Abstract
Objective: To investigate the value of combined detection of T-cell immunoglobulin mucin molecule 3 (sTim-3), pepsinogen (PG) and programmed death receptor 1 (PD-L1) in evaluating treatment efficacy and predicting the prognosis of immunosuppressant therapy in advanced gastric cancer. Methods: A retrospective study was conducted on the data of 90 patients with advanced gastric cancer who were treated at the First Affiliated Hospital of Changsha Medical University from January 2019 to February 2021. Patients were divided into effective and ineffective groups based on treatment response. Logistic regression was used to identify the factors affecting the efficacy of immune checkpoint inhibitors in treating progressive gastric cancer. ROC curves were drawn to assess the predictive value of serum sTim-3, PG, and PD-L1 alone or in combination. Serum levels of sTim-3, PG, PD-L1 were compared between the survival and death groups, and univariate and Cox proportional risk regression analyses were conducted to identify factors affecting prognosis of patients with progressive gastric cancer. Survival curves were plotted using the Kaplan-Meier method, and ROC curves were used to assess the prognostic value of combined and individual testing of sTim-3, PG, and PD-L1. Results: No statistically significant difference were observed between the effective and ineffective groups in terms of gender, age, body mass index, smoking history, alcohol consumption history, KPS score, tumor site, presence of ascites, or use of other therapies (all P>0.05). The levels of sTim-3, PG II, and PD-L1 were higher in the ineffective group than those in the effective group, while PG I was lower than that in the effective group (all P<0.05). Logistic regression analysis showed that sTim-3 (OR=2.408), PG I (OR=1.779) and PD-L1 (OR=1.844) were independent risk factors for treatment efficacy of immune checkpoint inhibitors in advanced gastric cancer (all P<0.05). The AUC value of combined detection of serum sTim-3, PG I and PD-L1 for treatment efficacy was higher than their individual detection (P<0.05). The serum levels of sTim-3 and PD-L1 in the death group were higher than those in the survival group, while PG I was lower than that in the survival group. The levels of sTim-3 (HR=2.686), PG I (HR=2.782) and PD-L1 (HR=2.018) were independent prognostic factors in patients with advanced gastric cancer. The AUC of the combined detection of sTim-3, PG I and PD-L1 in predicting the prognosis of patients with advanced gastric cancer was significantly higher than their individual detection (all P<0.05). Log-rank test showed that the 3-year survival rates of patients with high sTim-3 and PD-L1 levels were significantly higher than those with low levels (all P<0.05). Conclusion: sTim-3, PG and PD-L1 have significant clinical value in predicting treatment efficacy and prognosis of advanced gastric cancer patients undergoing immunosuppressive therapy.
Keywords: Advanced gastric cancer, Immunosuppressant, T cell immunoglobulin mucin molecule 3, pepsinogen, programmed death receptor 1
Introduction
Gastric cancer is a common malignant tumor with a low survival rate. Its pathogenesis remains unclear, and current treatments primarily include palliative surgery and adjuvant treatment of radiotherapy, which can improve patient survival to a certain extent [1,2]. However, existing biopsy and imaging methods have a low early detection rate, meaning most patients are diagnosed at advanced stages, missing the optimal time for surgical treatment, resulting in poor prognosis. The commonly used method for predicting treatment efficacy, the micro-tumor model, is limited by high establishment and maintenance costs, and a narrow range of detectable drugs.
With increasing research into tumor immunity, immune checkpoint inhibitors have emerged as a novel and effective option for antitumor therapy. Study [3] has shown that the use of immune checkpoint inhibitors in combination with chemotherapy as first-line treatment in progressive gastric cancer is more advantageous in prolonging the survival of patients, though some patients may not benefit due to individual differences. Therefore, finding effective molecular markers to effectively evaluate the efficacy and prognosis of immune checkpoint inhibitors in treating progressive gastric cancer is crucial for improving patient outcomes.
Tumor marker levels are commonly used to screen for malignant diseases. T-cell immunoglobulin mucin molecule 3 (sTim-3) is an immunosuppressive factor involved in immune regulation and inflammation or autoimmune responses [4]. sTim-3 is highly expressed in the serum of patients with a variety of malignant tumors and is closely associated with disease activity. Pepsinogen (PG), a precursor of pepsin found in the gastric fundus gland cells, is divided into PG I and PG II. Changes in PG I levels reflect the function of gastric secretory gland cells, while PG II is closely related to the function and structure of the gastric fundus mucosa.
Immune escape is one of the key mechanisms in tumorigenesis and progression [5]. Programmed death receptor 1 (PD-L1) inhibits T-cell activity to prevent over-activation of the immune response; however, its signaling pathway may lead to immune evasion, allowing tumor cells to escape immune surveillance and destruction [6]. To date, there are no reports clearly demonstrating the value of combined testing of sTim-3, PG, and PD-L1 in evaluating the clinical efficacy and prognosis of gastric cancer patients. The aim of this study was to investigate the changes in the levels of sTim-3, PG, and PD-L1 in progressive gastric cancer and their predictive value in assessing treatment outcomes and prognosis in patients receiving immune checkpoint inhibitors.
Materials and methods
General data
A retrospective analysis was conducted on the clinical data of 90 patients with advanced gastric cancer admitted to The First Affiliated Hospital of Changsha Medical University from January 2019 to February 2021. The patients were categorized into an effective group (n=34) and an ineffective group (n=56) based on their treatment efficacy. The study was approved by the First Affiliated Hospital of Changsha Medical University Ethics Committee.
Inclusion criteria: 1. Patients meeting the diagnostic criteria outlined in the esophageal and gastric cancer treatment guidelines [7]. 2. Patients with unresectable lesions confirmed by imaging and treated with immune checkpoint inhibitors. 3. Patients with good treatment adherence, having received at least 4 cycles of immune checkpoint inhibitor therapy. 4. Patients tested for PD-L1 expression prior to treatment with immune checkpoint inhibitors.
Exclusion criteria: 1. Patients unable to assess the treatment efficacy or unable to cooperate with follow-up visits. 2. Patients unable to tolerate chemotherapy or immunotherapy. 3. Patients with incomplete clinical information. 4. Patients with other primary malignant tumors. 5. Patients with digestive tract deformities or other digestive disorders.
Immune checkpoint inhibitor therapy
All patients received at least two cycles of immune checkpoint inhibitor therapy. Treatment approaches included immunotherapy, immunotherapy combined with chemotherapy, or targeted combination therapy. The drugs used in immunotherapy included PD-1/PD-L1 antibodies, Nivolumab or Pembrolizumab, (Shanghai Hengrui Pharmaceutical Co., Ltd.; 2019S00365/202303001). Additionally, 200 mg Xindilimumab (Xinda Biopharmaceutical Co, Ltd.; batch number: S20180016) diluted in 100 mL of 0.9% sodium chloride solution and administered as an intravenous infusion over 30 minutes. Each treatment cycle lasted 21 days, with two consecutive cycles administered.
Collection of clinical information
Clinical data of patients with progressive gastric cancer were collected, including gender, age, body mass index (BMI), smoking history, alcohol consumption history, and Karnofsky performance status (KPS) score for quality of life [8]. The KPS score, ranging from 0 to 100 points, assesses physical status and functional status, with higher score suggesting better quality of life.
Laboratory indicators
After admission, 5 mL of fasting venous blood was drawn from each patient in the morning. The blood samples were left to stand for 2 hours before centrifugation at 3500 r/min for 10 min, with a centrifugation radius of 10.6 cm. The upper layer of the serum supernatant was collected, and the serum levels of sTim-3 and PD-L1 were detected using enzyme-linked immunosorbent assay (ELISA). Serum PG I and PG II levels were detected using enhanced chemiluminescence.
Evaluation of treatment efficacy
Treatment efficacy was assessed based on the “Rating Criteria for Evaluation of Efficacy in Solid Tumor Treatment (RECIST)” [9] and classified into complete remission, partial remission, stable disease, and disease progression. The effective rate was calculated as (complete remission + partial remission)/total number of cases × 100%.
Follow up
Patients were followed for 3 years (up to February 2024) using WeChat, telephone, and outpatient visits. The patients were further categorized into survival and death groups based on their survival status. Death was defined as mortality caused by gastric cancer or its metastasis.
Statistical analysis
All data were analyzed using SPSS 22.0 statistical analysis software. Continuous data were expressed as mean ± standard deviation (x̅±s) and compared between groups using independent t-tests. Categorical data were expressed as percentages (n/%) and compared using chi-square tests. Factors influencing the clinical efficacy of immune checkpoint inhibitors in progressive gastric cancer were analyzed using logistic regression analysis. The predictive value of sTim-3, PG, and PD-L1, both individually and in combination, was assessed using receiver operating characteristic (ROC) curve analysis. Prognostic factors for patients with progressive gastric cancer were analyzed using univariate and Cox proportional hazards regression. Survival curves were generated using the Kaplan-Meier method. A p-value of less than 0.05 was considered significant.
Results
Therapeutic efficacy in the included subjects
After treatment with immune checkpoint inhibitors, among the 90 patients with progressive gastric cancer, there were 13 cases of complete remission, 21 cases of partial remission, 38 cases of stable disease, and 18 cases of disease progression, with an overall effective rate of 37.78%. Thus, the patients were grouped into an effective group with 34 cases and an ineffective group with 56 cases.
Comparison of clinical data and serologic indices between the effective and ineffective groups
There were no statistically significant differences between the two groups in terms of gender, age, BMI, smoking history, alcohol consumption history, KPS score, tumor site, presence of ascites, or use of additional therapies (all P>0.05). However, the ineffective group had more immunotherapy cycles, and higher levels of sTim-3, PG II, and PD-L1 than those of the effective group, while PG I levels were lower than that of the effective group (all P<0.05), as shown in Table 1.
Table 1.
Clinical data and serologic indices of the two groups
| Parameters | Effective group (n=34) | Ineffective group (n=56) | t/χ2 | P |
|---|---|---|---|---|
| Gender (Male/Female) | 19/15 | 34/22 | 0.204 | 0.651 |
| Age (years) | 58.31±9.12 | 58.34±9.34 | 0.015 | 0.988 |
| Body mass index (kg/m2) | 22.25±2.33 | 22.26±2.29 | 0.020 | 0.984 |
| Smoking history | 17 (50.00) | 25 (44.64) | 0.244 | 0.621 |
| Drinking history | 20 (58.82) | 38 (67.86) | 0.753 | 0.385 |
| KPS scores (<70/≥70) | 15/19 | 32/24 | 1.439 | 0.230 |
| Tumor site (Gastric/Gastroesophageal junction) | 26/8 | 45/11 | 0.192 | 0.661 |
| Presence of ascites | 23 | 41 | 0.319 | 0.572 |
| Number of immunotherapy lines (<2/≥2) | 20/14 | 16/40 | 8.067 | 0.005 |
| Use of other therapies | 26 | 47 | 0.768 | 0.381 |
| sTim-3 (mg/L) | 1.98±0.32 | 2.28±0.39 | 3.777 | <0.001 |
| PG I (ng/mL) | 43.77±8.43 | 37.58±7.97 | 3.495 | 0.001 |
| PG II (ng/mL) | 12.17±2.52 | 14.80±3.32 | 3.973 | <0.001 |
| PD-L1 (pg/mL) | 195.44±32.86 | 231.48±44.57 | 4.085 | <0.001 |
Note: KPS: Quality of Life Karnofsky Score; STim-3: immunoglobulin mucin molecule 3; PG: pepsinogen; PD-L1: programmed death ligand-1.
Identification of factors influencing the treatment effectiveness
Logistic regression analysis showed that sTim-3 (OR=2.408), PG I (OR=1.779), and PD-L1 (OR=1.844) were all risk factors affecting the treatment efficacy of immune checkpoint inhibitors in progressive gastric cancer patients (all P<0.05), as shown in Table 2.
Table 2.
Logistic regression analysis of factors affecting the treatment efficacy of immune checkpoint inhibitors in patients with progressive gastric cancer
| Parameters | β | SE | Wald/χ2 | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| Number of immunotherapy lines ≥2 | 0.654 | 0.351 | 3.472 | 0.062 | 1.923 | 0.967-3.827 |
| sTim-3 | 0.879 | 0.214 | 16.871 | <0.001 | 2.408 | 1.583-3.664 |
| PG I | 0.576 | 0.023 | 627.176 | <0.001 | 1.779 | 1.700-1.861 |
| PG II | 0.457 | 0.327 | 1.953 | 0.162 | 1.579 | 0.832-2.998 |
| PD-L1 | 0.612 | 0.213 | 8.256 | 0.004 | 1.844 | 1.215-2.800 |
Note: STim-3: immunoglobulin mucin molecule 3; PG: pepsinogen; PD-L1: programmed death ligand-1.
Predictive values of serum sTim-3, PG I, and PD-L1 alone or in combination for treatment efficacy
The results of ROC curve analysis showed that the AUC value of the combined detection of serum sTim-3, PG I, and PD-L1 in predicting the efficacy of immune checkpoint inhibitors in the treatment of progressive gastric cancer was significantly higher than their individual detection (all P<0.05), as shown in Table 3 and Figure 1.
Table 3.
Predictive value of serum sTim-3, PG I, and PD-L1 alone and in combination for treatment efficacy
| Parameters | AUC | Standard error | 95% CI | P | Cut-off | Youden index | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|---|---|
| sTim-3 | 0.788* | 0.048 | 0.689-0.867 | <0.001 | >2.171 mg/L | 0.613 | 64.29 | 97.06 |
| PG I | 0.661* | 0.059 | 0.554-0.758 | 0.005 | >46.970 ng/mL | 0.250 | 25.00 | 100.00 |
| PD-L1 | 0.712* | 0.054 | 0.607-0.802 | <0.001 | >205.805 pg/mL | 0.378 | 64.29 | 73.53 |
| Combine | 0.903 | 0.334 | 0.823-0.956 | <0.001 | - | 0.798 | 85.71 | 94.12 |
Note: STim-3: immunoglobulin mucin molecule 3; PG: pepsinogen; PD-L1: programmed death ligand-1.
P<0.05, compare with combined testing.
Figure 1.
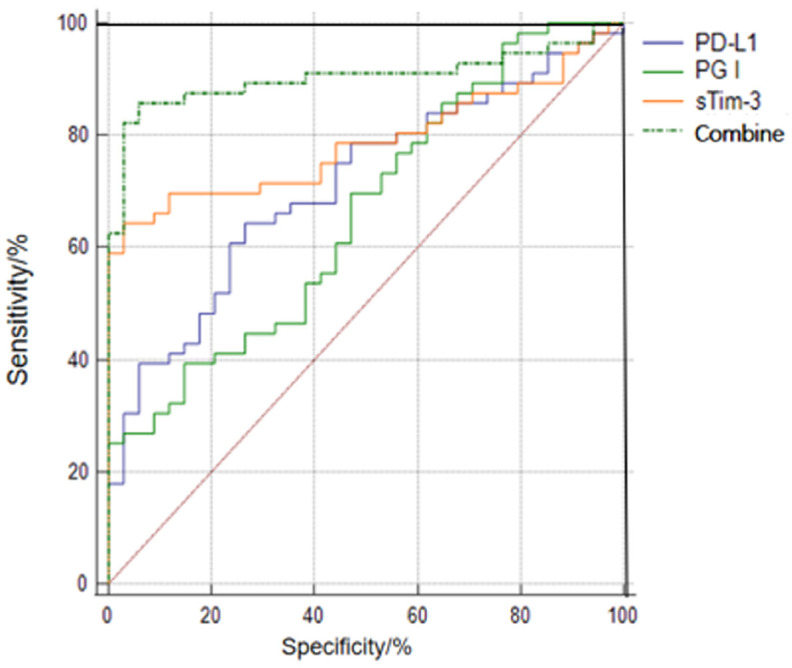
ROC curves of serum sTim-3, PG I, and PD-L1 alone and in combination for predicting treatment efficacy.
Comparison of serum levels of sTim-3, PG I, PD-L1 between the survival and death groups
Follow-up findings revealed 59 cases in the survival group and 31 cases in the death group. Serum sTim-3 and PD-L1 levels in the death group were significantly higher than those in the survival group, while PG I level was lower than that in the survival group (all P<0.05), as shown in Table 4.
Table 4.
Comparison of serum sTim-3, PG I, and PD-L1 levels between the survival and death groups
| Parameters | Survival group (n=59) | Death group (n=31) | t | P |
|---|---|---|---|---|
| sTim-3 (mg/L) | 2.35±0.46 | 2.87±0.53 | 4.833 | <0.001 |
| PG I (ng/mL) | 50.65±8.71 | 43.23±7.25 | 4.059 | <0.001 |
| PD-L1 (pg/mL) | 235.71±33.28 | 268.50±45.25 | 3.912 | <0.001 |
Note: STim-3: immunoglobulin mucin molecule 3; PG: pepsinogen; PD-L1: programmed death ligand -1.
Identification of factors influencing patient prognosis
Cox proportional hazard regression analysis revealed that the levels of sTim-3 (HR=2.686), PG I (HR=2.782) and PD-L1 (HR=2.018) were independent factors influencing the prognosis of patients with progressive gastric cancer (all P<0.05), as shown in Table 5.
Table 5.
Cox proportional risk regression analysis of factors influencing the prognosis of progressive gastric cancer treated with immune checkpoint inhibitors
| Parameters | β | SE | Wald/χ2 | P | HR | 95% CI |
|---|---|---|---|---|---|---|
| sTim-3 | 0.988 | 0.311 | 10.092 | 0.001 | 2.686 | 1.460-4.941 |
| PG I | 1.023 | 0.476 | 4.619 | 0.032 | 2.782 | 1.094-7.071 |
| PD-L1 | 0.702 | 0.264 | 7.071 | 0.008 | 2.018 | 1.203-3.385 |
Note: STim-3: immunoglobulin mucin molecule 3; PG: pepsinogen; PD-L1: programmed death ligand-1.
Predictive values of serum sTim-3, PG I, and PD-L1 alone or in combination for patient prognosis
The results of ROC curve analysis showed that the AUC of the combined test of sTim-3, PG I, and PD-L1 in predicting the prognosis of patients with progressive gastric cancer was significantly higher than their individual test (all P<0.05), as shown in Table 6 and Figure 2.
Table 6.
Predictive value of serum sTim-3, PG I, and PD-L1 alone or in combination for patient prognosis
| Parameters | AUC | Standard error | 95% CI | P | Cut-off | Youden index | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|---|---|
| sTim-3 | 0.776* | 0.050 | 0.676-0.857 | <0.001 | >2.889 mg/L | 0.445 | 58.06 | 86.44 |
| PG I | 0.683* | 0.683 | 0.576-0.777 | 0.001 | ≤46. 853 ng/mL | 0.325 | 61.29 | 71.19 |
| PD-L1 | 0.753* | 0.052 | 0.651-0.838 | <0.001 | >243.425 pg/mL | 0.381 | 83.87 | 54.24 |
| Combine | 0.878 | 0.036 | 0.792-0.937 | <0.001 | - | 0.622 | 77.42 | 84.75 |
Note: STim-3: immunoglobulin mucin molecule 3; PG: pepsinogen; PD-L1: programmed death ligand-1.
P<0.05, compare with combined testing.
Figure 2.
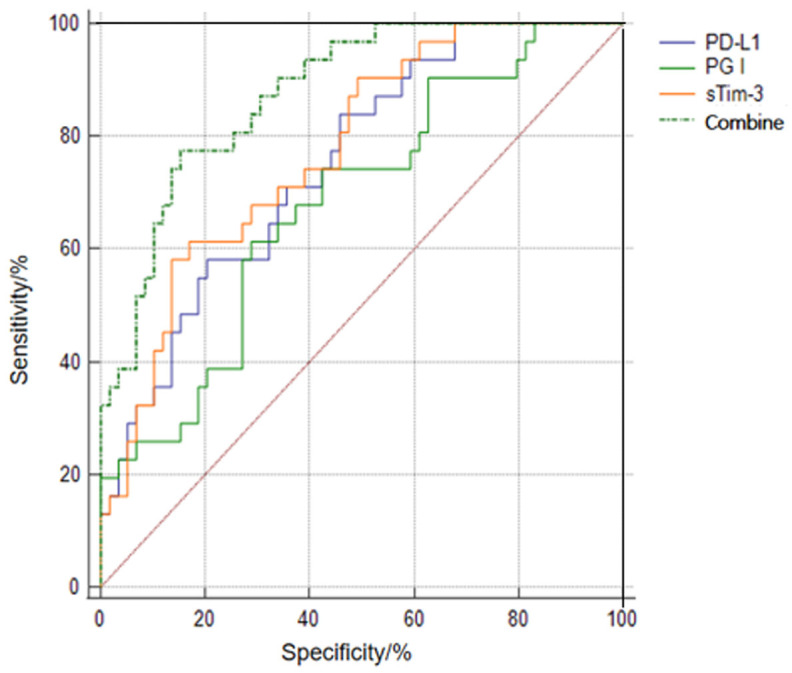
ROC curves of serum sTim-3, PG I, and PD-L1 alone and in combination for patient prognosis.
Comparison of survival time
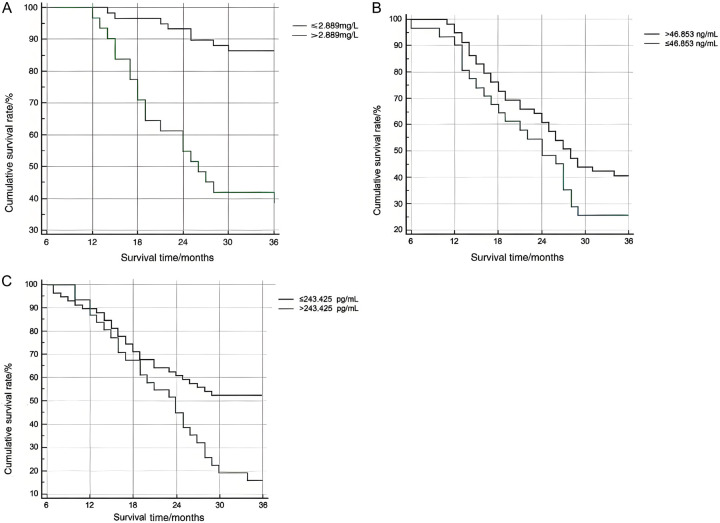
The patients were further sub-divided into high and low expression groups based on the cut-off values obtained from the ROC analysis. The log-rank test showed that the 3-year survival rate in patients with low sTim-3 (≤2.889 mg/L) and PD-L1 (≤243.425 pg/mL) expression levels were significantly higher than those with high sTim-3 (>2.889 mg/L) and PD-L1 (>243.425 pg/mL) levels (all P<0.05). However, the difference was not statistically significant between patients with low and high PG I expression levels (P>0.05), as shown in Figure 3.
Figure 3.
Comparison of 3-year survival rates in advanced cancer patients with different levels of sTim-3, PG I, and PD-L1 (A: Tim-3, B: PG I, C: PD-L1).
Discussion
Gastric cancer poses serious threats to patient life and health. The pathogenesis of gastric cancer remains unclear, though it is believed to be influenced by many factors, including diet, lifestyle, environmental conditions, and genetic predisposition. Abdominal pain, vomiting blood, wight loss, and anemia are the main clinical manifestations of gastric cancer [10]. Early clinical symptoms of gastric cancer are often non-specific, the disease typically progresses insidiously. By the time symptoms are significant, the opportunity for surgical treatment if often lost, leaving most patients reliant on conservative treatments [11]. Immune checkpoints play a crucial role in immune tolerance in gastric cancer, and immunosuppressants have been used to regulate immune function in tumor patients. Therefore, immune checkpoint inhibitor therapy has become the preferred therapeutic strategy for patients with progressive gastric cancer [12]. While the evaluation criteria for the efficacy of solid tumors are widely used to assess the efficacy of anticancer drugs, these criteria, which largely focus on changes in tumor size, may not fully reflect treatment response or predict prognosis accurately. Therefore, identifying more reliable tumor markers to better assess tumor presence and growth is essential for improving efficacy evaluation and prognosis prediction.
Tim-3, a member of the TIM family, is a surface inhibitory molecule on T cells that exists in two forms: soluble Tim-3 (sTim-3) and membrane-bound Tim-3. abnormal expression of s Tim-3 inhibits immune cell proliferation and promotes tumor growth [13,14]. Wang et al. [15] reported that sTim-3, as a tumor immune checkpoint molecule, was significantly elevated in patients with hepatocellular carcinoma, indicating that its level were closely related to the disease activity and prognosis. In a study by Zhang Xinxin et al. [16], the number of immunotherapy lines (≥2) was found to be an influencing factor for gastric cancer, though their analysis was limited by the small sample size. When gastric function is impaired, increased gastric acid secretion stimulates the acid-secreting glands, resulting in abnormal levels of PG expression. This suggests atrophy of the gastric fundus glandular ducts and the presence of intestinal epithelial metaplasia [17]. Study [18] has shown that PG is organ-specific and plays a critical role in the development and progression of gastric cancer, making it a valuable indicator to predict the extent of malignant proliferation of gastric cancer. PD-L1 is an inhibitory co-stimulatory molecule capable of activating T cells and B cells, but tumor cells can evade immune detection and destruction by binding to T cell surface receptors via PD-L1 [19,20]. This study showed that serum sTim-3 and PD-L1 levels were significantly higher while PG I levels were significantly lower in the death group than those in the survival group. Increased levels of sTim-3 and PD-L1 and decreased levels of PG I are risk factors affecting the prognosis of patients with progressive gastric cancer, suggesting a correlation between serum levels of sTim-3, PG I, and PD-L1 and the prognosis of patients with progressive gastric cancer treated with immune checkpoint inhibitors.
The results of ROC curve analysis showed that the AUC for the combined detection of serum sTim-3, PG I and PD-L1 in predicting the efficacy and prognosis of immunosuppressant treatment in progressive gastric cancer was higher than that of their individual test. Additionally, the 3-year survival rates in patients with high sTim-3 (>2.889 mg/L) and PD-L1 (>243.425 pg/mL) were notably shorter than those with low sTim-3 (≤2.889 mg/L) and PD-L1 (≤243.425 pg/mL) levels. These findings suggest that the combined detection of these markers has significant value in predicting both treatment response and prognosis in patients with progressive gastric cancer undergoing immune checkpoint inhibitors.
sTim-3 acts as a key inhibitor in tumor cell escape and plays a physiological role in regulating the immune response and inducing immune tolerance [21]. sTim-3 exerts anti-tumor immunity by accelerating T cell apoptosis, enhancing immunosuppression, and promoting tumor cell polarization. Additionally, sTim-3 can impair natural killer cell function and promote the growth of gastric cancer cells. Knockdown of sTim-3 gene in tumor cells significantly reduces cell proliferation and migration capacity. With the progression of gastric cancer, patients’ gastric mucosa undergoes atrophic changes, and the number of mucosal cells decrease, which adversely affects the gastric secretion function and leads to a decrease in PG I levels [22]. PD-L1 inhibits T-cell function by inhibiting T-cell differentiation and accelerating T-cell cytoplasmic phosphorylation, affecting the normal immune function of the body. This promotes tumor cell proliferation, accelerating tumor progression. PD-L1 is highly expressed in gastric cancer patients, and its expression increases due to immune escape mechanism, increasing the risk of poor prognosis [23]. In this study, we did not observe statistically significant differences in survival time between patients with different PG I expression levels, which may be due to the small sample size. Further research is needed to explore this relationship in more depth.
In conclusion, elevated levels of sTim-3 and PD-L1, along with decreased levels of PG I, are independent risk factors affecting the treatment efficacy and prognosis in patients with progressive gastric cancer treated with immune checkpoint inhibitors. The combined detection of sTim-3, PG I, and PD-L1 provides better predictive value for both treatment efficacy and prognosis. However, there are still some limitations to this study, such as a relatively small sample size and single institution investigation, which may limit the generalizability, reliability, and applicability of the research results. Future studies should aim to expand the sample size, extend the follow-up period, conduct multi-center studies, and incorporate additional indicators and methodologies to provide a more comprehensive analysis and improve the reliability of the research results.
Acknowledgements
This study was supported by The Hunan Provincial Natural Science Foundation of China (2021JJ40643, 2021JJ40642), Scientific Research Fund of Hunan Provincial Education Department (20A054, 23B0874) and Special Project on ESI Discipline Construction at Changsha Medical College (2022CYY026).
Disclosure of conflict of interest
None.
References
- 1.Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, Vogel A, Smyth EC ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Gastric cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:1005–1020. doi: 10.1016/j.annonc.2022.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Kim B, Cho SJ. Endoscopic screening and surveillance for gastric cancer. Gastrointest Endosc Clin N Am. 2021;31:489–501. doi: 10.1016/j.giec.2021.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Q, Zhou XZ, Cheng S, Meng T, Gao ZJ, Zhang DZ, Zhu SH. Clinical study of peripheral blood inflammatory indexes in the therapeutic effect and prognostic value of immune checkpoint inhibitors in advanced gastric cancer. Chin Gene Med. 2024;22:198–201. [Google Scholar]
- 4.Xiong B, He XZ, Ye XL, Wang DY, Zhang JL. The value of serum soluble Tim-3 in predicting the efficacy and prognosis of arterial chemoembolization therapy for hepatocellular carcinoma. Chin Clin Oncol. 2022;27:527–532. [Google Scholar]
- 5.Zhou S, Wang K, He JX, Yang XJ, Yang P. Analysis of the correlation between serum PG, LGR4 and the prognosis of laparoscopic D2 radical surgery in patients with gastric cancer. Prog Mod Biomed. 2022;22:3051–3054. [Google Scholar]
- 6.Zuo XL, Chen ZQ, Dong RY, Wang ZX, Cai J. The value of combined detection of LDHA and PD-L1 in the prediction of efficacy and prognostic assessment of PD-1 inhibitors in advanced gastric cancer. Chin Oncol. 2023;33:460–468. [Google Scholar]
- 7.Lu YY, Lu LG, Qian NY. Treatment guidelines for esophageal and stomach cancer. Chin J Gastroenter. 2012;17:173–175. [Google Scholar]
- 8.Ma LJ, Wang Y, Li DM. Analysis of the correlation and diagnostic value of serum CEA, CYFRA21-1, ALP, ALB and KPS score in lung cancer patients. Labora Med Clin. 2022;19:454–458. [Google Scholar]
- 9.Yang XN, Wu YL. Criteria for evaluating the efficacy of treatment for solid tumors - RECIST. J Evid Based Med. 2004;4:85–90. [Google Scholar]
- 10.Yang L, Qin LQ, Geng Q, Li DQ, Qi CJ, Jiang H. Predictive value of soluble immune checkpoints on the efficacy and prognosis of immunotherapy in gastric cancer patients. Chin J Diges Med Image. 2023;13:305–311. [Google Scholar]
- 11.Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer [J] CA Cancer J Clin. 2021;71:264–279. doi: 10.3322/caac.21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu X, Tan YQ. Progress of immune checkpoint inhibitors in progressive gastric cancer. Oncol Prog. 2023;21:1629–1631. [Google Scholar]
- 13.Guo Y, Wei XJ, Gao Q. Regulatory mechanism of Tim-3 aberrant expression in T cells on gastric cancer progression. Chin J Geront. 2021;41:4834–4837. [Google Scholar]
- 14.Sexton RE, Al Hallak MN, Diab M, Azmi AS. Gastric cancer: a comprehensive review of current and future treatment strategies. Cancer Metastasis Rev. 2020;39:1179–1203. doi: 10.1007/s10555-020-09925-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang ZY, Gao F. Advances in Tim-3/Galectin-9 signaling pathway in digestive tract malignancies. Mod Diges Intervent. 2022;27:118–122. [Google Scholar]
- 16.Zhang XX, Li S, Wu C, Hou XF. Clinical analysis of immunotherapy for advanced gastric esophageal gastric junction adenocarcinoma [J] . Chinese Journal of Oncology. 2021;48:1208–1214. [Google Scholar]
- 17.Su JQ, Yan HD, Zhou LX, Wang ML, Lin PY, Huang ZS, Li GB. Relationship between changes in serum PG I/PG II, TK1, CEA levels and prognosis in patients with gastric cancer. Hebei Pharmaceutical. 2023;45:3239–3244. [Google Scholar]
- 18.Fan JY, Yao Y, Feng L, Huang LL. The value of plasma miR-27b-3p, PG combined with G-17 in early gastric cancer screening. Lab Immun Clin Med. 2021;28:1556–1559. [Google Scholar]
- 19.Yu JJ, Zhang LK, Yu PP. Correlation of serum c-Myc and PD-L1 levels with clinicopathologic features and prognosis in gastric cancer patients. Guangdong Med J. 2024;45:220–224. [Google Scholar]
- 20.Xu Y, Tang Y, Li DY. Diagnostic and prognostic value of peripheral blood CTC, PD-L1 and S100A6BP levels in gastric cancer patients. J Mole Diag Thera. 2024;16:273–277. [Google Scholar]
- 21.Chen L, Hong J, Hu R, Yu X, Chen X, Zheng S, Qin Y, Zhou X, Wang Y, Zheng L, Fang H, Liu P, Huang B. Clinical value of combined detection of serum stim-3 and pepsinogen for gastric cancer diagnosis. Cancer Manag Res. 2021;13:7759–7769. doi: 10.2147/CMAR.S328312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng Y, Chen J. The value of serum IL-6 and pepsinogen I/II ratio in the prognostic assessment of elderly patients with gastric cancer. Geriatr Heal Care. 2022;28:120–124. [Google Scholar]
- 23.Zhang X, Fang N. Relationship between serum sTim-3 and YKL-40 levels and recurrence after endoscopic submucosal dissection for early gastric cancer. J Trop Med. 2024;24:97–101. [Google Scholar]