Abstract
Objective: To explore the influence of obesity on microvascular obstruction (MVO) and myocardial area at risk (AAR) in patients with ST-segment elevation myocardial infarction (STEMI). Methods: A retrospective analysis was performed on patients with first-episode STEMI hospitalized at Nanjing University of Traditional Chinese Medicine between May 2020 and May 2022. Patients were categorized into normal weight, overweight, and obese groups based on their body mass index (BMI). Baseline characteristics, blood biochemical indexes and cardiac magnetic resonance (CMR) parameters were compared among the groups. Pearson correlation analysis and binary logistic regression were performed to assess the correlation between MVO ratio and BMI in each subgroup and to identify the risk factors for MVO. Results: Of the 233 patients, 77 were of normal weight, 102 were overweight, and 54 were obese. Obese patients were younger than normal-weight and overweight patients. Both the obese and overweight groups had significantly higher rates of hypertension and hyperlipidemia compared to the normal-weight group. Hemoglobin (Hb), triglycerides (TAG), and high-density lipoprotein cholesterol (HDL-C) levels were higher in obese patients, while creatine kinase isoenzyme (CK-MB) levels were lower (all P<0.05). MVO ratio showed a negative correlation with BMI across all patients (r=-0.133, P<0.05). Binary logistic regression confirmed that BMI was an independent predictor of MVO [0.908 (0.838-0.984), P=0.019]. Additionally, the AAR ratio was significantly lower in obese patients compared to those of normal weight. Conclusions: Overweight and obese patients are strongly associated with a reduced risk of MVO, and BMI is an independent predictor of MVO. Obesity is significantly linked to a smaller AAR in myocardial infarction.
Keywords: Obesity, body mass index, myocardial infarction, microvascular obstruction, area at risk
Introduction
Coronary artery disease (CAD) primarily refers to coronary atherosclerosis, which leads to significant coronary artery stenosis and insufficient myocardial blood supply [1]. Acute ST-segment elevation myocardial infarction (STEMI) is the most severe manifestation of CAD. It involves a drastic reduction or interruption of blood flow to the coronary arteries due to pathological changes, resulting in severe and persistent acute myocardial ischemic necrosis, with high rates of morbidity and mortality [2]. In developing countries, the prevalence and incidence of cardiovascular diseases (CVD) are on the rise [3]. This increase can be attributed to longer life expectancy, along with lifestyle and dietary changes - particularly the consumption of saturated fats and refined sugars [4,5].
Obesity is now recognized as a chronic condition and a major risk factor for CVD [6,7]. It poses multiple health risks, impacts various organ systems, and is associated with the early onset of coronary heart disease and acute myocardial infarction (MI) [8,9]. The mechanisms through which obesity affects the cardiovascular system include adipokine dysregulation, inflammation, increased circulating free fatty acids, heightened oxidative stress, and adipose tissue hypoxia, all of which contribute to atherosclerosis and the formation of atherosclerotic plaques [10]. Studies have shown that the revascularization rate is 43% in overweight individuals and 24% in those with obesity [11]. However, the impact of obesity on the outcomes of acute MI remains controversial [12,13]. Some research suggests that obese patients have better prognoses than non-obese patients, regardless of whether they undergo percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) - a phenomenon referred to as the “obesity paradox” [14,15]. It has been proposed that this paradox may be due to unrecognized confounding factors, such as age, comorbidities, and treatment strategies [16,17].
Despite advances in PCI techniques improving the overall prognosis of STEMI patients, some patients still experience the “no-reflow” phenomenon during surgery. This condition results in poor perioperative outcomes and long-term adverse events, with myocardial microvascular obstruction (MVO) being the key pathophysiological mechanism [18]. Post-reperfusion MVO is reported to occur in 60% to 70% of STEMI patients, regardless of the reperfusion method used, including PCI and thrombolysis [19,20]. Cardiac magnetic resonance (CMR) imaging has been shown to effectively identify and quantitatively assess MVO using gadolinium-based contrast enhancement [21]. CMR provides a comprehensive evaluation of post-infarction patients, encompassing morphological, functional, and microvascular consequences. However, there is a lack of large-scale studies investigating the influence of body mass index (BMI) on MVO and the area at risk (AAR) in Chinese STEMI patients. This study aims to retrospectively analyze consecutive STEMI patients who underwent PCI and compare the effects of BMI across three groups - normal weight, overweight, and obese - on MVO and AAR.
Materials and methods
Study population
A retrospective analysis was performed on patients with first-episode STEMI who were hospitalized at Nanjing University of Traditional Chinese Medicine between May 2020 and May 2022. STEMI was diagnosed based on the diagnostic criteria established by the World Health Organization (WHO).
Inclusion criteria: (1) Patients with first-episode STEMI [22] successfully treated with PCI within 12 hours of symptom onset; (2) Patients who successfully underwent CMR examination; (3) Patients with a BMI>18.5; (4) Patients with complete medical records. Exclusion criteria: (1) Prior MI, revascularization, or congestive heart failure with left ventricular ejection fraction (LVEF) <40%; (2) Abnormal hemodynamic parameters such as blood pressure or heart rate; (3) Presence of arrhythmias such as atrial fibrillation; (4) Acute infectious or rheumatic diseases within the past 3 months; (5) Malignant tumors; (6) Poor CMR image quality; (7) Incomplete medical records. Based on these criteria, 235 patients were selected, and their clinical data were collected.
According to the guidelines for the Prevention and Control of Overweight and Obesity in Chinese Adults [13], BMI (BMI = weight/height2 [kg/m2]) was used as the evaluation index, and patients were categorized into normal weight (18.5≤BMI<24.0), overweight (24.0≤BMI<28.0), and obesity (BMI≥28.0) groups. This study was approved by the Ethics Committee of Nanjing University of Traditional Chinese Medicine.
Data gathering
(1) Baseline data: Baseline information was collected from patients’ medical records, including sex, age, height, weight, history of hypertension, diabetes, hyperlipidemia, smoking history, history of chest pain, time from symptom onset to reperfusion, heart rate and systolic blood pressure at admission, Killip classification, and time from symptom onset to CMR examination. (2) Laboratory data: Fasting venous blood (4 mL) was collected within 24 hours of admission, and a Roche COBAS INTEGRA 800 automated biochemical analyzer was used to measure C-reactive protein (CRP), white blood cell count (WBC), hemoglobin (Hb), platelets (PLT), and serum creatinine (Scr). Fasting venous blood was also collected the morning after admission, and serum was obtained by centrifugation. Total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were measured using biochemical analyzers, while serum creatine kinase isoenzyme (CK-MB) was assessed using an immunochemiluminescence method.
CMR
Echocardiographic data were obtained from echocardiograms performed on the same day as the CMR examination. The CMR procedure was conducted using a 3.0T scanner (Philips Intera, Best, The Netherlands) 5-7 days after PCI. Images were acquired using electrocardiogram gating and breath-holding at end-inspiration. CMR scans included vertical and horizontal planes in both long and short axes. Following acquisition of the plain scans, black-blood T2-weighted images were obtained using fast spin-echo sequences in the short-axis plane, with fat suppression applied. Late gadolinium enhancement imaging was performed 10 minutes after the intravenous injection of 0.2 mmol/kg of gadolinium contrast agent, using an inversion-recovery sequence. Data were independently analyzed by investigators using CMR software (MASS v7.2, Medis, Netherlands). The CMR parameters assessed included left ventricular ejection fraction (LVEF), myocardial infarct (MI) volume, and microvascular obstruction (MVO) ratio. The AAR was defined as the hyperenhanced region on T2-weighted images, while the MVO was identified as the hypoenhanced region within the MI area.
Statistical analysis
Data were analyzed using SPSS 22.0. As no specific hypothesis was tested, sample size estimation was not performed. After assessing the normality of continuous variables using the Kolmogorov-Smirnov test, data following a normal distribution were presented as mean ± standard deviation and compared between groups using one-way ANOVA. Non-normally distributed data were expressed as Median (P25, P75) and analyzed using non-parametric tests. Categorical data were described as percentages or composition ratios, with group comparisons made using the χ2 test. Binary logistic regression was used to identify risk factors for MVO. A two-tailed P-value of <0.05 was considered statistically significant.
Results
Comparison of baseline characteristics
A total of 233 patients were included in the analysis. Obese patients were younger than those in the normal and overweight groups, and the proportions of hypertension and hyperlipidemia were significantly higher in both the obese and overweight groups compared to the normal weight group (P<0.05). No other baseline characteristics showed statistically significant differences (all P>0.05). See Table 1.
Table 1.
Comparison of clinical baseline data
| Normal weight group (n=77) | Overweight group (n=102) | Obese group (n=54) | F | P | |
|---|---|---|---|---|---|
| Age (years old) | 59.91±9.66 | 59.05±9.89* | 55.13±8.47*,# | 4.406 | 0.013 |
| Sex (male) | 60 | 79 | 42 | 0.006 | 0.997 |
| BMI (kg/m2) | 21.98±1.07 | 25.76±0.94* | 30.67±1.50*,# | 933.7 | <0.001 |
| Diabetes mellitus | 20 (26.0) | 26 (25.5) | 13 (24.1) | 0.063 | 0.969 |
| Hypertension | 36 (46.8) | 60 (58.8) | 37 (68.5) | 6.362 | 0.042 |
| Hyperlipidemia | 5 (6.5) | 11 (10.8) | 13 (24.1) | 9.462 | 0.009 |
| Smoking history | 52 (67.5) | 62 (60.8) | 31 (57.4) | 1.546 | 0.462 |
| Drinking history | 26 (33.8) | 36 (35.3) | 20 (37.0) | 0.150 | 0.928 |
| Killip classification | 2.224 | 0.898 | |||
| I | 41 (53.2) | 54 (52.9) | 32 (59.3) | ||
| II | 28 (36.4) | 41 (40.2) | 17 (31.5) | ||
| III | 5 (6.5) | 5 (4.9) | 4 (7.4) | ||
| IV | 3 (3.9) | 2 (2.0) | 1 (1.9) | ||
| Heart rate at admission (bpm) | 74.09±9.75 | 74.75±17.14 | 76.20±18.97 | 0.296 | 0.744 |
| Systolic blood pressure at admission (mmHg) | 122.86±12.44 | 124.03±9.19 | 126.17±7.83 | 1.709 | 0.183 |
| Onset-to-reperfusion time (h) | 5.59±1.57 | 5.41±1.28 | 5.57±1.15 | 0.464 | 0.630 |
Notes: BMI, body mass index;
P<0.05 vs. normal weight group;
P<0.05 vs. overweight group.
Comparison of blood biochemical indexes
As shown in Table 2, significant differences were observed in Hb (P=0.001), TAG (P=0.030), HDL-C (P=0.001) and CK-MB (P<0.0001) among the three groups. Obese patients had higher Hb, TAG, and HDL-C levels, while CK-MB levels were lower compared to the other groups (all P<0.05).
Table 2.
Comparison of blood biochemical indexes
| Normal weight group (n=77) | Overweight group (n=102) | Obese group (n=54) | F | P | |
|---|---|---|---|---|---|
| WBC (×109/L) | 10.75 (8.97, 12.32) | 10.36 (8.51, 12.34) | 9.55 (8.67, 11.34) | 2.865 | 0.059 |
| Hb (g/L) | 137.03 (125.97, 150.11) | 145.75 (128.68, 151.18) | 147.83 (141.32, 152.06) | 6.037 | 0.001 |
| PLT (×1012/L) | 198.33 (178.33, 229.37) | 194.52 (176.18, 212.77) | 196.50 (174.49, 233.45) | 2.784 | 0.064 |
| Scr (μmol/L) | 78.06 (69.73, 98.62) | 81.68 (71.12, 101.03) | 79.13 (69.40, 90.00) | 0.440 | 0.644 |
| TAG (mmol/L) | 1.27 (0.94, 1.78) | 1.47 (1.01, 1.81) | 1.56 (1.12, 2.08) | 5.894 | 0.030 |
| TC (mmol/L) | 4.15 (3.45, 4.93) | 4.08 (3.71, 4.83) | 4.20 (4.00, 4.92) | 0.352 | 0.704 |
| LDL-C (mmol/L) | 2.84 (2.25, 3.15) | 2.88 (2.32, 3.09) | 2.91 (2.39, 3.41) | 2.715 | 0.068 |
| HDL-C (mmol/L) | 1.02 (0.86, 1.25) | 0.95 (0.88, 1.02) | 1.19 (0.83, 1.36) | 9.417 | 0.001 |
| CK-MB (ng/mL) | 217.78 (187.65, 227.48) | 194.85 (164.06, 211.39) | 185.73 (132.23, 234.53) | 12.54 | <0.0001 |
Notes: WBC, white blood cell count; Hb, hemoglobin; PLT, platelet; Scr, serum creatinine; TAG, triglyceride; TC, total cholesterol; HDL-C/LDL-C, high/low density lipoprotein cholesterol; CK-MB, creatine kinase isoenzyme.
Comparison of CMR parameters
The analysis of CMR parameters (Table 3) showed significant differences in left ventricular end-diastolic volume (LVEDV) and AAR among the three groups (all P<0.05). Obese patients had higher LVEDV and lower AAR ratios compared to the normal group (all P<0.05).
Table 3.
Comparison of CMR parameters
| Normal weight group (n=77) | Overweight group (n=102) | Obese group (n=54) | F | P | |
|---|---|---|---|---|---|
| Left ventricular end-diastolic volume (mL) | 132.52±19.61 | 142.66±26.20* | 149.22±29.14* | 7.578 | <0.001 |
| Left ventricular end-systolic volume (mL) | 67.88±13.19 | 71.89±18.54 | 73.39±11.85 | 2.361 | 0.097 |
| Left ventricular ejection fraction (%) | 43.35±6.89 | 42.61±7.15 | 44.22±7.35 | 0.922 | 0.399 |
| Myocardial infarction rate (%) | 21.71±3.15 | 21.40±3.94 | 20.94±3.87 | 0.695 | 0.500 |
| Areas at risk of infarction (%) | 34.60±7.91 | 32.36±13.46 | 26.72±10.48*,# | 8.082 | <0.001 |
| Microvascular obstruction (%) | 2.07±1.92 | 1.56±1.73 | 1.83±1.87 | 1.725 | 0.180 |
P<0.05 vs. normal weight group;
P<0.05 vs. overweight group.
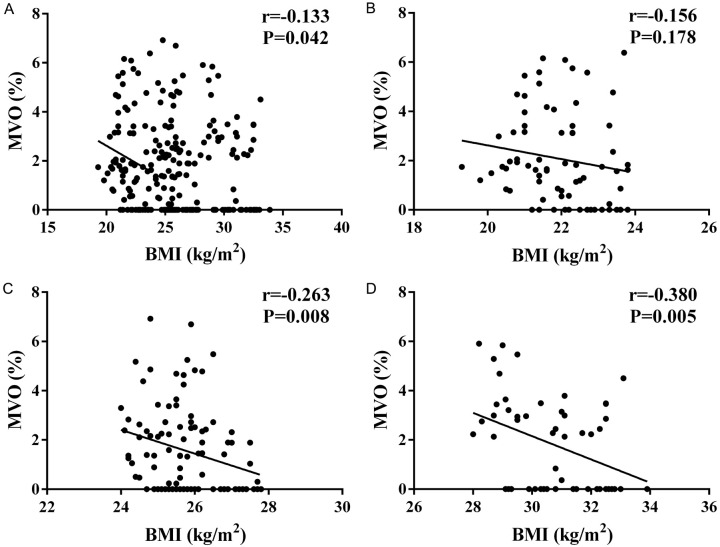
Correlation between BMI and MVO
Pearson correlation analysis (Figure 1) demonstrated an inverse relationship between BMI and MVO across all patients (P<0.05). However, BMI and MVO ratio were not significantly correlated in normal-weight patients (r=-0.156, P=0.178). In contrast, significant correlations were found in overweight (r=-0.263, P=0.008) and obese patients (r=-0.380, P=0.005).
Figure 1.
Correlation analysis between body mass index (BMI) and microvascular obstruction (MVO) ratio. A: Correlation of BMI with MVO in all patients; B: Correlation of BMI with MVO in the normal weight group; C: Correlation of BMI with MVO in overweight patients; D: Correlation of BMI with MVO in obese patients.
Analysis of influencing factors of MVO
The analysis of factors influencing MVO (Table 4) identified BMI, Killip classification, CK-MB, and LVEDV as independent influencing factors. BMI (HR: 0.908, P=0.019) and LVEDV (HR: 0.989, P=0.042) were found to be protective factors.
Table 4.
Binary logistic analysis of influencing factors of MVO
| B | S.E. | P | OR (95% CI) | |
|---|---|---|---|---|
| Age | 0.007 | 0.014 | 0.629 | 1.007 (0.979-1.036) |
| Sex | 0.149 | 0.328 | 0.649 | 1.161 (0.610-2.210) |
| BMI | -0.096 | 0.041 | 0.019 | 0.908 (0.838-0.984) |
| Diabetes mellitus | 0.000 | 0.318 | 0.999 | 1.000 (0.536-1.865) |
| Hypertension | 0.150 | 0.281 | 0.594 | 1.161 (0.670-2.014) |
| Hyperlipidemia | 0.539 | 0.458 | 0.239 | 1.714 (0.699-4.027) |
| Killip classification | 1.081 | 0.264 | 0.000 | 2.946 (1.755-4.946) |
| Hb (g/L) | 0.012 | 0.010 | 0.228 | 1.012 (0.992-1.033) |
| TAG (mmol/L) | 0.278 | 0.305 | 0.361 | 1.321 (0.727-2.402) |
| HDL-C (mmol/L) | 0.966 | 0.628 | 0.124 | 2.628 (0.767-9.000) |
| CK-MB (ng/mL) | 0.009 | 0.004 | 0.040 | 1.009 (1.000-1.017) |
| LVEDV (mL) | -0.011 | 0.005 | 0.042 | 0.989 (0.979-1.000) |
Notes: MVO, microvascular occlusion; BMI, body mass index; Hb, hemoglobin; TAG, triglyceride; HDL-C, high density lipoprotein; CK-MB, creatine kinase isoenzyme; LVEDV, left ventricular end-diastolic volume.
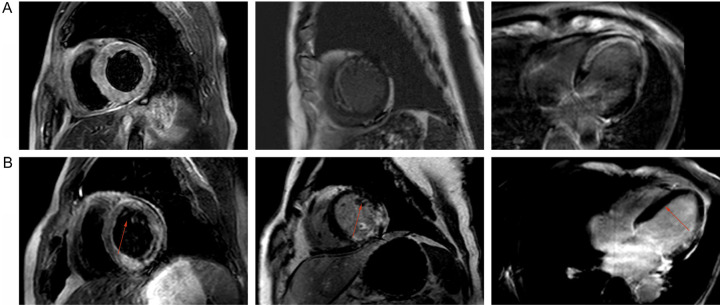
Comparison of ARR of infarction
AAR was significantly lower in obese patients compared to those with normal weight (P<0.05), while no significant difference was observed between overweight and normal-weight patients (P>0.05; Table 5). As shown in Figure 2, in a patient with a BMI of 23.2, the ventricular septum was slightly thickened, with a maximum diameter of 1.4 cm. No obvious abnormalities were found in the apical or septal myocardium signals during the plain scan, and first-pass perfusion was slightly reduced before the enhanced scan. In a patient with a BMI of 25.1, T2WI hyperintensities were observed in the apical and inferior wall myocardia, with poorly defined hyperintensity of adipose tissue. First-pass perfusion was reduced on enhanced scans, and delayed enhancement was noted.
Table 5.
Comparison of areas at risk of infarction
| Normal weight vs. Overweight | Normal weight vs. Obese | |||
|---|---|---|---|---|
|
|
|
|||
| Adjusted Difference (95% CI) | P | Adjusted Difference (95% CI) | P | |
| Area at risk (%) | -0.004 (-0.011, 0.002) | 0.195 | -0.040 (-0.056, -0.024) | 0.000 |
Figure 2.
CMR images of normal weight and overweight patients. A: Normal-weight (BMI=23.2) patients; B: Overweight (BMI=25.1) patients. Arrow indicates the location of the lesion.
Discussion
Obesity is currently a global health issue. It is not only a disease in its own right but also a major risk factor for type 2 diabetes, CVD, hypertension, stroke, and various cancers. As a result, the World Health Organization lists obesity as one of the top ten risk factors contributing to the global disease burden [23]. Numerous guidelines, both domestic and international, recommend body weight control for the prevention and treatment of cardio-cerebrovascular diseases. However, several epidemiological studies have highlighted the protective effects of obesity in certain conditions, such as end-stage renal failure, heart failure, atrial fibrillation, sudden cardiac death, and coronary heart disease - a phenomenon referred to as the “obesity paradox”. For example, an observational study of 10,142 patients undergoing PCI in Japan found that lower body weight was associated with an increased incidence of PCI-related complications, particularly bleeding, and identified BMI as an independent predictor of bleeding during PCI [11]. Nevertheless, not all research supports the obesity paradox. A German study involving 890 consecutive STEMI patients undergoing PCI did not observe the paradox.
Our findings support the presence of the “obesity paradox” in STEMI patients treated at our hospital. Among the 233 patients analyzed, obese patients were younger than those in the normal- and overweight groups, and the obesity and overweight groups had significantly higher rates of hypertension and hyperlipidemia than the normal-weight group. Additionally, Hb, TAG and HDL-C levels were higher in obese patients, while CK-MB levels were lower. An inverse association between MVO ratio and BMI was observed in all patients. Binary logistic regression further identified BMI, Killip classification, CK-MB, and LVEDV as independent factors influencing MVO. In the context of cardiovascular complications, microcirculatory disturbances and endothelial dysfunction precede atherosclerosis [24]. The difference in CK-MB levels suggests that MVO is associated with more severe myocardial and microvascular damage, aligning with current pathophysiological research and clinical experience [25]. Obese patients often report dyspnea, which may result from reduced left ventricular systolic or diastolic function and increased ventricular filling pressure. Obesity is linked to ventricular remodeling, which may normalize wall stress while increasing stroke volume to meet metabolic demands. It is also associated with elevated LVEDP, indicating a relationship with diastolic dysfunction [26]. Furthermore, several studies have demonstrated that the presence of MVO is associated with worse prognosis and more pronounced left ventricular remodeling compared to infarction without MVO [27,28].
We found a significant negative association between the MVO ratio and BMI in all patients. However, in the BMI subgroup analysis, there was no significant association between BMI and MVO ratio in the normal weight group, while a strong inverse relationship was observed in the overweight and obese groups. Some researchers suggest that MI patients with normal or low BMI may have a larger infarct size compared to obese patients [29]. Consistent with our findings, Lan et al. also reported a lower prevalence of MVO in overweight and obese patients [30]. However, the results of this study remain controversial. First, we used BMI to classify overweight and obesity. While BMI is easy to apply in clinical settings, it does not accurately reflect true obesity. It is hypothesized that more comprehensive measures of body fat composition could better correlate with cardiovascular risk and mortality than BMI alone [31]. Indicators such as waist circumference, body roundness index, and body shape index may more accurately reflect the differences in fat distribution and obesity-related pathophysiological conditions [32]. Additionally, some studies suggest that overweight and obesity may not be associated with infarct size or myocardial reperfusion injury in acute STEMI patients treated with PCI [33]. Therefore, the “obesity paradox” requires further investigation.
This study has several limitations. First, it is a single-center retrospective study with a relatively small sample size. Although this ensures consistency in interventional therapy techniques, future studies should include more patients and expand the sample size to improve statistical power. Second, we did not evaluate other obesity-related indicators apart from BMI, and the data did not include factors such as waist circumference, hip circumference, weight changes during follow-up, and daily activity, which can reflect body composition and fat distribution. The inclusion of these data would be valuable in future research. Moreover, this study did not analyze patients with low body mass. Future multi-center studies should aim to include more low body mass patients and assess additional obesity-related indicators beyond BMI.
In conclusion, BMI is associated with MVO and AAR in STEMI patients undergoing PCI. Additionally, obesity may be linked to a lower risk of MVO and AAR.
Acknowledgements
Study based on funding from the mTOR/NLRP3 signaling mechanism of electroacupuncture pretreatment to alleviate myocardial IR injury based on autophagy-inflammation regulation, No.: 81974583.
Disclosure of conflict of interest
None.
References
- 1.Malakar AK, Choudhury D, Halder B, Paul P, Uddin A, Chakraborty S. A review on coronary artery disease, its risk factors, and therapeutics. J Cell Physiol. 2019;234:16812–16823. doi: 10.1002/jcp.28350. [DOI] [PubMed] [Google Scholar]
- 2.Montecucco F, Carbone F, Schindler TH. Pathophysiology of ST-segment elevation myocardial infarction: novel mechanisms and treatments. Eur Heart J. 2016;37:1268–1283. doi: 10.1093/eurheartj/ehv592. [DOI] [PubMed] [Google Scholar]
- 3.Vogel B, Claessen BE, Arnold SV, Chan D, Cohen DJ, Giannitsis E, Gibson CM, Goto S, Katus HA, Kerneis M, Kimura T, Kunadian V, Pinto DS, Shiomi H, Spertus JA, Steg PG, Mehran R. ST-segment elevation myocardial infarction. Nat Rev Dis Primers. 2019;5:39. doi: 10.1038/s41572-019-0090-3. [DOI] [PubMed] [Google Scholar]
- 4.Gersh BJ, Sliwa K, Mayosi BM, Yusuf S. Novel therapeutic concepts: the epidemic of cardiovascular disease in the developing world: global implications. Eur Heart J. 2010;31:642–648. doi: 10.1093/eurheartj/ehq030. [DOI] [PubMed] [Google Scholar]
- 5.Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K, Alla F, Alvis-Guzman N, Amrock S, Ansari H, Ärnlöv J, Asayesh H, Atey TM, Avila-Burgos L, Awasthi A, Banerjee A, Barac A, Bärnighausen T, Barregard L, Bedi N, Belay Ketema E, Bennett D, Berhe G, Bhutta Z, Bitew S, Carapetis J, Carrero JJ, Malta DC, Castañeda-Orjuela CA, Castillo-Rivas J, Catalá-López F, Choi JY, Christensen H, Cirillo M, Cooper L Jr, Criqui M, Cundiff D, Damasceno A, Dandona L, Dandona R, Davletov K, Dharmaratne S, Dorairaj P, Dubey M, Ehrenkranz R, El Sayed Zaki M, Faraon EJA, Esteghamati A, Farid T, Farvid M, Feigin V, Ding EL, Fowkes G, Gebrehiwot T, Gillum R, Gold A, Gona P, Gupta R, Habtewold TD, Hafezi-Nejad N, Hailu T, Hailu GB, Hankey G, Hassen HY, Abate KH, Havmoeller R, Hay SI, Horino M, Hotez PJ, Jacobsen K, James S, Javanbakht M, Jeemon P, John D, Jonas J, Kalkonde Y, Karimkhani C, Kasaeian A, Khader Y, Khan A, Khang YH, Khera S, Khoja AT, Khubchandani J, Kim D, Kolte D, Kosen S, Krohn KJ, Kumar GA, Kwan GF, Lal DK, Larsson A, Linn S, Lopez A, Lotufo PA, El Razek HMA, Malekzadeh R, Mazidi M, Meier T, Meles KG, Mensah G, Meretoja A, Mezgebe H, Miller T, Mirrakhimov E, Mohammed S, Moran AE, Musa KI, Narula J, Neal B, Ngalesoni F, Nguyen G, Obermeyer CM, Owolabi M, Patton G, Pedro J, Qato D, Qorbani M, Rahimi K, Rai RK, Rawaf S, Ribeiro A, Safiri S, Salomon JA, Santos I, Santric Milicevic M, Sartorius B, Schutte A, Sepanlou S, Shaikh MA, Shin MJ, Shishehbor M, Shore H, Silva DAS, Sobngwi E, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Tadele Atnafu N, Tesfay F, Thakur JS, Thrift A, Topor-Madry R, Truelsen T, Tyrovolas S, Ukwaja KN, Uthman O, Vasankari T, Vlassov V, Vollset SE, Wakayo T, Watkins D, Weintraub R, Werdecker A, Westerman R, Wiysonge CS, Wolfe C, Workicho A, Xu G, Yano Y, Yip P, Yonemoto N, Younis M, Yu C, Vos T, Naghavi M, Murray C. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70:1–25. doi: 10.1016/j.jacc.2017.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6–10. doi: 10.1016/j.metabol.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Powell-Wiley TM, Poirier P, Burke LE, Després JP, Gordon-Larsen P, Lavie CJ, Lear SA, Ndumele CE, Neeland IJ, Sanders P, St-Onge MP American Heart Association Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Epidemiology and Prevention; and Stroke Council. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;143:e984–e1010. doi: 10.1161/CIR.0000000000000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cleven L, Krell-Roesch J, Nigg CR, Woll A. The association between physical activity with incident obesity, coronary heart disease, diabetes and hypertension in adults: a systematic review of longitudinal studies published after 2012. BMC Public Health. 2020;20:726. doi: 10.1186/s12889-020-08715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiebe N, Stenvinkel P, Tonelli M. Associations of chronic inflammation, insulin resistance, and severe obesity with mortality, myocardial infarction, cancer, and chronic pulmonary disease. JAMA Netw Open. 2019;2:e1910456. doi: 10.1001/jamanetworkopen.2019.10456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piché ME, Tchernof A, Després JP. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res. 2020;126:1477–1500. doi: 10.1161/CIRCRESAHA.120.316101. [DOI] [PubMed] [Google Scholar]
- 11.Numasawa Y, Kohsaka S, Miyata H, Kawamura A, Noma S, Suzuki M, Nakagawa S, Momiyama Y, Naito K, Fukuda K. Impact of body mass index on in-hospital complications in patients undergoing percutaneous coronary intervention in a Japanese real-world multicenter registry. PLoS One. 2015;10:e0124399. doi: 10.1371/journal.pone.0124399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banack HR, Kaufman JS. The obesity paradox: understanding the effect of obesity on mortality among individuals with cardiovascular disease. Prev Med. 2014;62:96–102. doi: 10.1016/j.ypmed.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Xia JY, Lloyd-Jones DM, Khan SS. Association of body mass index with mortality in cardiovascular disease: new insights into the obesity paradox from multiple perspectives. Trends Cardiovasc Med. 2019;29:220–225. doi: 10.1016/j.tcm.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Gupta T, Kolte D, Mohananey D, Khera S, Goel K, Mondal P, Aronow WS, Jain D, Cooper HA, Iwai S, Frishman WH, Bhatt DL, Fonarow GC, Panza JA. Relation of obesity to survival after in-hospital cardiac arrest. Am J Cardiol. 2016;118:662–667. doi: 10.1016/j.amjcard.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 15.Johnson AP, Parlow JL, Whitehead M, Xu J, Rohland S, Milne B. Body mass index, outcomes, and mortality following cardiac surgery in Ontario, Canada. J Am Heart Assoc. 2015;4:e001977. doi: 10.1161/JAHA.115.002140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elagizi A, Kachur S, Lavie CJ, Carbone S, Pandey A, Ortega FB, Milani RV. An overview and update on obesity and the obesity paradox in cardiovascular diseases. Prog Cardiovasc Dis. 2018;61:142–150. doi: 10.1016/j.pcad.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Habbu A, Lakkis NM, Dokainish H. The obesity paradox: fact or fiction? Am J Cardiol. 2006;98:944–948. doi: 10.1016/j.amjcard.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 18.De Maria GL, Alkhalil M, Wolfrum M, Fahrni G, Borlotti A, Gaughran L, Dawkins S, Langrish JP, Lucking AJ, Choudhury RP, Porto I, Crea F, Dall’Armellina E, Channon KM, Kharbanda RK, Banning AP. Index of microcirculatory resistance as a tool to characterize microvascular obstruction and to predict infarct size regression in patients With STEMI undergoing primary PCI. JACC Cardiovasc Imaging. 2019;12:837–848. doi: 10.1016/j.jcmg.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 19.Pu J, Ding S, Ge H, Han Y, Guo J, Lin R, Su X, Zhang H, Chen L, He B EARLY-MYO Investigators. Efficacy and safety of a pharmaco-invasive strategy with half-dose alteplase versus primary angioplasty in ST-segment-elevation myocardial infarction: EARLY-MYO trial (early routine catheterization after alteplase fibrinolysis versus primary PCI in acute ST-segment-elevation myocardial infarction) Circulation. 2017;136:1462–1473. doi: 10.1161/CIRCULATIONAHA.117.030582. [DOI] [PubMed] [Google Scholar]
- 20.He J, Kong LC, Zeng JT, Shi BZ, An DA, Chen BH, Ding S, Li Z, Yang F, Yang YN, Yan FH, Xiu JC, Wang HW, Xu JR, Ge H, Pu J. Comparison of direct stenting with conventional strategy on myocardial impairments in ST-segment elevation myocardial infarction: a cardiac magnetic resonance imaging study. Int J Cardiovasc Imaging. 2020;36:1167–1175. doi: 10.1007/s10554-020-01812-w. [DOI] [PubMed] [Google Scholar]
- 21.Wong DT, Leung MC, Richardson JD, Puri R, Bertaso AG, Williams K, Meredith IT, Teo KS, Worthley MI, Worthley SG. Cardiac magnetic resonance derived late microvascular obstruction assessment post ST-segment elevation myocardial infarction is the best predictor of left ventricular function: a comparison of angiographic and cardiac magnetic resonance derived measurements. Int J Cardiovasc Imaging. 2012;28:1971–1981. doi: 10.1007/s10554-012-0021-9. [DOI] [PubMed] [Google Scholar]
- 22.Hanna EB, Glancy DL. ST-segment elevation: differential diagnosis, caveats. Cleve Clin J Med. 2015;82:373–384. doi: 10.3949/ccjm.82a.14026. [DOI] [PubMed] [Google Scholar]
- 23.Aronne LJ. Classification of obesity and assessment of obesity-related health risks. Obes Res. 2002;10(Suppl 2):105S–115S. doi: 10.1038/oby.2002.203. [DOI] [PubMed] [Google Scholar]
- 24.Koller A, Balasko M, Bagi Z. Endothelial regulation of coronary microcirculation in health and cardiometabolic diseases. Intern Emerg Med. 2013;8(Suppl 1):S51–54. doi: 10.1007/s11739-013-0910-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abbas A, Matthews GH, Brown IW, Shambrook JS, Peebles CR, Harden SP. Cardiac MR assessment of microvascular obstruction. Br J Radiol. 2015;88:20140470. doi: 10.1259/bjr.20140470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cai A, Liu L, Zhou D, Zhou Y, Tang S, Feng Y. The patterns of left ventricular alteration by adipose tissue distribution: implication for heart failure prevention. ESC Heart Fail. 2021;8:3093–3105. doi: 10.1002/ehf2.13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hombach V, Grebe O, Merkle N, Waldenmaier S, Höher M, Kochs M, Wöhrle J, Kestler HA. Sequelae of acute myocardial infarction regarding cardiac structure and function and their prognostic significance as assessed by magnetic resonance imaging. Eur Heart J. 2005;26:549–557. doi: 10.1093/eurheartj/ehi147. [DOI] [PubMed] [Google Scholar]
- 28.Baks T, van Geuns RJ, Biagini E, Wielopolski P, Mollet NR, Cademartiri F, van der Giessen WJ, Krestin GP, Serruys PW, Duncker DJ, de Feyter PJ. Effects of primary angioplasty for acute myocardial infarction on early and late infarct size and left ventricular wall characteristics. J Am Coll Cardiol. 2006;47:40–44. doi: 10.1016/j.jacc.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Pingitore A, Di Bella G, Lombardi M, Iervasi G, Strata E, Aquaro GD, Positano V, De Marchi D, Rossi G, L’Abbate A, Rovai D. The obesity paradox and myocardial infarct size. J Cardiovasc Med (Hagerstown) 2007;8:713–717. doi: 10.2459/JCM.0b013e328011c984. [DOI] [PubMed] [Google Scholar]
- 30.Lan DH, Zhang Y, Hua B, Li JS, He Y, Chen H, Li WP, Li HW. Impact of obesity on microvascular obstruction and area at risk in patients after ST-segment-elevation myocardial infarction: a magnetic resonance imaging study. Diabetes Metab Syndr Obes. 2022;15:2207–2216. doi: 10.2147/DMSO.S369222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gadde KM, Martin CK, Berthoud HR, Heymsfield SB. Obesity: pathophysiology and management. J Am Coll Cardiol. 2018;71:69–84. doi: 10.1016/j.jacc.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Endukuru CK, Gaur GS, Dhanalakshmi Y, Sahoo J, Vairappan B. Cut-off values and clinical efficacy of body roundness index and other novel anthropometric indices in identifying metabolic syndrome and its components among Southern-Indian adults. Diabetol Int. 2022;13:188–200. doi: 10.1007/s13340-021-00522-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reinstadler SJ, Metzler B. Myocardial damage after primary PCI: does obesity really matter? JACC Cardiovasc Interv. 2020;13:973–975. doi: 10.1016/j.jcin.2020.02.037. [DOI] [PubMed] [Google Scholar]