Abstract
We investigated the effect of overexpressing a pumpkin gibberellin (GA) 20-oxidase gene encoding an enzyme that forms predominantly biologically inactive products on GA biosynthesis and plant morphology in transgenic lettuce (Lactuca sativa cv Vanguard) plants. Lettuce was transformed with the pumpkin GA 20-oxidase gene downstream of a strong constitutive promoter cassette (El2–35S-Ω). The transgenic plants in which the pumpkin gene was detected by polymerase chain reaction were dwarfed in the T2 generation, whereas transformants with a normal growth phenotype did not contain the transgene. The result of Southern-blot analysis showed that the transgene was integrated as a single copy; the plants segregated three dwarfs to one normal in the T2 generation, indicating that the transgene was stable and dominant. The endogenous levels of GA1 and GA4 were reduced in the dwarfs, whereas large amounts of GA17 and GA25, which are inactive products of the pumpkin GA 20-oxidase, accumulated in these lines. These results indicate that a functional pumpkin GA 20-oxidase is expressed in the transgenic lettuce, resulting in a diversion of the normal pathway of GA biosynthesis to inactive products. Furthermore, this technique may be useful for controlling plant stature in other agricultural and horticultural species.
The gibberellin (GA) plant hormones play important roles in plant development, controlling such processes as seed germination, internode elongation, flower development, and fruit set in higher plants (Crozier, 1983). Control of such processes is achieved, at least in part, by the development-dependent and organ-specific adjustment of the concentrations of biologically active GAs, so that the biosynthetic enzymes are potential regulators of plant development. Furthermore, manipulation of these enzymes by genetic engineering could be used to control plant growth and thereby introduce agronomically useful traits (Hedden and Kamiya, 1997; Lange, 1998).
In higher plants, GA biosynthesis requires the conversion of geranylgeranyl diphosphate to ent-kaurene via copalyl diphosphate catalyzed by the diterpene cyclases, and further converted to biologically active GAs through a series of oxidative steps catalyzed by cytochrome P450-dependent monooxygenases and 2-oxoglutarate-dependent dioxygenases (Hedden and Kamiya, 1997). One of the dioxygenase, GA 20-oxidase, which catalyzes the sequential oxidation and elimination of C-20, has been shown to be a regulatory enzyme. In recent studies, cDNA clones encoding the GA 20-oxidase gene have been isolated from several higher plants (Lange et al., 1994; Phillips et al., 1995; Wu et al., 1996; Toyomasu et al., 1997) and the expression of the gene has been shown to be controlled by photoperiod (Wu et al., 1996; Xu et al., 1997), by GA in a type of feedback regulation (Phillips et al., 1995; Xu et al., 1995; Martin et al., 1996; Carrera et al., 1999), and according to organ or developmental stage (Garcia-Martinez et al., 1997; Rebers et al., 1999). Thus, expression of GA 20-oxidase genes controls the production of biologically active GA in response to developmental and environmental stimuli. Furthermore, overexpression of GA 20-oxidase genes in Arabidopsis has been shown to increase GA production and stimulate growth, so confirming that GA 20-oxidase activity is a limiting factor in both processes (Huang et al., 1998; Coles et al., 1999).
Among the GA 20-oxidases known to date, only that from developing pumpkin seeds has been shown to produce biologically inactive GAs as the major products; the pumpkin enzyme converts the aldehyde intermediates GA24 and GA19 to the tricarboxylic acids, GA25 and GA17, respectively (Lange, 1998). Overexpression of the pumpkin GA 20-oxidase gene in transgenic plants could, therefore, result in a reduction of biologically active GAs by diverting the pathway to the tricarboxylic acids. Thus, the gene is a potentially useful tool for controlling plant growth.
In the present study, we tested this hypothesis by overexpressing the pumpkin GA 20-oxidase gene in lettuce (Lactuca sativa cv Vanguard) and we demonstrated that the approach can result in a reduction in active GA content and growth.
RESULTS
Transformation and Selection of Lettuce Plants Overexpressing the Pumpkin GA 20-Oxidase Gene
In a preliminary study, lettuce plants harboring the pumpkin GA 20-oxidase gene driven by the cauliflower mosaic virus (CaMV) 35S promoter did not show an altered phenotype (data not shown). Therefore, in subsequent experiments a stronger promoter cassette containing a translational enhancer (El2–35S-Ω) was used to enhance the pumpkin GA 20-oxidase expression (Fig. 1). Sixteen transformants were obtained by kanamycin selection, one of which (SGΩ-4) showed a dwarf phenotype with smaller leaves compared with wild-type plants. In the T2 generation obtained by selfing SGΩ-4, dwarf plants were obtained in which hypocotyls, internodes, and leaves were reduced in length by 60%, 70%, and 40%, respectively, compared with wild-type plants (Table I). However, no remarkable differences were observed comparing the normal-type T2 plants from SGΩ-4 with wild-type plants (Table I). The dwarfs segregated 3:1 from plants with a normal phenotype, indicating that the transgene was stable and dominant in transgenic plants.
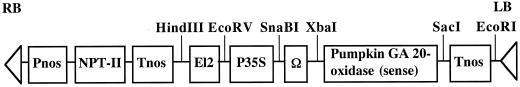
Figure 1.
Structure of T-DNA region of pSGΩ. Pnos, 5′-upstream region of nopaline synthase gene. NPT-II, Coding region of nopaline synthase gene. Tnos, Polyadenylation region of nopaline synthase gene. El2, 5′-upstream region of CaMV 35S promoter (−419 to −90) × 2. P35S, 5′-upstream region of CaMV promoter (−90 to −1). Ω, 5′-Untranslated region of tobacco mosaic virus.
Table I.
Phenotypic characterization of SGΩ-4 plants
| Phenotype | No. of Plants | Segregation Ratio | Hypocotyl Lengtha | Internode Lengthab | Leaf Lengtha | PCR Band |
|---|---|---|---|---|---|---|
| cm | ||||||
| SGΩ-4 plants | ||||||
| Dwarf type | 67 | 3 | 0.99 ± 0.30 | 0.10 ± 0.01 | 6.66 ± 1.41 | + |
| Normal type | 24 | 1 | 2.72 ± 0.31 | 0.32 ± 0.05 | 12.88 ± 1.50 | − |
| Wild-type plants | 24 | – | 2.44 ± 0.61 | 0.32 ± 0.03 | 11.37 ± 2.12 | − |
Seeds of T2 generation were germinated on soil in pots and hypocotyl length was measured at 2 weeks after sowing. After growing for 1 month, leaf and internode length were measured. Genomic DNA was extracted from a leaf of the seedlings and the integrated pumpkin GA 20-oxidase gene was detected by PCR using a specific primer.
Values are means ± se.
Average values of primary to fourth leaf.
Molecular Analysis of SGΩ-4 Plants
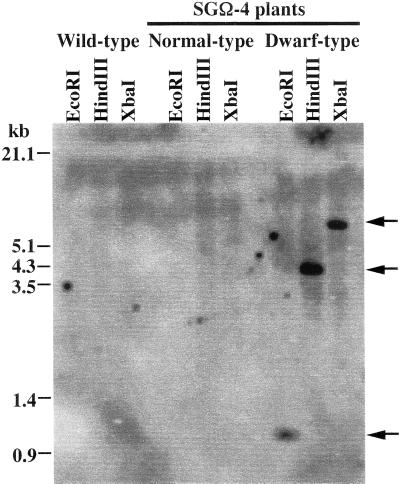
The presence of the integrated pumpkin GA 20-oxidase gene was tested by PCR, and the product of this was sequenced to ensure that it corresponded to the transgene. The transgene was detected only in genomic DNA from dwarf SGΩ-4 plants; transformants with a normal phenotype did not contain the gene (Table I). The result of Southern-blot analysis is shown in Figure 2. A single band was present in all lanes of genomic DNA from dwarf plants digested with each of three restriction enzymes, suggesting that the transgene is integrated as a single copy. The probe used for the Southern-blot analysis did not cross-hybridize with endogenous GA 20-oxidase genes in lettuce and is assumed to be specific for the transgene.
Figure 2.
Southern-blot analysis of transgene in SGΩ-4 plants. Genomic DNA extracted from wild-type or SGΩ-4 plants was digested with EcoRI, HindIII, or XbaI, respectively, and 10 μg of digested DNA was loaded per lane. An EcoRI-digested PCR fragment of the pumpkin GA 20-oxidase gene, including a 3′-specific region (368 bp), was used as a probe.
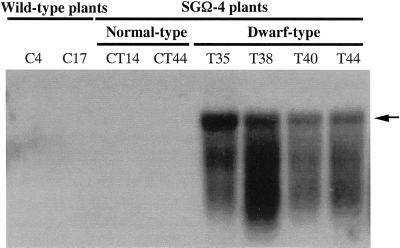
As shown by northern-blot analysis, large amounts of the transcript for the transgene were detectable only in the dwarf SGΩ-4 plants (Fig. 3). These results, together with the observed dominance of the dwarfism conferred by the GA 20-oxidase transgene, confirm that the gene is being transcribed and that the observed dwarf phenotype is definitely linked to overexpression of the transgene.
Figure 3.
Northern-blot analysis of transgene expression. Total RNA was extracted from mature leaves of wild-type and SGΩ-4 plants, and 15 μg of total RNA per lane was blotted onto a membrane. Probe for hybridization was as described in Figure 2.
Morphological Characterization of SGΩ-4 Plants
Morphological differences between wild-type and SGΩ-4 plants are shown in Figure 4. In addition to reduced hypocotyl length (Table I), dwarf seedlings contained more lateral roots than the tall plants (Fig. 4A). Leaves of mature dwarf plants maintained the folded morphology of normal-type transformants and wild-type plants, but were shorter and narrower (Fig. 4B). Furthermore, flowering of the dwarf plants that had short, thin floral axes and fewer branches was delayed by about 2 weeks compared with the wild-type plants (data not shown). Seed production was reduced in the dwarfs, although no differences in seed germination between dwarfs and wild-type plants were observed (data not shown).
Figure 4.
Morphological changes in SGΩ-4 plants. A, Seedlings (3 weeks after sowing) of wild-type (right) and SGΩ-4 plants (normal, center and dwarf type, left). B, Mature plants (wild type, right. SGΩ-4 plants: normal, center and dwarf type, left).
Inhibitory Effect of Uniconazol (UCZ) on Plant Growth of Lettuce Plants
GA is known to stimulate elongation of floral axes and to accelerate flower production, and, hence, it is likely that the observed morphological changes in the transgenic dwarf-type plants are due to a reduction in the content of biologically active GAs. As shown in Figure 5, seedlings of wild-type plants treated with UCZ were dwarfed to the same degree as transgenic dwarf-type plants with shorter and narrower leaves. In contrast, when 100 ng of GA3 was applied to the dwarfs, the length and width of newly developed leaves and internode length were restored to the same extent of that of wild-type plants (data not shown). These results indicate that the plants overexpressing the pumpkin GA 20-oxidase gene would have a reduced content of biologically active GAs.
Figure 5.
Inhibitory effect of UCZ treatment on plant growth of wild-type lettuce plants. Seedlings of lettuce plants (dwarf type of SGΩ-4, left and wild type, center-left, center-right, and right) were treated with 0.001% (w/v, center-right) and 0.01% (w/v, right) or without (left and center-left) UCZ. Each concentration of 10 mL of UCZ solution was added to the soil. The plants were photographed at 1 month after the treatment.
Analysis of Endogenous GA Levels in Dwarf Lettuce Plants
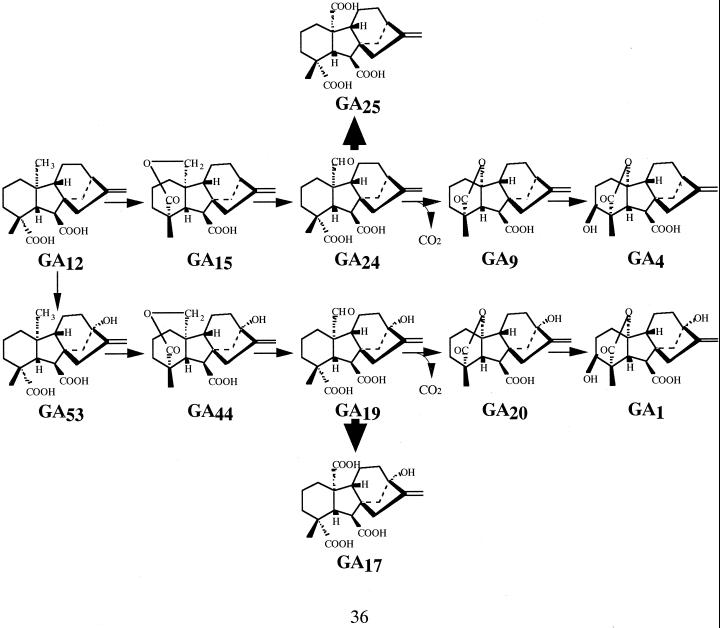
We analyzed endogenous GA levels in the transgenic plants by gas chromatography-mass spectrometry to confirm that GA biosynthesis is altered in the dwarf plants and to determine which steps of the GA biosynthetic pathway are affected. The pumpkin GA 20-oxidase is known to produce inactive tricarboxylic acid GAs of no known physiological function (Lange, 1998). As shown in Table II, for early-13-hydroxylated GAs as the major products in lettuce plants, the level of endogenous GA1 (a biologically active GA that is formed via GA19 and GA20) in the dwarfs was reduced to 15% to 25% of that in wild-type plants. In addition, the contents of GA20 and GA19 were also reduced in the dwarfs. However, large amounts of GA17, which is a biologically inactive GA formed as the major product from GA19 by the pumpkin GA 20-oxidase (Lange et al., 1994), accumulated in the dwarfs to levels that are >50-fold higher than in the wild-type plants (Table II). Furthermore, in the case of non-13-hydroxylated GAs that were massively minor products, it was the same tendency as early-13-hydroxylated GAs that a biologically active GA4 and its precursor GA24 were reduced and an inactive tricarboxylic acid GA25 was accumulated in the dwarfs. Thus, there is a massive increase in the ratios of inactive GA17 to active GA1 and GA25 to GA4 in the dwarfs, indicating that overexpression of the pumpkin GA 20-oxidase results in a switch of GA biosynthesis from GA19 to GA17 and GA24 to GA25 instead of GA19 to GA20 and GA24 to GA9 and following GA1 and GA4, respectively (Fig. 6).
Table II.
Levels of endogenous GAs in dwarf-type of SGΩ-4 plants and in wild-type plants
| Phenotype | 13-Hydroxylated GAs
|
Non-13-Hydroxylated GAs
|
||||||
|---|---|---|---|---|---|---|---|---|
| GA19 | GA20 | GA1 | GA17 | GA24 | GA9 | GA4 | GA25 | |
| pg g−1 fresh wt | ||||||||
| SGΩ-4 plants | ||||||||
| Dwarf type | 87.2 ± 10.0 | 14.3 ± 7.1 | 30.9 ± 4.1 | 11878.0 ± 718.0a | ND | ND | ND | 36.6 ± 10.4 |
| Wild-type plants | 175.0 ± 34.0 | 207.0 ± 26.0 | 177.0 ± 35.0 | 230.0 ± 54.0a | 1.9 ± 1.8 | ND | 7.7 ± 7.2 | ND |
ND, Not detected. All values are the means ± se of three independent experiments.
Values of the concentration of GA17 are relative to [2H2]GA19.
Figure 6.
GA biosynthesis pathway showing reactions catalyzed by GA 20-oxidase (GA53 to GA20 and GA12 to GA9) and 3β-hydroxylase (GA20 to GA1 and GA9 to GA4). Conversion of GA17 from GA19 and GA25 from GA24, catalyzed by the pumpkin GA 20-oxidase, are shown with heavy arrows.
DISCUSSION
Mutations in genes encoding GA-biosynthetic enzymes have been shown to cause dwarfism in many plant species (Martin et al., 1997; Hedden and Proebsting, 1999). In GA biosynthesis, GA 20-oxidase is a regulatory enzyme being controlled by developmental and environmental stimuli (Phillips et al., 1995; Xu et al., 1995; Garcia-Martinez et al., 1997; Tanaka-Ueguchi et al., 1998; Rebers et al., 1999), and as such, is a prime target for genetic manipulation. In Arabidopsis, overexpression of GA 20-oxidase genes produced a GA-overproduction phenotype with elongated hypocotyls and stem and early flowering (Huang et al., 1998; Coles et al., 1999). In a converse manner, antisense expression of a stem-specific 20-oxidase gene caused reduced stem elongation and delayed flowering in short days (Coles et al., 1999). In the present study we have used a different strategy to reduce GA content: we have introduced an enzyme, a GA 20-oxidase from developing pumpkin seed (Lange et al., 1994), that diverts the biosynthetic flux to an inactive by-product, thereby reducing the precursors available for biologically active GAs.
In transgenic lettuce plants, expression of the transgene, in terms of the presence of transcript, was associated with a dwarf phenotype. Furthermore, the dwarf plants had reduced levels of the biologically active GA1 and GA4, and their growth corresponded to UCZ-treated wild-type plants, which would be inhibited in GA biosynthesis. Thus, the introduced pumpkin GA 20-oxidase could have the expected function in the transgenic lettuce; the abnormally low levels of GA1 and its biosynthetic precursors, GA19 and GA20, were accompanied by elevated levels of the inactive tricarboxylic acid, GA17, which is the major product formed from GA19 in vitro by this enzyme (Lange et al., 1994).
It is well known that GA 20-oxidases can participate in two pathways to biologically active GAs. In the non-13-hydroxylation pathway, GA12 is converted by GA 20-oxidase to GA9, which is then 3β-hydroxylated to the biologically active GA4. GA1 is produced by the equivalent early-13-hydroxylation pathway (Fig. 6), which appears to predominate in lettuce plants (Waycott et al., 1991; Toyomasu et al., 1993). The pumpkin GA 20-oxidase converts non-hydroxylated substrates more efficiently than 13-hydroxylated GAs (Lange et al., 1994) and would, therefore, be expected to also influence the formation of GA4. The minor contents of non-13-hydroxylated GAs, including GA4, could not be detected, whereas GA25, which could not be detected in wild-type plants, accumulated in dwarf-type plants (Table II). However, in Arabidopsis, in which GA4 is the major biologically active GA, overexpression of the pumpkin GA 20-oxidase also resulted in reduced amounts of GA4, although GA1 levels were apparently not affected (Xu et al., 1999). It is surprising that there was only a slight height reduction in the transgenic Arabidopsis plants, prompting the authors to suggest that GA4 content was not limiting. Furthermore, in a recent study with Solanum dulcamara (Curtis et al., 2000), in which GA1 is the major biologically active GA as in lettuce, the plants overexpressing the pumpkin 20-oxidase gene were semi-dwarfed. In the case of S. dulcamara, the levels of the major biologically active GA1 were reduced, whereas GA4 was the same in stems or increased in the leaves.
It was suggested that the effects of overexpressing the pumpkin GA 20-oxidase gene were compromised by up-regulation of the endogenous 20-oxidase gene due to operation of the feedback control mechanism in GA biosynthesis (Xu et al., 1999; Curtis et al., 2000). In our experiments, expression of the transgene must override the feedback mechanism, possibly because of the use of a very strong promoter cassette (El2–35S-Ω) by which introduced pumpkin GA 20-oxidase could compete with the endogenous GA 20-oxidase and converts most of the available GA-precursors into inactive forms (Fig. 6). In preliminary experiments with a weaker promoter, no dwarf plants were obtained, suggesting that very high levels of the pumpkin enzyme may be needed to perturb the mechanism that maintains GA homeostasis.
Morphological changes observed in dwarf-type plants corresponded to the growth of wild-type plants that was inhibited in GA biosynthesis by treatment with UCZ (Fig. 5). In addition to reduced leaf size and stem height, the dwarf seedlings also contained thinner roots and more lateral roots as compared with wild-type plants (Fig. 4). Although GAs are not known to affect lateral root development, it is possible that the effect of reduced GA1 content on lateral root numbers is due to a change in hormone balance as a result of altered shoot morphology. Furthermore, we observed that flowering was delayed and seed production was reduced in the dwarf lettuce plants (data not shown), whereas in the case of S. dulcamara, semi-dwarfed plants overexpressing the pumpkin GA 20-oxidase gene flowered earlier and produced more fruit and seeds (Curtis et al., 2000).
In conclusion, we demonstrated that overexpression of the pumpkin GA 20-oxidase gene could result in a reduction of biologically active GAs causing production of dwarf lettuce plants. Furthermore, this method may be useful for controlling plant stature in other agricultural and horticultural species.
MATERIALS AND METHODS
Plant Material
Seeds of lettuce (Lactuca sativa cv Vanguard) and its transformants were germinated on soil in pots. The seedlings were grown in the greenhouse under natural daylight at 23°C during the day (10 h of light) and at 18°C at night (14 h of darkness).
Transformation of Lettuce Plants
Lettuce seeds were surface sterilized and germinated on Murashige and Skoog medium with 1% (w/v) Suc and 0.2% (w/v) gellan gum under continuous light at 25°C. Leaves of the seedlings were cut and pre-cultured on the medium for 1 d. Transformation of lettuce plants was performed by infection with Agrobacterium tumefaciens LBA4404 harboring a construct prepared by replacing the β-glucuronidase gene of pBI121 with the pumpkin GA 20-oxidase sequence in sense orientation as a XbaI-SacI fragment, and the CaMV 35S promoter sequence with the strong constitutive promoter cassette El2–35S-Ω from pBE2113 as a HindIII-XbaI fragment (Mitsuhara et al., 1996). After incubation in the conditioned medium, transformants were selected on the medium containing kanamycin.
Extraction of Genomic DNA and Analysis of Integrated Pumpkin GA 20-Oxidase Gene
Genomic DNA was extracted from leaves of lettuce seedlings using a DNA extraction kit (ISOPLANT, Wako Life Science, Japan). Extracted DNA was purified through a spin-column (Chroma spin-1000, CLONTECH Laboratories, Palo Alto, CA), and the integrated pumpkin GA 20-oxidase gene was detected by PCR using specific primers, including a 3′-specific region and an additional EcoRI site (forward: 5′-CGAGAATTCATAGAAATGATGGGC-3′ and reverse: 5′-GACGAATTCCAGCAACACATAAGA-3′). After agarose gel electrophoresis, the PCR fragment was recovered from the gel using a DNA purification kit (Toyobo, Japan). This fragment was inserted into EcoRV-digested pBluescript SK (-) and the DNA sequence was analyzed with a Dye terminator cycle sequencing kit and a DNA sequencer (PE-Applied Biosystems, Foster City, CA).
Southern-Blot Analysis
Genomic DNA was extracted in the same way as described above. For Southern-blot analysis, 10 μg of genomic DNA was digested with EcoRI, HindIII, or XbaI, respectively, and, after electrophoresis, it was blotted onto a nylon membrane (Hybond-N+, Amersham Pharmacia Biotech, Buckinghamshire, UK). The purified PCR fragment described above was digested with EcoRI, giving a pumpkin GA 20-oxidase gene-specific probe (368 bp), which was labeled with digoxigenin using digoxigenin-high prime DNA labeling kit (Boehringer Mannheim, Germany). Procedures for hybridization and detection were according to the manufacturer's instructions.
Extraction of Total RNA and Northern-Blot Analysis
Total RNA was extracted by the aurin tricarboxylic acid method (Gonzalez et al., 1980). For northern-blot analysis, 15 μg of total RNA per lane was subjected to electrophoresis and was blotted onto a nylon membrane (Hybond-N+, Amersham). The probe and the procedures for hybridization and detection were as for the Southern-blot analysis.
Treatment of Lettuce Plants with Uniconazol
Seedlings of wild-type lettuce plants were treated with UCZ by adding 10 mL of solution to the soil. The length of leaves was measured at 1 month after the treatment.
Analysis of Endogenous GA Levels
Leaves of 2-month-old lettuce plants (approximately 100 g of fresh weight) were extracted with 80% (v/v) methanol (MeOH). After addition of internal standards (100 ng of [2H2]GA1, 100 ng of [2H2]GA4, 100 ng of [2H2]GA9, 200 ng of [2H2]GA19, 100 ng of [2H2]GA20, 200 ng of [2H2]GA24, and 200 ng of [2H2]GA25), the extract was concentrated in vacuo and the aqueous residue was adjusted to pH 3 with HCl and was then extracted with ethyl acetate (EtOAc). The EtOAc fraction was extracted with 0.5 m phosphate buffer (pH 8.3), and 5% (w/v) of polyvinylpolypyrrolidone was added to the buffer fraction. After filtration, the fraction was adjusted to pH 3 and was extracted with EtOAc again. The EtOAc fraction was dried over anhydrous Na2SO4 and was evaporated in vacuo. The resulting residue was dissolved in 80% (v/v) MeOH and was passed through a Sep-Pak (ODS) cartridge (Waters, Milford, MA). The eluate was dissolved in MeOH and charged onto a Bondesil DEA column. The column was washed with MeOH and was then eluted with 0.5% (v/v) acetic acid (HOAc) in MeOH. The eluate was subjected to HPLC on an Inertsil-2 ODS column (25 × 0.46 cm i.d., Gasukuro Kogyo, Japan). Samples were eluted by linear gradient as follows: solvent, 0 to 2 min, 30% (v/v) MeOH (0.1% [v/v] HOAc); 2 to 10 min, 30% to 50% (v/v) MeOH (0.1% [v/v] HOAc); 10 to 50 min, 50% to 70% (v/v) MeOH (0.1% [v/v] HOAc); and 50 to 51 min, 70% to 100% (v/v) MeOH (0.1% [v/v] HOAc); flow rate, 0.6 mL min−1; column temperature, 40°C. The eluates were collected every 1 min and the fractions of retention time (Rt; 18 to 20 min for GA1, 27 to 29 min for GA20, and 32 to 35 min for GA17 and GA19) were used for early-13-hydroxylated GAs. For non-13-hydroxylated GAs, the fractions (Rt of 42–52 min) were combined and dissolved in MeOH containing 0.1% (v/v) HOAc and were then loaded onto a Senshu-Pak N(CH3)2 4151 n column (15 × 1.0 i.d. cm), which was eluted with the same solvent at a flow rate of 2 mL min−1 at 50°C. The elutes were collected every 2 min and the fractions of Rt 19 to 24 min (for GA4 and GA25), Rt 25 to 30 min (for GA9), and Rt 31 to 36 min (for GA24). All of those fractions were methylated with ethereal diazomethane, and were subsequently trimethylsilylated with N-methyl-N-(trimethylsilyl)- trifluoroacetamide at 80°C for 30 min. Gas chromatographyselected ion monitoring analysis was performed as described in Tanaka-Ueguchi et al. (1998) except that we used a capillary column (30 m × 0.25 mm i.d., HP-5, Hewlett-Packard, Palo Alto, CA), and ions were selected as follows: for early-13-hydroxylated GAs, m/z 506, 448, and 375 for GA1 MeTMSi; m/z 508, 450, and 377 for [2H2]GA1 MeTMSi; m/z 492, 432, 402, 373 for GA17 MeTMSi; m/z 434, 402, 374 for GA19 MeTMSi; m/z 436, 404, 376 for [2H2]GA19 MeTMSi; m/z 418, 403, and 375 for GA20 MeTMSi; m/z 420, 405, and 377 for [2H2]GA20 MeTMSi; for non-13-hydroxylated GAs, m/z 418, 386, 328, and 284 for GA4 MeTMSi; m/z 420, 338, 330, and 286 for [2H2]GA4 MeTMSi; m/z 330, 298, 270, and 243 for GA9; m/z 332, 300, 272, and 245 for [2H2]GA9 MeTMSi; m/z 374, 342, 314, and 286 for GA24; m/z 376, 344, 316, and 288 for [2H2]GA24 MeTMSi; m/z 404, 372, 312, and 286 for GA25; and m/z 406, 374, 314, and 288 for [2H2]GA25 MeTMSi. The levels of endogenous GA1, GA4, GA9, GA17, GA19, GA20, GA24, and GA25 were determined from the ratios of peak areas at m/z 506/508, 284/286, 298/300, 432/436, 434/436, 418/420, 314/316, and 312/314, respectively. Only GA17, whose existence was confirmed in the transgenic plants by full-mass scanning, was quantified relative to [2H2]GA19 because [2H2]GA17 has not been synthesized. The behavior of GA17 was very similar to that of GA19 on HPLC; however, GA17 and GA19 were fully separated on gas chromatography-mass spectrometry.
ACKNOWLEDGMENTS
We thank Dr. Y. Ohashi (National Institute of Agrobiological Resources, Tsukuba, Japan) for giving us the plasmid of pBE2113 to enhance the transgene expression and Prof. I. Yamaguchi (Tokyo University) for a gift of GA17.
LITERATURE CITED
- Carrera E, Jackson SD, Prat S. Feedback control and diurnal regulation of gibberellin 20-oxidase transcript levels in potato. Plant Physiol. 1999;119:765–773. doi: 10.1104/pp.119.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles JP, Phillips AL, Croker SJ, Garcia-Lepe R, Lewis MJ, Hedden P. Modification of gibberellin production and plant development in Arabidopsis by sense and antisense expression of gibberellin 20-oxidase genes. Plant J. 1999;17:547–556. doi: 10.1046/j.1365-313x.1999.00410.x. [DOI] [PubMed] [Google Scholar]
- Crozier A. The Biochemistry and Physiology of Gibberellins. Vol. 2. New York: Praeger; 1983. [Google Scholar]
- Curtis IS, Ward DA, Thomas SG, Phillips AL, Davey MR, Power JB, Lowe KC, Croker SJ, Lewis MJ, Magness SL. Induction of dwarfism in transgenic Solanum dulcamara by overexpression of a gibberellin 20-oxidase cDNA from pumpkin. Plant J. 2000;23:329–338. doi: 10.1046/j.1365-313x.2000.00784.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Martinez JL, Lopez-Diaz I, Sanchez-Beltran MJ, Phillips AL, Ward DA, Gaskin P, Hedden P. Isolation and transcript analysis of gibberellin 20-oxidase genes in pea and bean in relation to fruit development. Plant Mol Biol. 1997;33:1073–1084. doi: 10.1023/a:1005715722193. [DOI] [PubMed] [Google Scholar]
- Gonzalez RG, Haxo RS, Schleich T. Mechanism of action of polymeric aurintricarboxylic acid, a potent inhibitor of protein-nucleic acid interactions. Biochemistry. 1980;19:4299–4303. doi: 10.1021/bi00559a023. [DOI] [PubMed] [Google Scholar]
- Hedden P, Kamiya Y. Gibberellin biosynthesis: enzymes, genes and their regulation. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:431–460. doi: 10.1146/annurev.arplant.48.1.431. [DOI] [PubMed] [Google Scholar]
- Hedden P, Proebsting WM. Genetic analysis of gibberellin biosynthesis. Plant Physiol. 1999;119:365–370. doi: 10.1104/pp.119.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Raman AS, Ream JE, Fujiwara H, Cerny RE, Brown SM. Overexpression of 20-oxidase confers a gibberellin-overproduction phenotype in Arabidopsis. Plant Physiol. 1998;118:773–781. doi: 10.1104/pp.118.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange T. Molecular biology of gibberellin synthesis. Planta. 1998;204:409–419. doi: 10.1007/s004250050274. [DOI] [PubMed] [Google Scholar]
- Lange T, Hedden P, Graebe JE. Expression and cloning of a gibberellin 20-oxidase, a multifunctional enzyme involved in gibberellin biosynthesis. Proc Natl Acad Sci USA. 1994;91:8552–8556. doi: 10.1073/pnas.91.18.8552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DN, Proebsting WM, Hedden P. Mendel's dwarfing gene: cDNAs from the Le alleles and function of the expressed proteins. Proc Natl Acad Sci USA. 1997;94:8907–8911. doi: 10.1073/pnas.94.16.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DN, Proebsting WM, Parks TD, Dougherty WG, Lange T, Lewis MJ, Gaskin P, Hedden P. Feedback regulation of gibberellin biosynthesis and gene expression in Pisum sativum L. Planta. 1996;200:159–166. doi: 10.1007/BF00208304. [DOI] [PubMed] [Google Scholar]
- Mitsuhara I, Ugaki M, Hirochika H, Ohshima M, Murakami T, Gotoh Y, Katayose Y, Nakamura S, Honkura R, Nishimiya S. Efficient promoter cassettes for enhanced expression of foreign genes in dicotyledonous and monocotyledonous plants. Plant Cell Physiol. 1996;37:49–59. doi: 10.1093/oxfordjournals.pcp.a028913. [DOI] [PubMed] [Google Scholar]
- Phillips AL, Ward DA, Uknes S, Appleford NEJ, Lange T, Huttly AK, Gaskin P, Graebe JE, Hedden P. Isolation and expression of three gibberellin 20-oxidase cDNA clones from Arabidopsis. Plant Physiol. 1995;108:1049–1057. doi: 10.1104/pp.108.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebers M, Kaneta T, Kawaide H, Yamaguchi S, Yang YY, Imai R, Sekimoto H, Kamiya Y. Regulation of gibberellin biosynthesis genes during flower and early fruit development of tomato. Plant J. 1999;17:241–250. doi: 10.1046/j.1365-313x.1999.00366.x. [DOI] [PubMed] [Google Scholar]
- Tanaka-Ueguchi M, Itoh H, Oyama N, Koshioka M, Matsuoka M. Overexpression of a tobacco homeobox gene, NTH15, decreases the expression of a gibberellin biosynthetic gene encoding GA 20-oxidase. Plant J. 1998;15:391–400. doi: 10.1046/j.1365-313x.1998.00217.x. [DOI] [PubMed] [Google Scholar]
- Toyomasu T, Kawaide H, Sekimoto C, von Numers C, Phillips AL, Hedden P, Kamiya Y. Cloning and characterization of a cDNA encoding gibberellin 20-oxidase from rice (Oryza sativa L.) seedlings. Plant Physiol. 1997;99:111–118. [Google Scholar]
- Toyomasu T, Tsuji H, Yamane H, Nakayama M, Yamaguchi I, Murofushi N, Nakahashi N, Inoue Y. Light effects on endogenous levels of gibberellins in photoblastic lettuce seeds. J Plant Growth Regul. 1993;12:85–90. [Google Scholar]
- Waycott W, Smith VA, Gaskin P, MacMillan J, Taiz L. The endogenous gibberellin of dwarf mutants of lettuce. Plant Physiol. 1991;95:1169–1173. doi: 10.1104/pp.95.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K, Li L, Gage DA, Zeevaart JAD. Molecular cloning and photoperiod-regulated expression of gibberellin 20-oxidase from the long-day plant spinach. Plant Physiol. 1996;110:547–554. doi: 10.1104/pp.110.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y-L, Gage DA, Zeevaart JAD. Gibberellins and stem growth in Arabidopsis thaliana: effects of photoperiod on expression of the GA4 and GA5 loci. Plant Physiol. 1997;114:1471–1476. doi: 10.1104/pp.114.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y-L, Li L, Gage DA, Zeevaart JAD. Feedback regulation of GA5 expression and metabolic engineering of gibberellin levels in Arabidopsis. Plant Cell. 1999;11:927–935. doi: 10.1105/tpc.11.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y-L, Li L, Wu K, Peeters AJM, Gage DA, Zeevaart JAD. The GA5 locus of Arabidopsis thaliana encodes a multifunctional gibberellin 20-oxidase: molecular cloning and functional expression. Proc Natl Acad Sci USA. 1995;92:6640–6644. doi: 10.1073/pnas.92.14.6640. [DOI] [PMC free article] [PubMed] [Google Scholar]