Abstract
This study aims to report the oncological, surgical and functional outcomes in 15 patients with locally advanced penile cancer who underwent total penile amputation with perineal urethrostomy (PU). A single-center retrospective analysis was conducted from January 2018 to September 2023. Outcomes included postoperative complications, cancer-specific survival (CSS), and overall survival (OS). Functional outcomes and pre and postoperative quality of life (QoL) were assessed by the International Prostate Symptom Score (IPSS) validated questionnaire. The median follow-up period was 10 months (SD±7). The median age of patients was 73 years (IQR 63-77), with a median Body Mass Index (BMI) of 28.1 kg/m2 (IQR 25.0-29.9). Risk factors included lack of circumcision (60.0%), history of lichen sclerosus (33.3%), and obesity (26.7%). The primary symptom reported was pain (67.0%). Total penectomy with PU was the initial surgery in 40.0% of cases; the rest underwent surgery after recurrence after penile organ-sparing surgery or partial penile amputation. Median operative time was 170 minutes (IQR 142.5-211.5), and the median hospital stay was 6 days (IQR 5-10). No local recurrence occurred during follow-up. The overall complication rate was 33.4%, with one patient (6.7%) requiring surgical revision (Clavien ≥ III). CSS was 80.0% with a median time to death of 6 months (IQR 3-13), and OS was 60.0%. Urinary symptoms and QoL significantly improved postoperatively (P = 0.0005 and P = 0.0012, respectively). Total penile amputation with PU is a safe procedure with acceptable complications and favorable functional outcomes.
Keywords: Penile cancer, penile amputation, perineal urethrostomy, surgical outcomes, functional outcomes
Introduction
Penile cancer (PC) is a rare malignancy with a global incidence of less than one case per 100,000 men. Developing countries, in particular, have shown a higher occurrence of PC than other regions [1]. Despite its rarity, PC can be aggressive, characterized by early locoregional infiltration and a significant risk of metastatic spread. Nodal metastasis is particularly crucial in determining the prognosis for PC survival [2,3]. Historically, the approach to PC generally consisted of a total or subtotal penile amputation with a large (2 cm) surgical margin of healthy tissue. While this approach facilitated satisfactory oncological control, it has proven to be extremely demolishing and disabling from both functional and aesthetic perspectives [4-6]. Over time, advancements in organ-sparing techniques have been made to improve the quality of life (QoL) for patients while maintaining an excellent rate of oncological radicalization [7]. The principle underlying the development of a minimally of a minimally invasive approach for the management of localized or locally advanced PC was the newly established concept of a reduced safe surgical margin up to 5 mm [8,9]. In cases of local recurrence (up to 30%), patients may need to undergo revision surgery; however, no reduction in 5-year overall survival (OS) has been observed [7,10]. When local recurrence does not occur (in most cases), patients will benefit from preserved urinary and sexual function, as well as an improved aesthetic appearance, compared to a simple amputation approach [7,11-13]. However, when locally advanced PC is present, more aggressive surgical procedures are required. These may involve either partial or radical penectomy with urinary diversion. The latter is considered for patients in whom organ-sparing surgery, while maintaining safe margins, would not result in a cancer-free residual stump suitable for sexual penetration or voiding [14]. Perineal urethrostomy (PU) configuration is often utilized as the definitive form of urinary reconstruction in patients undergoing penile amputation. Despite its oncological effectiveness, amputation surgery with PU can result in disfigurement and alter the body image leading to decreased QoL and potential impairments in sexual and voiding functions [15,16].
In this study, we aimed to evaluate the oncological outcomes and to report the functional and surgical outcomes of patients treated with total penectomy and PU configuration for PC in a referral tertiary center.
Materials and methods
Study setting and patients
A retrospective analysis was conducted at a single center from January 2018 to September 2023. The study included 15 patients who underwent total penectomy with a PU configuration. This study was conducted following the ethical standards of the institutional and national research committees and the 1964 Helsinki Declaration and its later amendments, and all subjects provided written informed consent. The research protocol was reviewed and approved by the local Institutional Review Board of A.O.U. Città della Salute e della Scienza, University of Turin, approval number 604.532-00235/2021.
The study’s primary objectives were to analyze and report cancer-specific survival (CSS) and OS following PC surgery. Secondary endpoints included describing various aspects of the study population, such as demographic characteristics, the presence of risk factors, etiologies, pathologic tumor and node staging, intra- and postoperative complications, and functional outcomes related to urinary function. Patients’ baseline characteristics and postoperative complications occurring within one year after surgery were considered. PC staging followed the 8th edition of the American Joint Committee on Cancer (AJCC) Tumor-Node-Metastasis cancer staging system [17]. Postoperative complications were evaluated and classified for severity according to the Clavien-Dindo classification (within 90 days after surgery) [18]. Lower urinary tract symptoms were assessed preoperatively and 6 months after the surgery using the validated version of the International Prostate Symptom Score (IPSS) questionnaire [19,20]. The questionnaire was administered to every patient via email or during a follow-up consultation in a clinical setting. It consists of seven questions that pertain to voiding and urinary storage symptoms (symptom score: mild 0-7, moderate 8-19, and severe 20-30). Additionally, it includes one question inquiring about patient’s overall QoL (ranging from 0 - delighted to 6 - terrible). In this study, we did not focus on lymphatic staging or its associated outcomes.
Preoperative evaluation
All patients affected by PC were preoperatively conversed with by a multidisciplinary team, following our institutional protocol, to confirm the surgical approach for the primary lesion. The management of lymph nodes was determined subsequently based on definitive histological findings and staging imaging.
Surgical technique
The surgical technique has already been described and demonstrated in detail previously [21]. The fundamental surgical steps of total penectomy with PU are as follows.
The patient is placed in the lithotomy position under general anesthesia. A traction stitch is applied to the glans, and a circumferential skin incision is made at the base of the penis, followed by penile degloving, which progresses proximally until the fundiform ligament is reached and incised. The penile shaft is gradually released from the surrounding dartos tissues to allow complete mobilization. Once mobilization is complete, a perineal inverse U-shaped incision is fashioned according to Blandy’s flap technique [22], and progressive dissection is performed up to the bulbospongiosus muscle. The corpus spongiosum is then mobilized off the corpora cavernosa by sectioning the bulbospongiosus and ischiocavernosus muscles. Subsequently, the penis is transposed through the perineal access. The level of penile amputation is marked to ensure a safe surgical margin and sufficient urethral length for PU. The anatomical structures of the penis are then dissected and separated, and the urethra is progressively isolated from the corpora cavernosa. Once completely isolated, the urethra is suspended with an elastic loop. The transection of the corpora cavernosa is performed, completing the penile amputation with the transection of the urethra. The residual proximal stump of the corpora cavernosa is closed using a running 2-0 monofilament suture. After positioning the Scott retractor, the urethra is spatulated ventrally at the 6 o’clock position to allow the apex of the inverted U-shaped skin flap to be incorporated ventrally. The skin is then sutured to the urethra using a 3-0 and 4-0 monofilament sutures to configure the PU. The pubic and perineal access sites are closed with 2-0 multifilament absorbable sutures. A bladder catheter is placed, and a compressive dressing is applied (Figure 1).
Figure 1.
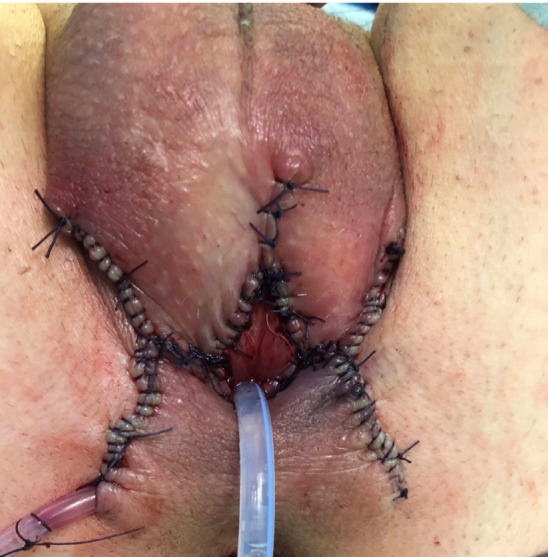
Penile amputation and perineal urethrostomy configuration.
Statistical analysis
Statistical analyses were performed using Stata 18 (StataCorp. 2009. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP.) and the Statistical Package for the Social Sciences (SPSS; v. 29; IBM, Chicago, USA), with a two-sided significance level set at P < 0.05. The normality of the distribution of variables was tested using the Kolmogorov-Smirnov test. Categorical variables were reported as frequencies and percentages. Continuous variables with normal distribution were described using mean and standard deviation (SD), while variables with non-normal distribution were described using the median and interquartile range (IQR). Differences between pre- and postoperative functional outcomes (IPSS scores) were assessed by the Mann-Whitney U test. CSS was estimated by Kaplan-Meier analysis.
Results
This study included a total of 15 patients who required total penectomy with PU. Table 1 presents the baseline and perioperative characteristics of the entire study population. The mean follow-up duration was 10 months (SD±7). The median age at the time of surgery was 73.0 years (IQR 63.0-77.0). The median length of hospital stay was 6 days (IQR 5-10). The identified main risk factors included lack of circumcision (60.0%), a history of Lichen Sclerosus (LS, 33.3%), and obesity (defined as a Body Mass Index, BMI, value ≥ 30 kg/m2, 26.7%). The primary symptom reported by patients was pain (67.0%), and 26.7% of patients had palpable nodes at the time of diagnosis.
Table 1.
Baseline clinical and demographic characteristics
| Variables | Patients (n = 15) |
|---|---|
| Age, years (IQR) | 73.0 (63.0-77.0) |
| Comorbidities: | |
| BMI, kg/m2 (IQR) | 28.1 (25.0-29.9) |
| Hypertension, n (%) | 9 (60.0) |
| Diabetes, n (%) | 3 (20.0) |
| Impaired Fasting Glucose, n (%) | 1 (6.7) |
| OSAS, n (%) | 0 (0) |
| Risk factors: | |
| Lack of previous circumcision, n (%) | 9 (60.0) |
| History of Lichen Sclerosus, n (%) | 5 (33.3) |
| Obesity (BMI ≥ 30 kg/m2), n (%) | 4 (26.7) |
| Current smoke, n (%) | 2 (13.3) |
| Genital lymphedema, n (%) | 2 (13.3) |
| Preoperative symptoms: | |
| Bleeding, n (%) | 5 (33.3) |
| Pain, n (%) | 10 (66.7) |
| Palpable nodes, n (%) | 4 (26.7) |
IQR = interquartile range; OSAS = Obstructive Sleep Apnea Syndrome; BMI = Body Mass Index.
Table 2 provides the surgical characteristics and complications. Total penectomy was the initial surgery for the primary tumor in 6 patients (40.0%), while in the remaining 60.0%, the surgery was secondary to a recurrence after penile organ-sparing surgery or partial penile amputation. The median operative time was 170 minutes (IQR 142.5-211.5). No intraoperative complications occurred. Positive resection margins were detected in 2 cases (13.3%), with no need for reoperation. The average catheterization time was 20 days (SD±10.9). No patients developed local recurrence. Four patients (26.7%) received adjuvant chemotherapy, while in two cases, adjuvant chemotherapy was not indicated due to poor performance status. Postoperative complications within 30 days were observed in a total of 5 patients (33.4%). Minor complications (Clavien < III) were observed in 26.7% of cases, including one case of wound dehiscence managed conservatively (grade I), one case of deep vein thrombosis requiring anticoagulants (grade II), one case of bleeding necessitating transfusion (grade II), and one case of urinary tract infection (UTI) requiring antibiotics administration (grade II). Surgical revision (Clavien ≥ III) was performed in one case for wound dehiscence. No cases of urethral stenosis, fistula, or acute urinary retention were registered (Figure 2).
Table 2.
Surgical outcomes and postoperative complications
| Variables | Patients (N = 15) |
|---|---|
| Operative time, minutes (IQR) | 170.0 (142.5-211.5) |
| Blood loss, cc (IQR) | 70.0 (50.0-100.0) |
| First surgical approach, n (%) | 6 (40.0) |
| Intraoperative complication, n (%) | 0 (0) |
| Post-operative complications, n (%) | 5 (33.4) |
| Clavien-Dindo classification, n (%) | |
| I | 1 (6.7) |
| II | 3 (20.0) |
| IIIa | 0 |
| IIIb | 1 (6.7) |
| IV | 0 |
| V | 0 |
| Re-operation for complication, n (%) | 0 (0) |
| Hospital stay, days (IQR) | 6 (5-10) |
| Duration of bladder catheterization, days (SD) | 20 (±10.9) |
| Positive surgical margins, n (%) | 2 (13.3) |
| Adjuvant chemotherapy, n (%) | 4 (26.7) |
| QoL: | |
| Good, n (%) | 6 (40.0) |
| Moderate, n (%) | 5 (33.3) |
| Bad, n (%) | 4 (26.7) |
| Recurrence, n (%) | 0 (0) |
| Re-operation for positive surgical margins, n (%) | 0 (0) |
| Re-operation for recurrence, n (%) | 0 (0) |
SD = Standard Deviation; QoL = quality of life.
Figure 2.
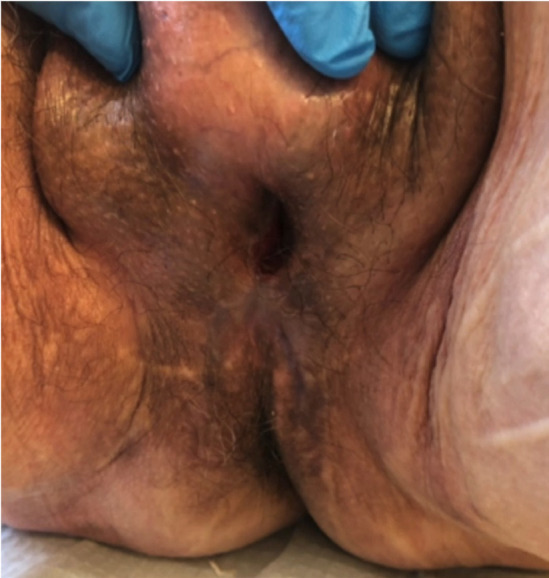
Surgical outcomes at 3-month follow-up.
In the histological examination (Table 3), all patients (100%) were diagnosed with a squamous subtype of PC. The most frequently observed pathological T (pT) stages were 2, 3, and 4 accounting for 33.3%, 46.7%, and 13.3% of patients, respectively. Perineural invasion was found in 53.3% of cases, and lymphovascular invasion was observed in 80% of cases. Positive inguinal nodes on histology were identified in 26.7% of patients (pN1 in 13.3%, pN2 in 6.7%, and pN3 in 6.7%).
Table 3.
Histological examination
| Variables | Patients (N = 15) |
|---|---|
| Histological subtype: | |
| Squamosus, n (%) | 15 (100) |
| Spinous, n (%) | 0 (0) |
| Urothelial, n (%) | 0 (0) |
| pT stage, n (%): | |
| pT1 | 1 (6.7) |
| pT2 | 5 (33.3) |
| pT3 | 7 (46.7) |
| pT4 | 2 (13.3) |
| Grading, n (%): | |
| G1, n (%) | 2 (13.3) |
| G2, n (%) | 8 (53.3) |
| G3, n (%) | 5 (33.3) |
| Keratinizing, n (%) | 11 (73.3) |
| Positive margins, n (%) | 2 (13.3) |
| Perineural invasion, n (%) | 8 (53.3) |
| Lymphovascular invasion, n (%) | 12 (80.0) |
| pN stage, n (%): | |
| pNx | 6 (40.0) |
| pN0 | 5 (33.3) |
| pN1 | 2 (13.3) |
| pN2 | 1 (6.7) |
| pN3 | 1 (6.7) |
pT stage = pathological T-stage; pN stage = pathological N-stage.
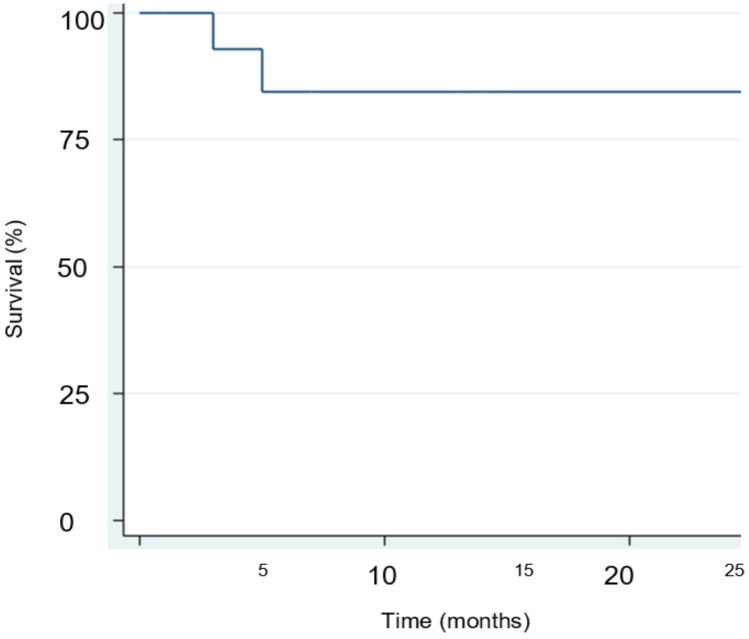
Table 4 illustrates the oncological outcomes after surgery. CSS rate at median follow-up was 80.0%, with a median time to death from the disease of 5 months (IQR 3-25). The OS rate was 60.0% at median follow-up (Figure 3).
Table 4.
Survival outcomes
| Variables | Patients (N = 15) |
|---|---|
| Cancer-specific survival, n (%) | 12 (80) |
| Time to death, months (IQR) | 6 (3-13) |
| Overall survival, n (%) | 9 (60) |
| Follow-up, months (SD) | 10 (±7) |
Figure 3.
Kaplan-Meyer cancer-specific survival analysis.
Nine patients reported functional outcomes (Table 5). Both urinary symptoms and QoL improved significantly after surgery (P = 0.0005 and P = 0.0012, respectively). The median preoperative IPSS score was 15 (IQR 10-17) with QoL score of 3 out of 6 points (IQR 3-4). In contrast, the median postoperative IPSS score was 6 (IQR 5-7) with QoL score of 2 out of 6 points (IQR 1-2).
Table 5.
Functional outcomes after total penectomy and perineal urethrostomy configuration for penile cancer
| Variables | Pre-op evaluation (N = 9) | Post-op evaluation (N = 9) | P value |
|---|---|---|---|
| IPSS, score (IQR) | 15 (10-17) | 6 (5-7) | 0.0005 |
| QoL, score (IQR) | 3 (3-4) | 1 (1-2) | 0.0012 |
IPSS = International Prostatic Symptoms Score. All p values were calculated by Mann-Whitney U test.
Discussion
PC is a rare malignancy, and the spectrum of its clinical manifestations can have a devastating impact on psychosocial, urinary, and sexual QoL [15,23,24]. To mitigate the negative impact of the PC on patients, there has been a growing interest in implementing various penile-sparing procedures [25]. However, in cases of locally advanced PC, partial or total penile amputation remains the primary oncologic treatment to ensure negative surgical margins [26]. Although this approach allows for local disease control, it has significant consequences on psychological well-being, QoL and functional aspects.
In general, for all patients affected by PC - particularly those with locally advanced cases - it is essential to provide psychological support to both the patient and their partner or family. Within our institution, the availability of psycho-oncology services plays a pivotal role in offering this support and aiding patients in coming to terms with their diagnosis and the potential drawbacks of PC treatment.
It is noteworthy that delays in seeking medical consultation often stem from embarrassment, fear, modesty, and the patient’s underestimation of the severity of their condition. These delays pose significant challenges in the optimal management of advanced PC, where less invasive approaches may be more suitable for less advanced conditions.
Furthermore, it is crucial that physicians, during counseling, effectively communicate to patients the necessity of more invasive surgical approaches, particularly in terms of enhancing QoL concerning local hygiene, urinary function, and the potential for local decontamination to facilitate the implementation of all medical therapies for controlling the disease.
In the current medical landscape, there has been an increased interest in investigating functional aspects following radical surgery. However, there is a paucity of published data examining urinary outcomes after total penectomy with PU configuration.
From a surgical perspective, penile amputation with urinary reconstruction is a challenging procedure, requiring a median operation time of 170 minutes in our center (IQR 142.5-211.5). Total penile amputation is typically recommended for pT3 and pT4 cases, but our series also included patients who, upon final histological report, were found to have pT1 or pT2. These patients were the ones who experienced local recurrence after a previously performed surgical approach for PC. Local recurrence, particularly after partial penile amputation, may make it impossible to preserve an adequate penile stump, leading to difficulties with standing or sitting during voiding, and causing urine leakage onto the scrotum. This complicates local management, genital hygiene, and results in a lower overall QoL. The surgical approach was thoroughly discussed with the patients, emphasizing the challenges of further partial amputation compared to the configuration of a PU, which is more hygienic and easier to manage following complete penile amputation, especially in cases where the residual penile stump is extremely short.
Like other procedures, penile amputation could be characterized by surgical complications such as surgical site infection (SSI), wound dehiscence or bleeding, lymphedema, urinary tract infection (UTI), and urethral stenosis when PU is fashioned. In our study, we observed an overall complication rate of 33.3%. Of these, only one case (6.7%) was classified as a high-grade complication (Clavien IIIb) requiring surgical revision for wound dehiscence. The remaining complications were classified as minor (Clavien I-II) and were mostly managed conservatively requiring antibiotic administration or transfusion. Compared to other published series, we observed a slightly higher incidence of overall complications, which can be attributed to the small sample size and the higher tumor stage. Specifically, 60.0% of patients in our study had a pT3 stage or higher, compared to the 44.4% reported by de Vries et al. [27].
In 2019, Velazquez et al. [28] developed a prognostic risk score to predict penectomy complications in the immediate postoperative period (within 30 days). They evaluated 11 variables and found that older age (> 75 years), systemic sepsis, longer operation times (> 110 minutes), current smoking status, chronic steroid use, metastatic disease, and an American Society of Anesthesiologists (ASA) score of 4 or 5 were associated with a higher rate of complications and unfavorable outcomes. Their study reported an overall complication rate of 19.7% following partial or total penectomy. While longer surgical intervention times are often linked to more complex cases, they have previously been associated with an increased risk of infections [29]. Similar findings were described in a recent international multicentric retrospective study involving 299 patients who underwent PU after total penectomy [27]. The overall complication rate was 19%, with SSI and wound dehiscence being the most common complications, and only 4.0% of patients experiencing major complications (Clavien III-V). Likewise, a study conducted by Lumen et al. [30] described complications following Johanson PU and Blandy PU in 11 and 25% of cases, respectively. However, it is worth noting that the study focused on benign diseases, which may explain the lower complication rate. They also reported a mean operation time of 112.6 minutes for the Blandy PU technique, suggesting a potentially longer learning curve for this procedure.
From a functional perspective, patients undergoing penile amputation with PU are limited to urinating while sitting. The current literature lacks studies that investigate the micturition outcomes following total penectomy with PU and compare these results with those of patients who do not undergo urinary diversion. Patients undergoing PU due to a long history of urethral stricture disease often experience a significant impact on their QoL. These patients typically feel relief after the procedure, as it allows them to regain the ability to urinate, albeit in a sitting position. Similarly, patients undergoing PU following penectomy often have tumors that invade the corpora cavernosa and the corpus spongiosum, which leads to urinary problems. Consequently, the procedure is more likely to result in urinary satisfaction for these patients. These findings were confirmed in our study. We observed that the PU configuration led to an improvement in urinary function, as evidenced by a significant reduction in the IPSS score (P = 0.0005). Additionally, there was a significant improvement in the QoL during the post-operative period (P = 0.0012).
Regarding urinary complications associated with PU, the main issues include urethral stenosis (particularly at the neo-meatus), fistula and acute urinary retention. The incidence of these complications varies depending on numerous factors such as the vascularization of the neo-meatus, length of the urethra spatulation, tissue tension and duration of catheterization [31]. In some cases, these complications may require surgical revision, dilatation, or permanent catheterization [30]. The choice depends on patient’s preference, the severity of the stenosis, disease state, and comorbidities. However, in our study, none of the patients developed stenosis or fistula. Another study by de Vries et al. [27] described an overall stenosis incidence rate of 12%, which primarily occurred within 18 months after treatment and required surgical revision (74.0%).
Another important aspect following surgery in men affected by PC is its influence on sexual life, body image, and general QoL. Depression and psychiatric symptoms are also relatively common among these patients due to the mutilating nature of the primary treatment [32]. Unfortunately, our study did not focus on these aspects. However, previous research has shown that up to 37.5% to 40% of patients experience impaired well-being following PC surgery [32,33]. The Hospital Anxiety and Depression Score (HADS) was used in studies by D’Ancona et al. [5] and Romero et al. [4], revealing pathological anxiety in 0 and 31% of patients, respectively. Additionally, Ficarra et al. [33] reported the onset of signs and symptoms of mental illness in 53.3% of patients. In 2016, Sosnowski et al. [34] conducted a study involving 10 patients who had undergone total penectomy with PU. They employed several assessment tools to evaluate various aspects of QoL, including global QoL [34], self-esteem, and sexual function [35,36]. The findings revealed that total penile amputation with PU significantly impacted patients’ sexual life and overall QoL, particularly among younger and highly educated individuals. Despite these results, the surgery did not appear to negatively affect aspects such as relationship with the partner, self-assessment, or perception of masculinity. A year later, in another study run by the same authors [23], it was emphasized that as the aggressiveness of the surgery increased, patients’ evaluations of their global QoL and physical functioning significantly decreased (P < 0.05 for both). Similar findings were described by Opjordsmoen et al. [6], suggesting that more radical treatments had the most significant impact on patients’ sexual life. In 2018, Draeger et al. [16] introduced a new, unvalidated Quality of Life Questionnaire - Penile Cancer - Rostock (HRO-PE29) - designed to address specific issues faced by PC patients, such as changes in sexuality, genital sensitivity, and lymphedema, across different disease states and treatment forms. These studies collectively highlighted the negative impact of PC surgery on affected men and underscored the importance of early diagnosis, appropriate counseling, and consistent postoperative follow-up. However, the quality of data in these studies was limited. Most of the studies were retrospective, with small sample sizes, and employed various measurement tools, leading to limitations in the reliability of their findings.
The most important prognostic factor for survival is the presence and the extent of nodal metastases [2]. In our study, CSS at follow-up was 80.0% with a median time to death from disease of 5 months (IQR 3-25). All patients who died due to PC had a positive pN status, supporting existing literature findings.
Our study has several limitations. First, it is based on a relatively small sample size, which is partly due to the rarity of this disease. Additionally, it is a single-center study, and the data analysis was conducted retrospectively. Our follow-up data is insufficient for an analysis of long-term outcomes. Furthermore, the study lacks a control group. The preliminary data we reported should be confirmed through future multicenter randomized trials with a larger number of patients and more extensive follow-up.
Conclusions
In conclusion, the use of penile amputation combined with PU has proven to be a reliable and effective approach in managing invasive PC, ensuring satisfactory urinary outcomes for patients. However, further research is needed to evaluate the impact on QoL and functional outcomes. This would help in offering more comprehensive counseling and management strategies for patients post-treatment.
Disclosure of conflict of interest
None.
References
- 1.Fu L, Tian T, Yao K, Chen XF, Luo G, Gao Y, Lin YF, Wang B, Sun Y, Zheng W, Li P, Zhan Y, Fairley CK, Grulich A, Zou H. Global pattern and trends in penile cancer incidence: population-based study. JMIR Public Health Surveill. 2022;8:e34874. doi: 10.2196/34874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horenblas S, van Tinteren H. Squamous cell carcinoma of the penis. IV. Prognostic factors of survival: analysis of tumor, nodes and metastasis classification system. J Urol. 1994;151:1239–1243. doi: 10.1016/s0022-5347(17)35221-7. [DOI] [PubMed] [Google Scholar]
- 3.Cindolo L, Spiess PE, Bada M, Chipollini JJ, Nyirády P, Chiodini P, Varga J, Ditonno P, Battaglia M, De Nunzio C, Tema G, Veccia A, Antonelli A, Musi G, De Cobelli O, Conti A, Micali S, Álvarez-Maestro M, Olarte JQ, Diogenes E, Lima MVA, Tracey A, Guruli G, Autorino R, Sountoulides P, Schips L. Adherence to EAU guidelines on penile cancer translates into better outcomes: a multicenter international study. World J Urol. 2019;37:1649–1657. doi: 10.1007/s00345-018-2549-3. [DOI] [PubMed] [Google Scholar]
- 4.Romero FR, Romero KR, Mattos MA, Garcia CR, Fernandes Rde C, Perez MD. Sexual function after partial penectomy for penile cancer. Urology. 2005;66:1292–1295. doi: 10.1016/j.urology.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 5.D’Ancona CA, Botega NJ, De Moraes C, Lavoura NS Jr, Santos JK, Rodrigues Netto N Jr. Quality of life after partial penectomy for penile carcinoma. Urology. 1997;50:593–596. doi: 10.1016/s0090-4295(97)00309-9. [DOI] [PubMed] [Google Scholar]
- 6.Opjordsmoen S, Fosså SD. Quality of life in patients treated for penile cancer. A follow-up study. Br J Urol. 1994;74:652–657. doi: 10.1111/j.1464-410x.1994.tb09200.x. [DOI] [PubMed] [Google Scholar]
- 7.Philippou P, Shabbir M, Malone P, Nigam R, Muneer A, Ralph DJ, Minhas S. Conservative surgery for squamous cell carcinoma of the penis: resection margins and long-term oncological control. J Urol. 2012;188:803–808. doi: 10.1016/j.juro.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman MA, Renshaw AA, Loughlin KR. Squamous cell carcinoma of the penis and microscopic pathologic margins: how much margin is needed for local cure? Cancer. 1999;85:1565–1568. [PubMed] [Google Scholar]
- 9.Minhas S, Kayes O, Hegarty P, Kumar P, Freeman A, Ralph D. What surgical resection margins are required to achieve oncological control in men with primary penile cancer? BJU Int. 2005;96:1040–1043. doi: 10.1111/j.1464-410X.2005.05769.x. [DOI] [PubMed] [Google Scholar]
- 10.Parnham AS, Albersen M, Sahdev V, Christodoulidou M, Nigam R, Malone P, Freeman A, Muneer A. Glansectomy and split-thickness skin graft for penile cancer. Eur Urol. 2018;73:284–289. doi: 10.1016/j.eururo.2016.09.048. [DOI] [PubMed] [Google Scholar]
- 11.Burnett AL. Penile preserving and reconstructive surgery in the management of penile cancer. Nat Rev Urol. 2016;13:249–257. doi: 10.1038/nrurol.2016.54. [DOI] [PubMed] [Google Scholar]
- 12.Scarberry K, Angermeier KW, Montague D, Campbell S, Wood HM. Outcomes for organ-preserving surgery for penile cancer. Sex Med. 2015;3:62–66. doi: 10.1002/sm2.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Preto M, Falcone M, Blecher G, Capece M, Cocci A, Timpano M, Gontero P. Functional and patient reported outcomes following total glans resurfacing. J Sex Med. 2021;18:1099–1103. doi: 10.1016/j.jsxm.2021.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Brouwer OR, Albersen M, Parnham A, Protzel C, Pettaway CA, Ayres B, Antunes-Lopes T, Barreto L, Campi R, Crook J, Fernández-Pello S, Greco I, van der Heijden MS, Johnstone PAS, Kailavasan M, Manzie K, Marcus JD, Necchi A, Oliveira P, Osborne J, Pagliaro LC, Garcia-Perdomo HA, Rumble RB, Sachdeva A, Sakalis VI, Zapala Ł, Sánchez Martínez DF, Spiess PE, Tagawa ST. European Association of Urology-American Society of Clinical Oncology Collaborative Guideline on Penile Cancer: 2023 update. Eur Urol. 2023;83:548–560. doi: 10.1016/j.eururo.2023.02.027. [DOI] [PubMed] [Google Scholar]
- 15.Kieffer JM, Djajadiningrat RS, van Muilekom EA, Graafland NM, Horenblas S, Aaronson NK. Quality of life for patients treated for penile cancer. J Urol. 2014;192:1105–1110. doi: 10.1016/j.juro.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 16.Draeger DL, Sievert KD, Hakenberg OW. Cross-sectional patient-reported outcome measuring of health-related quality of life with establishment of cancer- and treatment-specific functional and symptom scales in patients with penile cancer. Clin Genitourin Cancer. 2018;16:e1215–e1220. doi: 10.1016/j.clgc.2018.07.029. [DOI] [PubMed] [Google Scholar]
- 17.Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67:93–99. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 18.Clavien PA, Barkun J, De Oliveira ML, Vauthey JN, Dindo D, Schulick RD, De Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The clavien-dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–96. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 19.Barry MJ, Fowler FJ Jr, O’Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, Cockett AT. The American Urological Association symptom index for benign prostatic hyperplasia. J Urol. 1992;148:1549–57. doi: 10.1016/s0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 20.Badía X, García-Losa M, Dal-Ré R. Ten-language translation and harmonization of the International Prostate Symptom Score: developing a methodology for multinational clinical trials. Eur Urol. 1997;31:129–140. doi: 10.1159/000474438. [DOI] [PubMed] [Google Scholar]
- 21.Falcone M, Preto M, Ferro I, Cirigliano L, Peretti F, Plamadeala N, Scavone M, Lavagno F, Oderda M, Gontero P. Surgical and functional outcomes of penile amputation and perineal urethrostomy configuration in invasive penile cancer. Urology. 2023;177:227. doi: 10.1016/j.urology.2023.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Blandy JP, Singh M, Tresidder GC. Urethroplasty by scrotal flap for long urethral strictures. Br J Urol. 1968;40:261–267. doi: 10.1111/j.1464-410x.1968.tb09886.x. [DOI] [PubMed] [Google Scholar]
- 23.Sosnowski R, Wolski JK, Kulpa M, Ziętalewicz U, Kosowicz M, Kalinowski T, Demkow T. Assessment of quality of life in patients surgically treated for penile cancer: Impact of aggressiveness in surgery. Eur J Oncol Nurs. 2017;31:1–5. doi: 10.1016/j.ejon.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Sansalone S, Silvani M, Leonardi R, Vespasiani G, Iacovelli V. Sexual outcomes after partial penectomy for penile cancer: results from a multi-institutional study. Asian J Androl. 2017;19:57–61. doi: 10.4103/1008-682X.168690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baumgarten A, Chipollini J, Yan S, Ottenhof SR, Tang DH, Draeger D, Protzel C, Zhu Y, Ye DW, Hakenberg OW, Horenblas S, Watkin NA, Spiess PE. Penile sparing surgery for penile cancer: a multicenter international retrospective cohort. J Urol. 2018;199:1233–1237. doi: 10.1016/j.juro.2017.10.045. [DOI] [PubMed] [Google Scholar]
- 26.Ornellas AA, Kinchin EW, Nóbrega BL, Wisnescky A, Koifman N, Quirino R. Surgical treatment of invasive squamous cell carcinoma of the penis: Brazilian National Cancer Institute long-term experience. J Surg Oncol. 2008;97:487–495. doi: 10.1002/jso.20980. [DOI] [PubMed] [Google Scholar]
- 27.de Vries HM, Chipollini J, Slongo J, Boyd F, Korkes F, Albersen M, Roussel E, Zhu Y, Ye DW, Master V, Le TL, Johnstone PA, Muneer A, Brouwer OR, Spiess PE. Outcomes of perineal urethrostomy for penile cancer: a 20-year international multicenter experience. Urol Oncol. 2021;39:500.e9–500.e13. doi: 10.1016/j.urolonc.2021.04.023. [DOI] [PubMed] [Google Scholar]
- 28.Velazquez N, Press B, Renson A, Wysock JS, Taneja S, Huang WC, Bjurlin MA. Development of a novel prognostic risk score for predicting complications of penectomy in the surgical management of penile cancer. Clin Genitourin Cancer. 2019;17:e123–e129. doi: 10.1016/j.clgc.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 29.Daley BJ, Cecil W, Clarke PC, Cofer JB, Guillamondegui OD. How slow is too slow? Correlation of operative time to complications: an analysis from the tennessee surgical quality collaborative. J Am Coll Surg. 2015;220:550–558. doi: 10.1016/j.jamcollsurg.2014.12.040. [DOI] [PubMed] [Google Scholar]
- 30.Lumen N, Beysens M, Van Praet C, Decaestecker K, Spinoit AF, Hoebeke P, Oosterlinck W. Perineal urethrostomy: surgical and functional evaluation of two techniques. Biomed Res Int. 2015;2015:365715. doi: 10.1155/2015/365715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myers JB, Porten SP, McAninch JW. The outcomes of perineal urethrostomy with preservation of the dorsal urethral plate and urethral blood supply. Urology. 2011;77:1223–1227. doi: 10.1016/j.urology.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 32.Maddineni SB, Lau MM, Sangar VK. Identifying the needs of penile cancer sufferers: a systematic review of the quality of life, psychosexual and psychosocial literature in penile cancer. BMC Urol. 2009;9:8. doi: 10.1186/1471-2490-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ficarra V, Righetti R, D’Amico A, Pilloni S, Balzarro M, Schiavone D, Malossini G, Mobilio G. General state of health and psychological well-being in patients after surgery for urological malignant neoplasms. Urol Int. 2000;65:130–134. doi: 10.1159/000064857. [DOI] [PubMed] [Google Scholar]
- 34.Sosnowski R, Kulpa M, Kosowicz M, Wolski JK, Kuczkiewicz O, Moskal K, Szymański M, Kalinowski T, Demkow T. Quality of life in penile carcinoma patients - post-total penectomy. Cent European J Urol. 2016;69:204–211. doi: 10.5173/ceju.2016.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC. The European organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 36.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–830. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]