Abstract
Cardiovascular-kidney-metabolic syndrome is an emerging entity that connects cardiovascular diseases, chronic kidney disease and diabetes. The non-steroidal mineralocorticoid receptor antagonist finerenone has been studied in three prospective randomized clinical trials of patients with cardiovascular-kidney-metabolic syndrome: FIDELIO-DKD, FIGARO-DKD and FINEARTS-HF. In light of the strong epidemiological overlap and shared mechanistic drivers of clinical outcomes across cardiovascular-kidney-metabolic syndrome, we summarize the efficacy and safety of finerenone on cardiovascular, kidney and mortality outcomes in this pre-specified participant-level pooled analysis. The three trials included 18,991 participants (mean age 67 ± 10 years; 35% women). During 2.9 years of median follow-up, the primary outcome of cardiovascular death occurred in 421 (4.4%) participants assigned to finerenone and 471 (5.0%) participants assigned to placebo (hazard ratio (HR): 0.89; 95% confidence interval (CI): 0.78–1.01; P = 0.076). Death from any cause occurred in 1,042 (11.0%) participants in the finerenone arm and in 1,136 (12.0%) participants in the placebo arm (HR: 0.91; 95% CI: 0.84–0.99; P = 0.027). Finerenone further reduced the risk of hospitalization from heart failure (HR: 0.83; 95% CI: 0.75–0.92; P < 0.001) and the composite kidney outcome (HR: 0.80; 95% CI: 0.72–0.90; P < 0.001). While in this pooled analysis the reduction in cardiovascular death was not statistically significant, finerenone reduced the risks for deaths of any cause, cardiovascular events and kidney outcomes. PROSPERO identifier: CRD42024570467.
Subject terms: Heart failure, Chronic kidney disease, Diabetes
This participant-level pooled analysis of the three phase 3 trials that have tested the non-steroidal mineralocorticoid receptor antagonist finerenone in patients with heart failure and chronic kidney disease with type 2 diabetes provides an integrated view of the mortality, cardiovascular and renal effects of this treatment.
Main
Cardiovascular diseases, chronic kidney disease (CKD) and metabolic conditions frequently occur in the same individual and may share common pathophysiological pathways of disease onset and progression1–3. This cardiovascular-kidney-metabolic (CKM) overlap is increasingly recognized, and there is potential for individual therapies to simultaneously improve multiple adjacent disease states4. Activation of the mineralocorticoid receptor is a well-recognized driver of systemic and target organ inflammation and fibrosis in these disease states5–7. Steroidal mineralocorticoid receptor antagonists, such as spironolactone and eplerenone, target these shared pathways, but their widespread use has been limited, especially in patients with CKM multimorbidity8. Gaps in implementation of steroidal mineralocorticoid receptor antagonists in heart failure (HF) are, in part, related to safety concerns due to hyperkalemia and worsening kidney function9.
Finerenone is a selective and potent non-steroidal mineralocorticoid receptor antagonist5,10–13 that may have lower risks of hyperkalemia and worsening kidney function compared to spironolactone14. Finerenone has been shown to reduce the risk of cardiovascular events and kidney failure in patients with CKD with type 2 diabetes (T2D)15,16 and has recently been shown to reduce worsening HF events in patients with HF with mildly reduced or preserved ejection fraction17. However, none of these trials was individually powered to evaluate treatment effects on less frequent cardiovascular-kidney outcomes, such as cardiovascular death or efficacy in key subgroups, including those with overlapping CKM conditions. A previous pooled analysis of the CKD with T2D trials showed that finerenone reduced major adverse cardiovascular events by 14% and a kidney composite outcome by 22% but remained underpowered in the evaluation of mortality outcomes18. Broadening this pooled population to encompass participants from the recently completed FINEARTS-HF trial will enhance precision on a range of safety and efficacy outcomes and allow evaluation of previously understudied subpopulations.
In light of the strong epidemiological overlap and shared mechanistic drivers, pooled integrated assessment of these CKM trials was pre-specified in a participant-level analysis of three phase 3 global, multicenter, double-blind, placebo-controlled randomized clinical trials of finerenone (FINE-HEART).
Results
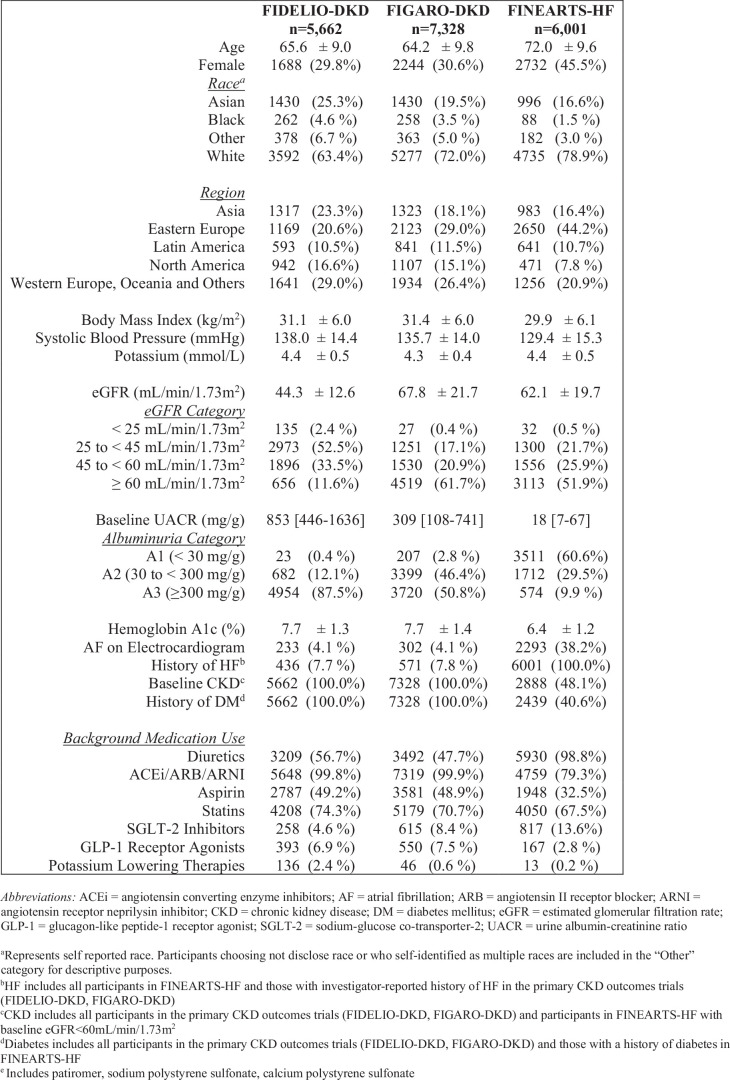
Overall, FINE-HEART comprised 18,991 participants from these three trials. Baseline clinical profiles and treatment patterns are summarized for the overall pooled population (Table 1) and by individual trial (Extended Data Table 4). Mean age was 67 ± 10 years; 35.1% were women; and participants were enrolled across all major geographic regions. Participants were at high risk for CKD progression (Extended Data Fig. 2) with either reduced estimated glomerular filtration rate (eGFR) (30.1% with eGFR < 45 and 26% with eGFR 45–60 ml min−1 1.73 m−2) and/or albuminuria (30.8% with ‘A2’ urine albumin creatinine ratio (UACR) 30–299 mg g−1 and 49.2% with ‘A3’ UACR ≥ 300 mg g−1). In total, 2,307 (12.1%) participants had all CKM conditions (HF, CKD and diabetes) (Extended Data Fig. 3). At baseline, 1,690 (8.9%) participants were co-treated with a sodium–glucose co-transporter-2 (SGLT2) inhibitor, and 1,110 (5.8%) participants were co-treated with a glucagon-like peptide-1 receptor agonist (GLP-1RA). Baseline characteristics and concurrent medical management were well balanced between treatment arms (Table 1). Most participants were titrated to a final dose of 20 mg (4,645 (69.8%) in the placebo arm and 4,248 (63.6%) in the finerenone arm), and some (exclusively in FINEARTS-HF) were titrated to 40 mg (920 (13.8%) in the placebo arm and 832 (12.5%) in the finerenone arm).
Table 1.
Baseline characteristics
| Finerenone | Placebo | |
|---|---|---|
| n = 9,501 | n = 9,490 | |
| Age | 67.0 ± 10.0 | 67.1 ± 10.2 |
| Female | 3,390 (35.7%) | 3,274 (34.5%) |
| Racea | ||
| Asian | 1,910 (20.1%) | 1,946 (20.5%) |
| Black | 300 (3.2 %) | 308 (3.2 %) |
| Other | 476 (5.0 %) | 447 (4.7 %) |
| White | 6,815 (71.7%) | 6,789 (71.5%) |
| Region | ||
| Asia | 1,808 (19.0%) | 1,815 (19.1%) |
| Eastern Europe | 3,001 (31.6%) | 2,941 (31.0%) |
| Latin America | 1,041 (11.0%) | 1,034 (10.9%) |
| North America | 1,259 (13.3%) | 1,261 (13.3%) |
| Western Europe, Oceania and Others | 2,392 (25.2%) | 2,439 (25.7%) |
| Body mass index (kg m-2) | 30.9 ± 6.1 | 30.9 ± 6.0 |
| Systolic blood pressure (mmHg) | 134.5 ± 14.9 | 134.4 ± 15.0 |
| Potassium (mmol L-1) | 4.4 ± 0.5 | 4.4 ± 0.5 |
| eGFR (mL min-1 1.73 m-2) | 58.9 ± 21.0 | 59.1 ± 21.3 |
| eGFR category | ||
| <25 mL min-1 1.73 m-2 | 100 (1.1%) | 94 (1.0%) |
| 25 to <45 mL min-1 1.73 m-2 | 2,742 (28.9%) | 2,782 (29.3%) |
| 45 to <60 mL min-1 1.73 m-2 | 2,513 (26.5%) | 2,469 (26.0%) |
| ≥60 mL min-1 1.73 m-2 | 4,145 (43.6%) | 4,143 (43.7%) |
| UACR (mg g-1) | 283 (46–836) | 293 (47–855) |
| Albuminuria category | ||
| A1 (<30 mg g-1) | 1,885 (20.1%) | 1,856 (19.8%) |
| A2 (30 to <300 mg g-1) | 2,910 (31.0%) | 2,883 (30.7%) |
| A3 (≥300 mg g-1) | 4,602 (49.0%) | 4,646 (49.5%) |
| Hemoglobin A1c (%) | 7.3 ± 1.4 | 7.3 ± 1.4 |
| AF on electrocardiogram | 1,449 (15.3%) | 1,379 (14.5%) |
| History of HFb | 3,488 (36.7%) | 3,520 (37.1%) |
| Baseline CKDc | 7,949 (83.7%) | 7,929 (83.6%) |
| History of DMd | 7,715 (81.2%) | 7,714 (81.3%) |
| Background medication use | ||
| Diuretics | 6,291 (66.2%) | 6,340 (66.8%) |
| ACEi/ARB/ARNI | 8,866 (93.3%) | 8,860 (93.4%) |
| Aspirin | 4,145 (43.6%) | 4,171 (44.0%) |
| Statins | 6,687 (70.4%) | 6,750 (71.1%) |
| SGLT-2 inhibitors | 829 (8.7%) | 861 (9.1%) |
| GLP-1 receptor agonists | 576 (6.1%) | 534 (5.6%) |
| Potassium-lowering therapiese | 99 (1.0%) | 96 (1.0%) |
A1, A2 and A3, albuminuria categories; ACEi, angiotensin-converting enzyme inhibitors; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; DM, diabetes mellitus.
aRepresents self-reported race. Participants choosing not to disclose race or who self-identified as multiple races are included in the ‘Other’ category for descriptive purposes.
bHF includes all participants in FINEARTS-HF and those with investigator-reported history of HF in the primary CKD outcomes trials (FIDELIO-DKD and FIGARO-DKD).
cCKD includes all participants in the primary CKD outcomes trials (FIDELIO-DKD and FIGARO-DKD) and participants in FINEARTS-HF with baseline eGFR of <60 ml min−1 1.73 m−2.
dDiabetes includes all participants in the primary CKD outcomes trials (FIDELIO-DKD and FIGARO-DKD) and those with a history of diabetes in FINEARTS-HF.
eIncludes patiromer, sodium polystyrene sulfonate and calcium polystyrene sulfonate.
Extended Data Table 4.
Baseline characteristics by trial
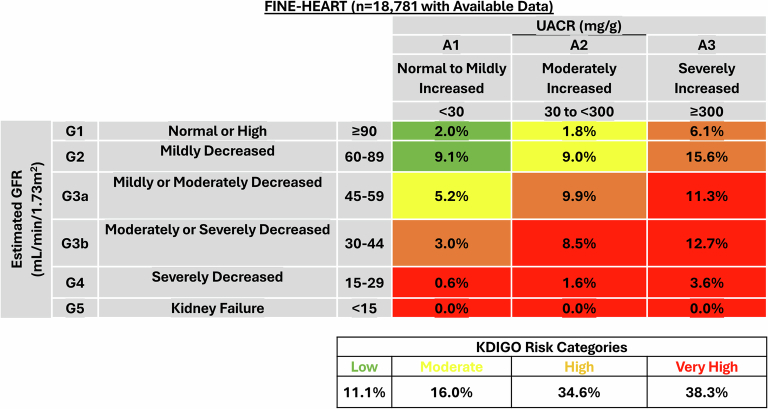
Extended Data Fig. 2.
KDIGO Risk Distribution at Baseline in FINE-HEART. Participants were at high risk for chronic kidney disease progression with either reduced estimated glomerular filtration rate or elevated levels of urine albumin creatinine ratio.
Extended Data Fig. 3.
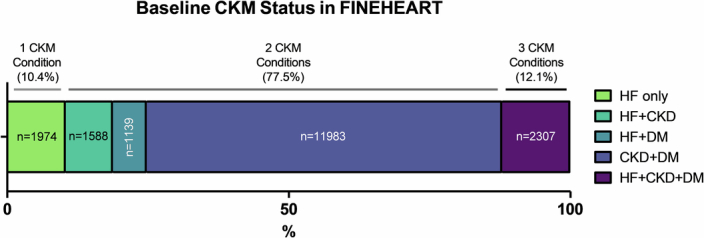
Baseline Cardio-Kidney-Metabolic Overlap in FINE-HEART. Participants included in this pooled analysis had a high degree of cardio-kidney-metabolic overlap with 90% having at least 2 conditions (heart failure, chronic kidney disease, or diabetes).
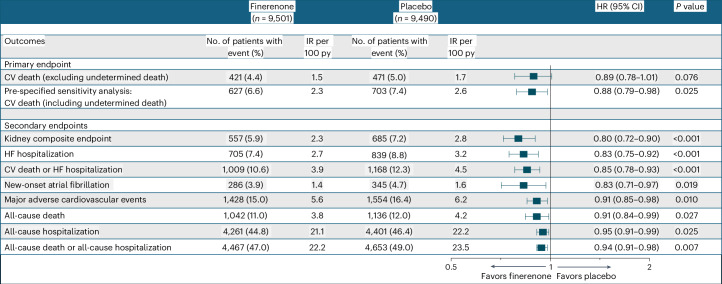
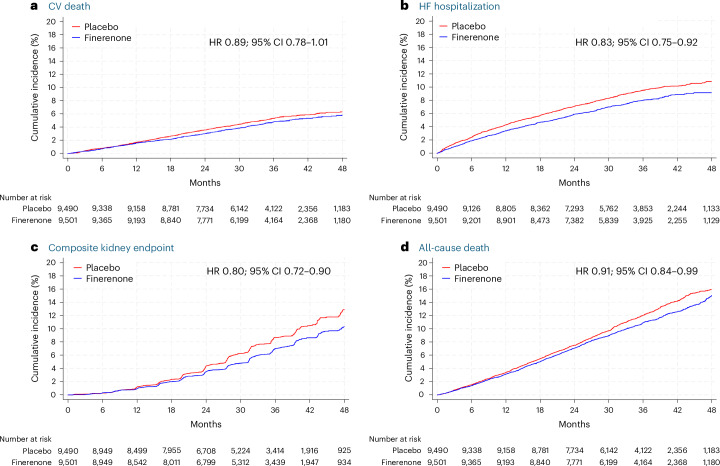
Median duration of follow-up was 2.6 years (FIDELIO-DKD), 3.4 years (FIGARO-DKD) and 2.6 years (FINEARTS-HF). Median follow-up of the pooled patient population was 2.9 years. The primary endpoint of cardiovascular death occurred in 421 (4.4%) participants in the finerenone arm and in 471 (5.0%) participants in the placebo arm (hazard ratio (HR): 0.89; 95% confidence interval (CI): 0.78–1.01; P = 0.076) with consistent findings in pre-specified sensitivity analysis including both cardiovascular deaths and undetermined deaths (6.6% versus 7.4%; HR: 0.88; 95% CI: 0.79–0.98; P = 0.025) (Fig. 1). Effects on cardiovascular death were consistent across individual trials: FIDELIO-DKD (HR: 0.90; 95% CI: 0.65–1.23); FIGARO-DKD (HR: 0.81; 95% CI: 0.62–1.04); and FINEARTS-HF (HR: 0.93; 95% CI: 0.78–1.11); Pinteraction = 0.68 (Extended Data Fig. 4). Deaths from any cause occurred in 1,042 (11.0%) participants in the finerenone arm and in 1,136 (12.0%) participants in the placebo arm (HR: 0.91; 95% CI: 0.84–0.99; P = 0.027) (Fig. 2). A complete accounting of cause-specific death by treatment arm is shown in Table 2.
Fig. 1. Efficacy outcomes.
The primary efficacy outcome was cardiovascular death (deaths of undetermined causes were excluded). Pre-specified sensitivity analysis of the primary efficacy outcome considered deaths of undetermined causes as having a cardiovascular cause. The kidney composite outcome was defined as a sustained decrease in eGFR to ≥50% from baseline, sustained decline in eGFR to <15 ml min−1 1.73 m−2, kidney failure and death due to kidney failure. Major adverse cardiovascular events included cardiovascular death or a non-fatal cardiovascular event (HF hospitalization, myocardial infarction or stroke). The composite of all-cause death or all-cause hospitalization was defined post hoc. All primary and secondary outcomes were analyzed as time to first outcomes using a stratified Cox proportional hazards model including the study intervention group as a fixed effect and stratified by geographic region and individual trial. All two-sided P values are reported without adjustment for multiple comparisons. The bars represent 95% CIs around each treatment effect point estimate. CV, cardiovascular; IR, incidence rate.
Extended Data Fig. 4. Key Efficacy Outcomes in Each Individual Trial.
The bars represent 95% confidence intervals around each treatment effect point estimate.
Fig. 2. Cumulative incidence of key efficacy outcomes.
Incidence of cardiovascular (CV) death (primary outcome) (a); HF hospitalization (b); kidney composite outcome (sustained decrease in eGFR to ≥50% from baseline, sustained decline in eGFR to <15 ml min−1 1.73 m−2, kidney failure and death due to kidney failure) (c); and all-cause death (d).
Table 2.
Cause-specific death
| Cause of death | Finerenone | Placebo | Total | |||
|---|---|---|---|---|---|---|
| n | Rate per 100 py | n | Rate per 100 py | n | Rate per 100 py | |
| All-cause death | 1,042 | 3.8 | 1,136 | 4.2 | 2,178 | 4.0 |
| Cardiovascular causes | ||||||
| Sudden | 188 | 0.7 | 230 | 0.9 | 418 | 0.8 |
| Heart failure | 92 | 0.3 | 113 | 0.4 | 205 | 0.4 |
| Stroke | 47 | 0.2 | 59 | 0.2 | 106 | 0.2 |
| Myocardial infarction | 35 | 0.1 | 37 | 0.1 | 72 | 0.1 |
| Cardiovascular procedural | 12 | 0.0 | 7 | 0.0 | 19 | 0.0 |
| Other cardiovascular related | 47 | 0.2 | 25 | 0.1 | 72 | 0.1 |
| Non-CV causes | ||||||
| Infection | 194 | 0.7 | 188 | 0.7 | 382 | 0.7 |
| Malignancy | 121 | 0.4 | 149 | 0.6 | 270 | 0.5 |
| Renal | 4 | 0.0 | 6 | 0.0 | 10 | 0.0 |
| Other non-cardiovascular related | 96 | 0.3 | 90 | 0.3 | 186 | 0.3 |
| Undetermined | 206 | 0.8 | 232 | 0.9 | 438 | 0.8 |
py, patient years.
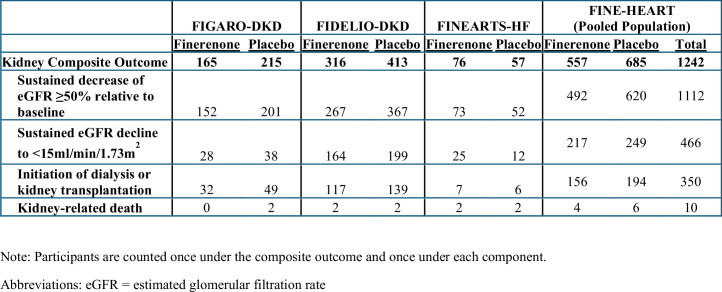
Finerenone reduced the risk of the composite kidney outcome whether defined inclusive of a sustained decrease in eGFR to ≥50% from baseline (HR: 0.80; 95% CI: 0.72–0.90; P < 0.001) or a sustained decrease in eGFR to ≥57% from baseline (HR: 0.79; 95% CI: 0.70–0.91; P < 0.001). These kidney effects appeared to be driven by FIDELIO-DKD and FIGARO-DKD (Extended Data Fig. 4). The incidences of the individual components of the kidney composite endpoint are displayed in Extended Data Table 5. Finerenone reduced the risk of hospitalizations due to HF alone (HR: 0.83; 95% CI: 0.75–0.92; P < 0.001) and the composite of cardiovascular death or HF hospitalization (HR: 0.85; 95% CI: 0.78–0.93; P < 0.001). Hospitalizations of any cause were also lower with finerenone compared to placebo (HR: 0.95; 95% CI: 0.91–0.99; P = 0.025). Finerenone further reduced the risk of the composite of all-cause death or all-cause hospitalization (HR: 0.94; 95% CI: 0.91–0.98; P = 0.007). Additional risk reductions were observed for the prevention of new-onset atrial fibrillation and major adverse cardiovascular events (Fig. 1).
Extended Data Table 5.
Components of the kidney composite endpoint
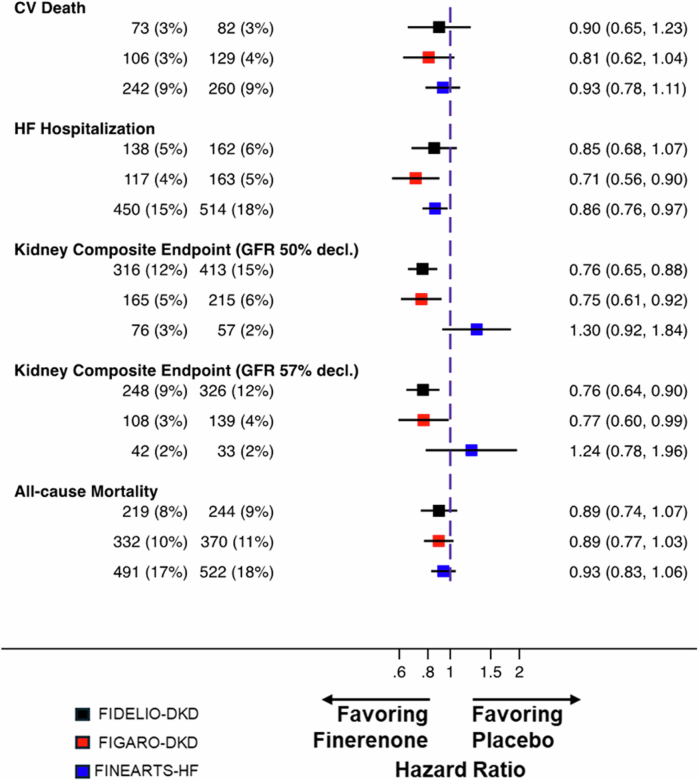
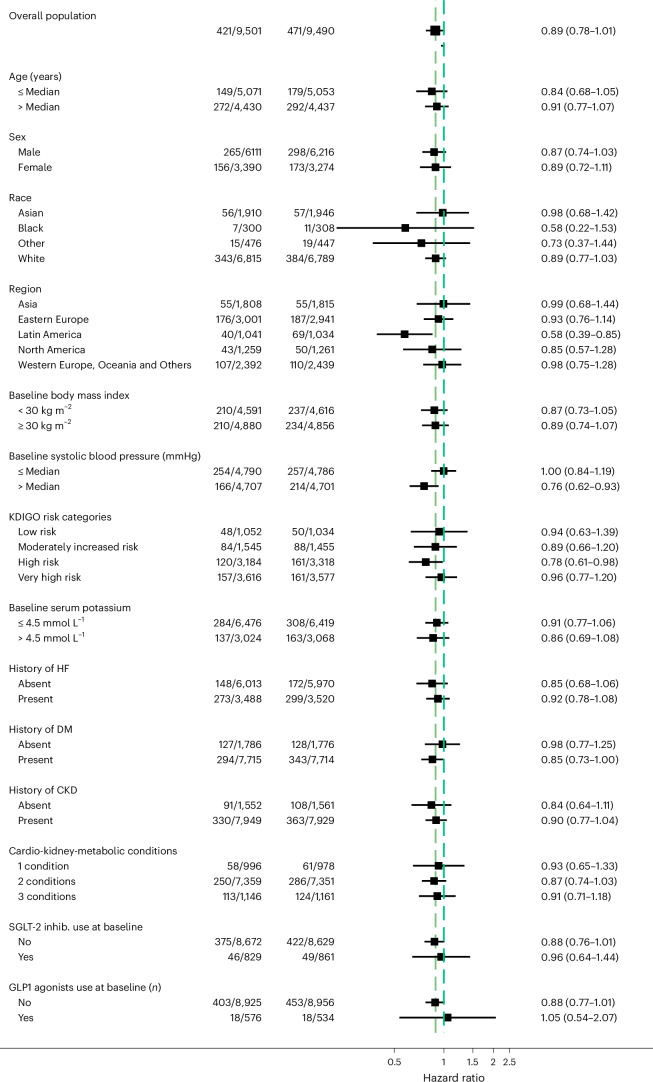
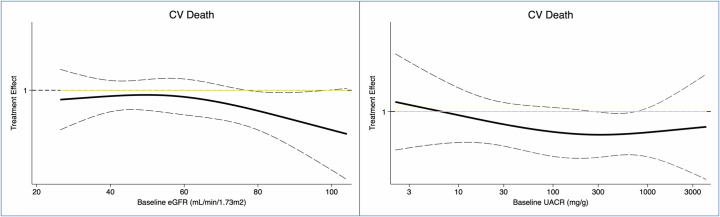
Treatment effects on cardiovascular death were generally consistent across the 16 subgroups examined (Fig. 3). The efficacy of finerenone on cardiovascular death was consistent across the range of eGFR (Pinteraction = 0.32) and UACR (Pinteraction = 0.55) (Extended Data Fig. 5). Treatment effects on cardiovascular death were also consistent across a range of CKM disease burden: one condition (HR: 0.93; 95% CI: 0.65–0.1.33); two conditions (HR: 0.87; 95% CI: 0.74–1.03); and three conditions (HR: 0.91; 95% CI: 0.71–1.18); Pinteraction = 0.94.
Fig. 3. Subgroup forest plot for primary outcome (CV death).
The median age was 68 years, and the median systolic blood pressure was 135 mmHg. For each patient, the presence of CKM conditions was summed for the following comorbidities: CKD, HF and/or DM. The bars represent 95% CIs around each treatment effect point estimate. CV, cardiovascular; DM, diabetes mellitus.
Extended Data Fig. 5. Treatment Effect on Primary Outcome of CV Death Across Baseline eGFR (Left Panel) and UACR (Right Panel).
The solid line represents the treatment effect estimate and the dashed lines represent 95% confidence intervals. Abbreviations: CV = cardiovascular; eGFR = estimated glomerular filtration rate; UACR = urine albumin creatinine ratio.
Incidences of any serious adverse event were lower with finerenone than placebo (34.6% versus 36.6%), although incidences of serious adverse events leading to drug discontinuation were slightly higher with finerenone (5.4% versus 4.6%). Laboratory-defined hyperkalemia was increased, whereas laboratory-defined hypokalemia was decreased, with finerenone. Incidences of investigator-reported hyperkalemia leading to permanent treatment discontinuation (1.3% versus 0.5%) and hyperkalemia-related hospitalization (0.8% versus 0.2%) were higher with finerenone. There were no deaths related to hyperkalemia and no between-group differences in incidences of acute kidney injury (Table 3).
Table 3.
Safety outcomes
| Finerenone | Placebo | |
|---|---|---|
| n = 9,482 | n = 9,467 | |
| Any serious adverse event | 3,283 (34.6%) | 3,463 (36.6%) |
| Any adverse event leading to treatment discontinuation | 515 (5.4 %) | 434 (4.6 %) |
| Any potassium >5.5 mmol L−1 (a) | 1,535 (16.5%) | 714 (7.7 %) |
| Any potassium >6.0 mmol L−1 (a) | 311 (3.3 %) | 133 (1.4 %) |
| Any potassium <3.5 mmol L−1 (a) | 448 (4.8 %) | 938 (10.1%) |
| Hyperkalemiab | 1,216 (12.8%) | 586 (6.2 %) |
| Hyperkalemia leading to treatment discontinuationb | 123 (1.3 %) | 43 (0.5 %) |
| Hyperkalemia leading to hospitalizationb | 80 (0.8 %) | 17 (0.2 %) |
| Hyperkalemia leading to deathb | 0 (0.0 %) | 0 (0.0 %) |
| Acute kidney injury | 345 (3.6 %) | 316 (3.3 %) |
| Acute kidney injury leading to treatment discontinuation | 15 (0.2 %) | 12 (0.1 %) |
| Acute kidney injury leading to hospitalization | 143 (1.5 %) | 116 (1.2 %) |
| Systolic blood pressure < 100 mmHg | 1,040 (11.1%) | 651 (7.0%) |
| Gynecomastia or breast hyperplasia | 16 (0.2%) | 19 (0.2%) |
Treatment-emergent adverse events are defined as any adverse event occurring in any patient who has received at least one dose of study drug and within 3 d of permanent discontinuation. This safety table includes one patient who was randomized to placebo but who actually received finerenone.
aBased on central laboratory measurements of potassium levels.
bBased on investigator-reported adverse events.
Discussion
This participant-level pooled analysis, FINE-HEART, represents, to our knowledge, the largest analysis of the efficacy and safety of the non-steroidal mineralocorticoid receptor antagonist finerenone across the CKM spectrum. Although this pooled analysis did not demonstrate a significant reduction in the primary outcome of cardiovascular death, this result was sensitive to the definition of cardiovascular death based on the classification of deaths of undetermined causes. As such, we placed greater confidence in the outcome of all-cause death, which was reduced with finerenone with nominal significance. This mortality signal with finerenone was further substantiated by clinically relevant benefits observed across a broad range of other cardiovascular-kidney outcomes, including kidney disease progression, HF hospitalizations, all-cause hospitalizations, new-onset atrial fibrillation and major adverse cardiovascular events. Treatment effects were consistent across all tested clinical subgroups, including those with multiple, intersecting CKM conditions. No new or unexpected safety signals were uncovered in this pooled analysis with a well-characterized modestly higher risk of hyperkalemia but overall lower incidences of serious adverse events and no excess risk of acute kidney injury with finerenone. Taken together, these data suggest the potential of finerenone to prevent or delay morbidity and mortality across a wide range of CKM conditions while being safe and well tolerated.
Finerenone is approved for use in patients with CKD and T2D. Although several major multi-specialty guidelines strongly recommend finerenone to delay CKD progression and prevent HF events in people with CKD with T2D19–21, the latest Kidney Disease: Improving Global Outcomes (KDIGO) guideline22 has offered a class 2A recommendation potentially related to residual uncertainties regarding the mortality effects of finerenone in the FIDELIO-DKD and FIGARO-DKD trials. Furthermore, according to the guideline, its use is to be considered in patients already on standard-of-care therapies, such as maximally tolerated renin–angiotensin system inhibitors and/or SGLT2 inhibitors. These pooled data did not identify heterogeneity with finerenone’s effects on cardiovascular death by background use of SGLT2 inhibitors or GLP-1RA, although the statistical power of these subgroup findings was limited. This pooled analysis bolsters recent calls for the combination use of finerenone alongside these therapies as foundational ‘pillars’ of care to maximize improvements in cardiovascular-kidney outcomes23,24; additional evidence will be needed to inform the expected efficacy and safety of various combinations of risk-lowering therapies.
Finerenone was shown to robustly reduce the kidney composite outcome by 20% in this pooled analysis, driven by benefits observed in FIDELIO-DKD and FIGARO-DKD. FINEARTS-HF enrolled a primary HF population with some co-existing kidney disease but with relatively low levels of albuminuria; therefore, CKD progression over a relatively short period of follow-up was difficult to evaluate. It is reassuring that finerenone did not show an increase in reports of acute kidney injury, despite the high baseline kidney risk profile of the patient population and the varied clinical care settings of therapeutic initiation.
Although each trial had broad eligibility criteria, some groups were understudied in individual trials. For instance, both FIGARO-DKD and FIDELIO-DKD exclusively enrolled participants with CKD and T2D with albuminuria. FINEARTS-HF provides complementary evidence related to finerenone’s therapeutic effects in previously understudied populations, including those without diabetes (~60% of trial enrollment), those without CKD and those without significant urinary albumin excretion (>60% of the trial with UACR <30 mg g−1). FINEARTS-HF exclusively enrolled patients with symptomatic HF across clinical care settings, whereas FIDELIO-DKD and FIGARO-DKD specifically excluded patients with symptomatic HF with reduced ejection fraction. It is noteworthy that FIDELIO-DKD and FIGARO-DKD did enroll 5–10% of patients with HF with mildly reduced or preserved ejection fraction, emphasizing the overlap across these trials. These pooled data demonstrate that finerenone’s benefits extend to patients with different degrees of CKM multimorbidity and across broad patient profiles and enhance precision of estimates of risk reductions beyond previous analyses18. The diverse spectrum of benefits on HF, arrhythmia, atherosclerotic risk and kidney disease progression also underscores the systemic actions of finerenone in attenuating the adverse multi-organ effects of mineralocorticoid receptor overactivation5.
A key strength of this pooled analysis was access to individual participant-level data from all phase 3 trials conducted to date with finerenone, which allowed harmonization of data elements related to baseline clinical characteristics, outcomes and subgroups. Major efficacy outcomes were independently adjudicated, and safety assessments were conducted with aligned definitions in a standardized fashion. As undetermined deaths are variably handled across trials of CKM conditions, including in the three trials examined in this pooled study, separate analyses were carried out for cardiovascular death excluding and including these deaths.
This pooled analysis is subject to several limitations. The findings were derived from randomized clinical trials with specific inclusion and exclusion criteria and, thus, may not be generalizable to all populations treated in clinical practice. Despite the large global population studied, enrollment of select groups, such as Black patients, remained limited. Certain data elements were not consistently available across trials to allow for pooling. For instance, urgent HF visits, which were included as a part of the FINEARTS-HF primary outcome, were not collected in FIDELIO-DKD and FIGARO-DKD. Background use of SGLT2 inhibitors and GLP-1RA was only modest, limiting firm conclusions of the additive effects of finerenone. No adjustment was made for testing of multiple comparisons. Finally, not all trials contributed equally to each of the subgroups examined; for instance, only FINEARTS-HF included patients who did not have diabetes.
While in this pooled analysis the reduction in cardiovascular death was not statistically significant, finerenone reduced the risks for deaths of any cause, cardiovascular events and kidney outcomes while being safe and well tolerated. The totality of the evidence thus supports the disease-modifying potential of finerenone in broad, high-risk patient populations encompassing cardiovascular, kidney and metabolic diseases.
Methods
Search strategy and trial selection
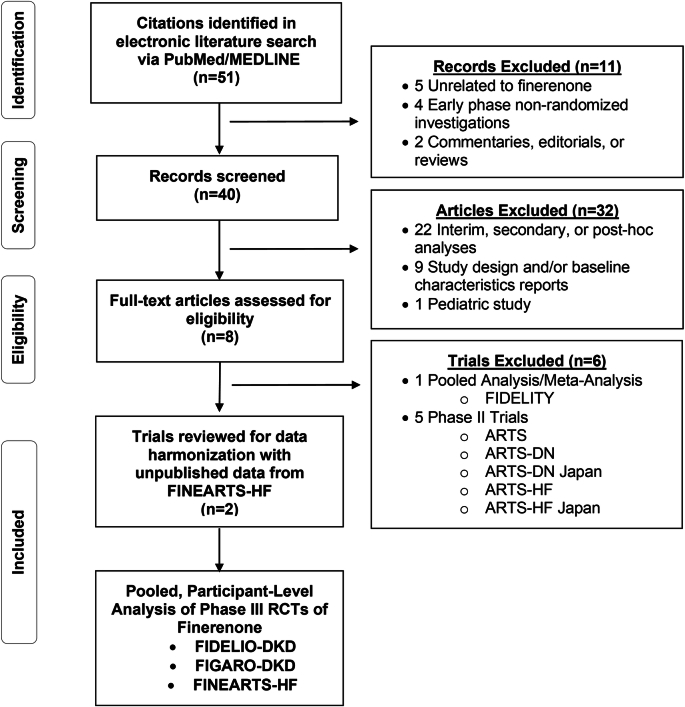
We conducted a participant-level pooled analysis of two trials of CKD and T2D (FIDELIO-DKD (Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease; NCT02540993) and FIGARO-DKD (Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease; NCT02545049)) and a trial of patients with HF (FINEARTS-HF (FINerenone trial to investigate Efficacy and sAfety superioR to placebo in paTientS with Heart Failure; NCT04435626)) that included patients with and without diabetes. The designs25–27 and primary results15–17 of each of the three trials have been published. Key design elements of each of the trials are summarized in Extended Data Table 1. We further conducted a systematic review of the literature using PubMed and MEDLINE to ensure that other relevant trials were not missed. We searched for randomized clinical trials of finerenone published from database inception to 24 July 2024. The following string was used in the PubMed/MEDLINE pre-specified search query to identify potential studies to be included in the meta-analysis:
Extended Data Table 1.
Key study design features
(‘finerenone’[Extended Data Concept] OR ‘finerenone’[All Fields]) AND (randomizedcontrolledtrial[Filter]). The pre-specified search query, which was run on 24 July 2024, did not identify any additional phase 3 trials that met criteria for inclusion (Extended Data Fig. 1). Data from the FINEARTS-HF trial were unpublished at the time of analysis and were included with permission from the steering committee and trial sponsor.
Extended Data Fig. 1.
Systematic Search of the Literature to Identify Eligible Trials for Inclusion in FINE-HEART. We conducted a systematic review of the literature using PubMed and MEDLINE to ensure that relevant trials were not missed to be included in this pooled analysis.
Design of FIDELIO-DKD and FIGARO-DKD
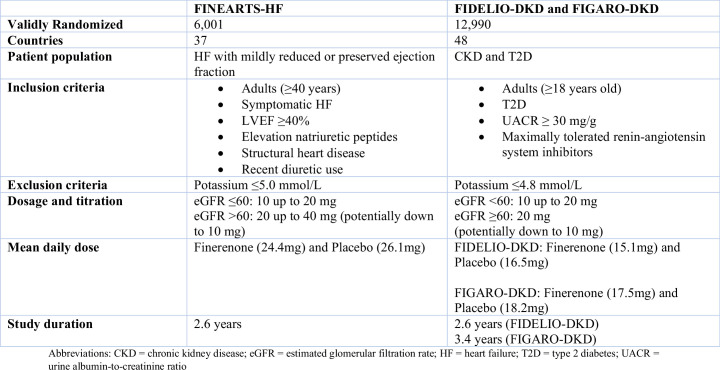
In brief, both FIDELIO-DKD and FIGARO-DKD trials enrolled adults (≥18 years) with T2D and CKD across 48 countries. FIDELIO-DKD required a UACR of 30 to <300 mg g−1, an eGFR of 25 to <60 ml min−1 1.73 m−2 and a history of diabetic retinopathy or a UACR of 300–5,000 mg g−1 and an eGFR of 25 to <75 ml min−1 1.73 m−2. FIGARO-DKD required a UACR of 30 to <300 mg g−1 and an eGFR of 25–90 ml min−1 1.73 m−2 or a UACR of 300–5,000 mg g−1 and an eGFR of ≥60 ml min−1 1.73 m−2. Both trials required a serum potassium level of ≤4.8 mmol L−1 for enrollment. Renin–angiotensin system inhibitor use and dosing were optimized before screening during run-in phases (lasting 4–16 weeks) in both trials. Patients with symptomatic HF with reduced ejection fraction were excluded, but those with HF and higher ejection fraction were eligible.
Design of FINEARTS-HF
FINEARTS-HF enrolled adults (≥40 years) with symptomatic HF with mildly reduced or preserved ejection fraction across 37 countries. Key inclusion criteria included left ventricular ejection fraction ≥40%, elevated natriuretic peptides (adjusted based on atrial fibrillation status and clinical setting of screening), evidence of structural heart disease and recent diuretic use for at least 30 d. Patients were required to have an eGFR of ≥25 ml min−1 1.73 m−2 and a serum potassium level of ≤5.0 mmol L−1 for enrollment. Participants could be enrolled regardless of clinical care setting (whether hospitalized, recently hospitalized or ambulatory).
All participants were randomly allocated to finerenone or placebo with initial dosing determined based on kidney function. The initial dose was 10 mg for patients with an eGFR of <60 ml min−1 1.73 m−2, titrated up to a target dose of 20 mg once daily as tolerated. Participants with an eGFR of ≥60 ml min−1 1.73 m−2 were started at the target dose of 20 mg once daily. In FINEARTS-HF, participants with an eGFR of >60 ml min−1 1.73 m−2 were started on 20 mg and could be titrated up to 40 mg once daily as tolerated, whereas 20 mg was the target dose for patients with an eGFR of ≤60 ml min−1 1.73 m−2. As dose-dependent effects of finerenone have been observed on natriuretic peptide levels in the preceding phase 2 program of patients with worsening HF28, FINEARTS-HF examined a higher target maintenance dose of 40 mg (in those with an eGFR of >60 ml min−1 1.73 m−2) than in FIDELIO-DKD and FIGARO-DKD. The trial protocols were approved by ethics committees or institutional review boards at all participating sites, and all patients provided explicit written informed consent.
FINE-HEART pooling strategy
Individual participant-level data were accessed and pooled with harmonized data elements for baseline characteristics and clinical outcomes (Extended Data Table 2). All participants randomized in each of the three trials were considered for this pooled analysis with only patients with critical Good Clinical Practice violations excluded. A total of 160 randomized patients (60 patients in FIDELIO-DKD, 85 patients in FIGARO-DKD and 15 patients in FINEARTS-HF) were prospectively excluded before database lock from all analyses because of critical Good Clinical Practice violations or due to re-randomization of the same subject. In addition, 36 participants in FIDELIO-DKD and FIGARO-DKD were confirmed to have critical Good Clinical Practice violations after database lock. These 196 participants were excluded from all efficacy and safety analysis in FINE-HEART. The final sample size was 18,991 participants.
Extended Data Table 2.
Harmonization of key outcome measures and their assessment across trials
FINE-HEART pooled analysis outcomes
All efficacy outcomes were analyzed in randomized patients under intention-to-treat principles, and all safety outcomes were analyzed in randomized patients who had taken at least one dose of the study drug. All deaths were adjudicated by independent clinical endpoint committees in each of the respective trials included in this pooled analysis (specific adjudication criteria included in the Supplementary Methods).
The pre-specified primary outcome for FINE-HEART was time to cardiovascular death. The definition of cardiovascular death differed slightly among the three trials and was harmonized for FINE-HEART as time to cardiovascular death (excluding undetermined deaths) (Extended Data Table 2). Other pre-specified outcomes included a kidney composite outcome (defined as a sustained decrease in eGFR to ≥50% from baseline, sustained decline in eGFR to <15 ml min−1 1.73 m−2, kidney failure and death due to kidney failure), HF hospitalization, composite of cardiovascular death or HF hospitalization, new-onset atrial fibrillation, major adverse cardiovascular events (a composite of non-fatal myocardial infarction, non-fatal stroke, HF hospitalization or cardiovascular death), all-cause death and hospitalization for any reason. The kidney composite endpoint inclusive of a sustained decrease in eGFR to ≥57% from baseline (corresponding to a doubling of serum creatinine) was additionally reported. The composite of all-cause death or all-cause hospitalization was defined post hoc to describe the total burden or morbidity and mortality. Select treatment-emergent adverse events related to hyperkalemia, acute kidney injury, hypotension and gynecomastia were additionally reported. The primary outcome (cardiovascular death) was assessed across key subgroups, including age, sex, race, region, baseline body mass index, baseline systolic blood pressure, KDIGO risk, baseline serum potassium levels, baseline eGFR, baseline UACR, history of HF, history of diabetes, presence of CKD, number of CKM conditions (CKD, HF and/or diabetes) and baseline use of SGLT2 inhibitors or GLP-1RA.
Statistical analysis
All primary and secondary outcomes were analyzed as time to first outcomes using a stratified Cox proportional hazards model including the study intervention group as a fixed effect and stratified by geographic region and individual trial (Extended Data Table 2). All treatment effect estimates are presented as HRs with associated 95% CIs. Select primary and secondary outcomes were additionally graphically displayed using Kaplan–Meier methods. There was no multiple testing strategy; namely, testing for secondary outcomes continued even if results for the primary outcome were neutral or negative. A pre-specified sensitivity analysis was conducted for the primary outcome that considered deaths of undetermined causes to be cardiovascular deaths in all three trials. Treatment effects on cardiovascular death were assessed across all pre-specified subgroups. Incidence rates of cardiovascular death as a function of baseline eGFR and log-transformed UACR were estimated separately for each treatment arm using Poisson regression models, allowing for potentially nonlinear relationships using restricted cubic splines with three knots. The treatment effect of finerenone was then estimated as the ratio of these two group-specific estimates.
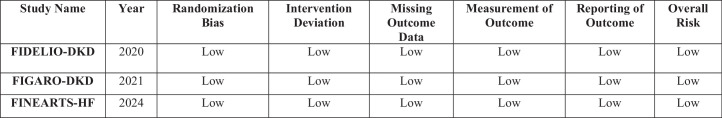
The statistical analysis plan for this pooled analysis was pre-specified, and the protocol was prospectively registered in the International Prospective Register of Systematic Reviews (PROSPERO CRD42024570467) before unblinding the FINEARTS-HF trial. All three trials were assessed as high quality with a low risk of bias before pooling (Extended Data Table 3). The trials included in this pooled analysis were funded by Bayer AG. Trial steering committees designed and oversaw their conduct in collaboration with the sponsor. However, the primary analyses, interpretation of the data and initial manuscript drafting were conducted independently by the academic teams. Statistical analyses were conducted using STATA version 18 software.
Extended Data Table 3.
Risk of bias assessment using the revised tool to assess risk of bias in randomized trials (RoB 2.0)
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41591-024-03264-4.
Supplementary information
Acknowledgements
This work is dedicated to the memory of Dr. George Bakris whose career was devoted to improving the care of patients living with cardiovascular and kidney disease. FIDELIO-DKD, FIGARO-DKD and FINEARTS-HF were funded by Bayer AG. Steering committees of each of the trials designed and oversaw their conduct in collaboration with the sponsor. However, the primary analyses, interpretation of the data and initial manuscript drafting were conducted independently by the academic teams.
Extended data
Author contributions
All authors made substantial contributions to the conceptualization and design of the study. B.L.C. and P.S.J. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. P.S. and J.L.-F. cross-verified all analytic output. M.V. wrote the first draft of the manuscript. All authors contributed to data interpretation and writing of the final version of the manuscript, and all authors were responsible for the decision to submit the manuscript for publication.
Peer review
Peer review information
Nature Medicine thanks Johannes Mann, Katherine Tuttle and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Michael Basson, in collaboration with the Nature Medicine team.
Data availability
For each of the three clinical trials (FIDELIO-DKD, FIGARO-DKD and FINEARTS-HF), Bayer (the sponsor) commits to sharing, upon reasonable request from qualified scientific and medical researchers, patient-level clinical trial data, study-level clinical trial data and protocols. Interested researchers can use https://vivli.org/ to request access to anonymized patient-level data and supporting documents from clinical studies. Data access will be granted to anonymized patient-level data, protocols and clinical study reports after approval by an independent scientific review panel, with scope and conditions laid out as on https://vivli.org/ourmember/bayer/.
Competing interests
M.V. has received research grant support, served on advisory boards or had speaker engagements with American Regent, Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, Bristol Myers Squibb, Boehringer Ingelheim, Chiesi, Cytokinetics, Fresenius Medical Care, Idorsia Pharmaceuticals, Lexicon Pharmaceuticals, Merck, Milestone Pharmaceuticals, Novartis, Novo Nordisk, Pharmacosmos, Relypsa, Roche Diagnostics, Sanofi and Tricog Health and participates on clinical trial committees for studies sponsored by AstraZeneca, Galmed, Novartis, Bayer AG, Occlutech and Impulse Dynamics. G.F. has received lecture fees from Bayer, Boehringer Ingelheim, Servier and Novartis; trial committee membership fees from Bayer, Boehringer Ingelheim, Servier, Impulse Dynamics, Vifor and Medtronic; consulting fees from Cardior and Novo Nordisk; and research grants from the European Union. B.L.C. has received personal consulting fees from Alnylam, Bristol Myers Squibb, Cardior, Cardurion, Corvia, CVRx, Eli Lilly, Intellia and Rocket and has served on a data and safety monitoring board (DSMB) for Novo Nordisk. A.S.S. has received institutional research grants (to Brigham and Women’s Hospital) from Abbott, Alnylam, AstraZeneca, Bayer, Novartis and Pfizer as well as personal consulting fees from Abbott, Alnylam, AstraZeneca, Bayer, Biofourmis, Boston Scientific, Medpace, Medtronic, Merck, Novartis, Parexel, Porter Health, Regeneron, River2Renal, Roche, Veristat, Verily and Zydus. P.S.J. reports speaker fees from AstraZeneca, Novartis, Alkem Metabolics, ProAdWise Communications and Sun Pharmaceuticals; advisory board fees from AstraZeneca, Boehringer Ingelheim and Novartis; and research funding from AstraZeneca, Boehringer Ingelheim, Analog Devices and Roche Diagnostics. P.S.J.’s employer, the University of Glasgow, has been remunerated for clinical trial work from AstraZeneca, Bayer AG, Novartis and Novo Nordisk. Director of the Global Clinical Trial Partners Ltd. A.D. has no financial conflicts to report. M.B. is a full-time employee of Bayer AG. P.K. is a full-time employee of Bayer AG. He is also a co-inventor of finerenone and holds US and European patents relating to finerenone (US8436180B2 and EP2132206B1). P.S. is an employee of Bayer AG. J.L.-F. is a full-time employee of Bayer plc, Research & Development, Pharmaceuticals. P.V. is an employee of Bayer AG. C.S.P.L. has received research support from NovoNordisk and Roche Diagnostics; has received consulting fees from Alleviant Medical, Allysta Pharma, AnaCardio AB, Applied Therapeutics, AstraZeneca, Bayer, Biopeutics, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, CardioRenal, CPC Clinical Research, Eli Lilly, Impulse Dynamics, Intellia Therapeutics, Ionis Pharmaceutical, Janssen Research & Development, Medscape/WebMD Global, Merck, Novartis, Novo Nordisk, Prosciento, Quidel Corporation, Radcliffe Group, Recardio ReCor Medical, Roche Diagnostics, Sanofi, Siemens Healthcare Diagnostics and Us2.ai; and is a co-founder and non-executive director of Us2.ai. M.S. has served on advisory boards for and has received consultancy fees and honoraria from Novartis, Abbott, Merck, Merck Sharp & Dohme, Vifor, AstraZeneca, Cardurion, Novonordisk, Bayer and Boehringer Ingelheim. S.J.S. has received research grants from the National Institutes of Health (NIH) (U54 HL160273, X01 HL169712, R01 HL140731 and R01 HL149423), the American Heart Association (AHA) (24SFRNPCN1291224), AstraZeneca, Corvia and Pfizer and consulting fees from Abbott, Alleviant, AstraZeneca, Amgen, Aria CV, Axon Therapies, Bayer, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cyclerion, Cytokinetics, Edwards Lifesciences, Eidos, Imara, Impulse Dynamics, Intellia, Ionis, Eli Lilly, Merck, MyoKardia, Novartis, Novo Nordisk, Pfizer, Prothena, Regeneron, Rivus, Sardocor, Shifamed, Tenax, Tenaya and Ultromics. A.A.V.ʼs employer received consultancy fees and/or research support from Adrenomed, Anacardio, AstraZeneca, Bayer AG, Bristol Myers Squibb, Boehringer Ingelheim, Corteria, Eli Lilly, Merck, Moderna, Novartis, Novo Nordisk, Roche Diagnostics and SalubrisBio. F.Z. reports personal fees from 89Bio, Abbott, Acceleron, Applied Therapeutics, Bayer, Betagenon, Boehringer, Bristol Myers Squibb, CVRx, Cambrian, Cardior, Cereno Pharmaceutical, Cellprothera, CEVA, Inventiva, KBP, Merck, NovoNordisk, Owkin, Otsuka, Roche Diagnostics, Northsea and USa2; having stock options at G3Pharmaceutical and equities at Cereno, Cardiorenal, Eshmoun Clinical Research; and being the founder of Cardiovascular Clinical Trialists. P.R. reports grants and payment of honoraria for lectures, educational events and steering group participation from AstraZeneca, Bayer and Novo Nordisk (all to the Steno Diabetes Center Copenhagen); payment of honoraria for lectures and participation in advisory boards from Boehringer Ingelheim, Sanofi, Gilead and Astellas (all to the Steno Diabetes Center Copenhagen); and receipt of study drugs for free for investigator-initiated studies from Bayer, Novo Nordisk and Lexicon. L.M.R. has no financial conflicts to report. S.D.A. reports grants from Vifor and Abbott; consulting fees from CVRx, Astra Zeneca, Bioventrix, Repairon, Novo Nordisk, Brahms, Novartis, Actimed Therapeutics, Faraday Pharmaceuticals, Cytokinetics, HeartKinetics, GlaxoSmithKline, Vectorious, Scirent, Sensible Medical, Edwards, Relaxera, Repairon, Regeneron Pharmaceuticals and Cordio; steering or advisory committee work for Vifor, Bayer AG, Boehringer Ingelheim, Medtronic, Abbott, Impulse Dynamics, Cardior, V-Wave, Pfizer, Cardiac Dimensions and Occlutech; and being named as a co-inventor of two patent applications regarding MR-proANP (DE 102007010834 and DE 102007022367), but he does not benefit personally from the related issued patents. B.P. has served as a consultant for Bayer, Astra Zeneca, Bristol Meyers Squibb, Boehringer Ingelheim, Lexicon, Anacardia and G3 Pharmaceuticvals. He has served as a consultant and received stock options or stocks from Sea Star Medical, Vifor, scPharmaceuticals, SQinnovations, KBP Biosciences, Sarfez, Cereno Scientific, Prointel and Brainstorm Medical. He holds a US patent (9931412: Site specific delivery of eplerenone to the myocardium) and has a US patent pending (63/045,783: Histone modulating agents for the prevention and treatment of organ damage). R.A. reports support from Bayer; royalties or licenses from UpToDate; consulting fees from Boehringer Ingelheim, Novartis, Akebia, Intercept Pharma and Alnylam; support for meetings from Boehringer Ingelheim, Novartis, Akebia and Vertex; and participation on DSMBs or advisory boards for Vertex, Eloxx and Chinook. J.J.V.M. reports payments through the University of Glasgow from work on clinical trials, consulting and grants from Amgen, AstraZeneca, Bayer, Cardurion, Cytokinetics, GlaxoSmithKline and Novartis; personal consultancy fees from Alynylam Pharmaceuticals, Amgen, AnaCardio, AstraZeneca, Bayer, Berlin Cures, Bristol Myers Squibb, Cardurion, Cytokinetics, Ionis Pharmaceuticals, Novartis, Regeneron Pharmaceuticals, River 2 Renal Corp., the British Heart Foundation, the NIH National Heart, Lung, and Blood Institute (NHLBI), Boehringer Ingelheim, SQ Innovations and Catalyze Group; personal lecture fees from Abbott, Alkem Metabolics, AstraZeneca, Blue Ocean Scientific Solutions, Boehringer Ingelheim, Canadian Medical and Surgical Knowledge, Emcure Pharmaceuticals, Eris Lifesciences, European Academy of CME, Hikma Pharmaceuticals, Imagica Health, Intas Pharmaceuticals, J.B. Chemicals & Pharmaceuticals, Lupin Pharmaceuticals, Medscape/Heart.Org., ProAdWise Communications, Radcliffe Cardiology, Sun Pharmaceuticals, The Corpus, Translation Research Group and Translational Medicine Academy; and DSMB for WIRB-Copernicus Group Clinical. He is also a director of Global Clinical Trial Partners, Ltd. S.D.S. has received research grants from Alexion, Alnylam, AstraZeneca, Bellerophon, Bayer, Bristol Myers Squibb, Boston Scientific, Cytokinetics, Edgewise, Eidos, Gossamer, GlaxoSmithKline, Ionis, Eli Lilly, MyoKardia, NIH/NHLBI, Novartis, NovoNordisk, Respicardia, Sanofi Pasteur, Theracos and US2.AI and has consulted for Abbott, Action, Akros, Alexion, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Boeringer Ingelheim, Bristol Myers Squibb, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi-Sankyo, GlaxoSmithKline, Eli Lilly, Merck, Myokardia, Novartis, Roche, Theracos, Quantum Genomics, Janssen, Cardiac Dimensions, Tenaya, Sanofi-Pasteur, Dinaqor, Tremeau, CellProThera, Moderna, American Regent, Sarepta, Lexicon, Anacardio, Akros and Valo.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
10/28/2024
A Correction to this paper has been published: 10.1038/s41591-024-03372-1
Extended data
is available for this paper at 10.1038/s41591-024-03264-4.
Supplementary information
The online version contains supplementary material available at 10.1038/s41591-024-03264-4.
References
- 1.Aggarwal, R., Ostrominski, J. W. & Vaduganathan, M. Prevalence of cardiovascular-kidney-metabolic syndrome stages in US adults, 2011–2020. JAMA331, 1858–1860 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostrominski, J. W. et al. Prevalence and overlap of cardiac, renal, and metabolic conditions in US adults, 1999–2020. JAMA Cardiol.8, 1050–1060 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ostrominski, J. W. et al. Cardiovascular-kidney-metabolic overlap in heart failure with mildly reduced or preserved ejection fraction: a trial-level analysis. J. Am. Coll. Cardiol.84, 223–228 (2024). [DOI] [PubMed] [Google Scholar]
- 4.Ndumele, C. E. et al. Cardiovascular-kidney-metabolic health: a presidential advisory from the American Heart Association. Circulation148, 1606–1635 (2023). [DOI] [PubMed] [Google Scholar]
- 5.Agarwal, R. et al. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur. Heart J.42, 152–161 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolkhof, P. & Borden, S. A. Molecular pharmacology of the mineralocorticoid receptor: prospects for novel therapeutics. Mol. Cell. Endocrinol.350, 310–317 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Kolkhof, P. & Bärfacker, L. 30 years of the mineralocorticoid receptor: mineralocorticoid receptor antagonists: 60 years of research and development. J. Endocrinol.234, T125–T140 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel, R. B. et al. Kidney function and outcomes in patients hospitalized with heart failure. J. Am. Coll. Cardiol.78, 330–343 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilstrap, L. G. et al. Reasons for guideline nonadherence at heart failure discharge. J. Am. Heart Assoc.7, e008789 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolkhof, P. et al. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist protects from rat cardiorenal injury. J. Cardiovasc. Pharmacol.64, 69–78 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Heinig, R., Kimmeskamp-Kirschbaum, N., Halabi, A. & Lentini, S. Pharmacokinetics of the novel nonsteroidal mineralocorticoid receptor antagonist finerenone (BAY 94-8862) in individuals with renal impairment. Clin. Pharmacol. Drug Dev.5, 488–501 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Gerisch, M. et al. Biotransformation of finerenone, a novel nonsteroidal mineralocorticoid receptor antagonist, in dogs, rats, and humans, in vivo and in vitro. Drug Metab. Dispos.46, 1546–1555 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Berger, M. et al. FIGARO-BM, a biomarker study of FIGARO-DKD, reveals new insights into the mode of action of finerenone. https://www.asn-online.org/education/kidneyweek/2023/program-abstract.aspx?controlId=3931413 (2023).
- 14.Pitt, B. et al. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur. Heart J.34, 2453–2463 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bakris, G. L. et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N. Engl. J. Med.383, 2219–2229 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Pitt, B. et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N. Engl. J. Med.385, 2252–2263 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Solomon, S. D. et al. Finerenone in heart failure with mildly reduced or preserved ejection fraction. N. Engl. J. Med. (in the press). [DOI] [PubMed]
- 18.Agarwal, R. et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur. Heart J.43, 474–484 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Diabetes Association Professional Practice Committee. Chronic kidney disease and risk management: Standards of Care in Diabetes—2024. Diabetes Care47, S219–S230 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marx, N. et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur. Heart J.44, 4043–4140 (2023). [DOI] [PubMed] [Google Scholar]
- 21.McDonagh, T. A. et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J.44, 3627–3639 (2023). [DOI] [PubMed] [Google Scholar]
- 22.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int.105, S117–S314 (2024). [DOI] [PubMed] [Google Scholar]
- 23.Neuen, B. L. et al. Estimated lifetime cardiovascular, kidney, and mortality benefits of combination treatment with SGLT2 inhibitors, GLP-1 receptor agonists, and nonsteroidal MRA compared with conventional care in patients with type 2 diabetes and albuminuria. Circulation149, 450–462 (2024). [DOI] [PubMed] [Google Scholar]
- 24.Naaman, S. C. & Bakris, G. L. Diabetic nephropathy: update on pillars of therapy slowing progression. Diabetes Care46, 1574–1586 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bakris, G. L. et al. Design and baseline characteristics of the finerenone in reducing kidney failure and disease progression in diabetic kidney disease trial. Am. J. Nephrol.50, 333–344 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruilope, L. M. et al. Design and baseline characteristics of the Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease trial. Am. J. Nephrol.50, 345–356 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaduganathan, M. et al. Finerenone in patients with heart failure with mildly reduced or preserved ejection fraction: rationale and design of the FINEARTS-HF trial. Eur. J. Heart Fail.26, 1324–1333 (2024). [DOI] [PubMed] [Google Scholar]
- 28.Filippatos, G. et al. A randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur. Heart J.37, 2105–2114 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
For each of the three clinical trials (FIDELIO-DKD, FIGARO-DKD and FINEARTS-HF), Bayer (the sponsor) commits to sharing, upon reasonable request from qualified scientific and medical researchers, patient-level clinical trial data, study-level clinical trial data and protocols. Interested researchers can use https://vivli.org/ to request access to anonymized patient-level data and supporting documents from clinical studies. Data access will be granted to anonymized patient-level data, protocols and clinical study reports after approval by an independent scientific review panel, with scope and conditions laid out as on https://vivli.org/ourmember/bayer/.