Abstract
By using the X-ray-diffraction results reported previously for sodium heparan sulphate, a twofold helical conformation with an axially projected disaccharide repeat (h) equal to 0.93 nm has been examined in detail. On the basis of a repeating sequence of 1,4-alpha-D-glucosamine and 1,4-beta-D-glucuronic acid, trial and stereochemically feasible molecular models were computer-generated. An optimum twofold helical conformation is proposed, incorporating stabilizing intra-chain hydrogen bonds across both glycosidic linkages.
Full text
PDF
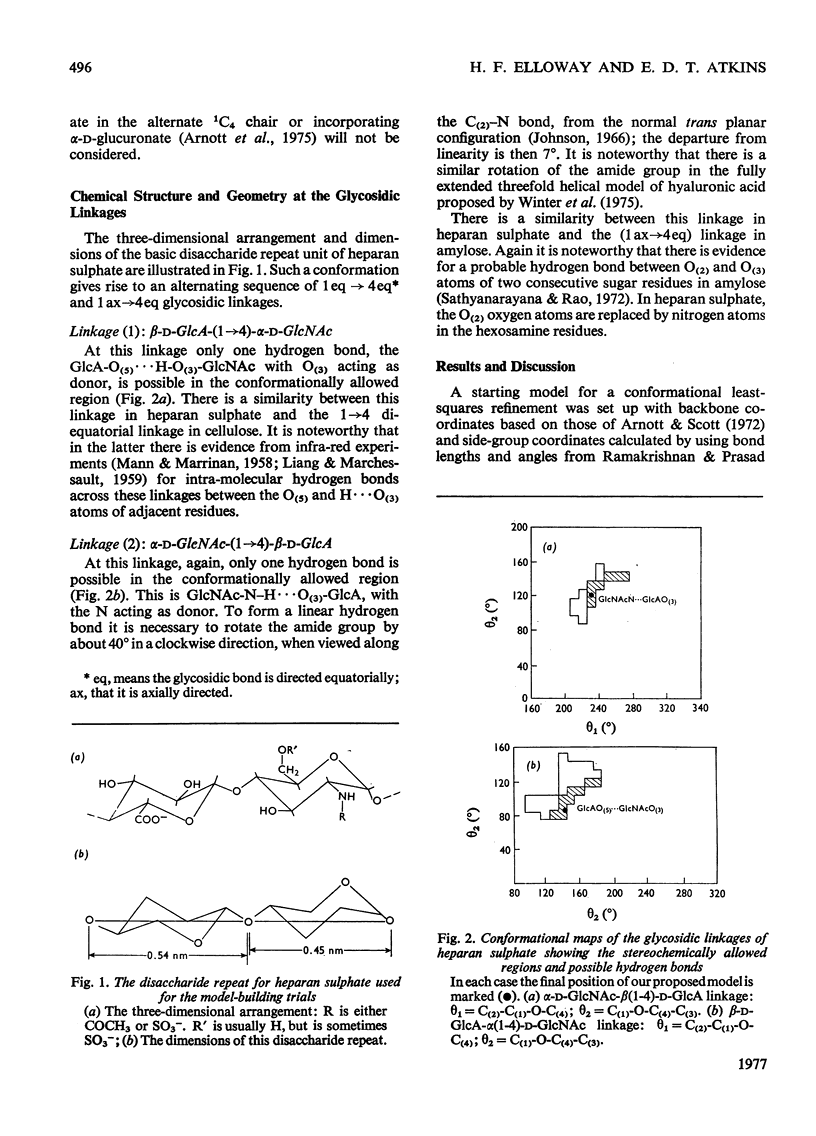
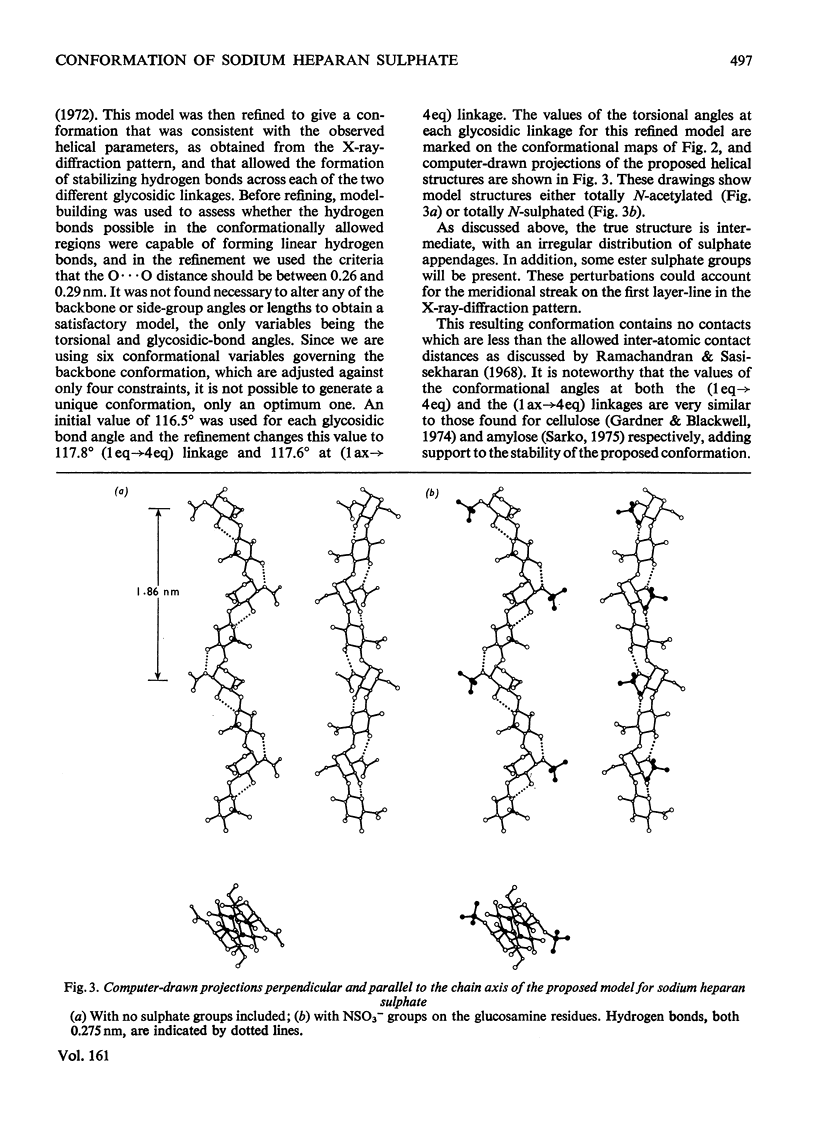

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins E. D., Laurent T. C. X-ray-diffraction patterns from chondroitin 4-sulphate, dermatan sulphate and heparan sulphate. Biochem J. 1973 Jul;133(3):605–606. doi: 10.1042/bj1330605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich C. P., De Oca H. M. Production of heparin related mucopolysaccharides by mammalian cells in culture. Proc Soc Exp Biol Med. 1970 Sep;134(4):955–962. doi: 10.3181/00379727-134-34920. [DOI] [PubMed] [Google Scholar]
- Helting T., Lindahl U. Occurrence and biosynthesis of beta-glucuronidic linkages in heparin. J Biol Chem. 1971 Sep 10;246(17):5442–5447. [PubMed] [Google Scholar]
- Hovingh P., Linker A. The disaccharide repeating-units of heparan sulfate. Carbohydr Res. 1974 Oct;37(1):181–192. doi: 10.1016/s0008-6215(00)87073-1. [DOI] [PubMed] [Google Scholar]
- Hök M., Lindahl U., Iverius P. H. Distribution of sulphate and iduronic acid residues in heparin and heparan sulphate. Biochem J. 1974 Jan;137(1):33–43. doi: 10.1042/bj1370033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson L., Lindahl U. Evidence for the existence of a multichain proteoglycan of heparan sulphate. Biochem J. 1970 May;117(4):699–702. doi: 10.1042/bj1170699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. N. The crystal structure of N-acetyl-alpha-D-glucosamine. Acta Crystallogr. 1966 Dec 10;21(6):885–891. doi: 10.1107/s0365110x66004146. [DOI] [PubMed] [Google Scholar]
- Kraemer P. M. Heparan sulfates of cultured cells. I. Membrane-associated and cell-sap species in Chinese hamster cells. Biochemistry. 1971 Apr 13;10(8):1437–1445. doi: 10.1021/bi00784a026. [DOI] [PubMed] [Google Scholar]
- Kraemer P. M. Heparan sulfates of cultured cells. II. Acid-soluble and -precipitable species of different cell lines. Biochemistry. 1971 Apr 13;10(8):1445–1451. doi: 10.1021/bi00784a027. [DOI] [PubMed] [Google Scholar]
- Kraemer P. M., Smith D. A. High molecular-weight heparan sulfate from the cell surface. Biochem Biophys Res Commun. 1974 Jan 23;56(2):423–430. doi: 10.1016/0006-291x(74)90859-6. [DOI] [PubMed] [Google Scholar]
- Obrind B., Pertoft H., Iverius P. H., Laurent The effect of calcium on the macromolecular properties of heparan sulfate. Connect Tissue Res. 1975;3(2):187–193. doi: 10.3109/03008207509152178. [DOI] [PubMed] [Google Scholar]
- Ramachandran G. N., Sasisekharan V. Conformation of polypeptides and proteins. Adv Protein Chem. 1968;23:283–438. doi: 10.1016/s0065-3233(08)60402-7. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan C., Prasad N. Rigid-body refinement and conformation of -chitin. Biochim Biophys Acta. 1972 Jan 28;261(1):123–135. doi: 10.1016/0304-4165(72)90321-2. [DOI] [PubMed] [Google Scholar]
- Winter W. T., Smith P. J., Arnott S. Hyaluronic acid: structure of a fully extended 3-fold helical sodium salt and comparison with the less extended 4-fold helical forms. J Mol Biol. 1975 Dec 5;99(2):219–235. doi: 10.1016/s0022-2836(75)80142-2. [DOI] [PubMed] [Google Scholar]


