Abstract
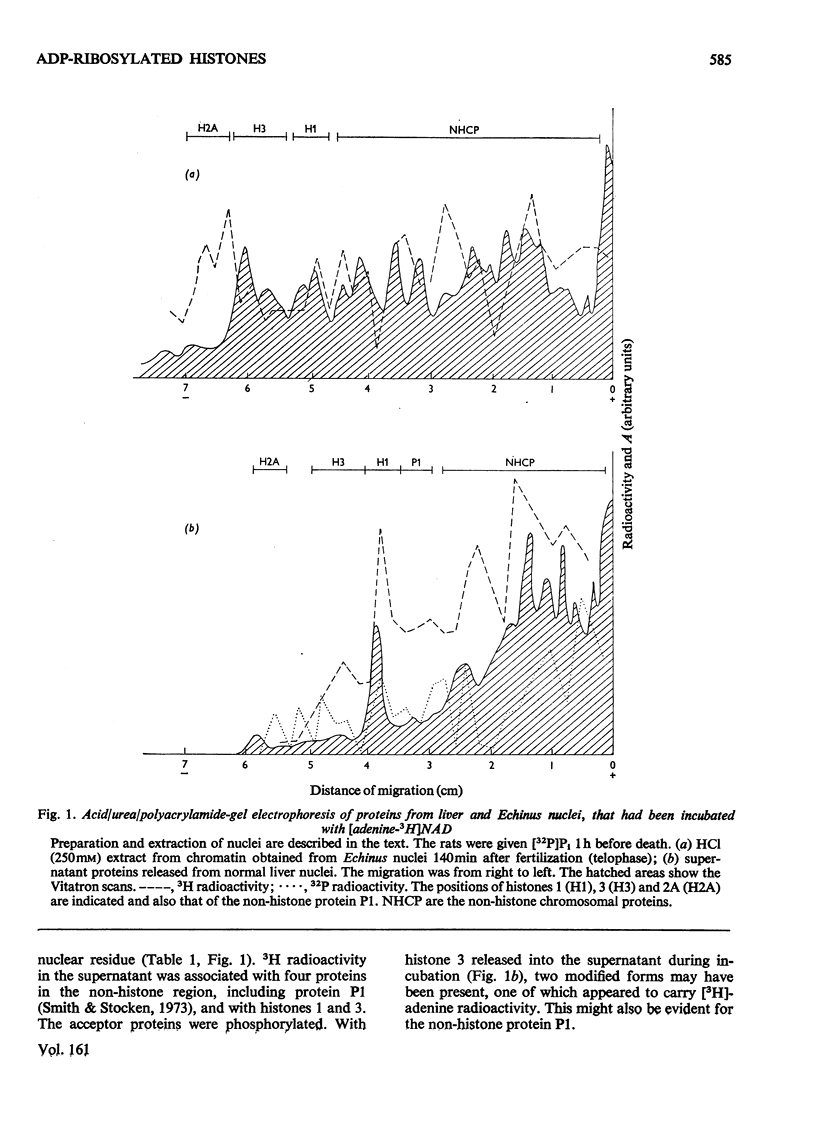
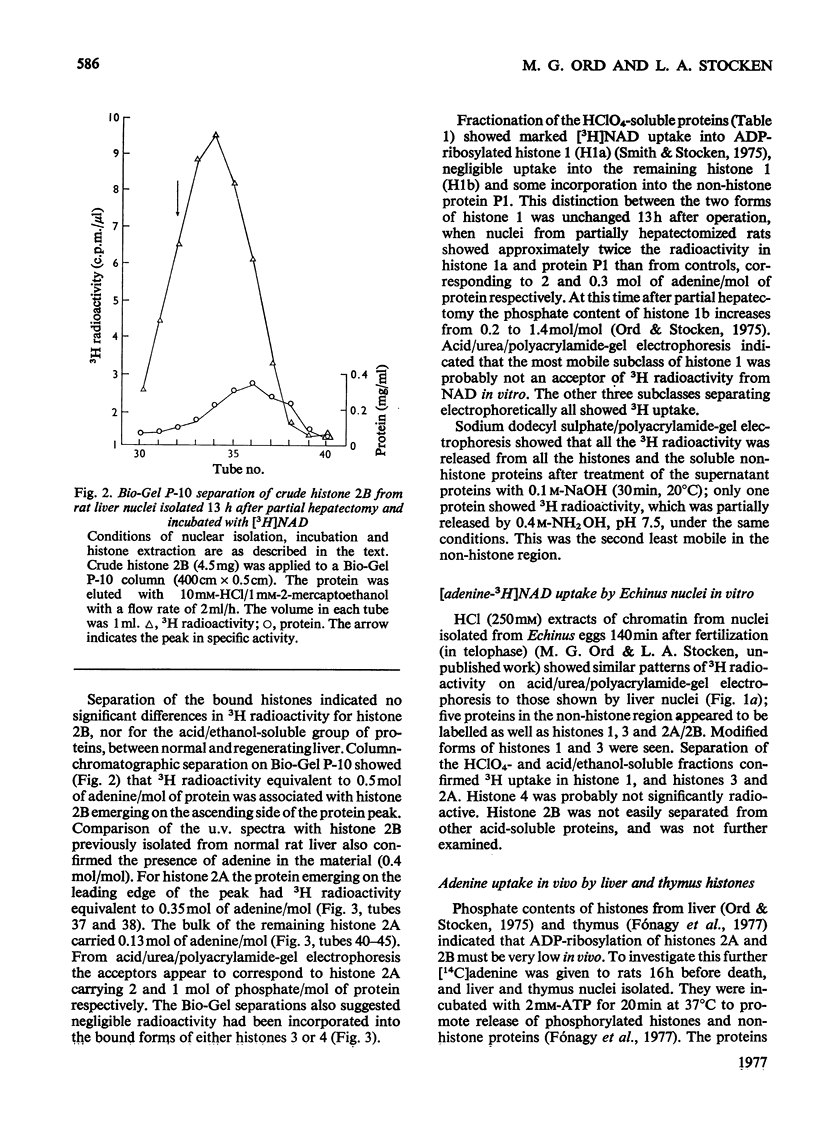
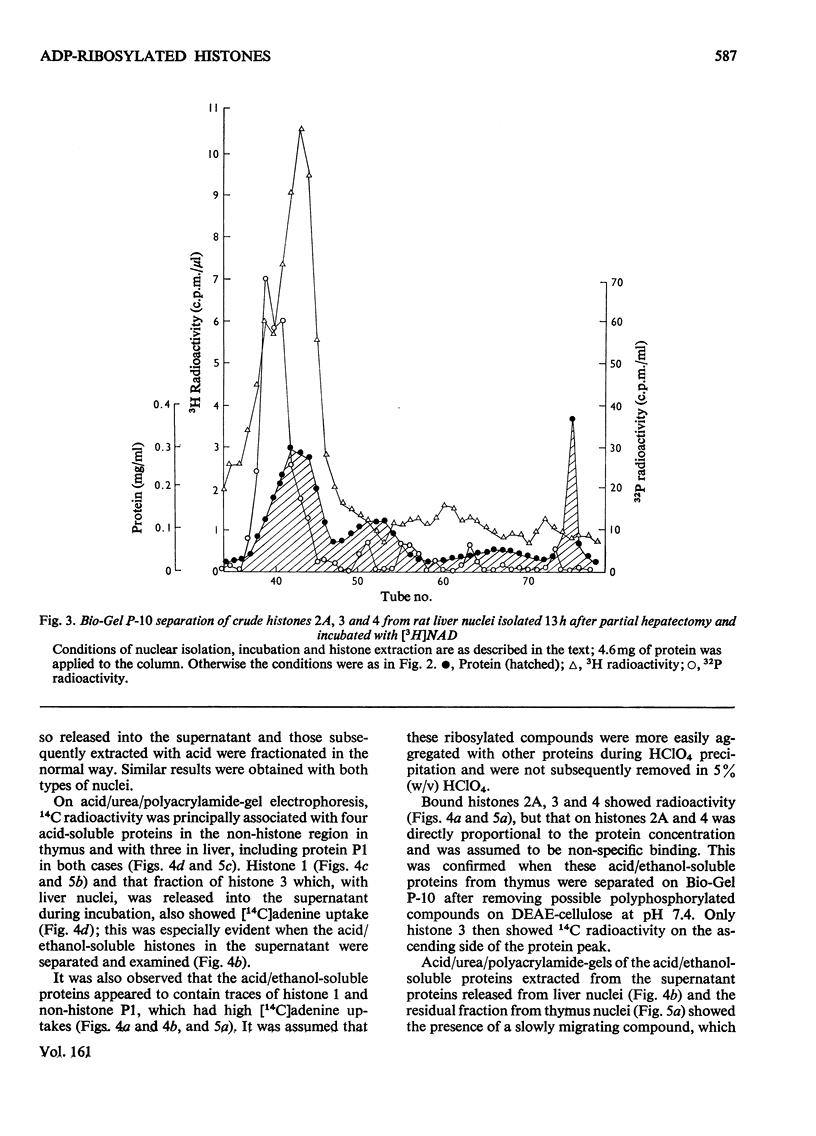
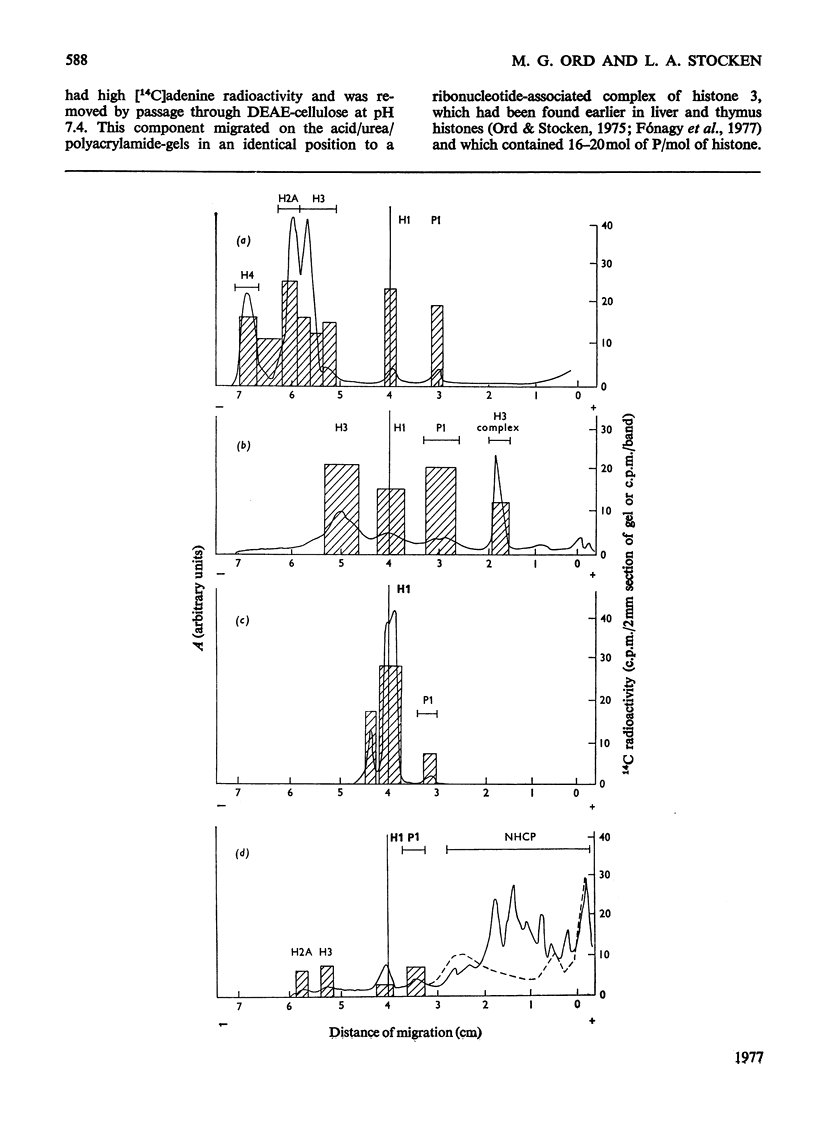
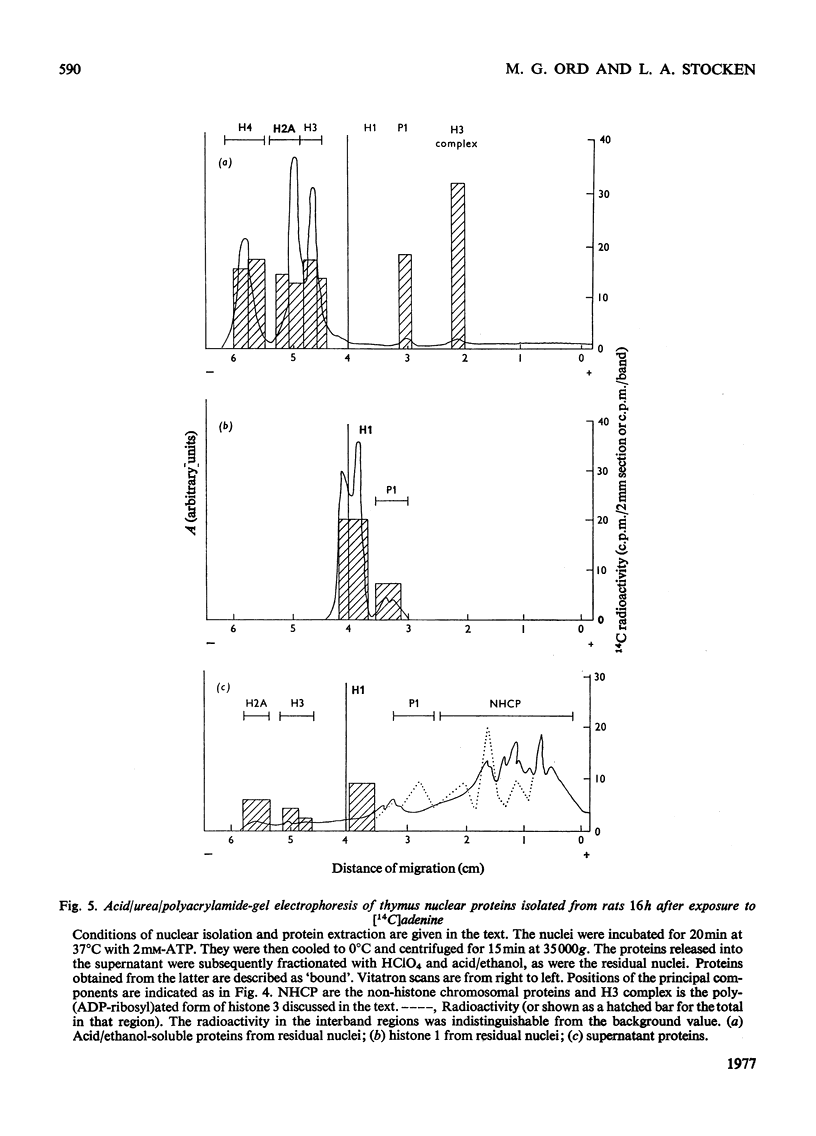
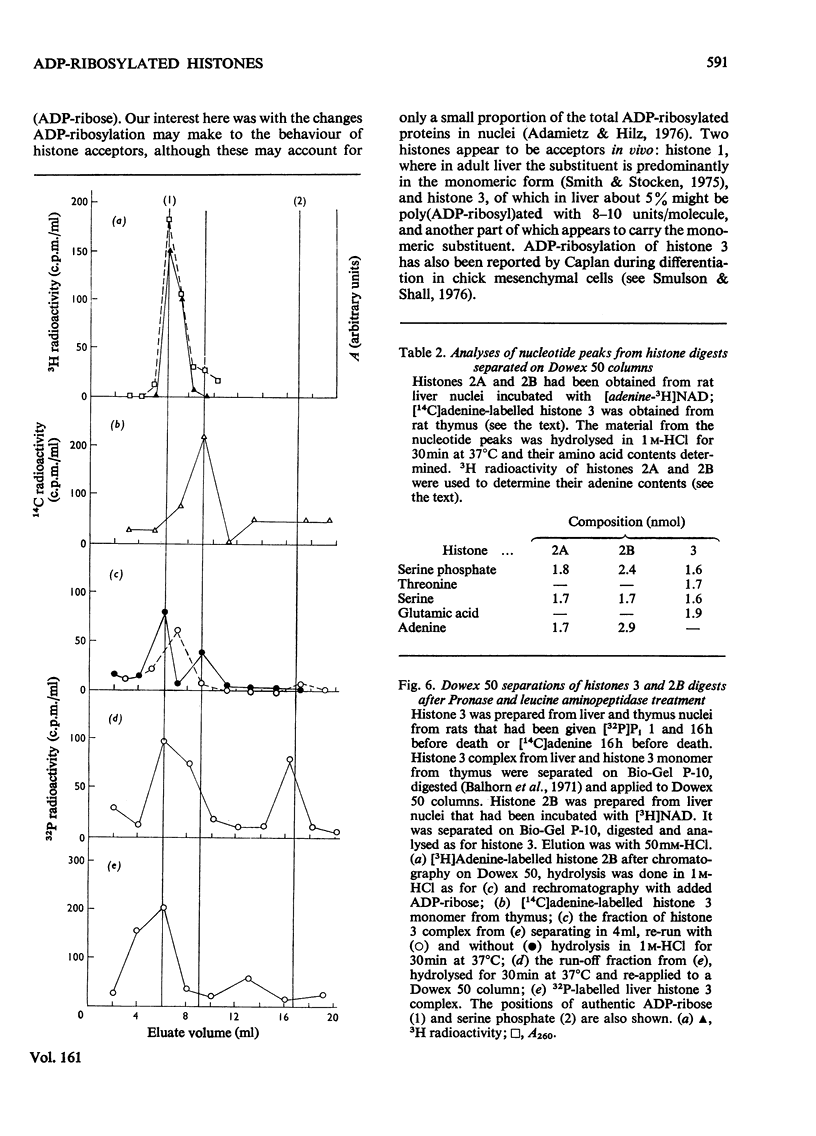
When rat liver nuclei were incubated with [adenine-3H]NAD, besides histone 1, histone 2A and especially histone 2B accepted 3H radioactivity. 3H radioactivity was also found on the non-histone proteins and on the small amounts of histones 1 and 3 released into the supernatant during incubation. [14C]Adenine uptake in vivo by liver and thymus nuclei showed radioactivity in histones 1 and 3. After digestion with Pronase and leucine aminopeptidase 14C- or 32P-labelled histone 3 released a serine phosphate-containing nucleotide, which on acid hydrolysis yielded ADP-ribose and serine phosphate. Serine phosphate was also found in the material from the nucleotide peaks from histones 2A and 2B. ADP-ribosylated histones 1 and 3 were more easily released from nuclei than their unmodified forms and showed higher [32P]Pi and [3H]lysine uptakes in vivo [Ord & Stocken (1975) FEBS Meet. Proc. 34, 113-125].
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamietz P., Hilz H. Poly(adenosine diphosphate ribose) is covalently linked to nuclear proteins by two types of bonds. Hoppe Seylers Z Physiol Chem. 1976 Apr;357(4):527–534. doi: 10.1515/bchm2.1976.357.1.527. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balhorn R., Rieke W. O., Chalkley R. Rapid electrophoretic analysis for histone phosphorylation. A reinvestigation of phosphorylation of lysine-rich histone during rat liver regeneration. Biochemistry. 1971 Oct 12;10(21):3952–3959. doi: 10.1021/bi00797a024. [DOI] [PubMed] [Google Scholar]
- Fónagy A., Ord M. G., Stocken L. A. Phosphorylation of rat thymus histones, its control and the effects thereon of gamma-irradiation. Biochem J. 1977 Jan 15;162(1):171–181. doi: 10.1042/bj1620171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto E., Takeda M., Nishizuka Y. Phosphorylated sites of calf thymus histone H2B by adenosine 3',5'-monophosphate-dependent protein kinase from silkworm. Biochem Biophys Res Commun. 1975 Sep 16;66(2):547–555. doi: 10.1016/0006-291x(75)90545-8. [DOI] [PubMed] [Google Scholar]
- Ord M. G., Stocken L. A. Metabolic properties of histones from rat liver and thymus gland. Biochem J. 1966 Mar;98(3):888–897. doi: 10.1042/bj0980888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ord M. G., Stocken L. A. Thymidine uptake by Paracentrotus eggs during the first cellcycle after fertilization. Exp Cell Res. 1974 Feb;83(2):411–414. doi: 10.1016/0014-4827(74)90357-7. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. The heterogeneity of histones. I. A quantitative analysis of calf histones in very long polyacrylamide gels. Biochemistry. 1969 Oct;8(10):3972–3979. doi: 10.1021/bi00838a013. [DOI] [PubMed] [Google Scholar]
- Roberts J. H., Stard P., Giri C. P., Smulson M. Cytoplasmic poly(ADP-ribose) polymerase during the HeLa cell cycle. Arch Biochem Biophys. 1975 Nov;171(1):305–315. doi: 10.1016/0003-9861(75)90037-5. [DOI] [PubMed] [Google Scholar]
- Shlyapnikov S. V., Arutyunyan A. A., Kurochkin S. N., Memelova L. V., Nesterova M. V., Sashchenko L. P., Severin E. S. Investigation of the sites phosphorylated in lysine-rich histones by protein kinase from pig brain. FEBS Lett. 1975 May 15;53(3):316–319. doi: 10.1016/0014-5793(75)80045-7. [DOI] [PubMed] [Google Scholar]
- Smith J. A., Stocken L. A. Chemical and metabolic properties of adenosine diphosphate ribose derivatives of nuclear proteins. Biochem J. 1975 Jun;147(3):523–529. doi: 10.1042/bj1470523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. A., Stocken L. A. The characterization of a non-histone protein isolated from histone F1 preparations. Biochem J. 1973 Apr;131(4):859–861. doi: 10.1042/bj1310859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura T. Poly(adenosine diphosphate ribose). Prog Nucleic Acid Res Mol Biol. 1973;13:127–151. doi: 10.1016/s0079-6603(08)60102-6. [DOI] [PubMed] [Google Scholar]
- Ueda K., Omachi A., Kawaichi M., Hayaishi O. Natural occurrence of poly(ADP-ribosyl) histones in rat liver. Proc Natl Acad Sci U S A. 1975 Jan;72(1):205–209. doi: 10.1073/pnas.72.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]



