) epidermis (Fig. 1, B and C). BAMT protein was found exclusively in epidermal cells of the petal. However, the amount of BAMT differed between the inner and outer epidermis of the lobe (Fig. 1D). BAMT was mainly localized in the conical cells of petals where the signal appears as green fluorescence within the cells under a fluorescence microscope. Although the outer epidermis of lobes bore numerous multicellular hairs, no signal was detected within these hairs. BAMT activity was also absent in the purified hairs when it was measured in the sonicated extracts of petal hairs and compared with the corresponding specific activities from whole petal homogenates (N. Gorenstein and N. Dudareva, unpublished data). No signal was observed when the section was incubated with preimmune serum from the same rabbit (Fig. 1E) and also when the cross sections of lower petal lobes of 1-d-old flowers were treated with the anti-BAMT polyclonal antibodies (Fig. 1F). One-day-old flowers were used as an additional negative control since it has been shown that they produce very low amounts of methylbenzoate and contain an amount of BAMT protein undetectable by immunoblotting (Dudareva et al., 2000).
Immunolocalization of BAMT in Corolla Tube
The five petals of snapdragon are classified into three types: two dorsals, two laterals, and one ventral. All five are fused for part of their length into a tube that ends in a sharp border with the petal lobes (Fig. 1A). Since some BAMT activity was previously found in corolla tube (Dudareva et al., 2000), it was of interest to determine the exact cellular distribution of BAMT expression within the tube. When corolla tube cross sections from 7-d-old snapdragon flowers (Fig. 1G) were treated with anti-BAMT antibodies, green fluorescence signal was detected exclusively in epidermal cell layers (Fig. 1H). The signal was concentrated in the inner epidermis of the tube with little BAMT protein detected in the outer epidermis. The negative control performed with the preimmune serum instead of primary antibodies showed no signal (Fig. 1I).
Within the tube, there are two stripes of yellow hairs on the internal surface located to either side of the boundaries between ventral and lateral petals. Environmental scanning electron microscopy and light microscopy revealed that these hairs are unicellular glands and they consist of stalks and heads (Fig. 2, A–D). Since these glands are on the bee's way to nectar, we examined whether they are involved in production of methylbenzoate. Cross sections of single-cell hairs (Fig. 2, E and H) were incubated with BAMT antiserum and a green fluorescent signal was detected at the surface of the hair (Fig. 2, F and I). Preimmune serum used as a negative control did not show any signal (Fig. 2, G and J).
Figure 2.
Immunofluorescence localization of BAMT in snapdragon flower glands. A, Environmental scanning electron micrograph of glands within corolla tube of 7-d-old snapdragon flower. B, Environmental scanning electron micrograph showing the gland's head. C, Light microscopy photograph of glands within corolla tube. Fresh samples of snapdragon corolla tube were hand cut. D, Transverse section of glands within corolla tube of 7-d-old snapdragon flower. E, Cross-section through gland head within corolla tube of 7-d-old snapdragon flower. F, Cross-section through gland head within corolla tube of 7-d-old snapdragon flower treated with anti-BAMT antibodies and visualized by fluorescent FITC-conjugated secondary antibodies. G, Control section corresponding to F. Cross-section through gland head within corolla tube of 7-d-old snapdragon flower treated with preimmune serum and visualized by fluorescent FITC-conjugated secondary antibodies. H, Cross-section through gland stalk within corolla tube of 7-d-old snapdragon flower. I, Cross-section through gland stalk within corolla tube of 7-d-old snapdragon flower treated with anti-BAMT antibodies and visualized by fluorescent FITC-conjugated secondary antibodies. J, Control section corresponding to I. Cross-section through gland stalk within corolla tube of 7-d-old snapdragon flower treated with preimmune serum and visualized by fluorescent FITC-conjugated secondary antibodies. Scale bars = 100 μm (A and D), 20 μm (B, E–G, I, and J), 300 μm (C), and 10 μm (H).
Subcellular Localization of BAMT Protein
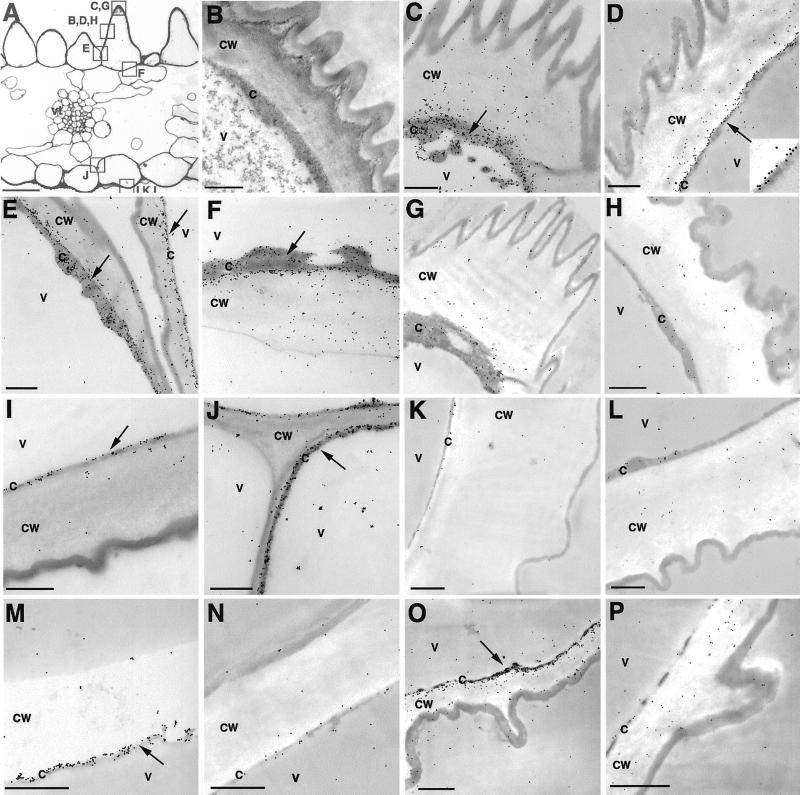
To determine the intracellular distribution of BAMT in the epidermal cells of the petal, immunoelectron microscopy was carried out with transverse sections of the lower lobes (Fig. 3, A and B), tubes, and glands of 7-d-old snapdragon flowers. Substantial immunogold labeling was found within the cytoplasm of epidermal cells, adjacent to the primary cell wall (Fig. 3, C–F, I, and J), suggesting the cytoplasmic localization of BAMT protein. Such a distribution pattern of gold particles was regularly observed within inner (Fig. 3, C–F) and outer epidermal cells of lobes (Fig. 3, I and J), tubes (Fig. 3M) and glands (Fig. 3O). There was no evidence for an ordered localization within the cytoplasm as would be predicted for the distribution of endoplasmic-reticulum-associated proteins (Shorrosh et al., 1993). No specific immunolabeling was observed in control sections treated with preimmune serum instead of anti-BAMT antibodies (Fig. 3, G, H, K, L, N, and P).
Figure 3.
Immunogold localization of BAMT in snapdragon flower. A, Light microscopy photograph of transverse section of 7-d-old snapdragon lower petal lobe. Boxes with letters indicate positions where the following corresponding pictures were taken. B, Fine structure of conical cell of 7-d-old snapdragon lower petal lobe. C through F, Transmission electron microscopy (TEM) of conical cells of 7-d-old snapdragon lower petal lobe labeled with anti-BAMT antibodies and gold-conjugated goat anti-rabbit antibodies. D, The region marked with an arrow was magnified three times to show gold particles (see insert). G and H, Control sections corresponding to C through F. G, TEM of conical cells of 7-d-old snapdragon lower petal lobe treated with preimmune serum and gold-conjugated goat anti-rabbit antibodies. H, TEM of conical cells of 1-d-old snapdragon lower petal lobe labeled with anti-BAMT antibodies and gold-conjugated goat anti-rabbit antibodies. I and J, TEM of outer epidermal cell of 7-d-old snapdragon lower petal lobe labeled with anti-BAMT antibodies and gold-conjugated goat anti-rabbit antibodies. K and L, Control sections corresponding to I and J. K, TEM of outer epidermal cell of 7-d-old snapdragon lower petal lobe treated with preimmune serum and gold-conjugated goat anti-rabbit antibodies. L, TEM of outer epidermal cell of 1-d-old snapdragon lower petal lobe labeled with anti-BAMT antibodies and gold-conjugated goat anti-rabbit antibodies. M, TEM of inner epidermal cell of corolla tube of 7-d-old snapdragon flower labeled with anti-BAMT antibodies and gold-conjugated goat anti-rabbit antibodies. N, Control section corresponding to M. TEM of inner epidermal cell of corolla tube of 7-d-old snapdragon flower treated with preimmune serum and gold-conjugated goat anti-rabbit antibodies. O, TEM of gland within corolla tube of 7-d-old snapdragon flower labeled with anti-BAMT antibodies and gold-conjugated goat anti-rabbit antibodies. P, Control section corresponding to O. TEM of gland within corolla tube of 7-d-old snapdragon flower treated with preimmune serum and gold-conjugated goat anti-rabbit antibodies. c, Cytosol; cw, cell wall; v, vacuole; vt, vascular tissue. Scale bars = 50 μm (A) and 1 μm (B–P). Arrows point to regions of BAMT localization.
DISCUSSION
Methylbenzoate, a major component of the scent produced by the bee-pollinated snapdragon, is synthesized and emitted mostly from the upper and lower lobes of petals where bumblebees come in contact with the flower (Dudareva et al., 2000). This research shows that BAMT gene expression is epidermis specific and that the two epidermal petal layers, the inner and the outer, are differentially involved in floral scent biosynthesis. Methylbenzoate is produced predominantly in the conical cells of the inner epidermal layer and to a much lesser extent in the epidermal cells of the outer epidermis lining the surface exterior of the corolla lobes (Fig. 1D). In addition, methylbenzoate is also made in the inner epidermis of the corolla tube with little production in the outer epidermis (Fig. 1H). These results coupled with the data previously obtained in C. breweri (Dudareva et al., 1996; Dudareva and Pichersky, 2000) strongly suggest that scent biosynthetic genes are expressed almost exclusively in the epidermal cells of floral organs.
Vegetative essential oil production in plants is generally associated with the presence of specialized secretory structures such as oil and resin cells, glandular trichomes, oil and resin ducts, or glandular epidermis (Fahn, 1988; Wagner, 1991; Croteau, 1992). Such structures contain a variety of natural products, including terpenoids, phenolics, Suc esters, and organic acids (Croteau and Johnson, 1984; Gershenzon et al., 1992; McCaskill et al., 1992; Gang et al., 2001). Such structures are thought to be necessary to protect metabolically active cells from these toxic compounds (Fahn, 1979; Croteau, 1992).
Structures that were named “scent glands,” or osmophores, have been found in flowering plants such as the different genera and species of orchids (Curry et al., 1988, 1991) and boronia flowers, Boronia megastigma Nees (Mactavish and Menary, 1997). These structures appear to contain high concentrations of lipophylic substances (Fahn, 1979). In snapdragon flowers, we found no unusual grandular structures on the petal surface, but the outer epidermis of lobes contained numerous multicellular hairs. Although these hairs increase surface area and might enhance scent volatilization from the petals, they do not appear to be involved in scent production in snapdragon flowers. No signal was found when the cross sections of lower petal lobes were treated with the anti-BAMT polyclonal antibodies. However, BAMT gene expression was found in the yellow hairs within the tube located on the bee's way to the nectar (Fig. 2, F and I).
To date little is known about the intracellular biosynthesis of volatile compounds and their trafficking from the site of synthesis to their eventual emission from floral tissues. The evidence that monoterpenes have a plastidic origin came largely from biochemical studies with vegetative tissue (McGarvey and Croteau, 1995) and immunogold localization of limonene synthase in leucoplasts of oil gland secretory cells of peppermint leaves (Turner et al., 1999). Moreover, all the known monoterpene synthases sequences apparently possess plastid-targeting sequences (Bohlmann et al., 1998). In floral tissue, monoterpene biosynthesis was indirectly localized in the plastids by showing that isolated chromoplasts from daffodil petals were capable of catalyzing the production of several monoterpenes, including limonene, myrcene, ocimene, and linalool (Mettal et al., 1988). In contrast to monoterpene biosynthesis, the intracellular location of the synthesis of volatile phenylpropanoid scent compounds has not been previously determined. Here we show that BAMT, the enzyme that catalyzes the formation of the volatile ester methylbenzoate, is found in the cytoplasm of the epidermal cells of snapdragon petals (Fig. 3, C–F, I, J, M, and O). An apparent association with the plasma membrane/cell wall region (Fig. 3, D, I, M, and O) may be due to the presence of large vacuoles, which would press the cytosol against the cell wall thereby compacting the immunogold labeling.
Flowers of many plant species attract pollinators by visual and olfactory cues. In bumblebee foraging, attraction to flowers from a distance is primarily a visual phenomenon (Heinrich, 1976), whereas landing depends upon both visual and olfactory cues (Lunau, 1991, 1992). There is an advantage for the plant to have its scent output at maximal levels only when the flower is ready for pollination and concomitantly when its potential pollinator is active. Young snapdragon flowers, which are not ready to function as pollen donors because their anthers have not yet dehisced, produce fewer odors and are less attractive to pollinators than are older flowers (Jones et al., 1998; Dudareva et al., 2000). Furthermore, snapdragon flowers release four times more volatile compounds during the daytime, when bumblebees are active, than at night (Dudareva et al., 2000). Our current results indicate that to increase pollination “advertisement efficiency,” snapdragon flowers concentrate floral scent on petal surfaces by producing methylbenzoate in the conical cells of upper and lower petal lobes (Fig. 1D), which face the pollinators during landing. In addition, the inner epidermis of the corolla tube, which surrounds the bee while it approaches the nectar, is also involved in floral scent production (Fig. 1H). Moreover, methylbenzoate is made in the yellow hairs within the tube located on the bee's way to nectar (Fig. 2, F and I). This pattern in scent production, in addition to informing pollinators of the reward status of the flower, may also help to minimize the biosynthetic cost of advertising for pollinators by restricting areas of scent production. It may also help to intensify odor production around the bee and thereby odor delivery to the nest, which in turn may increase the pollinator recruitment rate.
The conical cells of petal lobes (Fig. 1B), in addition to being major sources of floral scent production, also contain the highest concentrations of anthocyanins (Martin and Gerats, 1993). Moreover, the conical shape of the epidermal cells increases the proportion of incident light that enters the cells, thereby enhancing light absorption by the floral pigments, and thus the intensity of their color, especially in regions facing prospective pollinators (Kay et al., 1981; Gorton and Vogelmann, 1996). The conical shape of the epidermal cells also increases the emitting surface of the cells when compared with flat epidermal cells (Noda et al., 1994). In addition to conical shape, the ridges in the cell wall, cuticle, and wax layers (Figs. 1B and 3B) may play a part in light absorption and reflection, increase surface area, and provide tactile stimulation for pollinator-petal surface recognition. Contoured ridges of conical cells may also provide better traction for insect landing and movement on the petal surface. Overall, pigment and scent production in the same type of cells, along with the conical shape and ridged surface of these cells, are likely important parts of the mechanism by which plants increase the efficiency of their interaction with pollinators.
MATERIALS AND METHODS
Plant Material and Tissue Preparation
Snapdragons (Antirrhinum majus cv Maryland True Pink; Ball Seed Company, Chicago) were grown under standard greenhouse conditions, as previously described (Dudareva et al., 2000).
For structural analysis, sections from lower petal lobes and tubes of 1- and 7-d-old snapdragon flowers were fixed for 1 h at room temperature in one-half-strength Karnovsky's fixative, containing 2.5% (v/v) glutaraldehyde and 2% (v/v) formaldehyde in 0.05 m phosphate buffer, pH 6.8 (PB) (Karnovsky, 1965). Sections were washed with PB and post-fixed for 1 h in 2% (w/v) osmium tetroxide in 0.05 m PB. After washing with PB, the tissue slices were dehydrated through a gradient series of ethanol, infiltrated with Spurr's embedding medium (Electron Microscopy Science, Fort Washington, PA) and polymerized for 48 h at 60°C.
For immunogold labeling, sections from lower petal lobes of 1- and 7-d-old snapdragon flowers were fixed for 1 h at room temperature in 0.3% (v/v) glutaraldehyde and 3% (v/v) formaldehyde in 0.01 m PB, pH 6.8. After washing with 0.01 m PB, pH 6.8, the tissue slices were dehydrated through a gradient series of ethanol. The samples were then slowly infiltrated with LR White embedding medium (Electron Microscopy Science) through a graded series (resin:ethanol 1:3, 1:1, and 3:1) for 12 h each, followed by three 12-h incubations in pure resin, prior to polymerization for 48 h at 55°C.
Environmental Scanning Electron Microscopy
Fresh samples of snapdragon petals were placed on a Peltier stage cooled to 4°C. They were viewed using an Electronscan 2020 environmental scanning electron microscope (FEI Corporation, Hillsdale, OR) at 12-kV accelerating voltage and pressures of 5.4 to 5.9 Torr.
Immunofluorescence and Light Microscopy
Serial sections (0.5 μm thick) were prepared from LR White-embedded samples with glass knives, mounted in distilled water on glass slides (precleaned Gold Seal RITE-ON microslides, Gold Seal Products, Portsmouth, NH) with fast well silicon isolators (Fast Research Product International, Mount Prospect, IL), and air-dried overnight at 55°C. The sections were first incubated for 20 min at room temperature in blocking solution of Tris-buffered saline (TBS: 20 mm Tris-HCl, pH 7.4, with 150 mm NaCl) containing 0.3% (v/v) Tween 20 and 1% (w/v) bovine serum albumin (TBS-TB), followed directly by incubation with rabbit polyclonal antibodies raised against snapdragon BAMT (Dudareva et al., 2000). Incubation was for 16+ h at 4°C in TBS-TB solution containing either a 1:50 dilution of anti-BAMT polyclonal antibodies or a comparable dilution of preimmune serum. After washing with TBS-T (20 mm Tris-HCl, pH 7.4 with 150 mm NaCl and 0.3% [v/v] Tween 20) three times for 10 min each, sections on glass slide were incubated for 1 h at room temperature with goat anti-rabbit FITC-labeled IgG (Sigma, Milwaukee, WI), diluted 1:50 in TBS-TB buffer. Sections were then washed with TBS-T solution three times for 10 min each, followed by rinsing with distilled water, covered by glass cover slips, and analyzed under a Olympus-Vanox light microscope (Olympus America, Melville, NY) with fluorescent illuminator and green excitation selected using an exciter filter number BP545 (λ 480–545 nm) and barrier filter O 590. Pictures were taken using a Spot-RT digital camera (Diagnostic Instruments, Sterling Heights, MI).
For structural analysis, 0.5-μm-thick sections were prepared from samples embedded in Spurr's resin, mounted on glass slides, and stained with 1% toluidine blue (Polysciences, Warrington, PA). Toluidine blue-stained sections were observed under a conventional Olympus-Vanox light microscope (Olympus America, Millville, NY). Pictures were taken using a Spot-RT digital camera (Diagnostic Instruments).
Transmission Electron Microscopy and Immunogold Labeling
Ultrathin sections (80–100 nm) were prepared from LR White-embedded samples and mounted on carbon-coated Formvar 200-mesh nickel grids (Electron Microscopy Science). Sections on grids were incubated for 20 min at room temperature with TBS-TB blocking solution, followed directly by incubation for 16+ h at 4°C with anti-BAMT antibodies diluted 1:50 with TBS-TB solution. After washing with TBS-T buffer three times for 10 min each, sections on grids were incubated for 1 h at room temperature with goat anti-rabbit IgG conjugated to 10-nm gold (Ted Pella, Redding, CA) and diluted 1:50 in TBS-TB buffer. Sections were then washed with TBS-T buffer three times for 10 min each, followed by rinsing with distilled water for 30 s. Finally they were air-dried and stained with aqueous 2% (w/v) uranyl acetate for 1 min, and observed under an electron microscope (Philips EM-400; FEI Company). To increase visibility of small gold particles used to visualize the BAMT protein, selected electron micrographs were scanned into an Apple Macintosh Quadra 950 computer at a resolution of 600 dots per inch using an Epson expression 1600 flatbed scanner and video enhanced by adjusting contrast and brightness in Adobe Photoshop 5.5. The diameter of the gold particles was enlarged 2-fold. The image files were printed on a Mitsubishi CP210U dye-sublimation printer using Professional Output Manager software from Visual Business Systems (Atlanta, GA).
For ultrastructural analysis, ultrathin sections (80–100 nm) were prepared from Spurr's resin-embedded samples and mounted on carbon-coated Formvar 100-mesh copper grids (Electron Microscopy Science). Sections were air-dried, stained at room temperature with 2% (w/v) uranyl acetate for 3 min and then with lead citrate for 1 min, prepared according to Reynolds (1963) prior to viewing with an electron microscope Philips EM 400 (FEI Company).
Footnotes
This work was supported by the National Science Foundation (grant no. IBN–9904910) and by the Fred Gloeckner Foundation, Inc. This paper is contribution no. 16,446 from Purdue University Agricultural Experimental Station.
LITERATURE CITED
- Bohlmann J, Meyer-Gauen G, Croteau R. Plant terpenoid synthases: molecular biology and phylogenetic analysis. Proc Natl Acad Sci USA. 1998;95:4126–4133. doi: 10.1073/pnas.95.8.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau R. Biochemistry of monoterpenes and sesquiterpenes of the essential oils. In: Craker LE, Simon JE, editors. Herbs, Spices, and Medicinal Plants. New York: Food Product Press; 1992. pp. 81–133. [Google Scholar]
- Croteau R, Johnson MA. Biosynthesis of terpenoids in glandular trichomes. In: Rodriguez E, Healey PL, Mehta I, editors. Biology and Chemistry of Plant Trichomes. New York: Plenum Publishing; 1984. pp. 133–185. [Google Scholar]
- Curry KJ, McDowell LM, Judd WS, Stern WL. Osmophores, floral features, and systematics of Stanhopea (Orchidaceae) Am J Bot. 1991;78:610–623. [Google Scholar]
- Curry KJ, Stern WL, McDowell LM. Osmophore development in Stanhopea anfracta and S. pulla (Orchidaceae) Lindleyana. 1988;3:212–220. [Google Scholar]
- Dobson HEM. Floral volatiles in insect biology. In: Bernays E, editor. Insect-Plant Interactions. V. Boca Raton, FL: CRC Press; 1994. pp. 47–81. [Google Scholar]
- Dudareva N, Cseke L, Blanc VM, Pichersky E. Evolution of floral scent in Clarkia: novel patterns of S-linalool synthase gene expression in the C. breweri flower. Plant Cell. 1996;8:1137–1148. doi: 10.1105/tpc.8.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudareva N, Murfitt LM, Mann CJ, Gorenstein Nl, Kolosova N, Kish CM, Bonham C, Wood K. Developmental regulation of methyl benzoate biosynthesis and emission in snapdragon flowers. Plant Cell. 2000;12:949–961. doi: 10.1105/tpc.12.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudareva N, Pichersky E. Biochemical and molecular aspects of floral scents. Plant Physiol. 2000;122:627–634. doi: 10.1104/pp.122.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudareva N, Piechulla B, Pichersky E. Biogenesis of floral scent. Hortic Rev. 1999;24:31–54. [Google Scholar]
- Fahn A. Secretory Tissues in Plants. New York: Academic Press; 1979. [Google Scholar]
- Fahn A. Secretory tissues in vascular plants. New Phytol. 1988;108:229–257. doi: 10.1111/j.1469-8137.1988.tb04159.x. [DOI] [PubMed] [Google Scholar]
- Gang DR, Wang J, Dudareva N, Nam KH, Simon J, Lewinsohn E, Pichersky E. An investigation of the storage and biosynthesis of phenylpropenes in sweet basil (Ocimum basilicum L.) Plant Physiol. 2001;125:539–555. doi: 10.1104/pp.125.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershenzon J, McCaskill D, Rajaonarivony IM, Mihaliak C, Karp F, Croteau R. Isolation of secretory cells from plant glandular trichomes and their use in biosynthetic studies of monoterpenes and other gland products. Anal Biochem. 1992;200:130–138. doi: 10.1016/0003-2697(92)90288-i. [DOI] [PubMed] [Google Scholar]
- Gorton HL, Vogelmann TC. Effects of epidermal cell shape and pigmentation on optical properties of Antirrhinum petals at visible and ultraviolet wavelengths. Plant Physiol. 1996;112:879–888. doi: 10.1104/pp.112.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich B. The foraging specializations of individual bumblebees. Ecol Monog. 1976;46:105–128. [Google Scholar]
- Jones KN, Reithel JS, Irvin RE. A trade-off between the frequency and duration of bumblebee visits to flowers. Oecologia. 1998;117:161–168. doi: 10.1007/s004420050644. [DOI] [PubMed] [Google Scholar]
- Karnovsky MJ. A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. J Cell Biol. 1965;27:137A. [Google Scholar]
- Kay QON, Daoud HS, Stirton CH. Pigment distribution, light reflection and cell structure in petals. Bot J Linn Soc. 1981;83:57–84. [Google Scholar]
- Knudsen JT, Tollsten L, Bergstrom G. Floral scents: a checklist of volatile compounds isolated by head-space techniques. Phytochemistry. 1993;33:253–280. [Google Scholar]
- Lunau K. Innate flower recognition in bumblebees (Bombus terrestris, B. lucorum; Apidae): optical signals from stamens as landing reaction releasers. Ethology. 1991;88:203–214. [Google Scholar]
- Lunau K. Innate recognition of flowers by bumblebees: orientation of antennae to visual stamen signals. Can J Zool. 1992;70:2139–2144. [Google Scholar]
- Mactavish HS, Menary RC. Volatiles in different floral organs, and effect of floral characteristics on yield of extract from Boronia megastigma (Nees) Ann Bot. 1997;80:305–311. [Google Scholar]
- Martin C, Gerats T. Control of pigment biosynthesis genes during petal development. Plant Cell. 1993;5:1253–1264. doi: 10.1105/tpc.5.10.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaskill D, Gedrshenzon J, Croteau R. Morphology and monoterpene biosynthetic capabilities of secretory cell clusters isolated from glandular trichomes of peppermint (Mentha piperita L.) Planta. 1992;187:445–454. doi: 10.1007/BF00199962. [DOI] [PubMed] [Google Scholar]
- McGarvey DJ, Croteau R. Terpenoid metabolism. Plant Cell. 1995;7:1015–1026. doi: 10.1105/tpc.7.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettal U, Boland W, Beyer P, Kleinig H. Biosynthesis of monoterpene hydrocarbons by isolated chromoplasts from daffodil flowers. Eur J Biochem. 1988;170:613–616. doi: 10.1111/j.1432-1033.1988.tb13741.x. [DOI] [PubMed] [Google Scholar]
- Murfitt LM, Kolosova N, Mann CJ, Dudareva N. Purification and characterization of S-adenosyl-l-methionine:benzoic acid carboxyl methyltransferase, the enzyme responsible for biosynthesis of the volatile ester methyl benzoate in flowers of Antirrhinum majus. Arch Biochem Biophys. 2000;382:145–151. doi: 10.1006/abbi.2000.2008. [DOI] [PubMed] [Google Scholar]
- Noda K, Glover BJ, Linstead P, Martin C. Flower color intensity depends on specialized cell shape controlled by a Myb-related transcription factor. Nature. 1994;369:661–664. doi: 10.1038/369661a0. [DOI] [PubMed] [Google Scholar]
- Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorrosh BS, Subramaniam J, Schubert KR, Dixon RA. Expression and localization of plant protein disulfide isomerase. Plant Physiol. 1993;103:719–726. doi: 10.1104/pp.103.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner G, Gershenzon J, Nielson EE, Froehlich JE, Croteau R. Limonene synthase, the enzyme responsible for monoterpene biosynthesis in peppermint, is localized to leucoplasts of oil gland secretory cells. Plant Physiol. 1999;120:879–886. doi: 10.1104/pp.120.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GJ. Secreting glandular trichomes: more than just hair. Plant Physiol. 1991;96:675–679. doi: 10.1104/pp.96.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]