Abstract
Objectives: To investigate the factors influencing the cosmetic outcomes and prognosis of patients undergoing maxillofacial trauma reconstruction. Methods: A retrospective analysis was conducted on the clinical data of 335 patients who underwent maxillofacial trauma surgery criteria at Yunfu People’s Hospital from March 2016 to June 2023. The Face-Q facial cosmetic rating scale was utilized to evaluate outcomes, with scores above 60 deemed the good prognosis group (n=234) and scores below 60 as the poor prognosis group (n=101). Two groups were compared in terms of demographic data, type of trauma, clinical presentation, intraoperative indicators, postoperative serum parameters and nutritional levels, Hamilton Anxiety Scale (HAS), Pittsburgh Sleep Quality Index (PSQI) sleep quality scores. Postoperative recovery and the incidence of complications were documented. Correlation analysis was performed, and Logistic regression analysis was used to determine influencing factors. Results: Patients in the good prognosis group were significantly younger than those in the poor prognosis group (38.15 ± 10.32 vs. 46.69 ± 12.15, P < 0.001). Postoperative protein intake (65.81% vs. 33.66%, P < 0.001) and levels of anxiety (5.57 ± 1.52 vs. 6.61 ± 1.47, P < 0.001) were also better in the good prognosis group. There were significant differences in scar formation (5.57 ± 1.52 vs. 6.61 ± 1.47, P < 0.001), postoperative complications (2.56% vs. 8.91%, P=0.022) and scar hypertrophy (1.28% vs. 6.93%, P=0.015) between the two groups. Logistic regression analysis revealed that age (OR=1.07, 95% CI: 1.039-1.109), protein intake adequacy (OR=0.297, 95% CI: 0.141-0.625), HAS scores (OR=1.295, 95% CI: 1.011-1.658), infection (OR=11.579, 95% CI: 2.656-52.274), and Vancouver Scar Scale (VSS) score (OR=15.672, 95% CI: 7.379-33.285) were significantly associated with aesthetic outcomes. The ROC analysis showed that their combined prediction had an AUC of 0.920, indicating good predictive value. Conclusions: Younger age, adequate protein intake, lower anxiety scores, better scar assessment, and lower infection rates were associated with better prognosis. These findings emphasize the importance of addressing these factors to optimize outcome in craniofacial trauma reconstruction.
Keywords: Risk factors, maxillofacial injuries, plastic surgery, prognosis
Introduction
The craniofacial region is one of the most complex areas of the human body, consisting of various tissues and organs, including bones, muscles, nerves, blood vessels, teeth, and salivary glands. The craniofacial skeleton and sinuses are intricately structured, with teeth attached to the jawbone and the tongue occupying the oral cavity. The face contains facial muscles and nerves responsible for expressions, along with the temporomandibular joint and salivary glands, which support essential functions such as expressions, speech, chewing, swallowing, and breathing [1]. Disorders in the craniofacial region are highly complex and diverse, including dental problems, oral diseases, craniofacial anomalies, jaw fractures, temporomandibular joint disorders, and facial pain. As a result, craniofacial issues can have significant effects on overall health and quality of life [2-4].
Craniofacial traumas often result from sudden accidents such as traffic collisions or animal bites, posing significant risks. Trauma is the most common cause of injuries in the craniofacial region, often resulting in damage to bones, dental arches, and facial soft tissues [5,6]. The rising frequency and severity of craniofacial injuries may be linked to increased dependence on road transport and growing socioeconomic activity. The severity and type of trauma depend on the anatomical site of the injury, the force exerted, and the direction upon impact [7-9].
Craniofacial traumas are prone to infections, presenting with symptoms like redness, swelling, fever, and pain [6]. Treatment often involves cranial traction, affecting oral activities, eating habits, and oral hygiene. Additionally, these traumas can lead to damages in the neck, brain, and other anatomic structures, with the risk of nerve damage resulting in drooling, facial paralysis, and scarring on the face [10-12]. Jaw and facial injuries can lead to facial deformities impairing the appearance. Timely treatment with reconstructive techniques can reduce the likelihood of these adverse outcomes.
However, some patients still experience poor outcomes. While previous studies [13-15] have discussed the various treatment modalities and medication effects on postoperative recovery in craniofacial trauma patients, there is limited literature on factors influencing long-term cosmetic outcomes. This study aims to explore the key factors affecting the cosmetic outcomes in patients undergoing craniofacial trauma reconstruction.
Materials and methods
Study design and participants
This retrospective study included patients who underwent craniofacial surgery at Yunfu People’s Hospital from March 2016 to June 2023. Inclusion criteira: (1) Age between 18 and 65 years, both genders; (2) American Society of Anesthesiologists (ASA) physical status of I or II; (3) Underwent overnight endotracheal extubation. Exclusion criteria: (1) Acute myocardial infarction or severe heart failure; (2) Drug dependence, alcoholism, psychological illness, or severe cognitive dysfunction; (3) Pregnancy or lactation. The flow diagram of patient selection is shown in Figure 1.
Figure 1.
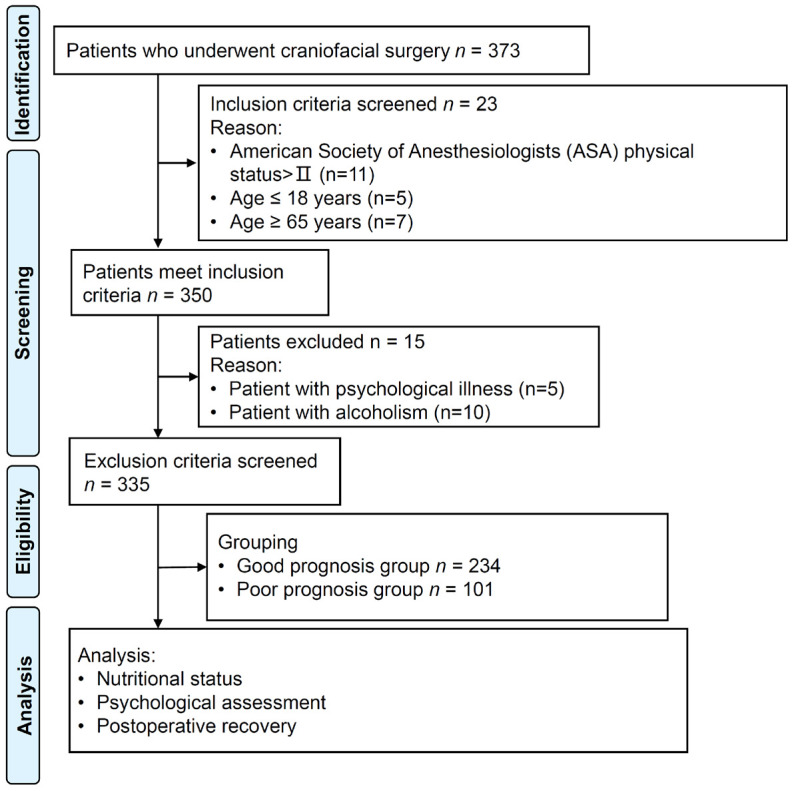
Flow diagram of study.
A total of 335 patients met the inclusion criteria and were included in the study. Patients were categorized into two groups based on their postoperative Face-Q aesthetic scale scores: good prognosis group (score ≥ 60) and poor prognosis group (score < 60). The scale consists of three categories: Health-related Quality of Life (10 scales), Appearance Appraisal (24 scales), and Adverse Effects (6 scales). Checklists serve a different function compared to the scales [16].
The study was approved by the Institutional Review Board and Research Ethics Committee of Yunfu People’s Hospital and was conducted in accordance with the tenets of the Declaration of Helsinki. Informed consent for this retrospective study was waived by the Institutional Review Board and Ethics Committee as the study only used de-identified patient data without potential harm or impact on patient care.
Data collection
Demographic information and clinical data
Before surgery, it is crucial for the physicians to obtain a detailed medical history and conduct a comprehensive craniofacial examination to select suitable surgical and anesthesia methods based on the patient’s condition. Suturing of incisions or bone cutting was performed as necessary, with attention to protecting surrounding soft tissues and crucial neurovascular structures to prevent damage. Adequate postoperative care was administered, including regular follow-up examinations to ensure the effectiveness of the surgery. Demographic data of patients, including age, gender, BMI, smoking history, alcohol consumption history, comorbidities like hypertension, diabetes, and hyperlipidemia, educational level, ethnicity, occupation status and monthly average income were collected and documented from the medical records system. Clinical data, including the cause of trauma, clinical manifestations, surgical duration, anesthesia method, intraoperative blood loss and postoperative drainage volume, were also recorded for both groups.
Laboratory data
One week postoperatively, the following data were examined. Five milliliters of venous blood were drawn in the early morning on an empty stomach, and the samples were processed by high-speed centrifugation to separate serum and plasma. Hemoglobin, white blood cell count (× 10^9/L), platelet count (× 10^9/L), and other parameters of complete blood count measures were analyzed using a fully automated coagulation analyzer (HC00608166, STA Compact, China).
Nutritional status
The nutritional status was assessed based on protein intake volume. Standard protein intake = Resting metabolic rate × Protein reference intake ratio (0.8 g/kg), where Resting metabolic rate was calculated using: Body weight × 24 × Basal metabolic rate (0.9). Meeting this standard indicated adequate protein intake.
Psychological assessment
Psychological scores were evaluated using Hamilton Anxiety Scale (H) before and after treatment. The scale includes 14 items that assess the severity of anxiety symptoms. Each item is rated on a 0 to 4 scale, with the following levels: 0= none; 1= mild; 2= moderate; 3= severe; 4= very severe. The Ham-A has demonstrated good internal consistency (Cronbach’s alpha =0.893) [17].
Sleep quality was evaluated using the Pittsburgh Sleep Quality Index (PSQI), developed by Buysse et al. in 1993, assesses subjective sleep quality over the past month. The PSQI comprises 19 self-rated questions and 5 questions rated by a sleep partner, with only the 19 self-rated questions scored. These items form 7 components, each rated from 0 to 3 points, with a total score ranging from 0 to 21, where higher scores indicates poorer sleep quality. The Chinese version of the PSQI has a Cronbach alpha of 0.71 [18].
Postoperative recovery
During the postoperative hospital stay, key recovery indicators such as the number of days with edema, wound healing time, and suture removal time were observed and recorded. One month postoperatively, wounds were examined for signs of infection and hypertrophic scarring. Wound healing was assessed three months postoperatively in both groups, using the Vancouver Scar Scale (VSS). The scale includes four evaluation criteria: pigmentation, thickness, pliability, and vascularity, with a total score ranging from 0 to 15. A higher score indicates more pronounced scarring and poorer wound healing. The Cronbach α reliability coefficient for the VSS is 0.84.
Statistical analysis
Statistical analysis was performed using SPSS 29.0 software (SPSS Inc., Chicago, IL, USA). Categorical data were presented as [n (%)] and analyzed using chi-square test. The normality of continuous variables was assessed using the Shapiro-Wilk method. Normally distributed variables were expressed as (X±s) and analyzed using the t-test with corrected variance. A two-tailed P value < 0.05 was considered statistically significant for differences. The relationship between detected indicators and prognosis was analyzed using Spearman correlation analysis. Significant indicators from difference and correlation analyses were further analyzed using multiple-factor logistic regression analysis and ROC analysis.
Logistic regression methods: ① Variable Inclusion and Exclusion Criteria. Variables with a p-value < 0.2 in univariate analysis and clinically relevant variables were included in the initial multivariate logistic regression model. Variables with a p-value > 0.2 in univariate analysis and those showing multicollinearity (VIF > 10) were excluded. ② Step-Wise Model. A step-wise backward elimination approach was used to refine the model. Initially, all variables that met the inclusion criteria were included, and at each step, the variable with the highest p-value (P > 0.05) was removed from the model. This process continued until all remaining variables had a p-value ≤ 0.05. Alternatively, a forward selection approach was considered, starting with no variables in the model. At each step, the variable with the lowest p-value (P < 0.05) was added to the model. This process continued until no additional variables could be added with a p-value < 0.05. ③ Final Model. The final model included variables that remained significant after the step-wise backward elimination process. The model was checked for goodness-of-fit using the Hosmer-Lemeshow test and for overall significance using the likelihood ratio test.
Results
General information
This retrospective study included a total of 335 patients, with 234 classified into the good prognosis group and 101 into the poor prognosis group. The two groups were comparable in general demographic characteristics such as gender (P > 0.05) (Table 1). However, there was a significant difference in age between the two groups (38.15 ± 10.32 vs. 46.69 ± 12.15, P < 0.001), indicating that patients in the good prognosis group were younger than the poor prognosis group.
Table 1.
Comparison of general information between two groups of patients
| Good prognosis group (n=234) | Poor prognosis group (n=101) | t/χ2 | p | |
|---|---|---|---|---|
| Age (years) | 38.15 ± 10.32 | 46.69 ± 12.15 | 6.17 | P < 0.001 |
| BMI (kg/m2) | 23.51 ± 3.41 | 23.17 ± 3.57 | 0.807 | 0.421 |
| Male/Female | 128 (54.7%)/106 (45.3%) | 58 (57.43%)/43 (42.57%) | 0.116 | 0.733 |
| Smoking history | 31 (13.25%) | 19 (18.81%) | 1.31 | 0.252 |
| Alcohol history | 42 (17.95%) | 24 (23.76%) | 1.162 | 0.281 |
| Diabetes | 24 (10.26%) | 13 (12.87%) | 0.261 | 0.61 |
| Hypertension | 26 (11.11%) | 15 (14.85%) | 0.604 | 0.437 |
| Educational level (high school or below/college or above) | 51 (21.79%)/183 (78.21%) | 25 (24.75%)/76 (75.25%) | 0.352 | 0.553 |
| Ethnicity (Han/Other) | 184 (78.63%)/50 (21.37%) | 79 (78.22%)/22 (21.78%) | 0.007 | 0.932 |
| Occupation status (Working/Not working) | 98 (41.88%)/136 (58.12%) | 38 (37.62%)/63 (62.38%) | 0.53 | 0.467 |
| Monthly average income (< 3000/3000-6000/> 6000) | 49 (20.94%)/129 (55.13%)/56 (23.93%) | 18 (17.82%)/53 (52.48%)/30 (29.7%) | 1.35 | 0.509 |
| Cause of trauma | 1.682 | 0.794 | ||
| Car accident injury | 81 (34.62%) | 40 (39.6%) | ||
| Incised wound | 67 (28.63%) | 31 (30.69%) | ||
| Laceration | 52 (22.22%) | 19 (18.81%) | ||
| Blast injury | 21 (8.97%) | 7 (6.93%) | ||
| Animal bite injury | 13 (5.56%) | 4 (3.96%) | ||
| Clinical manifestation | ||||
| Occlusal disorder | 89 (38.03%) | 37 (36.63%) | 0.014 | 0.905 |
| Restriction of mouth opening | 175 (74.79%) | 68 (67.33%) | 1.614 | 0.204 |
| Gum tear | 87 (37.18%) | 27 (26.73%) | 2.98 | 0.084 |
| Maxillofacial soft tissue injury | 156 (66.67%) | 62 (61.39%) | 0.649 | 0.421 |
| Maxillofacial fracture | 49 (20.94%) | 25 (24.75%) | 0.395 | 0.530 |
BMI: Body Mass Index.
Preoperative examination index
The preoperative nursing assessment indicators for both groups are summarized in Table 1. Statistical analysis revealed no significant differences between the two groups in terms of trauma cause or clinical manifestations (P > 0.05), suggesting that these factors do not significantly affect prognosis.
Intraoperative index
A comparative analysis of intraoperative indicators between the two groups of patients, including anesthesia method, medical experience, operation duration, intraoperative blood loss, and postoperative drainage, volume was conducted (Table 2; Figure 2). The results indicated that there were no significant differences in these intraoperative indicators between the two groups of patients (all P > 0.05).
Table 2.
Comparison of intraoperative indices between two groups of patients
| Characteristic | Good prognosis group (n=234) | Poor prognosis group (n=101) | t/χ2 | p |
|---|---|---|---|---|
| Anesthesia method | 0.002 | 0.965 | ||
| Regional anesthesia | 132 (56.41%) | 56 (55.45%) | ||
| General anesthesia | 102 (43.59%) | 45 (44.55%) | ||
| Medical experience | 1.16 | 0.282 | ||
| Experience > 5 years | 151 (64.53%) | 72 (71.29%) | ||
| Experience < 5 years | 83 (35.47%) | 29 (28.71%) |
Figure 2.
Comparison of intraoperative indices between two groups. A. Time of operation; B. Intraoperative blood loss; C. Postoperative drainage volume.
Postoperative blood tests
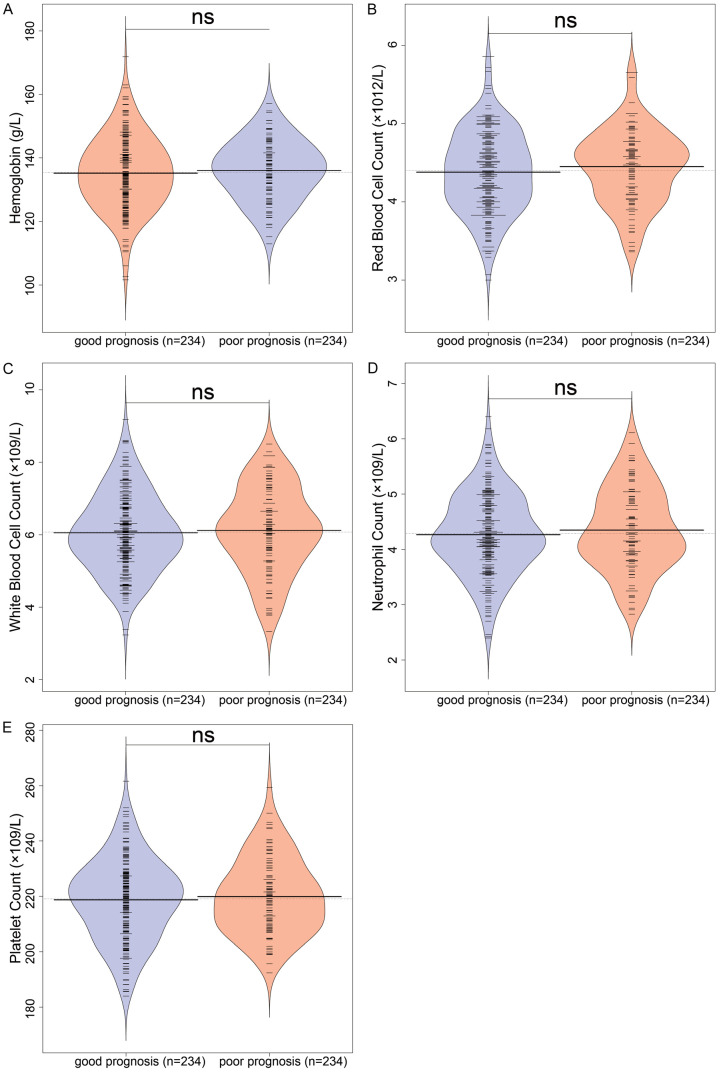
Following surgery, routine blood tests were conducted (Figure 3). The results indicated no significant differences in hemoglobin levels, red blood cell count, white blood cell count, neutrophils, and platelets between the two patient groups (all P > 0.05).
Figure 3.
Blood routine examination. A. Hemoglobin levels; B. Red blood cell count; C. White blood cell count; D. Neutrophils; E. Platelets. ns: no significant differences.
Postoperative examination
Postoperative comparisons were made regarding the protein intake, HAM-A, and PSQI scores of the patients (Table 3). The results revealed no significant difference in the PSQI score (P > 0.05) between the two groups. However, the good prognosis group exhibited significantly higher proportion of patients with adequate protein intake than the poor prognosis group (65.81% vs. 33.66%, P < 0.001), and significantly lower HAM-A score (5.57 ± 1.52 vs. 6.61 ± 1.47, P < 0.001). This indicates that patients in the good prognosis group had higher nutrition intake and better psychological well-being levels than those in the poor prognosis group.
Table 3.
Comparison of postoperative examination between two groups of patients
| Characteristic | Good prognosis group (n=234) | Poor prognosis group (n=101) | t/χ2 | p |
|---|---|---|---|---|
| Protein intake | 28.318 | P < 0.001 | ||
| Qualified | 154 (65.81%) | 34 (33.66%) | ||
| Unqualified | 80 (34.19%) | 67 (66.34%) | ||
| HAMA score | 5.57 ± 1.52 | 6.61 ± 1.47 | 5.9 | P < 0.001 |
| PQSI score | 6.12 ± 0.94 | 6.04 ± 0.78 | 0.76 | 0.448 |
HAMA: Hamilton Anxiety Scale; PQSI: Pittsburgh Sleep Quality Index.
Postoperative recovery
Postoperative recovery of the two groups was compared (Table 4). There were no significant differences between the two groups in terms of edema duration, wound healing time, or suture removal time (all P > 0.05). However, the scar assessment score in the good prognosis group was significantly lower than that of the poor prognosis group (2.98 ± 0.46 vs. 4.17 ± 0.94, P < 0.001). Among the 234 patients in the good prognosis group, there were 6 cases of infection and 3 cases of scar hyperplasia, while in the 101 patients in the poor prognosis group, there were 9 cases of infection and 7 cases of scar hyperplasia, showing significantly lower incidences of infection and hyperplasia in the good prognosis group (2.56% vs. 8.91%, P=0.022; 1.28% vs. 6.93%, P=0.015). This indicates that postoperative complications and scar recovery affect the outcome.
Table 4.
Comparison of postoperative recovery between two groups of patients
| Characteristic | Good prognosis group (n=234) | Poor prognosis group (n=101) | t/χ2 | p |
|---|---|---|---|---|
| Days of edema | 3.95 ± 0.74 | 4.02 ± 0.82 | 0.74 | 0.46 |
| Wound healing time (day) | 4.68 ± 1.03 | 4.76 ± 0.96 | 0.745 | 0.457 |
| Suture removal time (day) | 5.47 ± 1.05 | 5.61 ± 1.12 | 1.052 | 0.294 |
| VSS score | 2.98 ± 0.46 | 4.17 ± 0.94 | 12.099 | P < 0.001 |
| Infection | 6 (2.56%) | 9 (8.91%) | 5.243 | 0.022 |
| Cicatricial hyperplasia | 3 (1.28%) | 7 (6.93%) | 5.945 | 0.015 |
VSS: Vancouver Scar Scale.
Logistic regression analysis
In the univariate logistic regression analysis of factors influencing overall aesthetic outcomes in patients undergoing craniofacial trauma reconstruction for maxillofacial trauma wound healing, several variables showed significant associations with poor prognosis (Table 5). Older age (OR 1.074, 95% CI 1.050-1.102), HAM-A scores (OR 1.600, 95% CI 1.352-1.913), increased VSS scores (OR 16.121, 95% CI 8.896-32.256), lower protein intake (OR 0.264, 95% CI 0.160-0.429), presence of infection (OR 3.717, 95% CI 1.304-11.368), and development of cicatricial hyperplasia (OR 5.734, 95% CI 1.559-27.038) were all significantly associated with poor aesthetic outcomes. In the multivariate logistic regression analysis (Table 6), age (OR 1.073, 95% CI 1.039-1.109), HAM-A scores (OR 1.295, 95% CI 1.011-1.658), VSS scores (OR 15.672, 95% CI 7.379-33.285), lower protein intake (OR 0.297, 95% CI 0.141-0.625), presence of infection (OR 11.579, 95% CI 2.565-52.274), and cicatricial hyperplasia (OR 3.846, 95% CI 0.624-23.702) retained their significant associations with poor aesthetic outcomes. These results highlight the importance of considering these factors in the management of patients undergoing craniofacial trauma reconstruction for maxillofacial trauma wound healing.
Table 5.
Univariate logistic regression analysis of factors influencing a poor prognosis
| Coefficient | Std. Error | Wald | P Value | OR | 95% CI | |
|---|---|---|---|---|---|---|
| Age (years) | 0.072 | 0.012 | 5.814 | < 0.001 | 1.074 | 1.050-1.102 |
| HAMA score | 0.470 | 0.088 | 5.319 | < 0.001 | 1.600 | 1.352-1.913 |
| VSS score | 2.780 | 0.327 | 8.494 | < 0.001 | 16.121 | 8.896-32.256 |
| Protein intake | -1.333 | 0.252 | 5.298 | < 0.001 | 0.264 | 0.160-0.429 |
| Infection | 1.313 | 0.541 | 2.426 | 0.015 | 3.717 | 1.304-11.368 |
| Cicatricial hyperplasia | 1.746 | 0.701 | 2.492 | 0.013 | 5.734 | 1.559-27.038 |
HAMA: Hamilton Anxiety Scale; VSS: Vancouver Scar Scale.
Table 6.
Multivariate logistic regression analysis of influencing factors for poor prognosis
| Coefficient | Std. Error | Wald | P Value | OR | 95% CI | |
|---|---|---|---|---|---|---|
| Age (years) | 0.071 | 0.017 | 4.264 | < 0.001 | 1.073 | 1.039-1.109 |
| HAMA score | 0.258 | 0.126 | 2.047 | 0.041 | 1.295 | 1.011-1.658 |
| VSS score | 2.752 | 0.384 | 7.161 | < 0.001 | 15.672 | 7.379-33.285 |
| Protein intake | -1.213 | 0.379 | -3.201 | 0.001 | 0.297 | 0.141-0.625 |
| Infection | 2.449 | 0.769 | 3.185 | 0.001 | 11.579 | 2.565-52.274 |
| Cicatricial hyperplasia | 1.347 | 0.928 | 1.452 | 0.147 | 3.846 | 0.624-23.702 |
HAMA: Hamilton Anxiety Scale; VSS: Vancouver Scar Scale.
ROC analysis
ROC analysis was used to evaluate the predictive value of various factors for the prognosis of craniofacial reconstruction following maxillofacial trauma (Table 7). The VSS score demonstrated the highest area under the curve (AUC) of 0.88, with a sensitivity of 0.752 and a specificity of 0.936, resulting in a Youden index of 0.688 and an F1 score of 0.792. The HAM-A score showed an AUC of 0.691, with a sensitivity of 0.733 and a specificity of 0.585, leading to a Youden index of 0.318 and an F1 score of 0.544. Age had an AUC of 0.707, with a sensitivity of 0.733 and a specificity of 0.598, resulting in a Youden index of 0.331 and an F1 score of 0.55. Protein intake achieved an AUC of 0.661, with a sensitivity of 0.663 and a specificity of 0.658, leading to a Youden index of 0.321 and an F1 score of 0.235. In contrast, infection and cicatricial hyperplasia showed lower AUCs of 0.532 and 0.528, respectively, with sensitivities of 0.089 and 0.069, and specificities of 0.974 and 0.987, resulting in Youden indices of 0.063 and 0.056, and F1 scores of 0.155 and 0.126, respectively. These findings suggest that the VSS score and HAM-A score may be more reliable indicators for predicting outcomes, warranting further validation in larger cohorts. To improve predictive accuracy, a combined prediction model was created using these key indicators (Figure 4). The combined model achieved an AUC of 0.920, indicating a strong discriminatory power. An AUC value close to 1 represents excellent discrimination, while values closer to 0.5 indicate poor discrimination. The point labeled “0.299 (0.889, 0.832)” on the curve corresponds to a sensitivity of 0.889 and a specificity of 0.832 at a certain cut-off point, indicating the model’s ability to correctly differentiate between good and poor prognoses at that threshold. Overall, these results indicate that these important factors have a high predictive value for the prognosis in craniofacial reconstruction after maxillofacial trauma and may be useful in clinical practice.
Table 7.
Predictive value of various factors for the prognosis of craniofacial reconstruction following maxillofacial trauma
| Best threshold | Sensitivities | Specificities | AUC | Youden index | F1 score | |
|---|---|---|---|---|---|---|
| Age (years) | 41.02 | 0.733 | 0.598 | 0.707 | 0.331 | 0.55 |
| Protein intake (Qualified/Unqualified) | 0.5 | 0.663 | 0.658 | 0.661 | 0.321 | 0.235 |
| HAMA score | 5.885 | 0.733 | 0.585 | 0.691 | 0.318 | 0.544 |
| VSS score | 3.625 | 0.752 | 0.936 | 0.88 | 0.688 | 0.792 |
| Infection | 0.5 | 0.089 | 0.974 | 0.532 | 0.063 | 0.155 |
| Cicatricial hyperplasia | 0.5 | 0.069 | 0.987 | 0.528 | 0.056 | 0.126 |
HAMA: Hamilton Anxiety Scale; VSS: Vancouver Scar Scale.
Figure 4.
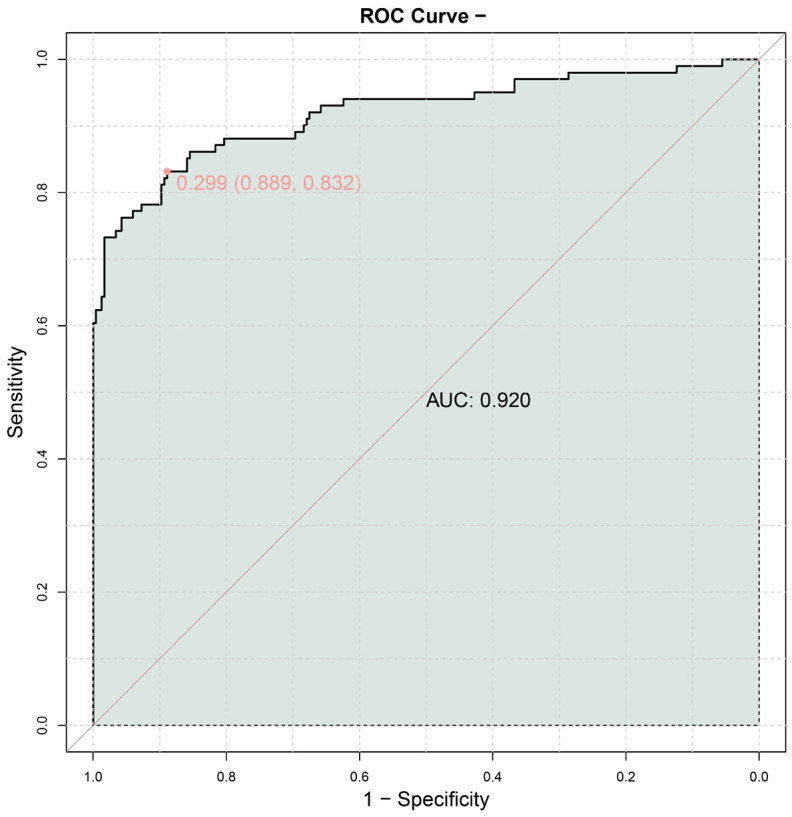
ROC curve for combined prediction.
Discussion
Maxillofacial trauma is a major cause of damage to the soft tissues and bones of the face. Maxillofacial trauma presents specific features, treatment modalities, and outcomes. Understanding the risk factors for the prognosis of maxillofacial plastic surgery is crucial for the effective treatment and management of facial injuries [19-21]. This retrospective study specifically explores the influencing factors on the prognosis in patients undergoing maxillofacial trauma plastic surgery. Factors such as age, postoperative nutritional status, anxiety scores, wound healing conditions, and complications significantly impact the overall cosmetic outcomes after facial trauma plastic surgery.
Increasing age is a significant risk factor that adversely affects the prognosis of maxillofacial trauma plastic surgery. This aligns with previous research findings suggesting that age may influence the healing rate of patients’ wounds. The underlying mechanism may be attributed to the gradual slowing of skin metabolism with aging, diminished circulation, and reduced synthesis of collagen and elastin fibers, leading to weakened skin repair abilities [22-24]. Additionally, older patients may have poorer physical fitness compared to younger individuals, with aging cells potentially losing their division capacity, resulting in slower wound healing.
Nutritional status is another risk factor, with this study measuring patients’ nutritional levels based on protein intake. Protein, a complex organic compound, plays a role in providing energy and participating in metabolism [25]. During wound healing, the body releases various growth factors, and proteins provides necessary raw materials for these factors, promoting cell growth and repair [26,27]. Thederan et al. revealed that protein intake could optimize nutritional status, thereby promoting wound healing, enhancing recovery, and improving prognosis [28]. These findings are consistent with our study results.
Moreover, numerous studies have shown that emotional states are closely linked to physiological conditions. Negative emotions, such as depression and anxiety, can decrease the quantity and vitality of immune cells, potentially leading to hormonal imbalances that can adversely impact postoperative recovery [29-31]. In such cases, healthcare providers should offer timely psychological counseling to patients and appropriately apply psychotherapy to alleviate anxiety.
Wound healing conditions and the occurrence of complications are also significant risk factors for poor prognosis in maxillofacial trauma plastic surgery. This study predominantly discusses postoperative complications such as infections and scar hypertrophy. The oral and maxillofacial regions are prone to infections due to the abundance of bacteria from nasal sinuses and cavities [32]. Scar hypertrophy, resulting from excessive fibrous connective tissue proliferation, manifests as raised, lumpy scars that are reddish, shiny, and often accompanied by dilated capillaries [33]. Both complications can lead to inferior prognostic outcomes, underscoring the importance of prompt intervention and management.
While this study introduced several risk factors associated with poor prognosis in maxillofacial trauma plastic surgery, it is important to acknowledge certain limitations. First, the retrospective design imposes inherent constraints on causal inferences, as clear causality between observed influencing factors and prognostic outcomes has not been established. Relying on retrospective data collection also introduces the possibility of information bias and confounding variables that may affect observed results. The use of subjective outcome measures, such as the Face-Q aesthetic scale, HAM-A scale, and PSQI sleep quality score, can introduce variability and potential bias. These scales rely on patient self-reporting, which can be influenced by individual perceptions and experiences. Additionally, the relatively small sample size of this study might restrict the generalizability of the findings to a broader patient population. In future research, we plan to conduct multicenter prospective studies to further investigate the risk factors for poor prognosis in maxillofacial trauma plastic surgery.
Conclusion
Younger age, adequate protein intake, lower anxiety scores, better scar assessment, and lower infection rates are associated with better prognosis of maxillofacial trauma plastic surgery. These findings emphasize the importance of addressing these factors in patient care to optimize outcomes in craniofacial trauma reconstruction.
Acknowledgements
This study was supported by the Research Foundation of Yunfu People’s Hospital (No. A20231005).
Disclosure of conflict of interest
None.
References
- 1.Sharifi F, Samieirad S, Grillo R, Da Graça Naclério-Homem M, Bardideh E, Manafi A, Mohammadi Z, Eshghpour M. The causes and prevalence of maxillofacial fractures in Iran: a systematic review. World J Plast Surg. 2023;12:1–11. doi: 10.52547/wjps.12.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.AlMofreh AlQahtani F, Bishawi K, Jaber M, Thomas S. Maxillofacial trauma in the gulf countries: a systematic review. Eur J Trauma Emerg Surg. 2021;47:397–406. doi: 10.1007/s00068-020-01417-x. [DOI] [PubMed] [Google Scholar]
- 3.Gokdogan O. Bone grafting in maxillofacial trauma. Curr Opin Otolaryngol Head Neck Surg. 2022;30:260–264. doi: 10.1097/MOO.0000000000000809. [DOI] [PubMed] [Google Scholar]
- 4.Othman A, Al-Mofreh Al-Qahtani F, Al-Qahtani H, Jaber M, Bishawi K, Hassan Khamis A, Al-Shanably A. Traumatic brain injuries and maxillofacial fractures: a systematic review and meta-analysis. Oral Maxillofac Surg. 2023;27:373–385. doi: 10.1007/s10006-022-01076-9. [DOI] [PubMed] [Google Scholar]
- 5.Sifuentes-Cervantes JS, Muzzi D, Castro-Núñez J, Guerrero LM, Cunningham LL. Maxillofacial trauma patterns in bare-knuckle fighting. J Oral Maxillofac Surg. 2022;80:1663–1669. doi: 10.1016/j.joms.2022.07.137. [DOI] [PubMed] [Google Scholar]
- 6.Bansod AV, Pisulkar SG, Dahihandekar C, Beri A. Rapid prototyping in maxillofacial rehabilitation: a review of literature. Cureus. 2022;14:e28969. doi: 10.7759/cureus.28969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar S, Kashyap S, Singh S, Sharma R, Singh YP, Naik HY. Maxillofacial trauma among Indians. Bioinformation. 2023;19:876–880. doi: 10.6026/97320630019876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan TU, Rahat S, Khan ZA, Shahid L, Banouri SS, Muhammad N. Etiology and pattern of maxillofacial trauma. PLoS One. 2022;17:e0275515. doi: 10.1371/journal.pone.0275515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaber MA, AlQahtani F, Bishawi K, Kuriadom ST. Patterns of maxillofacial injuries in the Middle East and North Africa: a systematic review. Int Dent J. 2021;71:292–299. doi: 10.1111/idj.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Omami G, Branstetter BF 4th. Imaging of maxillofacial injuries. Dent Clin North Am. 2024;68:393–407. doi: 10.1016/j.cden.2023.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Wang YH, Wang DR, Liu JY, Pan J. Local anesthesia in oral and maxillofacial surgery: a review of current opinion. J Dent Sci. 2021;16:1055–1065. doi: 10.1016/j.jds.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou HH, Lv K, Rong-TaoYang, Li Z, Li ZB. Maxillofacial injuries in pediatric patients. J Craniofac Surg. 2021;32:1476–1479. doi: 10.1097/SCS.0000000000007402. [DOI] [PubMed] [Google Scholar]
- 13.Kim EJ, Kim CH, Yoon JY, Byeon GJ, Kim HY, Choi EJ. Comparison of postoperative nausea and vomiting between Remimazolam and Propofol in patients undergoing oral and maxillofacial surgery: a prospective randomized controlled trial. BMC Anesthesiol. 2023;23:132. doi: 10.1186/s12871-023-02091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Preda F, Morgan N, Van Gerven A, Nogueira-Reis F, Smolders A, Wang X, Nomidis S, Shaheen E, Willems H, Jacobs R. Deep convolutional neural network-based automated segmentation of the maxillofacial complex from cone-beam computed tomography: a validation study. J Dent. 2022;124:104238. doi: 10.1016/j.jdent.2022.104238. [DOI] [PubMed] [Google Scholar]
- 15.Yanan J, Hui D, Jianwei G, Ronglin L, Lijuan Z, Jing Z. A comparative study on sedation efficacy between general and regional anesthesia with dexmedetomidine in patients under maxillofacial surgery. Curr Drug Metab. 2022;23:920–927. doi: 10.2174/1389200223666220413113412. [DOI] [PubMed] [Google Scholar]
- 16.Ottenhof MJ, Veldhuizen IJ, Hensbergen LJV, Blankensteijn LL, Bramer W, Lei BV, Hoogbergen MM, Hulst RRWJ, Sidey-Gibbons CJ. The use of the FACE-Q aesthetic: a narrative review. Aesthetic Plast Surg. 2022;46:2769–2780. doi: 10.1007/s00266-022-02974-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmerman M, Martinez JH, Young D, Chelminski I, Dalrymple K. Severity classification on the Hamilton Depression Rating Scale. J Affect Disord. 2013;150:384–388. doi: 10.1016/j.jad.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 18.Ho KY, Lam KKW, Xia W, Chung JOK, Cheung AT, Ho LLK, Chiu SY, Chan GCF, Li WHC. Psychometric properties of the Chinese version of the Pittsburgh Sleep Quality Index (PSQI) among Hong Kong Chinese childhood cancer survivors. Health Qual Life Outcomes. 2021;19:176. doi: 10.1186/s12955-021-01803-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pullos AN, Krishnan DG. Complicated maxillofacial fractures: pediatric and geriatric. Atlas Oral Maxillofac Surg Clin North Am. 2019;27:113–118. doi: 10.1016/j.cxom.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Wolfaardt JF, Brecht LE, Taft RM. The future of maxillofacial prosthodontics in North America: part I-journey to the present. J Prosthet Dent. 2022;127:345–350. doi: 10.1016/j.prosdent.2020.11.012. [DOI] [PubMed] [Google Scholar]
- 21.Wusiman P, Maimaitituerxun B, Guli, Saimaiti A, Moming A. Epidemiology and pattern of oral and maxillofacial trauma. J Craniofac Surg. 2020;31:e517–e520. doi: 10.1097/SCS.0000000000006719. [DOI] [PubMed] [Google Scholar]
- 22.Benton MJ, Hutchins AM. The relationship between resting metabolic rate and quality of life is moderated by age and body composition in women: a cross-sectional study. BMC Womens Health. 2024;24:235. doi: 10.1186/s12905-024-03085-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen K, Dai J, Klöting N, Cao X, Jin S, Chen L, Wang Y, Liu S, Hu Y, Jiang L, Liew CW, Blüher M, Wang X. Serum scEMC10 levels are negatively associated with resting metabolic rate and age in humans. J Clin Endocrinol Metab. 2023;108:e1074–e1081. doi: 10.1210/clinem/dgad214. [DOI] [PubMed] [Google Scholar]
- 24.Vásquez-Alvarez S, Bustamante-Villagomez SK, Vazquez-Marroquin G, Porchia LM, Pérez-Fuentes R, Torres-Rasgado E, Herrera-Fomperosa O, Montes-Arana I, Gonzalez-Mejia ME. Metabolic age, an index based on basal metabolic rate, can predict individuals that are high risk of developing metabolic syndrome. High Blood Press Cardiovasc Prev. 2021;28:263–270. doi: 10.1007/s40292-021-00441-1. [DOI] [PubMed] [Google Scholar]
- 25.Zhang W, Wang T. Understanding protein functions in the biological context. Protein Pept Lett. 2023;30:449–458. doi: 10.2174/0929866530666230507212638. [DOI] [PubMed] [Google Scholar]
- 26.Franco-Valencia K, Nóbrega IBC, Cantaruti T, Barra A, Klein A, Azevedo-Jr GM, Costa RA, Carvalho CR. Subcutaneous injection of an immunologically tolerated protein up to 5 days before skin injuries improves wound healing. Braz J Med Biol Res. 2022;55:e11735. doi: 10.1590/1414-431X2021e11735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He W, Xu J, Zheng Y, Chen J, Yin Y, Mosselhy DA, Zou F, Ma M, Liu X. Bacterial cellulose/soybean protein isolate composites with promoted inflammation inhibition, angiogenesis and hair follicle regeneration for wound healing. Int J Biol Macromol. 2022;211:754–766. doi: 10.1016/j.ijbiomac.2022.04.118. [DOI] [PubMed] [Google Scholar]
- 28.Thederan I, Zyriax BC, Heinzer H. Role of nutrition in urological prehabilitation. Urologie. 2023;62:1017–1024. doi: 10.1007/s00120-023-02192-4. [DOI] [PubMed] [Google Scholar]
- 29.Basu S, Goswami AG, David LE, Mudge E. Psychological stress on wound healing: a silent player in a complex background. Int J Low Extrem Wounds. 2022 doi: 10.1177/15347346221077571. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Hedayati J, Bagheri-Nesami M, Elyasi F, Hosseinnataj A. The effect of music therapy on the pain and anxiety levels of patients experiencing wound healing by suturing in the emergency wards. Anesth Pain Med. 2023;13:e132943. doi: 10.5812/aapm-132943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar N, Huda F, Mani R, Singla T, Kundal A, Sharma J, Gajula B. Role of hospital anxiety and depression on the healing of chronic leg ulcer: a prospective study. Int Wound J. 2020;17:1941–1947. doi: 10.1111/iwj.13485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henry A, Dawoud B, Kent S, McDonald C, Logan G, Hennedige A, Exely R, Regan A, Kulkarni R, Gilbert K, Basyuni S, Young D, Kyzas P, Morrison R, McCaul J List of collaborators who participated in the Cervicofacial Infection Study. Clinical features and presentation of cervicofacial infection: a maxillofacial trainee research collaborative (MTReC) study. Br J Oral Maxillofac Surg. 2021;59:433–438. doi: 10.1016/j.bjoms.2020.08.102. [DOI] [PubMed] [Google Scholar]
- 33.Zhu B, Tong G, Gao P, Yan M. Evaluating the impact of recombinant human epidermal growth factor on scar formation in oral and maxillofacial traumatic wound healing. Int Wound J. 2024;21:e14851. doi: 10.1111/iwj.14851. [DOI] [PMC free article] [PubMed] [Google Scholar]