Abstract
Cysteine (Cys) synthase [O-acetyl-l-Ser(thiol)-lyase, EC 4.2.99.8; CSase] is responsible for the final step in biosynthesis of Cys. Transgenic tobacco (Nicotiana tabacum; F1) plants with enhanced CSase activities in the cytosol and in the chloroplasts were generated by cross-fertilization of two transformants expressing cytosolic CSase or chloroplastic CSase. The F1 transgenic plants were highly tolerant to toxic sulfur dioxide and sulfite. Upon fumigation with 0.1 μL L−1 sulfur dioxide, the Cys and glutathione contents in leaves of F1 plants were increased significantly, but not in leaves of non-transformed control plants. Furthermore, the leaves of F1 plants exhibited the increased resistance to paraquat, a herbicide generating active oxygen species.
Environmental pollution by sulfur-containing compounds, e.g. sulfur dioxide (SO2), hydrogen sulfide (H2S), sulfite (SO32−), and sulfate ions (SO42−), is a serious problem for the global environment. In particular, gaseous SO2, which is emitted mainly by natural sources, i.e. microbial activities, volcanoes, etc. and by human activities, i.e. combustion of fossil fuels, industrial refining of sulfur-containing ores, etc., influences human health and the global ecological system of animals and plants (Wellburn, 1994; Murray, 1997). One of the most common types of visible injury to plants caused by SO2 is foliar damage such as chlorosis and necrosis. Moreover, when the gaseous SO2 encounters moisture, considerable amounts of SO2 are converted into sulfite and sulfate. These are important components of acid rain and haze (Wellburn, 1994).
Although sulfur-containing compounds, e.g. SO2 and SO32−, are toxic for plants at higher concentrations, sulfur is also an essential nutrition for plants. The inorganic sulfur in the environment (e.g. SO42− in the soil and SO2 in the air) is assimilated into Cys mainly by the Cys biosynthetic pathway in plants (Saito, 1999, 2000). Cys is incorporated into proteins and glutathione (GSH) or serves as the sulfur donor of Met and sulfur-containing secondary products in plants. Thus, the engineering of this Cys biosynthetic pathway may be promising for development of the transgenic plant tolerant to sulfur-containing pollutants. The Cys biosynthetic pathway involves several enzymatic reactions (Brunold and Rennenberg, 1997; Leustek and Saito, 1999). The SO42− is reduced to SO32− and then sulfide (S2−) through the sulfate reduction pathway. The final step of Cys biosynthesis is the incorporation of S2− into Cys. The reaction is catalyzed by Cys synthase [O-acetyl-l-Ser(thiol)-lyase, EC 4.2.99.8; CSase], which uses S2− and O-acetyl-l-Ser as the substrates. This final step of Cys biosynthesis seems to exist necessarily in three major compartments of plant cells, e.g. cytosol, chloroplasts, and mitochondria, since the presence of CSase has been demonstrated in these three compartments from several plants (Brunold and Suter, 1989; Lunn et al., 1990). In spinach leaves the major activity of CSase is localized in cytosol (44%) and chloroplasts (42%), and only 10% of the activity is present in mitochondria (Lunn et al., 1990).
We constructed transgenic tobacco (Nicotiana tabacum) carrying spinach cytosolic CSase A cDNA (Saito et al., 1992), designated 3F plants, or chimeric CSase A cDNA fused with the sequence for chloroplast-targeting transit peptide of pea RUBISCO, designated 4F plants (Saito et al., 1994). 3F and 4F showed enhanced CSase activity in the cytosol and in the chloroplasts, respectively. The leaf discs of these transgenic tobaccos showed partial tolerance to SO32− (Saito et al., 1994).
In the present study, to obtain transgenic plants highly tolerant to sulfur-containing pollutants, we crossed 3F plants with 4F plants to generate F1 transgenic tobacco in which CSase activities were enhanced in the cytosol and in the chloroplasts. We found that the tolerance of F1 plants to sulfur-containing pollutants was enhanced. These plants were also resistant to paraquat. We also discuss the mechanism of tolerance to SO2 in transgenic plants.
RESULTS
Expression of the CSase in Transgenic Tobacco
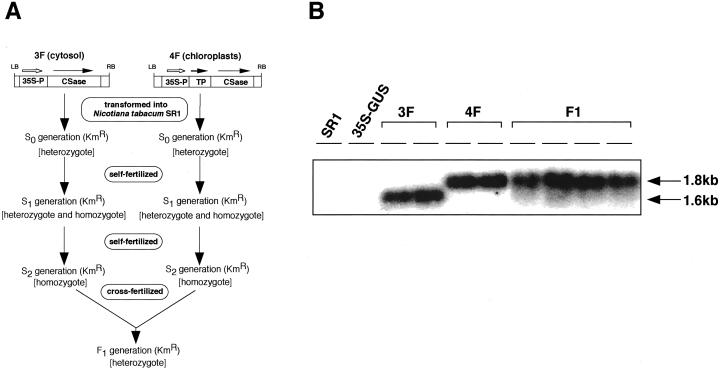
We previously constructed transgenic tobacco carrying spinach cytosolic CSase A cDNA (3F plants) or chimeric CSase A cDNA fused with the sequence for chloroplast-targeting transit peptide (4F plants) driven by the cauliflower mosaic virus 35S promoter (Saito et al., 1994). From these two classes of transgenic tobacco plants we selected two transgenic lines, 3F-24 and 4F-15 (Saito et al., 1994) as parents for mating because of their high levels of CSase activities. The homozygous plants of 3F-24 (abbreviated as 3F) and 4F-15 (abbreviated as 4F) were obtained by self-pollination. Then we crossed 3F with 4F to generate the plants of first filial generation, designated F1 (Fig. 1A). Southern-blot analysis of total DNA isolated from leaves of 3F, 4F, and F1 showed that 3F and 4F contained one copy and two copies of the introduced gene per haploid, respectively, and F1 contained three copies of the introduced gene derived from 3F and 4F (data not shown).
Figure 1.
A, Construction of transgenic plants overexpressing CSase. 35S-P, Cauliflower mosaic virus 35S RNA promoter; CSase, CSase transgene from spinach; TP, the chloroplast-targeting transit peptide of pea RUBISCO. B, Northern-blot analysis of total RNA of transgenic plants. SR1, Non-transformed tobacco (cv SR1); 35S-β-glucuronidase (GUS), tobacco plants transformed with a bacterial UidA gene encoding GUS using as a negative control.
To examine the expression of the CSase gene, total RNA isolated from leaves of transgenic tobacco was analyzed by northern hybridization analysis (Fig. 1B). Transcripts of 1.6 kb in length were detected in 3F. The 1.8-kb transcripts from the chimeric transit peptide/CSase gene were accumulated in 4F. F1 expressed 1.6- and 1.8-kb transcripts descended from 3F and 4F, although the expression level of 1.6-kb transcripts was considerably lower than that of 1.8-kb transcripts.
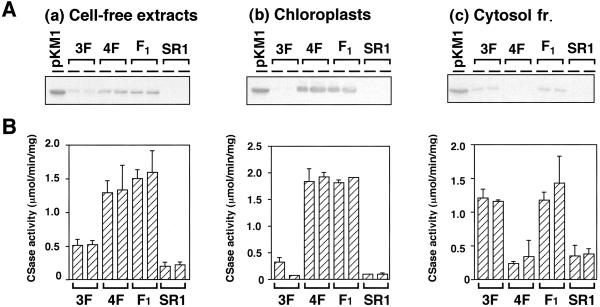
Cell-free extracts, chloroplast fractions, and cytosol fractions isolated from transgenic plants (3F, 4F, and F1) were analyzed by western blotting and assaying CSase activity. The accumulation of 34-kD CSase protein detected by western blotting was shown in the cell-free extracts of 3F, 4F, and F1 (Fig. 2A). The molecular mass of the immunoreactive CSase protein of 4F was the same as that of 3F, suggesting that the pre-CSase with the pea transit peptide was correctly processed in the transgenic tobacco cells. In the extracts of 3F and 4F, the 34-kD band specific to spinach CSase A was detected in cytosolic or in chloroplast fraction, respectively, whereas in the extract of F1, the 34-kD band was detected in cytosol and in chloroplasts. CSase activity in the cell-free extract of F1 was about 5-fold higher than that of non-transformant (SR1; Fig. 2B). In the chloroplast fraction, CSase activity of F1 was at the same level as that of 4F, and was about 6-fold higher than those of 3F and non-transformant. In the cytosol fraction, CSase activity of F1 was at the same level as that of 3F, and was about 4-fold higher than those of 4F and non-transformant. These results indicated that F1, in which CSase activities were enhanced in the chloroplasts and in the cytosol, had the highest activity of CSase in these transgenic tobacco plants.
Figure 2.
Expression analysis of spinach CSase in subcellular fractions of transgenic plants. A, For western blotting, 16 μg of protein were separated by 12% (w/v) SDS-PAGE, transferred onto an Immobilon P membrane, and then localized by immunostaining using rabbit anti-spinach CSase A serum. B, For the enzyme assay, the activities of CSase were determined in each extract from fully expanded leaves of transgenic plants. SR1, Non-transformed tobacco (cv SR1); pKM1/NK3, bacterial extract expressing Spinach CSase A as a positive control (CSase activity was not determined). Data are the means of triplicate analyses ± sd.
Tolerance to Sulfur-Containing Pollutants
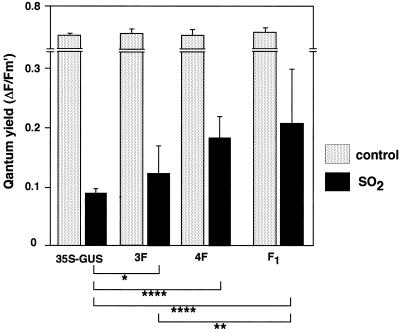
We examined the resistance of these transgenic plants to sulfur-containing pollutants. The transgenic plants were used for fumigation experiments with high levels of SO2. We measured a quantum yield of photosynthetic activity of leaves of transgenic plants before SO2 fumigation and after a 3-h fumigation with 1 μL L−1 SO2 (Fig. 3). Before fumigation, the quantum yields of the leaves were at the same level for all transgenic plants. After a 3-h fumigation of SO2, the quantum yields of F1 leaves were significantly higher than those of control (P < 0.005) and 3F (P < 0.05) plants. The quantum yield of F1 exhibited the higher trend than that of 4F plant, although the difference was not statistically significant.
Figure 3.
Photosynthetic quantum yield of transgenic plants exposed to SO2. Six transgenic plants from each transgenic line were exposed to 1 μL L−1 SO2 for 3 h. The third leaves from the top of transgenic plants were assayed for quantum yield. The differences in the quantum yield among transgenic plants were statistically significant by Student's t test (*, P < 0.1; **, P < 0.05; ****, P < 0.005). Data are the means of analyses of six plants ± sd. 35S-GUS, Tobacco plants transformed with a bacterial UidA gene encoding GUS using as a negative control.
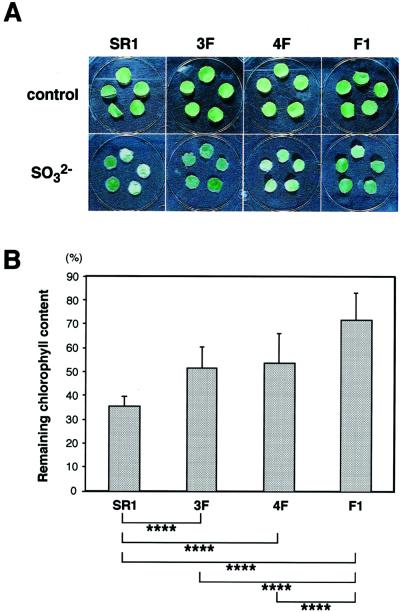
Since SO2 absorbed by plants changes into SO32− rapidly, the toxicity of SO2 can be reproduced by SO32−. Therefore, leaf discs of transgenic tobacco were cultured with 20 mm SO32− under constant illumination. After cultivation for 54 h, the leaf discs of F1 showed a tolerance to 20 mm SO32−, whereas the leaf discs of non-transformed tobacco (cv SR1) were seriously damaged, and those of 3F and 4F were partially damaged (Fig. 4A). Then we determined the remaining chlorophyll of each leaf disc after this treatment (Fig. 4B). The F1 plant showed significantly higher resistance to SO32− (higher percentage of residual chlorophyll) as compared with the non-transformed control and the parents (3F and 4F). Taken together, these results may suggest that F1 plants obtain the high tolerance to SO32− and SO2 by overexpressing CSase in cytosol and in chloroplasts.
Figure 4.
Resistance to SO32− toxicity of leaf discs of transgenic plants. A, Photographs of the leaf discs cultivated on A1 medium with 20 mm sodium sulfite (bottom layer) and on A1 medium (top layer) for 54 h at 25°C under light (25 μE m−2 s−1). SR1, Non-transformed tobacco (cv SR1). B, Resistance is shown as a percentage of remaining chlorophyll content in a leaf disc after the cultivation in the presence of 20 mm sodium sulfite. The differences in the remaining chlorophyll content among control and transgenic plants were statistically significant by Student's t test (****, P < 0.005). Data are the means of analyses of 10 leaf discs ± sd.
Analysis of Cys and GSH Levels in Transgenic Plants
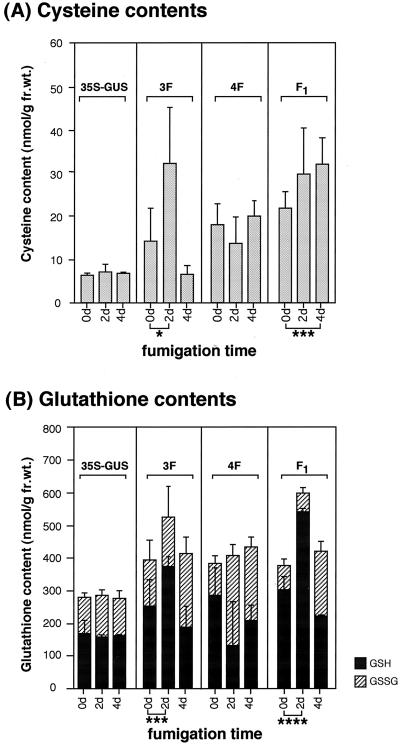
We exposed the transgenic plants to lower level of SO2, namely 0.1 μL L−1 for 4 d, and then measured the contents of Cys and GSH in leaves. After the fumigation with 0.1 μL L−1 SO2 for 4 d, there was no visible damage on the leaves of control or transgenic plants (data not shown). However, Cys contents in F1 plants after a 4-d fumigation was increased significantly (Fig. 5A). GSH contents in F1 and 3F plants were also increased significantly after a 2-d fumigation (Fig. 5B). Cys and GSH contents were not changed in control plants (Fig. 5, A and B). An unexpected finding was that Cys and GSH contents in 4F plants were not increased during the fumigation. These results suggest that transgenic plants overexpressing CSase, especially overexpressing in cytosol, can fix the atmospheric SO2 into Cys and GSH more efficiently than the control plant, since the contents of Cys and GSH in the control plant were unchanged during the fumigation with 0.1 μL L−1 SO2.
Figure 5.
Contents of Cys and GSH in transgenic plants exposed to 0.1 μL L−1 SO2. Six transgenic plants from each transgenic line were exposed to 0.1 μL L−1 SO2 for 4 d. The third leaves from the top of the transgenic plants were prepared and immediately subjected to quantification of the sulfhydryl compounds. The differences of the thiol content between 0- and 2- or 4-d treatment were analyzed statistically using Student's t test. (*, P < 0.1; ***, P < 0.025; ****, P < 0.005). Data are the means of triplicate analyses ± sd. 35S-GUS, Tobacco plants transformed with a bacterial UidA gene encoding GUS using as a negative control.
Effect of Photooxidative Stress on Transgenic Plants
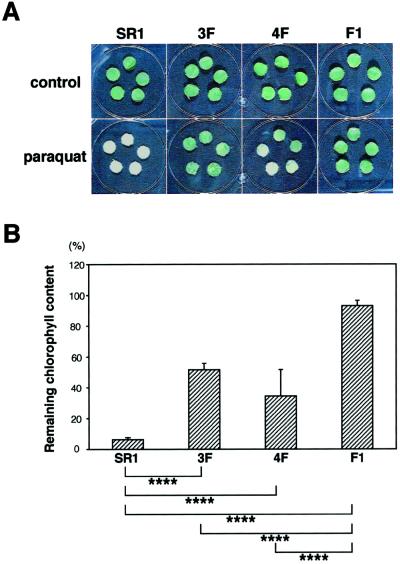
The toxicity of SO2 is thought to result from generation of active oxygen species (Shimazaki et al., 1980). To investigate the enhanced tolerance of transgenic plants to active oxygen species we treated the leaf discs of transgenic plants with paraquat. Paraquat is a reagent that generates active oxygen species in chloroplasts under constant illumination (Dodge, 1975). After a 40-h cultivation with 2 μm paraquat, the leaf discs of F1 again showed the highest resistance to paraquat (Fig. 6A). We determined the remaining chlorophyll of each leaf disc after the paraquat treatment (Fig. 6B). The F1 plant showed significantly higher resistance to paraquat (higher percentage of residual chlorophyll) as compared with the control and the parents (3F and 4F). The activities of enzymes involved in scavenging active oxygen species in plants (superoxide dismutase, catalase, and ascorbate peroxidase) were at the same level among control and CSase overexpressing plants; moreover, the activities of these enzymes never changed after a 12-h cultivation with 1 μm paraquat (data not shown).
Figure 6.
Effects of paraquat on leaf discs of transgenic tobacco. A, Photographs of the leaf discs cultivated on solutions with 2 μm paraquat (bottom layer) and without paraquat (top layer) for 40 h at 25°C under light (25 μE m−2 s−1). SR1, Non-transformed tobacco (cv SR1). B, Resistance is shown as a percentage of remaining chlorophyll content in a leaf disc after the treatment of 2 μm paraquat. The differences in the remaining chlorophyll content among control and transgenic plants were statistically significant by Student's t test (****, P < 0.005). Data are the means of analyses of five leaf discs ± sd.
DISCUSSION
We generated transgenic tobacco plants (F1) in which CSase activities were enhanced in the chloroplasts and in the cytosol. The F1 transgenic tobacco showed higher tolerance to sulfur-containing pollutants, i.e. SO32−, than the parent plants (3F and 4F) and the control tobacco.
To reveal the mechanism of resistance to SO2 in transgenic plants we exposed the plants to 0.1 μL L−1 SO2 and then measured the contents of Cys and GSH in the leaves. During the SO2 fumigation, Cys and GSH contents were increased significantly in F1 plants, but not in control plants (Fig. 5), suggesting that the F1 transgenic plants may fix the atmospheric SO2 into Cys and GSH more efficiently than the control plants. Thus, the tolerance to SO2 may be caused by the efficient assimilation of sulfur into Cys and GSH. After a fumigation time of 4 d, there is no difference in the GSH content between 3F, 4F, and F1. A 4-d fumigation of 0.1 μL L−1 SO2 may cause some physiological damage to these transgenic plants even though no visible damage was not observed on the leaves of transgenic plants after a 4-d-fumigation. Since the level of SO2 normally found in the atmosphere is less than 0.04 μL L−1, 0.1 μL L−1 SO2 may be sufficient to cause the physiological damage to the plants despite no visible damage observed.
Since SO2 is highly soluble in water, SO2 absorbed by plants changes into SO32− rapidly. Some part of SO32− is used for the biosynthesis of Cys, and the rest is changed into SO42− by oxidation in a light-dependent manner. Produced SO42− are stored in vacuoles and are subsequently used for the biosynthesis of Cys (Rennenberg, 1984). During oxidation of SO32− to SO42− in plant cells, active oxygen species are produced as secondary toxic substances (Cohen et al., 1973). Therefore, the toxicity of SO2 may be due to the generation of the active oxygen species. In fact, transgenic tobacco in which the ability to scavenge the active oxygen species was enhanced by expressing a bacterial GSH reductase gene in chloroplasts showed tolerance to SO2 (Aono et al., 1993). GSH reductase is involved in the ascorbate-GSH cycle. This cycle is an enzymatic-scavenging mechanism for the removal of active oxygen species (De Kok and Stulen, 1993).
F1 transgenic tobacco also showed high tolerance to paraquat, which generates active oxygen species. The activities of enzymes involved in scavenging active oxygen species, however, were not enhanced in F1 plant. GSH levels respond to the availability of Cys (Strohm et al., 1995), therefore, F1 possessing high activity of CSase was expected to have high ability to synthesize GSH. The increased GSH is most likely to be involved in the detoxification of active oxygen; accordingly, F1 showed a high tolerance to active oxygen species. Taken together, these results suggest that the tolerance of F1 plants to sulfur-containing pollutants may be caused not only by the efficient assimilation of sulfur into Cys, but also by the detoxification of active oxygen species by GSH.
A part of SO2 absorbed by plants is emitted as H2S (Stulen and De Kok, 1993); however, strictly speaking, this system may not be the detoxification mechanism of SO2 because H2S itself is a toxic substance to plants and animals (Wellburn, 1994). There is a report that the enhanced activity of cytosolic CSase resulted in the increased resistance to H2S (Youssefian et al., 1993). Therefore, the Cys biosynthetic pathway may be considered as the sole detoxification mechanism of SO2, and the enhancement of the ability of Cys synthesis in plants may be the best way to produce the transgenic plant tolerant to SO2. Our observations suggest that the transgenic plants in which the ability of Cys synthesis has been enhanced in the cytosol and in the chloroplasts by overexpressing CSase may be applied to produce the transgenic plants resistant to oxidative stress caused by the photochemical oxidant such as ozone.
MATERIALS AND METHODS
Plant Growth and Cross Fertilization
The transgenic plants were grown on A1 agar medium (one-half-strength Murashige and Skoog salts [Murashige and Skoog, 1962], 1% [w/v] Suc, and 0.8% [w/v] agar, pH5.7) containing kanamycin (100 mg/L) under 16-h light (25 μE m−2 s−1) and 8-h dark cycles at 25°C. For fertilization and fumigation of SO2, transgenic plants were transferred to vermiculite after a 4-week cultivation on A1 agar medium, and they were then watered with 1,000-fold diluted Hyponex 5-10-5 (Hyponex-Japan, Osaka). Cross-fertilization was performed with the pollen of homozygous plants of 3F-24 and the pistils of homozygous plants of 4F-15.
Northern-Blot Analysis
Isolation of total RNA from the leaves of 8-week-old plants and northern-blot analysis were performed as described previously (Saito et al., 1991). Twenty micrograms of total RNA was separated under denaturing conditions on a 1.2% (w/v) agarose gel containing formaldehyde, transferred to a nylon membrane, and then hybridized with a 32P-labeled DNA fragment of spinach CSase A coding region. The final washing was performed in 0.1× SSPE (1× SSPE = 0.18 m NaCl, 0.01 m sodium phosphate, pH 7.7, and 1 mm Na2-EDTA) and 0.1% (w/v) SDS at 65°C for 15 min (Sambrook et al., 1989).
Protein Extraction, Fractionation of Crude Chloroplasts and Crude Cytosol, and Expression Analysis
Leaves from 10-week-old plants were lightly homogenized in an extraction buffer (330 mm sorbitol, 1 mm MgCl2, 2 mm EDTA, 2 mm sodium isoascorbic acid, and 50 mm MES [2-(N-morpholino)-ethanesulfonic acid], pH 6.1), and then the resulting extracts were filtered with nylon mesh (508 mesh). The filtrate was used as cell-free extracts. Crude cytosol fraction was obtained from the cell-free extracts by centrifugation at 1,000g for 100 s. The resulting supernatant was used as the crude cytosol fraction, and the resulting precipitate was resuspended in a resuspension buffer (330 mm sorbitol, 50 mm HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid], pH 7.9). After the centrifugation at 1,000g for 100 s, the precipitate was resuspended in 200 mm K-Pi buffer, which was used as the crude chloroplast fraction. Protein concentrations were determined with a protein assay kit (Bio-Rad, Hercules, CA) using bovine serum albumin as the standard. Proteins (16 μg) in these extracts were used for western blotting. Western-blot and immunostaining analyses were carried out on an Immobilon P membrane (Millipore, Bedford, MA) as reported (Saito et al., 1991). The rabbit anti-spinach CSase A serum (Saito et al., 1992) was used at a 1:400 dilution as a primary antibody. The immunoreactive protein was visualized using phosphatase-labeled goat anti-rabbit IgG (Kirkegaard and Perry Laboratories, Gaithersburg, MD) and 5-bromo-4-chloro-3-indolylphosphate p-toluidine/nitroblue tetrazolium chloride (Gibco-BRL, Cleveland) as substrates. The enzymatic activity of CSase was determined in the reaction mixture (0.5 mL) containing 50 mm potassium phosphate, pH 8.0, 4 mm Na2S, 12.5 mm O-acetyl-l-Ser, and the enzyme solution. The incubation was performed at 30°C for 10 min and was terminated by the addition of 7.5% (w/v) trichloroacetic acid. The amount of Cys in the resulting mixture was spectrophotometrically determined by the acid-ninhydrin method at 560 nm (Gaitonde, 1967).
Exposure of Plants to SO2
Six-week-old transgenic plants were exposed to 1.0 μL L−1 SO2 for 7 h or 0.1 μL L−1 SO2 for 4 d under light (400 μE m−2 s−1) at 25°C with a relative humidity of 70%. The quantum yield was determined by PAM 2000 (Walz, Effeltrich, Germany).
Exposure of Leaf Discs to SO32−
Leaf discs (7 mm in diameter) from 16-week-old transgenic plants were cultured in A1 medium with 20 mm sodium sulfite for 54 h under constant illumination (25 μE m−2 s−1).
Determination of Chlorophyll
The contents of chlorophyll in the leaf discs were determined as described previously (Saito et al., 1989). Leaf disc was homogenized and extracted with 80% (w/v) ethanol. After centrifugation at 3,000 rpm for 5 min, the chlorophyll in the supernatant was quantified fluorophotometrically at an excitation wavelength of 413 nm and an emission wavelength of 672 nm. The values of remaining chlorophyll contents after the stress treatment were determined as follows: the amount of chlorophyll per leaf disc (7 mm in diameter) treated with SO32− or paraquat was divided by the amount of chlorophyll per leaf disc treated without sulfite or paraquat and was expressed as a percentage.
Determination of Cys and GSH
Quantitative analyses of monobromobimane derivatives of reduced forms of Cys and GSH were performed by HPLC (Anderson, 1985; Fahey and Newton, 1987). The frozen leaf was ground in a mortar. Then, 2 volumes of extraction buffer (0.1 n HCl containing 4 μm N-acetyl-Cys as an internal standard) were added and grinding was continued. Twenty microliters of extract was reacted with 2 μL of 1 mm monobromobimane in acetonitrile and 10 μL of 1 m N-ethylmorpholine for 20 min at 37°C in the dark. The labeling reaction was terminated by the addition of 8 μL of acetic acid, and then the resulting solution was subjected to HPLC analysis. HPLC was carried out as previously described (Saito et al., 1994). The oxidized form of GSH were determined using the following procedure. After the assay of the reduced form of GSH, the rest of extract from the leaves was reduced by dithiothreitol (1 mm final concentration), and was then labeled with monobromobimane and analyzed by HPLC. The difference between the quantity of GSH from this assay and the quantity of GSH that had been determined previously was considered as the quantity of oxidized form of GSH.
Treatment of Leaf Discs with Paraquat
Discs with a diameter of 7 mm excised from leaves of 16-week-old transgenic plants were soaked in the solution that contained 2 μm paraquat (methyl viologen, 1, 1′-dimethyl-4, 4′-bipyridinium dichloride; Sigma, St. Louis) and 0.1% (w/v) Tween 20. They were placed under light (25 μE m−2 s−1) at 25°C for 24 h, and they were then examined visually for damage.
ACKNOWLEDGMENTS
We thank Dr. Melinda Martin and Mr. David A. Heinlein for kindly correcting the English in the manuscript.
Footnotes
This work was supported by grants-in-aid for scientific research from the Ministry of Education, Science, Sports and Culture, Japan, by Core Research for Evolutional Science and Technology of Japan Science and Technology, by the Showa Shell Sekiyu Foundation, and by the Asahi Glass Foundation.
LITERATURE CITED
- Anderson ME. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- Aono M, Kubo A, Saji H, Tanaka K, Kondo N. Enhanced tolerance to photooxidative stress of transgenic Nicotiana tabacum with high chloroplastic glutathione reductase activity. Plant Cell Physiol. 1993;34:129–135. [Google Scholar]
- Brunold C, Rennenberg H. Regulation of sulfur metabolism in plants: first molecular approaches. Prog Bot. 1997;58:164–186. [Google Scholar]
- Brunold C, Suter M. Localization of enzymes of assimilatory sulfate reduction in pea root. Planta. 1989;179:228–234. doi: 10.1007/BF00393693. [DOI] [PubMed] [Google Scholar]
- Cohen HJ, Drew RT, Johnson JL, Rajagopalan KV. Molecular basis of the biological function of molybdenum: the relationship between sulfite oxidase and the acute toxicity of bisulfite and SO2. Proc Natl Acad Sci USA. 1973;70:3655–3659. doi: 10.1073/pnas.70.12.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kok LJ, Stulen I. Role of glutathione in plants under oxidative stress. In: De Kok LJ, Stulen I, Rennenberg H, Brunold C, Rauser WE, editors. Sulfur Nutrition and Assimilation in Higher Plants: Regulatory Agricultural and Environmental Aspects. The Hague, The Netherlands: SPB Academic Publishing; 1993. pp. 125–138. [Google Scholar]
- Dodge AD. Some mechanisms of herbicide action. Sci Prog Oxf. 1975;62:447–466. [Google Scholar]
- Fahey RC, Newton GL. Determination of low-molecular weight thiols using monobromobimane fluorescent labeling and high-performance liquid chromatography. Methods Enzymol. 1987;143:85–96. doi: 10.1016/0076-6879(87)43016-4. [DOI] [PubMed] [Google Scholar]
- Gaitonde MK. A spectrophotometric method for the direct measurement of cysteine in the presence of other naturally occurring amino acids. Biochem J. 1967;104:627–633. doi: 10.1042/bj1040627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leustek T, Saito K. Sulfate transport and assimilation in plants. Plant Physiol. 1999;120:637–643. doi: 10.1104/pp.120.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn JE, Droux M, Martin J, Douce R. Localization of ATP sulfurylase and O-acetylserine (thiol) lyase in spinach leaves. Plant Physiol. 1990;94:1345–1352. doi: 10.1104/pp.94.3.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–479. [Google Scholar]
- Murray F. Urban air pollution and health effects. In: Brune D, Chapman DV, Gwynne MD, Pacyna JM, editors. The Global Environment: Science, Technology and Management. Weinheim, Germany: Scandinavian Science Publisher; 1997. pp. 585–598. [Google Scholar]
- Rennenberg H. The fate of excess sulphur in higher plants. Annu Rev Plant Physiol. 1984;35:121–153. [Google Scholar]
- Saito K. Biosynthesis of cysteine. In: Singh BK, editor. Plant Amino Acids: Biochemistry and Biotechnology. New York: Marcel Dekker; 1999. pp. 267–291. [Google Scholar]
- Saito K. Regulation of sulfate transport and synthesis of sulfur-containing amino acids. Curr Opin Plant Biol. 2000;3:188–195. [PubMed] [Google Scholar]
- Saito K, Kurosawa M, Tatsuguchi K, Takagi Y, Murakoshi I. Modulation of cysteine biosynthesis in chloroplasts of transgenic tobacco overexpressing cysteine synthase [O-acetylserine (thiol) lyase] Plant Physiol. 1994;106:887–895. doi: 10.1104/pp.106.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Miura N, Yamazaki M, Hirano H, Murakoshi I. Molecular cloning and bacterial expression of cDNA encoding a plant cysteine synthase. Proc Natl Acad Sci USA. 1992;89:8078–8082. doi: 10.1073/pnas.89.17.8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Noji M, Ohmori S, Imai Y, Murakoshi I. Integration and expression of a rabbit liver cytochrome P-450 gene in transgenic Nicotiana tabacum. Proc Natl Acad Sci USA. 1991;88:7041–7045. doi: 10.1073/pnas.88.16.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Yamazaki M, Yamakawa K, Fujisawa S, Takamatsu S, Kawaguchi A, Murakoshi I. Lupin alkaloids in tissue culture of Sophora flavescens var. angustifolia: greening-induced production of matrine. Chem Pharm Bull. 1989;37:3001–3004. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Shimazaki K, Sakai T, Kondo N, Sugahara K. Active oxygen participation in chlorophyll destruction and lipid peroxidation in SO2-fumigated leaves of spinach. Plant Cell Physiol. 1980;21:1193–1204. [Google Scholar]
- Strohm M, Jouanin L, Kunert KJ, Pruvost C, Polle A, Foyer CH, Rennenberg H. Regulation of glutathione synthesis in leaves of transgenic poplar (Populus tremula × P. alba) overexpressing glutathione synthetase. Plant J. 1995;7:141–145. [Google Scholar]
- Stulen I, De Kok LJ. Whole plant regulation of sulfur metabolism a theoretical approach and comparison with current ideas on regulation of nitrogen metabolism. In: De Kok LJ, Stulen I, Rennenberg H, Brunold C, Rauser WE, editors. Sulfur Nutrition and Assimilation in Higher Plants: Regulatory Agricultural and Environmental Aspects. The Hague, The Netherlands: SPB Academic Publishing; 1993. pp. 77–91. [Google Scholar]
- Wellburn A. Air Pollution and Climate Change: The Biological Impact. Ed 2. Essex, UK: Longman Scientific and Technical; 1994. [Google Scholar]
- Youssefian S, Nakamura M, Sano H. Tobacco plants transformed with the O-acetylserine (thiol) lyase gene of wheat are resistant to toxic levels of hydrogen sulphide gas. Plant J. 1993;4:759–769. doi: 10.1046/j.1365-313x.1993.04050759.x. [DOI] [PubMed] [Google Scholar]