Abstract
Objective: Patients with chronic cervicitis are known to have an increased risk of infection with human papillomavirus (HPV), which is the primary cause of cervical cancer. Inhibition of cervical inflammation may reduce the risk of cervical cancer. This study investigated how human umbilical cord mesenchymal stem cell-derived exosomes (hucMSC-Ex) attenuated the lipopolysaccharide (LPS)-induced cervical inflammation. Methods: Human uterine squamous carcinoma (SiHa) cells were induced with LPS to construct an inflammatory model and treated with hucMSC-Ex. The expression levels of tumor necrosis factor α (TNF-α), and interleukins (IL)-1β, IL-6, IL-10 were analyzed by qRT-PCR. Western blot was used to detect the protein expression levels of cyclooxygenase-2 (COX-2) and proliferating cell nuclear antigen (PCNA) in cells, and CCK8 was used to examine cell proliferation, so as to explore the relieving effect of hucMSC-Ex on cell inflammation. The expression of epithelial-mesenchymal transition (EMT) markers in SiHa cells was also assessed by qRT-PCR and western blot to determine the effect of hucMSC-Ex on inflammation. Moreover, clinical cervical smears were collected to detect the expression of EMT markers in clinical exfoliated cell samples by immunofluorescence. Results: HucMSC-Ex treatment significantly reduced the expression of pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6 in the LPS-induced inflammation model, while increasing the level of anti-inflammatory cytokines, including IL-10, to reduce inflammation. HucMSC-Ex increased the expression level of epithelial markers (such as E-cadherin) while it decreased the expression of interstitial markers (such as N-cadherin), suggesting it inhibits EMT. Conclusions: Our results support that hucMSC-Ex alleviates LPS-induced cervical inflammation, possibly by inhibition of EMT.
Keywords: Cervicitis, HucMSC-Ex, EMT
Introduction
Cervicitis is a common inflammatory disease of the reproductive system in gynecology, with the inflammation mainly occurring in the cervical vagina or cervical mucosa. The course of the disease is often recurrent and can easily lead to complications such as urinary tract infections [1]. In the early stages of inflammation, there may be no obvious symptoms. However, as the inflammation spreads, it can develop into chronic cervicitis, cervical polyps, cervical hypertrophy, and other symptoms that pose a threat to female reproductive health. Cervical cancer is one of the most prevalent cancers among women worldwide and is a leading cause of cancer-related death. The mechanisms involved in the pathogenesis of cervical cancer are still largely unclear [2]. For the last few decades, prevention and treatment strategies for human papillomavirus (HPV) infection and its associated cervical cancer have become a major focus in gynecologic research [3]. While cervical inflammation itself does not typically lead to cancer, chronic inflammation combined with high-risk HPV infection can contribute to the development of cervical cancer over time [4]. Therefore, prompt and effective treatment of cervical inflammation may help to avoid cervical lesions turning into cervical cancer.
Mesenchymal stem cells (MSCs) are pluripotent and self-renewing stem cells with the ability to differentiate into multiple types of cells [5]. MSCs are primarily derived from mesoderm during early embryonic development and can be induced to differentiate into various cell types in vitro [6,7]. MSCs are also widely distributed and can be isolated and cultured from different tissues [8]. Due to their weak immunogenicity and origin in early embryonic mesoderm, MSCs have the capability to spread and migrate between ectodermal and endodermal layers [9]. Additionally, the stemness of MSCs exceeds that of stem cells originating in the proximal ectoderm, creating a greater potential for differentiation. These properties of MSCs suggest their broader application in tissue repair and gene therapy. Numerous studies have demonstrated that MSCs possess homing characteristics, allowing them to migrate directly to sites of inflammation and tissue damage [10]. It is well established that MSCs primarily exert their immunomodulatory function through paracrine pathways, with exosomes being one of the most prominent bioactive substances [11,12]. Exosomes are membrane vesicles of cellular origin found in almost all body fluids, carrying various biologically active substances such as cytoplasmic proteins, lipids, mRNA, miRNAs, and metabolites [13]. They play a role in transmitting information between cells, internalizing into target cells, participating in physiologic and pathologic processes, and regulating the genotype and phenotype of these receptor cells [14]. Furthermore, exosomes are abundant in sources and characterized by high stability and biocompatibility. HucMSCs-Ex, derived from human umbilical cord mesenchymal stem cells, exhibit targeted delivery to inflammatory and tumor sites and can deliver specific molecules to tumor cells [15]. This regulates tumor migration and proliferation by modulating related signaling pathways. In an inflammatory environment, MSC-Ex demonstrates immunosuppressive effects with anti-inflammatory properties, and can be utilized as a “non-cellular” therapeutic strategy for treating inflammation-related diseases [16]. Studies also indicate that hucMSC-Ex has the ability to reverse TGF-β1-induced EMT changes in renal tubular epithelial cells [17,18]. Our group has previously demonstrated that hucMSC-Ex inhibits TGF-β-induced proliferation, migration, and activation of human intestinal fibroblasts and alleviates DSS-induced IBD-associated intestinal fibrosis by reducing ERK phosphorylation [19]. These findings suggest that hucMSC-Ex may offer a novel approach for treating chronic cervical inflammation. MSC-Ex may hold great promise for the treatment of other inflammatory diseases.
Epithelial-mesenchymal transition (EMT) is a crucial event in the progression from chronic inflammation to cancer, and inflammatory mediators can promote EMT [20]. During this process, the expression of epithelial cell markers such as E-cadherin decreases, while interstitial cell markers such as N-cadherin and Vimentin increase [21]. Huang et al. utilized LPS to induce EMT in breast cancer cell lines and found that curcumin plays a significant role in inhibiting LPS-induced EMT in breast cancer cells by down-regulating NF-κB-Snail activity [22]. It has been shown that MSCs can reverse the LPS-induced EMT process by blocking the activation of the NF-κB and Hedgehog pathways [23]. Luo’s team previously reported that LPS promoted the metastasis of mesenchymal-like breast cancer cells with high expression of cyclin D1b, and subsequent experiments found that LPS up-regulated cyclin D1b expression in MCF-7 cells and induced EMT in breast cancer cells [24]. To best of our knowledge, there are no studies that report the relieving effects of hucMSC-Ex on cervical inflammation. As an inflammatory stimulator, LPS can activate inflammatory cells in the tumor microenvironment and alter cytokine levels, thereby promoting tumor cell proliferation, angiogenesis, invasion and metastasis, ultimately accelerating EMT in tumor cells.
Therefore, building upon previous experiments, this study takes the lead in investigating the effect and mechanism of hucMSC-Ex in alleviating LPS-induced cervical inflammation, which may provide a new therapy for the treatment of chronic cervicitis.
Materials and methods
Isolation of hucMSCs
Fresh umbilical cord tissue specimens were obtained from the Fourth People’s Hospital of Zhenjiang. After removing the artery and venous endothelium, the umbilical cord was cut into 1 mm3 pieces, placed in the 3.5 cm dishes, and cultured in an incubator containing 5% CO2 at 37°C for 2 h. After the tissue pieces adhered to the bottom of the small dish, α-MEM medium (Hyclone, Utah, USA) supplemented with 15% fetal bovine serum (FBS, Vazyme, Nanjing, China) was dropped into each small dish to completely cover the tissue blocks, and the solution was replaced every 3 days. When the cells grew into a whirlpool shape and fused to 80%, the tissue blocks were removed, and the cells were digested and cultured with α-MEM containing 10% exosome-free fetal bovine serum. When cells reached more than 90% confluence, they were passaged at a rate of 1:3. During this process, the cell culture supernatant was collected for extractions of exosomes. The study was approved by the Ethical Committee of Jiangsu University (2012258).
Exosomal extraction
Ultrafast centrifugation was used to extract exosomes from the supernatant of the collected MSCs, and the protein concentration of exosomes was analyzed by a BCA protein assay kit (GLPBIO, California, USA). The size distribution and particle number of exosomes were detected by NanoSight Nano Analyzer (Malvern Panalytical, Malvern, UK). Morphological characteristics of exosomes derived from MSCs were observed by transmission electron microscopy (Philips, Amsterdam, The Netherlands) and the expression of exosomal membrane protein surface markers Alix, Calnexin, CD9, and TSG101 were assessed by western blot.
Cell culture
SiHa cells were purchased from the Shanghai Institute of Biochemistry and Cell Biology and grown in Dulbecco’s Modified Eagle Medium (DMEM, Hyclone, Utah, USA) containing 10% FBS and 1% penicillin-streptomycin (Solarbio, Beijing, China). The cells were cultured under the condition of 37°C and 5% CO2. At 90% cell confluence, the cells were passaged at a density ratio of 1:3.
CCK-8
SiHa cells were inoculated into 96-well plates, with 5 compound pores in each experimental group and 1.5×103 cells in each well. The nutrient solution was discarded before testing, and 100 μl CCK-8 working solution (Vazyme) per well was added. Microplate reader (Thermo Fisher Scientific, USA) was used to measure the absorbance at 450 nm and the graph was statistically analyzed.
qRT-PCR analysis
RNA was extracted with the Trizol method (Vazyme) and dissolved in DEPC water. The quality of RNA was measured by a Microultraviolet-visible spectrophotometer (Thermo Fisher Scientific). cDNA was obtained by reverse transcription kit (Vazyme) and the transcript abundance of the genes with Synergy Brands (SYBR) Green detection (Vazyme) was detected by qRT-PCR. The relative expression was calculated using the 2-ΔΔCt method. The sequences of primers used are shown in Table 1.
Table 1.
Primers for genes
| Gene | Forward (5’-3’) | Reverse (3’-5’) |
|---|---|---|
| IL-10 | GTTGTTAAAGGAGTCCTTGCTG | TTCACAGGGAAGAAATCGATGA |
| IL-6 | TGGTGTTGCCTTCTGCCTTC | GCTGAGATCCGTCTAGGATG |
| IL-1β | GCCAGTGAAATGATGGCTTATT | AGGAGCACTTCATCTGTTTAGG |
| TNF-α | AAGGACACCATGAGCACTGAAAGC | AGGAAGGAGAAGAGGCTGAGGAAC |
| Vimentin | AAGGCGAGGAGAGCAGGATT | GAGTGGGTATCAACCAGAGGG |
| N-cadherin | AGCCAACCTTAACTGAGGAGT | GGCAAGTTGATTGGAGGGATG |
| E-cadherin | CGAGAGCTACACGTTCACGG | GGGTGTCG AGGGAAAAATAGG |
| Snail | TCGGAAGCCTAACTACAGCGA | AGATGAGCATTGGCAGCGAG |
| β-actin | GCACTCTTCCAGCCTTCCTTCC | GCGGATGTCCACGTCACACTTC |
Western blot
Protein samples and markers were added into the sample wells of 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the samples were separated by electrophoresis. The protein were transferred to a 0.45 μm PVDF membrane (Millipore, Billerica, USA) and blocked with 5% skim milk, followed by incubation with the primary antibodies: anti-Alix (1:500, Proteintech), anti-CD9 (1:500, Proteintech), anti-Calnexin (1:500; Abcam), anti-Tsg101 (1:1000, Proteintech), anti-N-cadherin (1:1000, Proteintech), anti-E-cadherin (1:4000, Proteintech), anti-Snail (1:1000, Proteintech), COX-2 (1:1000, abcam), PCNA (1:1000, abclonal), Claudin1 (1:1000, Proteintech), and anti-β-actin (1:1000, STARTER) at 4°C overnight. The HRP-conjugated secondary antibodies were incubated at room temperature for 1 h, and the PVDF membrane was cleaned with TBS-T. During exposure, an ECL detection reagent (Abbkine, Wuhan, China) was added for color development, and a gel imaging system (e-BLOT, Shanghai, China) was used to detect protein expression on the PVDF membrane.
Wound-healing assay
SiHa cells were cultured in 12-well plates with a cell count of 2.5×105/well. When the cell fusion reached 90% confluence, 3 straight lines were drawn on the plate with a gun tip of 10 µl, and then washed with PBS until no floating cells were found, followed by addition of 1% serum medium in the wells. LPS with a concentration of 4 µg/ml was added to the LPS and hucMSC-Ex groups, and hucMSC-Ex with a concentration of 300 µg/ml was added to the hucMSC-Ex group. The three fields of view were observed under an inverted microscope and photos were taken for 0 h and then returned to the incubator for 48 h. The scratches were viewed and photographed under a microscope and the distance between the scratches were measured.
Scratch healing rate = (0 hour width - 48 hour width)/0 hour width × 100%.
Immunofluorescence (IF)
Cervical scrape samples from clinical patients with cervical inflammation and healthy subjects were collected, and the samples were fixed with 95% anhydrous ethanol. The tissue was immersed in citrate buffer solution for hot repair for 30 min and blocked with 5% bovine serum albumin (BSA) solution for 1 h. Tissues were incubated with the primary antibodies anti-N-cadherin (1:100, Proteintech), anti-E-cadherin (1:200, Proteintech), and anti-Snail (1:100, Proteintech) at 4°C overnight. This was followed by FITC-conjugated secondary antibodies (1:100, Boster, China) at room temperature for 2 h. Finally, the tablets were sealed with anti-fluorescence quenching sealing tablets (Absin, Beijing, China) and observed under an inverted fluorescence microscope (Nikon, Japan).
Statistical analysis
Data were presented as mean ± standard deviation (SD). Statistical analysis was performed using GraphPad Prism9. Comparisons between multiple groups were assessed by one-way ANOVA and P<0.05 was considered significant.
Results
Characterization of hucMSC-derived exosomes
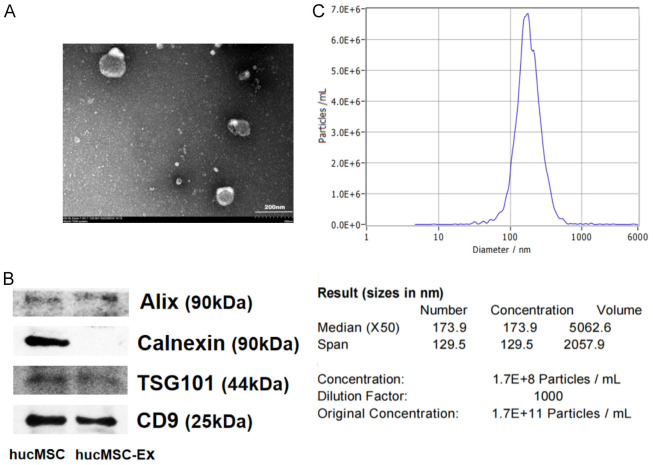
HucMSC-Exs were identified and characterized by transmission electron microscopy (TEM), western blot, and nanoparticle tracking analyses (NTA). TEM showed that hucMSC-Exs exhibit typical vesicle structure (Figure 1A) while western blot analysis showed that both hucMSCs and hucMSC-Exs expressed Alix, CD9, and TSG101, but hucMSC-Ex did not express Calnexin (Figure 1B). The results of NTA showed that the average diameter of exosome particles was approximately 173 nm (Figure 1C). These observations indicate that the isolates were hucMSC-Exs, which could be used for subsequent experiments.
Figure 1.
Identification of hucMSC-Ex. (A) The morphology of hucMSC-Ex was observed by transmission electron microscopy (TEM). (B) Western blot analysis of hucMSC-Ex protein markers. (C) The diameters and concentrations of hucMSC-Ex were determined using nanoparticle tracking analyzer (NTA). Bar = 200 nm (A).
LPS-induced cell inflammation model
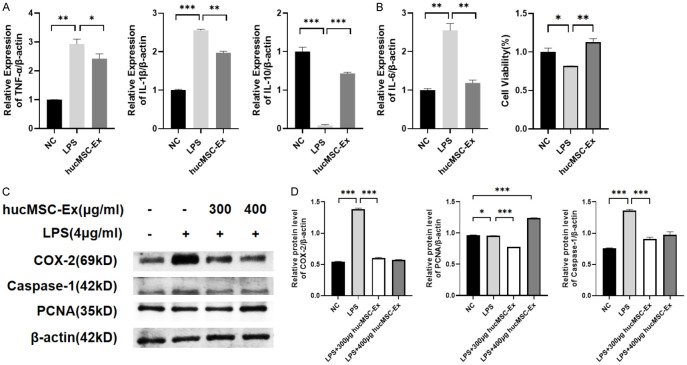
SiHa cells were induced by LPS to construct a cellular inflammation model by treating with a concentration gradient (0, 1, 2, 4, 6 μg/ml) for 18 hours. Western blot results showed that COX-2 expression was significantly enhanced at 4 μg/ml, while PCNA expression was significantly decreased (P<0.001) (Figure 2A, 2B). Subsequently, 4 μg/ml LPS was used to induce cellular inflammation.
Figure 2.
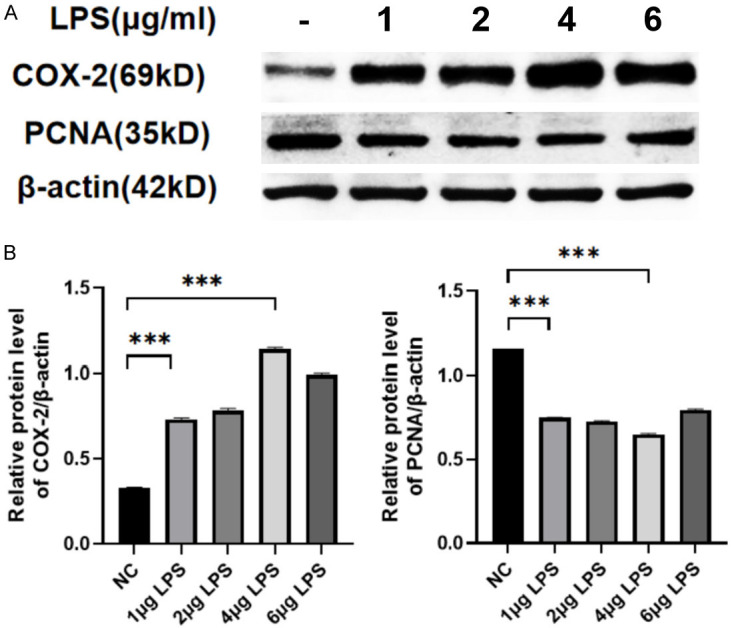
LPS-induced cell inflammation model. A. Western blot analysis of COX-2 and PCNA expression at different concentrations of LPS. B. Relative protein level of COX-2 and PCNA. Data are presented as mean ± SD and analyzed by one-way ANOVA. ***P<0.001.
HucMSC-Ex alleviates LPS-induced cell inflammation
A concentration of 300 μg/ml hucMSC-Ex was used to treat the LPS-induced cervical cancer cells (SiHa). qRT-PCR results showed that the expressions of pro-inflammatory factors TNF-α, IL-1β, and IL-6 were significantly increased (P<0.05, P<0.01) after LPS induction but significantly decreased after hucMSC-Ex treatment (P<0.001). Conversely, the anti-inflammatory factor IL-10 was significantly upregulated after treatment with hucMSC-Ex (Figure 3A). Subsequently, 300 μg/ml was selected as the optimal concentration, and cell proliferation was examined by the CCK-8 method. Cell proliferation was inhibited under LPS stimulation, while hucMSC-Ex treatment could significantly restore cell proliferation (Figure 3B). Western blot analysis of the expression of COX-2, PCNA, and Caspase-1 showed that hucMSC-Ex at 300 µg/ml could significantly down-regulated COX-2 and Caspase-1 expression compared with the LPS group, suggesting an anti-inflammatory role of hucMSC-Ex in LPS-induced inflammation (Figure 3C, 3D).
Figure 3.
HucMSC-Ex ameliorates LPS-induced inflammation in cervical cancer cells. A. The mRNA expression levels of pro-inflammatory factor TNF-α, IL-1β, IL-6, and anti-inflammatory factor IL-10 by qRT-PCR. B. CCK-8 of the different treatment groups. C. The expression of COX-2, PCNA, and Caspase-1 at different concentrations of hucMSC-Ex by western blot. D. Relative protein level of COX-2, PCNA, and Caspase-1. Data are presented as mean ± SD and analyzed by one-way ANOVA. *P<0.05, **P<0.01, ***P<0.001.
HucMSC-Ex reverses the cell migration promoted by LPS-induction
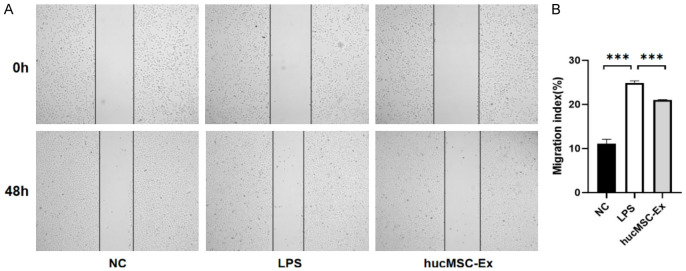
The scratch results showed that after 48 h of treatment, the wound healing degree of SiHa cells in the LPS group was significantly increased compared with that in the NC group, suggesting that inflammation occurred and promoted cell migration. Compared to the LPS group, the wound healing in the hucMSC-Ex group was reduced, indicating that hucMSC-Ex significantly inhibited the migratory stimulation of cells by LPS (Figure 4A, 4B).
Figure 4.
HucMSC-Ex reverses LPS-induced migration of cervical cancer cell. A. Effects of hucMSC-Ex on the migration of SiHa induced by LPS. B. Migration index. Data are presented as mean ± SD and analyzed by one-way ANOVA. ***P<0.001.
HucMSC-Ex inhibits EMT in LPS-induced inflammation
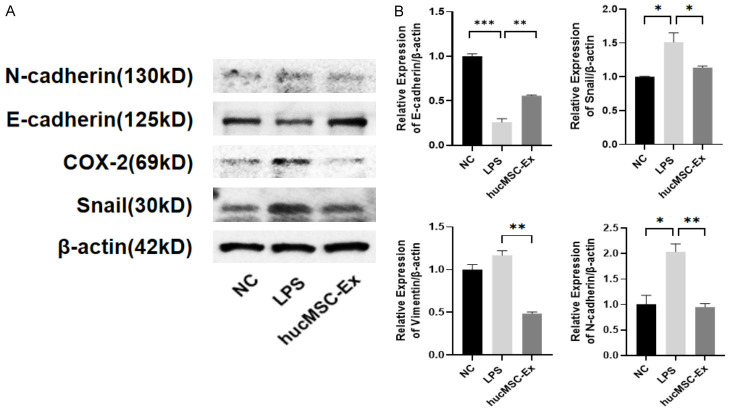
Next, the effect of hucMSC-Ex on the markers’ expression of EMT was examined. Western blot results showed that the levels of interstitial markers N-cadherin and the related transcription factor Snail1 were significantly up-regulated under LPS induction but reduced after hucMSC-Ex treatment. On the other hand, hucMSC-Ex upregulated the protein levels of E-cadherin, an epithelial cell marker in SiHa cells in the inflammatory model (Figure 5A). qRT-PCR results confirmed that LPS treatment of SiHa resulted in increased expression of N-cadherin and Snail1, and decreased expression of E-cadherin, while hucMSC-Ex reversed this trend, which is consistent with the results of western blot (Figure 5B). These results show that LPS can induce EMT in SiHa cells and hucMSC-Ex can inhibit the LPS-induced EMT in these cells.
Figure 5.
HucMSC-Ex inhibits EMT of inflammatory cells. A. Western blot detection of N-cadherin, E-cadherin, COX-2, and Snail expression. B. The expression levels of N-cadherin, E-cadherin, Snail, and Vimentin mRNA by qRT-PCR. Data are presented as mean ± SD and analyzed by one-way ANOVA. *P<0.05, **P<0.01, ***P<0.001.
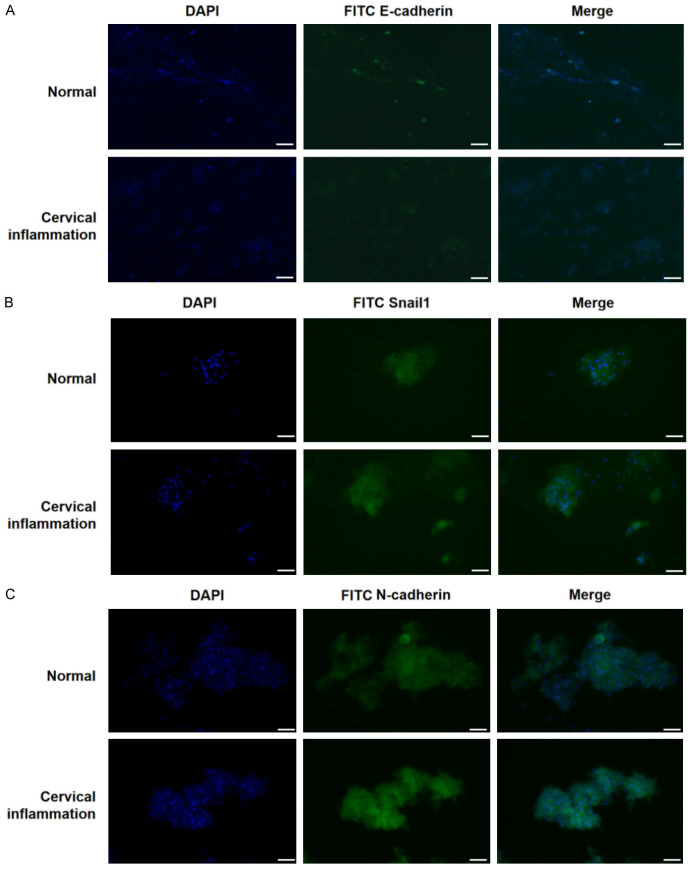
Validation in clinical specimens of cervical inflammation
Cervical scrape samples collected from clinical patients with cervical inflammation and healthy subjects were fixed and the expression of EMT markers was assessed by Immunofluorescence (IF). The results showed that the fluorescence of epithelial cell markers E-cadherin was significantly weakened (Figure 6A), while fluorescence of the interstitial cell markers N-cadherin and Snail1 was enhanced in patients with cervical inflammation (Figure 6B, 6C), suggesting that the inflammatory environment promotes the EMT of epithelial cells.
Figure 6.
Validation in clinical specimens of cervical inflammation. (A) The expression of E-cadherin is low in cervical smears of patients with cervical inflammation. (B) The expression of Snail1 is high in cervical smears of patients with cervical inflammation. (C) The expression of N-cadherin is high in cervical smears of patients with cervical inflammation. Bar = 50 μm (A-C).
Discussion
Mesenchymal stem cells (MSCs) have great potential in regenerative medicine, and exosomes secreted by MSCs are known to have the capacity to regulate the tumor microenvironment and participate in the regulation of many tumor behaviors, such as proliferation, metastasis, and EMT. Exosomes can also play an immunomodulatory role in the inflammatory environment and target anti-inflammatory [25,26]. It has previously been shown that hucMSC-Exs successfully reversed the increased inflammatory response of burned rats or macrophages exposed to LPS, and that hucMSC-Exs with miR-181c overexpression could inhibit TLR4 signaling pathway more effectively and thus reduce inflammation in burned rats [27]. EMT is a well-known biological process that allows epithelial cells to acquire mesenchymal properties, causing them to lose their polarity and adhesion properties, giving them migratory and invasive properties. There exists a significant correlation between inflammation and the metastasis of tumors, as tumors are infiltrated with a large number of inflammatory and immune cells, and inflammation is an effective inducer of EMT in tumors [28-30]. The content current study was focused on the inhibition effect of hucMSC-Ex on inflammation and EMT of SiHa under LPS-induction.
Previous studies have demonstrated that LPS can induce EMT through JAK/STAT and NF-κB signaling pathways [31]. In our study, LPS served as a potent inflammatory stimulus that triggered EMT in cervical epithelial cells SiHa. Some studies have shown that stimulation of SiHa cells with LPS leads to the production of pro-inflammatory mediators and increased protein levels of COX-2 and iNOS. We showed that COX-2, a good indicator of inflammatory response, is lowly expressed under normal physiologic conditions, but when cells are stimulated by inflammation, COX-2 is significantly increased. Firstly, we verified the inflammatory effect of LPS in cervical cancer cells by western blot detection of COX-2 and PCNA expression, and selected the most inflammatory dose concentration according to the concentration gradient of LPS (Figure 2). Western blot results showed that COX-2 expression was significantly enhanced at 4 μg/ml, while PCNA expression was significantly decreased (P<0.001). Subsequently, we used 4 μg/ml LPS to induce SiHa inflammation. We added a certain concentration of hucMSC-Exs to relieve the inflammation of SiHa, and detected the mRNA levels of inflammatory factors TNF-α, IL-1β, IL-6, and IL-10 by qRT-PCR (Figure 3). TNF-α and IL-6 are the main innate immune cytokines, which are produced in the first few minutes after inflammation induction by innate immune cells [32]. In vitro, epithelial cells produce high levels of TNF-α, IL-6 and IL-1β under conditions that induce inflammation [33]. The results showed that hucMSC-Ex could effectively reduce the increase of pro-inflammatory factor expression caused by LPS, and enhance the expression of anti-inflammatory factor IL-10. After hucMSC-Ex treatment, we found that hucMSC-Ex could relieve LPS-induced cell inflammation and restore LPS inhibition of cell proliferation by upregulating PCNA (Figure 3). To further explore how hucMSC-Ex alleviates cervical inflammation, this study further assessed the expression of EMT markers and found that after LPS stimulation, the expression of the epithelial marker E-cadherin decreased significantly, while the expression of interstitial markers N-cadherin and vimentin increased (Figure 5). The hucMSC-Ex treatment reversed both trends (P<0.05). Immunofluorescence experiments on cervical cells collected from the clinic showed a consistent EMT pattern as SiHa cells under inflammation (Figure 6), providing a potential hucMSC-Ex therapeutic target to inhibit the progression of EMT.
Similar to this study, Burcin et al. investigated the potential of wharton jelly-derived mesenchymal stem cell exosomes (WJ-MSC-Ex) as a carrier of anti-cancer drugs such as paclitaxel for cervical cancer cells [34]. The research showed that when paclitaxel was loaded into WJ-MSC-Ex, it could affect Hela cells at lower concentrations, induce cervical cancer cell apoptosis, and inhibit chemotherapy resistance by acting on EMT-related proteins.
The specific mechanisms underlying this phenomenon still require further exploration. Comprehending these pathways might offer insights into how inflammation affects cellular behavior and contributes to disease progression. Furthermore, although the preliminary outcomes from cell culture experiments are encouraging, they still need validation through animal models to confirm their relevance in vivo. Future research may also need to elucidate the specific signaling pathways, such as the TGF-β/Smad2 pathway and the Wnt/β-catenin pathways, involved in the EMT processes triggered by LPS exposure, and the mechanism behind for hucMSC-Ex alleviating the LPS-induced inflammation within cervical epithelial cells.
Conclusion
Human umbilical cord mesenchymal stem cells derived-exosomes alleviates LPS-induced cervical inflammation, possibly by inhibition of epithelial-mesenchymal transition (EMT).
Acknowledgements
This study was supported by the Zhenjiang Key Research and Development Plan (social development project) (Grant No. SH2023050), Jiangsu Province Engineering Research Center of Surface and Interface Functional Composites, and Jiangsu Higher Vocational College Engineering Research Center of Green Energy and Low Carbon Materials.
Disclosure of conflict of interest
None.
References
- 1.Ortiz-de la Tabla V, Gutiérrez F. Cervicitis: etiology, diagnosis and treatment. Enferm Infecc Microbiol Clin (Engl Ed) 2019;37:661–667. doi: 10.1016/j.eimc.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Shrestha AD, Neupane D, Vedsted P, Kallestrup P. Cervical cancer prevalence, incidence and mortality in low and middle income countries: a systematic review. Asian Pac J Cancer Prev. 2018;19:319–324. doi: 10.22034/APJCP.2018.19.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park NJ, Choi Y, Lee D, Park JY, Kim JM, Lee YH, Hong DG, Chong GO, Han HS. Transcriptomic network analysis using exfoliative cervical cells could discriminate a potential risk of progression to cancer in hpv-related cervical lesions: a pilot study. Cancer Genomics Proteomics. 2023;20:75–87. doi: 10.21873/cgp.20366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gong M, Chen C, Zhao H, Guo L, Sun M, Song M. Analysis of distributions of HPV infection in females with cervical lesions in the western district of Beijing Chaoyang Hospital. J Healthc Eng. 2022;2022:5422748. doi: 10.1155/2022/5422748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Z, Hu X, Zhong JF. Mesenchymal stem cells: characteristics, function, and application. Stem Cells Int. 2019;2019:8106818. doi: 10.1155/2019/8106818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 7.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 8.Lan T, Luo M, Wei X. Mesenchymal stem/stromal cells in cancer therapy. J Hematol Oncol. 2021;14:195. doi: 10.1186/s13045-021-01208-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 10.Fox JM, Chamberlain G, Ashton BA, Middleton J. Recent advances into the understanding of mesenchymal stem cell trafficking. Br J Haematol. 2007;137:491–502. doi: 10.1111/j.1365-2141.2007.06610.x. [DOI] [PubMed] [Google Scholar]
- 11.Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C, Wang Y. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14:493–507. doi: 10.1038/s41581-018-0023-5. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Liu T, Ran C, Wang W, Piao F, Yang J, Tian S, Li L, Zhao D. Immunoregulatory paracrine effect of mesenchymal stem cells and mechanism in the treatment of osteoarthritis. Front Cell Dev Biol. 2024;12:1411507. doi: 10.3389/fcell.2024.1411507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrell CR, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells. 2019;8:1605. doi: 10.3390/cells8121605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu J, Sane S, Kim JE, Yun S, Kim HJ, Jo KB, Wright JP, Khoshdoozmasouleh N, Lee K, Oh HT, Thiel K, Parvin A, Williams X, Hannon C, Lee H, Kim DK. Biogenesis and delivery of extracellular vesicles: harnessing the power of EVs for diagnostics and therapeutics. Front Mol Biosci. 2023;10:1330400. doi: 10.3389/fmolb.2023.1330400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J, Li Z, Yue C, Ma J, Cao L, Lin J, Zhu D, An R, Lai J, Guo Y, Gu B. Human umbilical cord mesenchymal stem cell-derived exosomes carrying miR-1827 downregulate SUCNR1 to inhibit macrophage M2 polarization and prevent colorectal liver metastasis. Apoptosis. 2023;28:549–565. doi: 10.1007/s10495-022-01798-x. [DOI] [PubMed] [Google Scholar]
- 16.Baghaei K, Tokhanbigli S, Asadzadeh H, Nmaki S, Reza Zali M, Hashemi SM. Exosomes as a novel cell-free therapeutic approach in gastrointestinal diseases. J Cell Physiol. 2019;234:9910–9926. doi: 10.1002/jcp.27934. [DOI] [PubMed] [Google Scholar]
- 17.Li T, Yan Y, Wang B, Qian H, Zhang X, Shen L, Wang M, Zhou Y, Zhu W, Li W, Xu W. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013;22:845–854. doi: 10.1089/scd.2012.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu B, Hu D, Zhou Y, Yu Y, Shen L, Long C, Butnaru D, Timashev P, He D, Lin T, Xu T, Zhang D, Wei G. Exosomes released by human umbilical cord mesenchymal stem cells protect against renal interstitial fibrosis through ROS-mediated P38MAPK/ERK signaling pathway. Am J Transl Res. 2020;12:4998–5014. [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Zhang Y, Lu B, Xi J, Ocansey DKW, Mao F, Hao D, Yan Y. hucMSC-Ex Alleviates IBD-associated intestinal fibrosis by inhibiting ERK phosphorylation in intestinal fibroblasts. Stem Cells Int. 2023;2023:2828981. doi: 10.1155/2023/2828981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suarez-Carmona M, Lesage J, Cataldo D, Gilles C. EMT and inflammation: inseparable actors of cancer progression. Mol Oncol. 2017;11:805–823. doi: 10.1002/1878-0261.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zsiros V, Kiss AL. Cellular and molecular events of inflammation induced transdifferentiation (EMT) and regeneration (MET) in mesenteric mesothelial cells. Inflamm Res. 2020;69:1173–1179. doi: 10.1007/s00011-020-01400-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, Zhang X, Cai X, Dong J, Chi Y, Chi Z, Gu HF. Effects of curcumin on high glucose-induced epithelial-to-mesenchymal transition in renal tubular epithelial cells through the TLR4-NF-κB signaling pathway. Diabetes Metab Syndr Obes. 2021;14:929–940. doi: 10.2147/DMSO.S296990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao K, He W, Guan W, Hou F, Yan P, Xu J, Zhou T, Liu Y, Xie L. Mesenchymal stem cells reverse EMT process through blocking the activation of NF-κB and Hedgehog pathways in LPS-induced acute lung injury. Cell Death Dis. 2020;11:863. doi: 10.1038/s41419-020-03034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo BP, Luo J, Hu YB, Yao XW, Wu FH. Cyclin D1b splice variant promotes αvβ3-mediated EMT induced by LPS in breast cancer cells. Curr Med Sci. 2018;38:467–472. doi: 10.1007/s11596-018-1902-5. [DOI] [PubMed] [Google Scholar]
- 25.Lin Z, Wu Y, Xu Y, Li G, Li Z, Liu T. Mesenchymal stem cell-derived exosomes in cancer therapy resistance: recent advances and therapeutic potential. Mol Cancer. 2022;21:179. doi: 10.1186/s12943-022-01650-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ha DH, Kim HK, Lee J, Kwon HH, Park GH, Yang SH, Jung JY, Choi H, Lee JH, Sung S, Yi YW, Cho BS. Mesenchymal stem/stromal cell-derived exosomes for immunomodulatory therapeutics and skin regeneration. Cells. 2020;9:1157. doi: 10.3390/cells9051157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Liu L, Yang J, Yu Y, Chai J, Wang L, Ma L, Yin H. Exosome derived from human umbilical cord mesenchymal stem cell mediates MiR-181c attenuating burn-induced excessive inflammation. EbioMedicine. 2016;8:72–82. doi: 10.1016/j.ebiom.2016.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Lollo V, Canciello A, Peserico A, Orsini M, Russo V, Cerveró-Varona A, Dufrusine B, El Khatib M, Curini V, Mauro A, Berardinelli P, Tournier C, Ancora M, Cammà C, Dainese E, Mincarelli LF, Barboni B. Unveiling the immunomodulatory shift: epithelial-mesenchymal transition alters immune mechanisms of amniotic epithelial cells. iScience. 2023;26:107582. doi: 10.1016/j.isci.2023.107582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sengez B, Carr BI, Alotaibi H. EMT and inflammation: crossroads in HCC. J Gastrointest Cancer. 2023;54:204–212. doi: 10.1007/s12029-021-00801-z. [DOI] [PubMed] [Google Scholar]
- 30.Jain SM, Deka D, Das A, Paul S, Pathak S, Banerjee A. Role of interleukins in inflammation-mediated tumor immune microenvironment modulation in colorectal cancer pathogenesis. Dig Dis Sci. 2023;68:3220–3236. doi: 10.1007/s10620-023-07972-8. [DOI] [PubMed] [Google Scholar]
- 31.He A, Ji R, Shao J, He C, Jin M, Xu Y. TLR4-MyD88-TRAF6-TAK1 complex-mediated NF-κB activation contribute to the anti-inflammatory effect of V8 in LPS-induced human cervical cancer SiHa cells. Inflammation. 2016;39:172–181. doi: 10.1007/s10753-015-0236-8. [DOI] [PubMed] [Google Scholar]
- 32.Bahramabadi R, Yousefi-Daredor H, Rezaeinejad S, Rezayati M, Arababadi MK. Down-regulation of transforming growth factor-beta and interleukin-6 serum levels in the idiopathic chronic obstructive pulmonary disease. Am J Clin Exp Immunol. 2022;11:45–50. [PMC free article] [PubMed] [Google Scholar]
- 33.Xu J, Chen C, Yin J, Fu J, Yang X, Wang B, Yu C, Zheng L, Zhang Z. Lactate-induced mtDNA accumulation activates cGAS-STING signaling and the inflammatory response in Sjögren’s syndrome. Int J Med Sci. 2023;20:1256–1271. doi: 10.7150/ijms.83801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abas BI, Demirbolat GM, Cevik O. Wharton jelly-derived mesenchymal stem cell exosomes induce apoptosis and suppress EMT signaling in cervical cancer cells as an effective drug carrier system of paclitaxel. PLoS One. 2022;17:e0274607. doi: 10.1371/journal.pone.0274607. [DOI] [PMC free article] [PubMed] [Google Scholar]