Abstract
Developing cotton (Gossypium hirsutum) fibers, cultured in vitro with their associated ovules, were used to compare the effects of two herbicides that inhibit cellulose synthesis: 2,6-dichlorobenzonitrile (DCB) and an experimental thiatriazine-based herbicide, CGA 325′615. CGA 325′615 in nanomolar concentrations or DCB in micromolar concentrations causes inhibition of synthesis of crystalline cellulose. Unlike DCB, CGA 325′615 also causes concomitant accumulation of non-crystalline β-1,4-glucan that can be at least partially solubilized from fiber walls with ammonium oxalate. The unusual solubility of this accumulated glucan may be explained by its strong association with protein. Treatment of the glucan fraction with protease changes its size distribution and leads to precipitation of the glucan. Treatment of the glucan fraction with cellulase digests the glucan and also releases protein that has been characterized as GhCesA-1 and GhCesA-2—proteins that are believed to represent the catalytic subunit of cellulose synthase. The fact that cellulase treatment is required to release this protein indicates an extremely tight association of the glucan with the CesA proteins. In addition, CGA 325′615, but not DCB, also causes accumulation of CesA protein and a membrane-associated cellulase in the membrane fraction of fibers. In addition to the effects of CGA 325′615 on levels of both of these proteins, the level of both also shows coordinate regulation during fiber development, further suggesting they are both important for cellulose synthesis. The accumulation of non-crystalline glucan caused by CGA 325′615 mimics the phenotype of the cellulose-deficient rsw1 mutant of Arabidopsis that also accumulates an apparently similar glucan (T. Arioli, L. Peng, A.S. Betzner, J. Burn, W. Wittke, W. Herth, C. Camilleri, H. Hofte, J. Plazinski, R. Birch et al. [1998] Science 279: 717).
Progress in understanding the process of cellulose synthesis in plants has been hampered for years by the inability to obtain substantial rates of in vitro synthesis of the microfibrillar product using isolated membrane preparations. For this reason, identification of a family of genes called CesA, which are proposed to encode the catalytic subunit of the cellulose synthase, has opened new approaches to the study of this process (for review, see Delmer, 1999). These genes, first identified in cotton (Gossypium hirsutum; Pear et al., 1996), encode proteins that share, with their bacterial homologs, important conserved motifs around three conserved Asp (D) residues that are proposed to be important for binding of the substrate UDP-Glc and for catalysis of glucan chain elongation (Saxena et al., 1995; Charnock and Davies, 1999). Using the cotton GhCesA-1 gene, Pear et al. (1996) showed that a recombinant protein that spans these conserved motifs is capable of binding the predicted substrate, and that deletion of the region surrounding D1 leads to loss of capacity to bind the substrate. Furthermore, the expression pattern of these genes strongly argues for an important role in the process of secondary wall cellulose synthesis in cotton fibers. Critical genetic evidence that the CesA genes are involved in cellulose synthesis comes from the finding that mutations in CesA genes from Arabidopsis lead to phenotypes that show reduced deposition of cellulose in specific tissues. These include RSW1, also referred to as AtCesA-1 (Arioli et al., 1998), IRX3, also called AtCesA7 (Taylor et al., 1999), IRX1, also called AtCesA-8 (Taylor et al., 2000), and PROCUSTE1, also called AtCesA-6 (Fagard et al., 2000). Additional strong support for the role of CesA genes comes from the recent finding that an antibody directed against a CesA protein shows a reaction that localizes the protein to rosettes, the structures believed to represent cellulose synthase complexes (Kimura et al., 1999). At least in Arabidopsis and maize, surveys of genomic and cDNA sequences indicate that there are at least 10 distinct CesA genes (Holland et al., 2000; Richmond and Somerville, 2000), and from sequence comparisons and expression patterns, it appears that some of these are co-expressed within the same cell type, with some groups being expressed in tissues undergoing primary wall cellulose synthesis and others expressed uniquely in cell types undergoing secondary wall cellulose deposition (Fagard et al., 2000; Holland et al., 2000; Taylor et al., 2000).
In spite of all this accumulated evidence supporting a role for CesA genes in cellulose synthesis, it has been difficult to prove without question that the CesA proteins do catalyze the process of glucan chain elongation, although they certainly contain motifs characteristic of family 2 glycosyltransferases (Campbell et al., 1997). The genetic evidence, protein localization, and gene expression patterns, and ability to bind UDP-Glc, collectively, strongly argue for this possibility, but in sum, these results still only provide evidence that CesA genes encode proteins that are somehow important for the process. It is unfortunate that there are many gaps in our knowledge of the mechanism of glucan chain polymerization. For example, it is not known whether a primer is required; if so, this would also require the action of a glycosyltransferase. There is also debate about whether elongation occurs from one or two distinct active sites on the same or different proteins (Koyama et al., 1997; Carpita and Vergara, 1998), and we know nothing about how chain termination is effected. Other recent results indicating that a membrane-associated cellulase may be important for the process also indicate that we have much still to learn about the mechanistic details involved in cellulose synthesis (Nicol et al., 1998; H. Hofte, personal communication). Conclusive proof for the proposed CesA catalytic function might come from showing that glucan chain elongation occurs in a heterologous host upon expression of a CesA gene. In this regard we have succeeded to express the GhCesA-1 gene in yeast and green monkey kidney cells; although the protein is integrated into membranes in high levels, no cellulose production was observed in these systems (Y. Kawagoe, D. Grubb, A. Spicer, and D.P. Delmer, unpublished data). Such negative findings may only indicate that a single CesA gene product is not sufficient for assembly of the complete synthase structures that have the capacity to synthesis microfibrils; they also indicate that other approaches are needed to shed more light on the function of CesA proteins.
The use of mutants or specific chemical inhibitors can often provide additional evidence about a biosynthetic pathway, especially when they lead to accumulation of intermediates. In this regard it has been reported that a non-crystalline form of β-1,4-glucan accumulates in the temperature-sensitive rsw1 mutant when crystalline cellulose formation is impaired at high temperatures (Arioli et al., 1998; Peng, 1999), although it is also clear that this mutant accumulates significant amounts of starch as well (Peng, 1999; Peng et al., 2000). Since high temperatures also led to rosette disintegration in rsw1, these authors suggested that CesA proteins in isolation might make glucan chains that are incapable of assembly into crystalline microfibrils. In terms of specific inhibitors, cellulose is a potentially attractive target for herbicide action, and several inhibitors of the process have been identified, the two most well known being 2,6-dichlorobenzonitrile (DCB) and Isoxaben (for review, see Delmer, 1999; Sabba and Vaughn, 1999). At present, the mode of action of DCB is not clear, although an 18-kD DCB-binding polypeptide was identified in cotton fibers (Delmer et al., 1987), but the potential role of this protein in cellulose synthesis has never been clarified. Two distinct CesA genes (AtCesA-3 and AtCesA-6) have recently been shown to map to the two distinct Isoxaben-resistant loci of Arabidopsis (H. Hofte and C. Somerville, personal communication), indicating that Isoxaben may act by direct interaction with CesA proteins; if so, this would provide further evidence for the importance of CesA proteins in the process.
The structure of a Novartis experimental thiatriazine herbicide called CGA 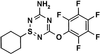 325′615 is shown below:
325′615 is shown below:
A recent patent (Stoller et al., 1996) describes the chemistry of synthesis and properties of this herbicide and other similar derivatives. This information, as well as other studies (A. Stoller and K. Kreuz, unpublished data), indicates that this herbicide acts specifically (IC50 about 5 nm) to inhibit synthesis of crystalline cellulose in cultured soybean cells and in a variety of other plants tested. In soybean cells its specificity has been tested further, and it has no effect on rates of protein, RNA, DNA, or lipid synthesis, nor does it inhibit respiration, photosynthesis (including the Hill reaction), synthesis of non-cellulosic polysaccharides, or cytoskeletal organization.
We have used CGA 325′615 to study its effects on cellulose synthesis in developing cotton fibers with hopes that it might provide further insights into the mechanism of cellulose synthesis in plants. The data presented herein show that this herbicide, unlike DCB, causes accumulation of a non-crystalline form of β-1,4-glucan concomitant with inhibition of crystalline cellulose formation. The fact that this glucan is also intimately associated with elevated levels of cotton fiber CesA proteins provides further evidence that these proteins are involved in the process of glucan chain synthesis.
RESULTS
Comparison of the Effects of CGA 325′615 and DCB on Cell Wall Synthesis
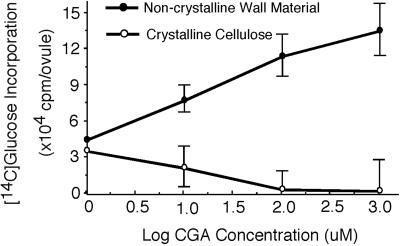
For studies with CGA 325′615 we have used cultured cotton ovules with their associated fibers. Development in culture mimics the events that occur in the intact plant in which fibers undergo an elongation phase where a thin primary wall is deposited followed by onset of massive secondary wall cellulose synthesis (Meinert and Delmer, 1977). Such fibers, cultured for 21 d post-anthesis (DPA) and engaged in secondary wall cellulose synthesis, were supplied with [U-14C]Glc as carbon source and the effect of CGA 325′615 on synthesis of cellulose was determined. In this study we define “crystalline cellulose” as that fraction of the cell wall that is resistant to digestion with acetic-nitric reagent (Updegraff, 1969), a fraction that has previously been shown to consist almost exclusively of glucan in β-1,4-linkage (Meinert and Delmer, 1977). In this system we find that synthesis of crystalline [14C]cellulose from supplied [U-14C]Glc is inhibited by CGA 325′615 with an IC50 of approximately 5 nm (Fig. 1). In other experiments with younger fibers we have found a similar inhibition of primary wall synthesis (not shown). The potency of this herbicide compares well with that of Isoxaben, which also effectively inhibits the synthesis of cellulose when applied in nanomolar concentrations (Heim et al., 1990), whereas the IC50 for DCB with cotton fibers is much higher, at approximately 1 μm (Montezinos and Delmer, 1980).
Figure 1.
The effect of CGA 325′615 on incorporation of [U-14C]Glc into cell wall material in cultured cotton ovules with their associated fibers. The experiment was conducted using 21 DPA fibers and the incubation time in labeled Glc with or without herbicide was 4 h. Crystalline cellulose is defined as that material resistant to digestion by acetic-nitric reagent (Updegraff, 1969); radioactivity solubilized by this treatment is represented as the non-crystalline wall material. Each data point represents the average of triplicate samples.
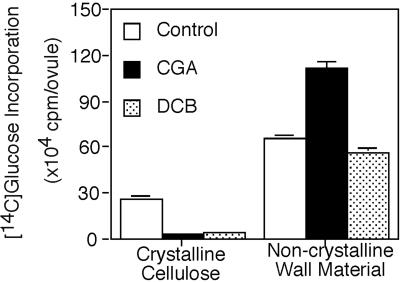
We also observe that CGA 325′615 causes a concomitant accumulation of radioactivity in the non-crystalline cell wall fraction (Fig. 1). In separate experiments we compared the effects of CGA 325′615 with that of DCB, and we found that the accumulation of the non-crystalline wall material is only characteristic of CGA 325′615 and is not observed with DCB (Fig. 2).
Figure 2.
Comparison of the effect of CGA 325′615 and DCB on cell wall synthesis. The experiment was conducted as described in “Materials and Methods” and in the legend to Figure 1. The concentration of CGA 325′615 was 10 nm and the concentration of DCB was 25 μm.
Characterization of the Non-Crystalline Wall Fraction
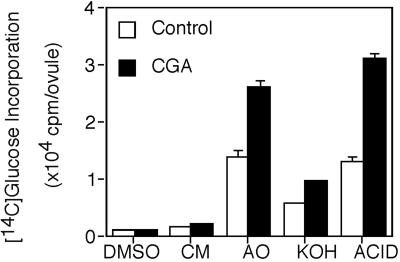
Sequential extraction of fiber walls indicates that a substantial portion of the material that accumulates in the presence of CGA 325′615 can be extracted by ammonium oxalate (AO), whereas the remainder requires harsher extraction conditions (Fig. 3). To avoid artifacts due to degradation by harsh treatments, we have analyzed only the AO fraction further.
Figure 3.
Non-crystalline wall material solubilized by sequential extraction of cotton fiber cell walls. CGA-treated fibers had been incubated for 4 h in 10 nm CGA 325′615. Details of cell wall preparation and extractions are given in “Materials and Methods.” DMSO, Dimethylsulfoxide; CM, chloroform:methanol; ACID, material solubilized by acetic-nitric treatment following KOH extraction.
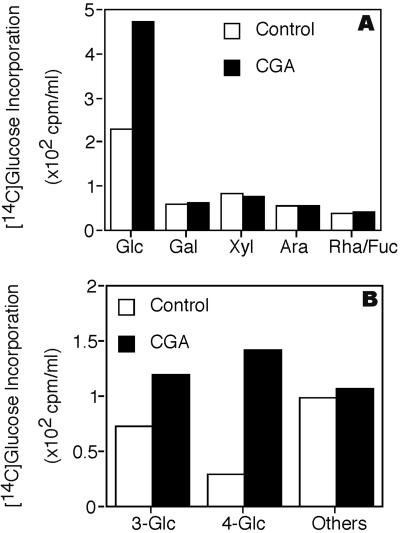
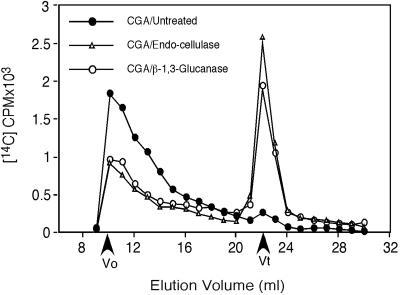
Analysis of the accumulated AO material indicates it contains some callose, as well as a form of non-crystalline β-1,4-glucan (NCG). Following acid hydrolysis, radioactivity is predominantly in [14C]Glc (Fig. 4A). Methylation analysis (Fig. 4B) indicates only two derivatives that show significant CGA 325′615-induced accumulation. One is 3-linked Glc in levels that are variable in repeated experiments and most likely come from callose, known to be synthesized transiently in fibers at this stage of development (Maltby et al., 1979) and may be enhanced by the stress of CGA 325′615 treatment. In the experiment shown in Figure 4, a substantial amount of the radioactivity is found in 3-linked Glc, but in repeats of these experiments we have found the level of this derivative to be quite variable. By contrast, the linkage that shows consistent elevation in all experiments compared with controls is 4-linked Glc. The total AO fraction that accumulates in the presence of CGA 325′615 is completely resistant to treatment with a lichenase (Megazyme, Bray, Ireland) that degrades glucans with mixed 1,3- and 1,4-β-linkages, providing evidence that these two linkages most likely do not co-exist in the same glucan. By contrast, we find that treatment of AO with a Trichoderma exo-1,3-β-glucanase partially alters the size distribution of the total AO fraction on Superdex 200 (Pharmacia, Uppsala; Fig. 5), and the material converted to low Mr is found by thin-layer chromatography (TLC) or gas-liquid chromatography (GLC) to be Glc (not shown). Treatment with Trichoderma endo-1,4-β-glucanase results also in conversion of a substantial portion of the radioactivity to low Mr material (Fig. 5) that migrates coincident with standards of Glc and cellobiose on TLC (not shown). We also find that the entire AO fraction is resistant to degradation by α-amylase (not shown); coupled with the fact that cotton fibers make no detectable starch at this stage of development, this indicates that no starch is present. Taken together, these data support the notion that callose (1,3-β-glucan) and NCG co-exist in the AO fraction. Although callose and a limited amount of NCG are present in controls lacking herbicide, we always find consistent elevation of the NCG, with variable elevation in the level of callose after herbicide treatment.
Figure 4.
Sugar composition and linkage analysis of AO fraction. CGA treatment prior to wall analyses was for 4 h at 10 nm CGA 325′615. A, Monosaccharide composition as determined by GLC of radioactive alditol acetate derivatives. B, Analysis of partially methylated, partially acetylated radioactive derivatives by GLC. “Others” refers to the sum of radioactivity in all other derivatives, none of which was significantly enhanced by CGA 325′615.
Figure 5.
Glucanase treatments alter the size distribution of the radioactive material in the AO fraction. Chromatography was on Superdex 200 using 0.1% (w/v) AO as solvent. The fraction was obtained from walls of fibers treated for 4 h with 10 nm CGA 325′615 and was applied to the column untreated or following digestion with exo-1,3-β-glucanase or endo-1,4-β-glucanase as detailed in “Materials and Methods.” Equal amounts of radioactivity (cpm) were loaded for control and treated samples. Vo represents the void volume of the column, and Vt the position where totally included molecules such as Glc and cellobiose would elute.
The Solubility of the Accumulated β-1,4-Glucan May Be Explained by Association with the CesA Protein
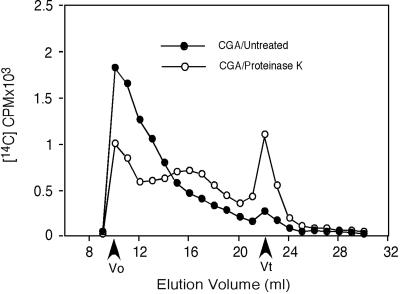
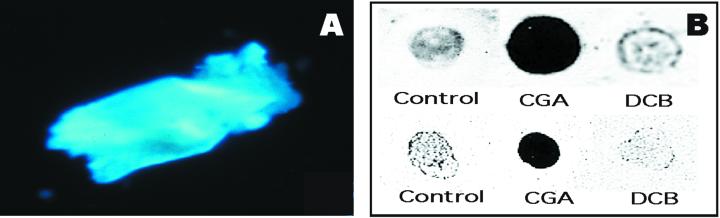
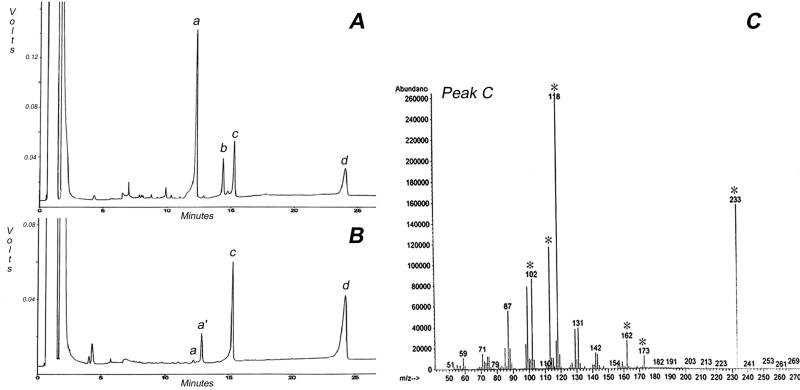
The solubility properties of this NCG require explanation since cellodextrins beyond seven residues are insoluble (Tonnessen and Ellefsen, 1971). The following evidence indicates that association with CesA protein may explain this unusual solubility. Upon gel filtration on Superdex 200, the total AO fraction has a high and heterogeneous molecular weight, and in addition to the effects of glucanases on size distribution (Fig. 5), we observe that treatment with Proteinase K also notably changes the size distribution (Fig. 6). The experiment shown in Figure 6 was carried out immediately following protease digestion; however, we have observed that if the protease-treated material is incubated overnight at 4°C, material that stains with the β-glucan-interacting dye Calcofluor precipitates (Fig. 7A); this precipitated material also interacts with a CBD that recognizes crystalline or non-crystalline domains (Fig. 7B). Methylation analysis of this precipitated material results in only one major peak on GLC that migrates coincident with a 4-linked Glc standard (Fig. 8B, peak c); this peak, when subjected to mass spectrometry (MS) analysis, shows fragments that confirm its identity as a 4-linked hexose (Fig. 8C). In the experiment shown in Figure 8B, a very small peak (a) corresponding to t-Glc is detected. In addition, another peak (labeled a′) was also detected; in another experiment, peak a′ was barely detectable. MS of peak a′ indicated it is a terminal hexose, but its elution time indicates it is not t-Glc; given that the level is variable, it remains uncertain if this t-hexose is really derived from the glucan or some other contaminant in the preparation. The change in size distribution of radioactivity following protease treatment (Fig. 6) suggests that the degree of polymerization of the NCG is heterogeneous and certainly must be longer than seven residues because of its limited solubility after protease treatment. Although the very low levels of t-Glc could indicate an average degree of polymerization of >20, one cannot exclude the possibility that the unidentified t-hexose is at the non-reducing end or an alternate possibility that the non-reducing termini of the glucan chains are blocked by association with some other component.
Figure 6.
Protease treatment alters the size distribution of the radioactive material in the AO fraction. All conditions were as in the legend to Figure 5, except that digestion was carried out by treatment with Proteinase K as detailed in “Materials and Methods.” Equal amounts of radioactivity (cpm) were loaded for control and treated samples.
Figure 7.
Characteristics of glucan that precipitates from the AO fraction following digestion with protease and chilling. A, The precipitate stains in fluorescence microscopy with the glucan-binding dye Calcofluor. B, Binding on polyvinylidene difluoride membrane of the insoluble glucan from CGA 325′615-treated fibers to a cellulose-binding protein (CBD) detected by probing with anti-CBD antibody (top), and an autoradiogram showing that this glucan retains its radioactivity upon precipitation (bottom).
Figure 8.
Methylation analysis of protease-treated glucan that precipitates upon chilling. A, GLC trace showing elution patterns for standards. a, t-Glc; b, 3-Glc; c, 4-Glc; and d, inositol. B, GLC trace of the partially methylated, partially acetylated derivatives from the precipitated glucan. The major peak (c) runs coincident with the 4-Glc standard. Peak a represents retention time for t-Glc, whereas peak a′ represents an unidentified terminal hexose, and peak d is the inositol standard. C, Mass spectrum of peak c that contains ions diagnostic of 4-Glc (indicated by asterisks).
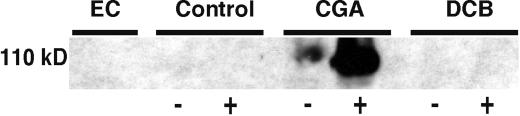
Following digestion with endocellulase and SDS-PAGE, a polypeptide of approximately 110 kD is released, a size in the range of cotton fiber CesA protein, and this, and only this, polypeptide reacted in western blots with antibody prepared against the N-terminal zinc finger domain of cotton CesA-1 (Fig. 9). In the experiment shown in Figure 9, no such polypeptide is released from the AO fraction from controls lacking herbicide or from DCB-treated fibers, although in occasional experiments and very long exposure of the western blots, we do detect minor amounts. It is clear that the one situation where large amounts of CesA protein are detected is the case where the CGA 325′615-induced AO fraction is treated with cellulase; minor amounts of a similar polypeptide of slightly higher Mr are also detected in samples that were not cellulase treated. Silver staining of such gels revealed only a strong band at 55 kD (the added cellulase), plus a faint smear throughout the lane for the control and DCB-treated samples; for the CGA 325′615 sample, we detected, in addition, a band that migrated in position of the band that reacted with the anti-CesA antibody (not shown).
Figure 9.
Western blot of proteins derived from the AO fraction of fibers incubated without (control) or with 10 nm CGA 325′615 or 25 μm DCB. Protein was detected by use of an antibody directed against the zinc-finger domain of GhCesA-1. EC, Endocellulase enzyme alone. AO samples not treated (−) or treated (+) with endocellulase prior to electrophoresis. Protein levels were too low to quantify accurately, and so equivalent volumes of the AO fractions from the same number of control or herbicide-treated ovules/fibers were loaded. This represented <2 μg of protein in both cases.
The identity of the cellulase-treated protein as CesA is further confirmed by matrix-assisted laser-desorption ionization time of flight (MALDI-TOF) MS of peptides released by tryptic digestion of the band that migrates as CesA in SDS-PAGE (Table I, column labeled AO). This experiment led to identification of at least seven prominent fragments of mass predicted within experimental error for tryptic digestion of GhCesA-1 or GhCesA-2 proteins, both of which are expressed in fibers at this stage of development (Pear et al., 1996). Note that for one peptide that spans the first conserved Asp (D1), we also detect fragments with Mr consistent with having some Glc residues attached; the predicted number is three to six residues for the cellulase-treated CesA protein from the AO fraction. In a similar experiment we also analyzed the tryptic fragments of derived from CesA (not treated with cellulase) from the crude microsomal membrane fraction (Table I, column labeled “Microsomal Membranes”). Fragments of predicted mass for CesAs were detected, including fragments with two or three predicted Glc residues. Since this latter protein fraction was not cellulase treated, it may indicate that at least some of the CesA within the membranes (as opposed to that in the AO fraction) has a few residues of Glc associated with it that would not be expected to alter its migration significantly in SDS-PAGE. In this regard we have also noted some radioactive terminal Glc residues associated with the membrane fraction after CGA 325′615 treatment (not shown), but we have not characterized these further at this point.
Table I.
MALDI-TOF MS identification of putative CesA peptides
| Massa
|
Residues | MOb | Predicted Peptide Sequence | AO | Microsonal Membranes | |
|---|---|---|---|---|---|---|
| Predicted | Observed | |||||
| CesA1 | ||||||
| 3,099.555 | 3,099.220 | 613–642 | 0 | TFGLSSVFIESTLMENGGVAESANPSTLIK | − | + |
| 2,874.319 | 2,874.383 | 472–494 | 2 | EAMCFLMDPQVGRDVCYVQFPQR | + | + |
| 2,704.340 | 2,705.016 | 445–468 | 1 | VSAVLTNAPFILNLDCD2HYVNNSK | + | + |
| 2,282.043 | 2,283.211 | 50–68 | 2 | ACLRCGSPYDENLLDDVEK | + | − |
| 728.466 | 728.577 | 364–370 | 0 | INALVAK | + | + |
| 3,152.689 | 3,153.031 | 286–311 | 3 | VSCYISDD1GAAMLTFESLVETADFAR + Glc2 | − | + |
| 3,313.760 | 3,313.296 | 286–311 | 3 | VSCYISDD1GAAMLTFESLVETADFAR + Glc3 | + | + |
| 3,679.107 | 3,678.683 | 286–311 | 1 | VSCYISDD1GAAMLTFESLVETADFAR + Glc5 | + | − |
| 3,810.282 | 3,810.565 | 286–311 | 2 | VSCYISDD1GAAMLTFESLVETADFAR + Glc6 | + | − |
| CesA2 | ||||||
| 2,299.056 | 2,299.145 | 418–437 | 0 | KPEEGWVMQDGTPWPGNNTR | + | − |
| 1,516.663 | 1,516.731 | 516–528 | 0 | EAMCFLMDPQFGK | + | + |
| 3,640.042 | 3,640.369 | 330–355 | 0 | VCCYVSDD1GASMLLFDSLSETAEFAR + Glc5 | + | − |
Fragments were identified by MALDI-TOF MS from trypsin digestion of CesA proteins released by cellulase digestion of the AO fraction (AO) or from non-cellulase-treated crude microsomal membranes derived from fibers treated for 4 h with 10 nm CGA. The released proteins were subjected to SDS-PAGE and the region containing a mixture of CesA-1 and CesA-2 polypeptides was excised and subjected to in-gel trypsin digestion, peptide purification, and MS.
All predicted and observed masses are monoisotopic and mass accuracy is ∼120 ppm.
MO, Modification occurrence: 0, non-modification; 1, all cysteines treated with iodoacetamide to form carboxyamidomethyl cysteine; or 2, with acrylamide adducts; 3, methionines oxidized to form methionine sulfoxide.
CGA 325′615 also Causes Accumulation of Membrane-Associated CesA and Cellulase
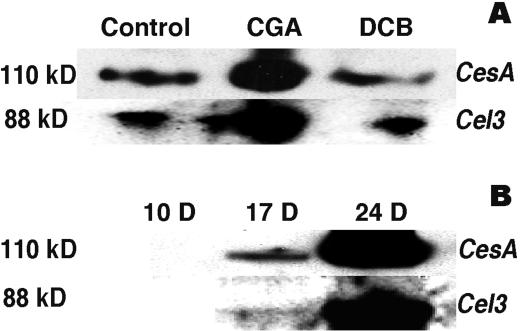
Note that the CesA protein that is released by cellulase treatment is derived from the AO cell wall fraction. Yet, as the proposed catalytic subunit of the cellulase synthase, CesA would normally be expected to reside in the plasma membrane. When membrane fractions were examined by western blotting, we found that CGA 325′615 treatment also leads to elevation in levels of CesA protein when compared with membranes from control or DCB-treated fibers (Fig. 10A). However, in this case, no cellulase treatment is required for entry into the gel system, a situation in marked contrast to the CesA found in the AO fraction. This would also be in accord with our finding tryptic peptides predicted to contain no more than two or three Glc residues without cellulase treatment, a level that would not substantially alter CesA migration in SDS-PAGE.
Figure 10.
Changes in level of CesA and Cel3/Kor proteins in CesA and cellulase (Kor) proteins during development or in response to herbicide treatment. A, Cultured ovules with fibers were incubated for 4 h without herbicide or with 10 nm CGA 325′615 or 25 μm DCB prior to isolation of crude microsomal membranes. Ten micrograms of membrane protein was loaded per lane. The top lane was probed with the antibody against the zinc-finger domain of GhCesA-1. The bottom lane was probed using antibody against the tomato Cel3 (Kor) protein. B, Western blot performed as above but showing changes in level of CesA and Cel3/Kor protein in crude microsomal membranes (10 μg/lane) derived from plant-grown fibers during cotton fiber development. Ten d post-anthesis represents stage of primary wall synthesis; 17 DPA transition to secondary wall synthesis, and fibers at 24 DPA are fully engaged in secondary wall synthesis.
It is of interest that we also detected, by western blotting, marked enhancement by CGA 325′615 treatment of the level of a protein that reacts with antibody against the tomato membrane-associated cellulase compared with levels in control and DCB-treated fibers (Fig. 10A). No such protein was detected in the AO fraction, with or without cellulase treatment (not shown). This type of cellulase (called Cel3) was first identified in tomato by Brummell et al. (1997); in Arabidopsis, the locus encoding this protein is referred to as Korrigan (Kor), and mutations in this gene lead to altered cell wall structures (Nicol et al., 1998) that contain less cellulose (H. Hofte, personal communication). We find that the level of this protein, detected at the predicted Mr during western blotting, increases in level in the membranes of plant-grown cotton fibers in parallel with the increase in CesA protein (Fig. 10B). The fact that treatment with CGA 325′615 causes a parallel accumulation of both proteins in the fiber suggests that the level of these proteins may be coordinately regulated.
DISCUSSION
These findings provide new information regarding the mechanism of action of a new cellulose synthesis inhibitor and also offer new insights into the process of cellulose synthesis. These studies show that CGA 325′615, like DCB, clearly inhibits synthesis of crystalline cellulose in cotton fibers. In terms of potency, CGA 325′615 is effective in nanomolar concentrations, whereas DCB requires micromolar concentrations to be similarly effective (Montezinos and Delmer, 1980). Another distinct difference between these two herbicides comes from our finding that CGA 325′615, but not DCB, also causes accumulation of NCG and, to a variable extent in repeated experiments, also causes some elevation in synthesis of callose. The surprising finding that the CesA proteins GhCesA-1 and GhCesA-2 appear to be intimately associated with this glucan may at least partially explain the unusual solubility properties of this glucan. A substantial portion of the NCG and associated CesA proteins can be extracted from walls by AO, whereas the remainder requires alkali and/or digestion with acetic-nitric reagent to become solubilized. We use the term “AO-soluble” with caution; this fraction of NCG remains soluble after centrifugation at 15,000g for 15 min, passes through a 0.2-μm filter, and is recoverable from a Superdex 200 column. However, the material is clearly of high Mr, with most eluting near and just after the void volume (Figs. 5 and 6), and one cannot exclude aggregation of the material in forms that do not drastically alter its solubility. The fact that the β-1,4-glucan precipitates after protease digestion and chilling suggests that the protein may influence solubility and also that the glucan chains in the NCG must exceed seven Glc residues, a result supported by our methylation analyses. The change in size distribution on Superdex 200 after protease treatment suggests the glucan chains are of quite heterogeneous length. In addition, the fact that additional NCG requires alkali or strong acid for extraction from the walls suggests an even broader distribution in size of the NCG chains.
The change in size distribution and solubility of the NCG after protease treatment suggests that it may be covalently linked to, or at least strongly associated with, protein. Our finding that cotton CesA proteins exist in the NCG and can only be released and detected on SDS-PAGE following cellulase digestion suggests an association between CesA proteins and the NCG. In this regard, during our MALDI-TOF MS analyses of cellulase-released, tryptic-digested CesA, in addition to the predicted fragments shown in Table I, we also detected four other fragments of mass consistent with three to six Glc residues attached to predicted GhCesA-1 or GhCesA-2 peptides of sequences VS/CCYI/VSDD1GAA/SMLT/LFE/DSLV/ SETAD/EFAR that span the region of the first conserved D1. Thus, these peptides might represent a potential site of glucan attachment to CesA. It is unfortunate that these have to date resisted sequence analysis, and further work will be necessary to prove the nature of any true covalent linkage that might exist. It is difficult to envision that a covalent linkage naturally exists between CesA and its acceptor chains as a transient intermediate in glucan chain elongation. Retaining glycosyltransferases may contain a transient covalent linkage between an Asp of the enzyme and the reducing end of the growing glycan chain (Unligil and Rini, 2000). Since CesA is proposed to function as an inverting enzyme that does not have such a predicted intermediate, the D1 in the above peptide does not seem a likely candidate for attachment.
An alternate explanation might be that CGA 325′615 causes some abnormal linkage to be created such as that which occurs in a mutant family 1 β-glucosidase. In this case, the mutant lacks the catalytic nucleophile residue, and the mutant occasionally makes a stable covalent linkage of Glc to a Tyr residue (Gebler et al., 1995). This type of linkage is attractive, as it would be base-stable during methylation analysis and a Tyr (Y) residue is found in the peptides described above from CesA. Yet here again, this linkage was formed in a glucosidase that normally forms a transient covalent intermediate—something that is not expected for family 2 glycosyltransferases such as CesA. We note that Tyr is also the site of attachment of Glc at a non-catalytic site in glycogenin (Rodriguez and Whelan, 1985; Roach and Skurat, 1997). With these uncertainties, one still cannot rule out a very strong non-covalent interaction between CesA and glucan—yet it is hard to imagine any such interaction that would resist disruption in sample buffers for SDS-PAGE. In this regard we do note that the association between Acetobacter xylinum CesA protein and its cellulose product is extremely strong in product-entrapped preparations (Mayer et al., 1991). This CesA is not released by extensive washing at high or low ionic strength, by changes in pH, or by sonication, and it is only partially released by a two-step treatment with cellulase. It is clear that determination of the precise nature of these interactions could shed more light on the mechanism and function of these CesA proteins.
In spite of these uncertainties, there is clearly a very intimate association between the NCG and CesA protein, and the levels of each in the cell wall are clearly enhanced greatly by treatment with CGA 325′615. This close association further adds to the genetic arguments in favor of a role for CesA proteins in cellulose synthesis, and particularly enhances the case for a role chain elongation. The fact that this CesA is found in the wall may indicate that its close association with the glucan has detached it from the plasma membrane during our isolation procedures. In addition to this form of CesA, we have also made the important finding of substantially elevated levels of CesA in the membrane fraction that may only have no more than two Glc residues attached to it, along with elevation in the level of the membrane-associated cellulase (Cel3/Kor) that is believed to play a role in cellulose synthesis. In addition, we have shown that the level of the Cel3/Kor protein (and mRNA; Y. Kawagoe, T.A. Wilkins, and D.P. Delmer, unpublished data) increases during the onset of secondary wall cellulose synthesis in fibers in a manner quite parallel to that of the CesA protein. These findings further support the notion that this protein plays some as-yet-unidentified role in cellulose synthesis in plants. The effect of CGA 325′615 on levels of both proteins also suggests the interesting possibility of some coordinate feedback regulation of the levels of these proteins in plants. We note that our findings are in contradiction to another report (Nakagawa and Sakurai, 1998) that claimed DCB causes an accumulation of CesA protein in tobacco Bright Yellow-2 cells. We have never observed this effect of DCB in cotton fibers or in Bright Yellow-2 cells, and we have no explanation at present for the differences in these results. Nor do our results shed any light on the finding that long-term exposure of tobacco protoplasts to DCB resulted in the accumulation of increased capacity for cellulose synthesis upon removal of the inhibitor (Galbraith and Shields, 1982).
There is a striking analogy between the effect of CGA 325′615 and that which occurs in the rsw1(AtcesA-1) mutant of Arabidopsis that also accumulates at least a small amount a non-crystalline glucan (Arioli et al., 1998; Peng, 1999). However, the rsw1 mutant also accumulates starch as well (Peng et al., 2000)—something that we do not see in cotton fibers treated with the herbicide. However, the analogy with rsw1 is extended further by the recent observation that CGA 325′615 also causes rosette disintegration (W. Herth, K. Kreuz, unpublished data). Thus, CGA 325′615 may act to alter CesA interactions within the synthase complex and may thereby prevent extensive chain elongation and/or further association of chains to form crystalline microfibrils. Why growing glucan chains should be “trapped” by rosette disassembly is not yet clear; one intriguing possibility is that chain elongation occurs by coordinated action of active sites on two adjacent CesA proteins within the complex, a mechanism that might support a modified version of the concept proposed for a two active site model for this enzyme (Koyama et al., 1997; Carpita and Vergara, 1998). (The original model proposed two sites within the same subunit, which does not seem consistent with the number of catalytic motifs present in one CesA polypeptide.) We also note that CesA paralogs expressed within a tissue type often exist in similar, but nonidentical, pairs (e.g. GhCesA-1 and -2 in fibers, AtCesA-4 and -8, and PtCesA-1 and -2 in vascular tissue; Holland et al., 2000). If CGA 325′615 disrupts these active site interactions, further chain elongation may be prevented. In an alternate manner, rosette formation may require nonidentical subunits, but each subunit, when interaction is disrupted can function alone to produce non-crystalline glucan. The concept that two or three nonidentical CesA proteins may be required for cellulose synthesis has also now been raised from genetic evidence in Arabidopsis (Fagard et al., 2000; Taylor et al., 2000).
Thus, use of such a new inhibitor raises many new questions and provides avenues for future experimentation. We have also recently made the surprising finding that cellulase treatment of the AO-NCG also releases steryl-glucoside and acylated sterylglucoside (L. Peng and D.P. Delmer, unpublished data). Such a finding suggests the intriguing possibility that these compounds may serve as primers for cellulose synthesis, a possibility that is under further investigation in our laboratory. Development of the ability to reproducibly synthesize cellulose in vitro still remains an important priority for further extension of the new ideas about the synthetic process suggested by the accumulation of this unusual NCG in response to treatment with CGA 325′615.
MATERIALS AND METHODS
In Vivo Labeling of Cultured Cotton Fibers
Ovules were harvested from fertilized 2 DPA flowers of cotton (Gossypium hirsutum Coker 130) and were cultured for an additional 19 d with associated developing fibers (Roberts et al., 1992). For herbicide treatments, the fibers/ovules were transferred to culture medium in which unlabeled Glc was replaced by [U-14C]Glc (16 μm; 5 μCi mL−1) and 10 mg mL−1 penta-erythritol was also added as osmotic protectant. Stocks of herbicides were dissolved in DMSO, and all samples, including controls, contained the same final concentration (0.01%, v/v) of DMSO. For the experiments shown in Figures 1 and 2, following incubation for 4 h at 30°C, fibers and ovules were washed three times sequentially with water, CM (2:1, v/v), methanol, and acetone. The tissue was extracted after 1 h with acetic-nitric reagent (Updegraff, 1969) at 100°C, and was then filtered to collect acid-soluble (non-crystalline wall material) and acid-insoluble (crystalline cellulose) fractions.
Cell Wall Preparation and Extraction
Labeled ovules plus fibers were washed extensively in water and were then disrupted using an Omni homogenizer using 50 mm sodium phosphate buffer, pH 7.5. The homogenate was centrifuged at 2,800g for 10 min, and the resulting pellet was used for cell wall isolation. This pellet was washed three times in water, three times in CM (1:1), one time in methanol, one time in cold acetone, and two times in water. The walls were then extracted overnight under nitrogen with 90% (v/v) DMSO. The resulting wall pellet was washed three times in water, was extracted for 1 h at 100°C in 0.5% (w/v) AO, and was then centrifuged at 2,800g for 10 min. This supernatant is referred to as the AO fraction and the pellet was again washed three times in water (the first wash being combined with the AO supernatant, the other washes discarded). The pellet was then extracted for 1 h at 25°C in 4 m KOH containing NaBH4 at 1 mg mL−1. Following centrifugation, the supernatant was neutralize, and all soluble fractions were dialyzed extensively against water at 4°C. The wall pellet was then treated with acetic-nitric reagent (Updegraff, 1969) for 1 h at 100°C, centrifuged, and the supernatant was then referred to as the AN-soluble fraction. The final pellet was washed three times in water and is referred to as the crystalline cellulose fraction.
Analytical Methods
Total sugars were quantified by the phenol-sulfuric acid method of Dubois et al. (1956). Protein was quantified using a protein assay kit (Bio-Rad, Hercules, CA) and alditol acetate (Blakeney et al., 1983) or partially-methylated, partially acetylated derivatives of neutral sugars were separated using a capillary column (DB-225, J&W Scientific, Folsom, CA) on a GLC (6890, Hewlett-Packard, Palo Alto, CA); when GLC/MS was carried out, a similar configuration was used coupled to a mass spectrometer (HP 5973, Hewlett-Packard). For collection of radioactive derivatives, a megabore column equipped with a stream splitter was used (DB-225, Hewlett-Packard). Methylation of polysaccharides was performed as described by Needs and Selvendran (1993) using sodium borodeuteride for the reduction step. For TLC, trifluoroacetic acid-released neutral sugars were separated on Silica Gel G thin-layer plates using ethyl acetate:isopropanol:water (7:4:2, v/v) as solvent. Standard sugars were detected by heating plates after spraying with 10% (w/v) H2SO4 in ethanol. Glucanase digestion of the AO fraction was carried out in 50 mm sodium acetate, pH 4.7, containing 2 units mL−1 of Trichoderma endo-1,4-β-glucanase or 1.4 units mL−1 of exo-1,3-β-glucanase (Megazyme). Digestion of the AO fraction with Proteinase K (Boehringer Mannheim, Basel; 2.5 units mL−1) was carried out in 10 mm Tris/HCl buffer, pH 8.0. Following incubation at 37°C overnight, samples were lyophilized and resuspended in water for TLC or GLC in sample buffer for SDS-PAGE and western blotting (see below), or in 0.1% (w/v) AO, and were passed through a 0.2-μm filter for use in gel filtration chromatography using a Superdex 200 column at a flow rate of 1 mL min−1 with 0.1% (w/v) AO as solvent. This Proteinase K preparation was tested in overnight incubations under the conditions used in these experiments, and it displayed no detectable ability to hydrolyze crystalline cellulose or carboxymethylcellulose.
MALDI-TOF MS
Unless otherwise noted, all chemicals were purchased from Sigma (St. Louis) and were of analytical grade. a-Cyano-4-hydroxycinnamic acid was from Aldrich Chemical Company (Milwaukee, WI). MilliQ water (Millipore, Bedford, MA) was used to prepare all solutions. For mass spectrometric analysis and preparation of digests, HPLC-grade methanol and acetonitrile were used. Trypsin was sequencing grade and was from Boehringer Mannheim (Germany). For trypsin digestion, proteins from cellulase-digested AO fraction were separated by SDS-PAGE. The region corresponding to the mixture of CesA-1 and CesA-2 polypeptides was excised and subjected to an in-gel digestion procedure as previously described by Matsui et al. (1997). The procedure includes washing and drying of gels, reduction and alkylation, rehydration with a trypsin buffer solution, incubation for 12 to 16 h at 37°C, and peptide extraction. Unseparated in-gel tryptic digests were further desalted using C18 ZipTips (Millipore) and were eluted in 3 μL of an elution buffer containing 10 mm a-cyano-4-hydroxycinnamic acid dissolved with 60% (v/v) acetonitrile in 0.1% (v/v) trifluoroacetic acid and purified by HPLC. MALDI samples were prepared using the overlay method (Vorm et al., 1994; Edmondson and Russell, 1996). In brief, 0.5 to 1 μL of a saturated solution of a-cyano-4-hydroxycinnamic acid in methanol was deposited on the MALDI sample plate and the solvent was evaporated to form a thin matrix base layer. ZipTiped digest (0.5 μL) was placed onto the previously prepared matrix base layer and was allowed to air dry. All MALDI-TOF mass spectra were acquired using a MALDI TOF (BIFLEX III, Bruker Instruments, Billerica, MA) equipped with a pulse nitrogen laser (337 nm) and operated in the reflectron mode. Signals from 200 laser shots were averaged to increase the signal to noise ratio of each mass spectrum. All mass spectra were internally calibrated using the Angiotensin II (M + H) + signal (m/z = 1046.5417) and trypsin autoproteolysis signal (m/z = 2211.1046).
SDS-PAGE, Antibody Reactions, and Western Blotting
The resulting precipitated glucan resulting from Proteinase K treatment of the AO fractions from control or herbicide-treated fibers (10 nm CGA 325/615 or 25 μm DCB for 4 h) was resuspended in water and was spotted onto a polyvinylidene difluoride membrane. These were then probed for binding with 5 μg of a purified CBD protein that recognizes crystalline or non-crystalline domains of cellulose (Ziv Shani, CBD Technologies, Rehovot, Israel) followed by reaction with rabbit anti-CBD serum (1:1,000) and detection by enhanced chemiluminescence using goat anti-rabbit peroxidase as second antibody.
Membrane-associated proteins were prepared by centrifugation of the low-speed supernatants used for cell wall isolation (see above) at 100,000g for 1 h at 4°C. Equal amounts of protein (10 μg) were separated by SDS-PAGE using Nu-Page 4% to 12% gradient gels (Invitrogen Corp., Carlsbad, CA) and were then blotted onto nitrocellulose. Similar techniques were used for the proteins from the AO fraction before or after endocellulase treatment; in this case, protein levels were too low to quantify accurately, and so equivalent volumes (<2 μg of protein excluding the cellulase) of the AO fractions from the same number of control or herbicide-treated ovules/fibers were loaded. Rabbit polyclonal antibody against tomato membrane-associated cellulase (Cel3) was provided by Alan Bennett (University of California, Davis) and was used at a 1:1,000 dilution. CesA proteins were detected by use of a rat polyclonal antibody (1:3,000) prepared by our laboratory that was directed against the purified His-tagged recombinant zinc-finger domain of GhCesA-1. Second antibodies used were purchased from Sigma and were included goat anti-rat (for CesA) or anti-rabbit (for cellulase) IgG coupled to peroxidase; detection was by enhanced chemiluminescence.
ACKNOWLEDGMENTS
We thank Pat Hogan, Ravit Eshed, Alison Roberts, Debbie Wilk, and Monika Doblin for providing stimulating advice, Alan Bennett for generously providing the Cel3 antibody, and Ziv Shani (CBD Technologies) for providing CBDs and antibodies against CBDs.
Footnotes
This work was supported by Novartis Crop Protection and by the U.S. Department of Energy (grant no. DE–FG–03–963–ER 20238 to D.P.D.).
LITERATURE CITED
- Arioli T, Peng L, Betzner AS, Burn J, Wittke W, Herth W, Camilleri C, Hofte H, Plazinski J, Birch R. Molecular analysis of cellulose biosynthesis in Arabidopsis. Science. 1998;279:717–720. doi: 10.1126/science.279.5351.717. [DOI] [PubMed] [Google Scholar]
- Blakeney AB, Harris PJ, Henry RJ, Stone BA. Simple and raid preparation of alditol acetates for monosaccharide analysis. Carbohydr Res. 1983;113:291–299. [Google Scholar]
- Brummell DA, Catala C, Lashbrook CC, Bennett AB. A membrane-anchored E-type endo-1,4-beta-glucanase is localized on Golgi and plasma membranes of higher plants. Proc Natl Acad Sci USA. 1997;94:4794–4799. doi: 10.1073/pnas.94.9.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JA, Davies GJ, Bulone V, Henrissat B. A classification of nucleotide-diphospho-sugar glycosyltransferases based on amino acid sequence similarities. Biochem J. 1997;326:929–942. doi: 10.1042/bj3260929u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita NC, Vergara C. A recipe for cellulose. Science. 1998;279:672–673. doi: 10.1126/science.279.5351.672. [DOI] [PubMed] [Google Scholar]
- Charnock SJ, Davies GJ. Structure of the nucleotide-diphospho-sugar transferase, SpsA from Bacillus subtilis, in native and nucleotide-complexed forms. Biochemistry. 1999;38:6380–6385. doi: 10.1021/bi990270y. [DOI] [PubMed] [Google Scholar]
- Delmer DP. Cellulose synthesis: exciting times for a difficult field. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:245–276. doi: 10.1146/annurev.arplant.50.1.245. [DOI] [PubMed] [Google Scholar]
- Delmer DP, Read SM, Cooper G. Identification of a protein receptor in cotton fibers for the herbicide 2,6-dichlorobenzonitrile. Plant Physiol. 1987;84:415–420. doi: 10.1104/pp.84.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- Edmondson RD, Russell DH. Evaluation of matrix-assisted laser desorption ionization-time-of-flight mass measurement accuracy by using delayed extraction spectrometry. J Am Soc Mass Spectrom. 1996;7:995–1080. doi: 10.1016/1044-0305(96)00027-X. [DOI] [PubMed] [Google Scholar]
- Fagard M, Desnos T, Desprez T, Goubet F, Réfrégier G, Mouille G, McCann M, Rayon C, Vernhettes S, Höfte H. PROCUSTE1 encodes a cellulose synthase required for normal cell elongation specifically in roots and dark-grown hypocotyls of Arabidopsis. Plant Cell. 2000;12:2409–2423. doi: 10.1105/tpc.12.12.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith DW, Shields BA. The effects of inhibitors of cell wall synthesis on tobacco protoplast development. Physiol Plant. 1982;55:25–30. [Google Scholar]
- Gebler JC, Trimbur DE, Warren RA, Aebersold R, Namechuk M, Withers SG. Substrate-induced inactivation of a crippled β-glucosidase mutant: identification of the labeled amino acid and mutagenic analysis of its role. Biochemistry. 1995;34:14547–14553. doi: 10.1021/bi00044a033. [DOI] [PubMed] [Google Scholar]
- Heim DR, Skomp JR, Tschabold EE, Larrinua IM. Isoxaben inhibits the synthesis of acid insoluble cell wall materials in Arabidopsis thaliana. Plant Physiol. 1990;93:695–700. doi: 10.1104/pp.93.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland N, Holland N, Helentjaris T, Dhugga KS, Xoconostle-Cazares B, Delmer DP. A comparative analysis of the plant cellulose synthase (CesA) gene family. Plant Physiol. 2000;123:1313–1323. doi: 10.1104/pp.123.4.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S, Laosinchai W, Itoh T, Cui X, Linder CR, Brown RM., Jr Immunogold labeling of rosette terminal cellulose-synthesizing complexes in the vascular plant Vigna angularis. Plant Cell. 1999;11:2075–2086. doi: 10.1105/tpc.11.11.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama M, Helbert W, Imai R, Sugiyama J, Henrissat B. Parallel-up structure evidences the molecular directionality during biosynthesis of bacterial cellulose. Proc Natl Acad Sci USA. 1997;94:9091–9095. doi: 10.1073/pnas.94.17.9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltby DM, Carpita N, Montezinos D, Kulow C, Delmer DP. β-1,3-Glucan in developing cotton fibers. Plant Physiol. 1979;63:1158–1164. doi: 10.1104/pp.63.6.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui NM, Smith DM, Clauser KR, Fichmann J, Andrews LE, Sullivan CM, Burlingame AL, Epstein LB. Immobilized pH gradient two-dimensional gel electrophoresis and mass spectrometric identification of cytokine-regulated proteins in ME-180 cervical carcinoma cells. Electrophoresis. 1997;18:409–417. doi: 10.1002/elps.1150180315. [DOI] [PubMed] [Google Scholar]
- Mayer RM, Ross P, Weinhouse H, Amikam D, Volman G, Ohana P, Calhoon RD, Wong HC, Emerick AW, Benziman M. Polypeptide composition of bacterial cyclic diguanylic acid-dependent cellulose synthase and the occurrence of immunologically cross-reacting proteins in higher plants. Proc Natl Acad Sci USA. 1991;88:5472–5476. doi: 10.1073/pnas.88.12.5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinert M, Delmer DP. Changes in biochemical composition of the cell wall of the cotton fiber during development. Plant Physiol. 1977;59:1088–1097. doi: 10.1104/pp.59.6.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montezinos D, Delmer DP. Characterization of inhibitors of cellulose synthesis in cotton fibers. Planta. 1980;148:305–311. doi: 10.1007/BF00388116. [DOI] [PubMed] [Google Scholar]
- Nakagawa N, Sakurai N. Increase in the amount of celA-1 protein in tobacco BY-2 cells by a cellulose biosynthesis inhibitor, 2,6-dichlorobenzonitrile. Plant Cell Physiol. 1998;39:779–785. doi: 10.1093/oxfordjournals.pcp.a029434. [DOI] [PubMed] [Google Scholar]
- Needs PW, Selvendran RR. An improved methylation procedure for the analysis of complex polysaccharides including resistant starch and critique of the factors which lead to undermethylation. Phytochem Anal. 1993;4:210–216. [Google Scholar]
- Nicol F, His I, Jauneau A, Vernhettes S, Canut H, Hofte H. A plasma membrane-bound putative endo-1,4-β-d-glucanase is required for normal wall assembly and cell elongation in Arabidopsis. EMBO J. 1998;17:5563–5576. doi: 10.1093/emboj/17.19.5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pear JR, Kawagoe Y, Schreckengost W, Delmer DP, Stalker DM. Higher plants contain homologs of the bacterial CelA genes encoding the catalytic subunit of the cellulose synthase. Proc Natl Acad Sci USA. 1996;93:12637–12642. doi: 10.1073/pnas.93.22.12637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L, Hocart CH, Redmond JW, Williamson RE. Fractionation of carbohydrates in Arabidopsis root cell walls shows that three radial swelling loci are specifically involved in cellulose production. Planta. 2000;211:406–414. doi: 10.1007/s004250000301. [DOI] [PubMed] [Google Scholar]
- Peng LC. Characterization of cellulose biosynthesis in Arabidopsis thaliana. PhD thesis. Canberra, Australia: Australian National University; 1999. [Google Scholar]
- Richmond TA, Somerville CR. The cellulose synthase superfamily. Plant Physiol. 2000;124:495–498. doi: 10.1104/pp.124.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach PJ, Skurat AV. Self-glucosylating initiator proteins and their role in glycogen biosynthesis. Nucleic Acids Res. 1997;57:289–315. doi: 10.1016/s0079-6603(08)60284-6. [DOI] [PubMed] [Google Scholar]
- Roberts EM, Nunna RR, Huang JY, Trolinder NL, Haigler CH. Effects of cycling temperatures on fiber metabolism in cultured cotton ovules. Plant Physiol. 1992;100:979–986. doi: 10.1104/pp.100.2.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez IR, Whelan WJ. A novel glycosyl-amino acid linkage: rabbit-muscle glycogen is covalently linked to a protein via tyrosine. Biochem Biophys Res Commun. 1985;132:829–836. doi: 10.1016/0006-291x(85)91206-9. [DOI] [PubMed] [Google Scholar]
- Sabba RP, Vaughn KC. Herbicides that inhibit cellulose biosynthesis. Weed Sci. 1999;47:757–763. [Google Scholar]
- Saxena IM, Brown RM, Jr, Fevre M, Geremia RA, Henrissat B. Multidomain architecture of β-glycosyltransferases: implications for mechanism of action. J Bacteriol. 1995;177:1419–1424. doi: 10.1128/jb.177.6.1419-1424.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoller A, Haake M, Zondler H, inventors June 26, 1995. Preparation of 3-amino-1,2,4,6-thiatriazines asherbicides. Patent Cooperation Treaty International Application No. WO 9601814
- Taylor NG, Laurie S, Turner SR. Multiple cellulose synthase catalytic subunits are required for cellulose synthesis in Arabidopsis. Plant Cell. 2000;12:2529–2539. doi: 10.1105/tpc.12.12.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NG, Scheible W, Cutler S, Somerville CR, Turner SR. The irregular xylem3 locus of Arabidopsis encodes a cellulose synthase required for secondary wall synthesis. Plant Cell. 1999;11:769–779. doi: 10.1105/tpc.11.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnessen BA, Ellefsen O. Submicroscopical investigations. In: Bikales NM, Segal L, editors. Cellulose and Cellulose Derivatives. New York: Wiley-Interscience; 1971. pp. 265–304. [Google Scholar]
- Unligil UM, Rini JM. Glycosyltransferase structure and mechanism. Curr Opin Struct Biol. 2000;10:510–517. doi: 10.1016/s0959-440x(00)00124-x. [DOI] [PubMed] [Google Scholar]
- Updegraff DM. Semi-micro determination of cellulose in biological materials. Anal Biochem. 1969;32:420–424. doi: 10.1016/s0003-2697(69)80009-6. [DOI] [PubMed] [Google Scholar]
- Vorm O, Roepstorff P, Mann M. Improved resolution and very high sensitivity in MALDI TOF of matrix surfaces made by fast evaporation. Anal Chem. 1994;66:3281–3287. [Google Scholar]