Abstract
Objective: To analyze the clinical effects of platelet-rich plasma (PRP) combined with arthroscopic microfracture (MF) in patients with knee cartilage injury. Methods: Eighty cases of knee cartilage injury treated in Lu’an Hospital of PKU HealthCare during July 2019 and July 2021 were selected for this study. Patients were divided into a control group (CG, treated with MF alone, n=36) and an observation group (OG, treated with MG+PRP, n=44) based on their intervention regimen. Outcomes were compared between the two groups, including Hospital for Special Surgery (HSS) score, Tegner Activity Scale (TAS) score, Berg Balance Scale (BBS) score (joint balance function), Visual Analogue Scale (VAS) score, and Short-Form 36 Item Health Survey (SF-36) score, as well as levels of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and matrix metalloproteinase (MMP)-13. Furthermore, a binary logistic regression analysis was conducted to identify the factors influencing treatment efficacy in patients with knee cartilage injuries. Results: The overall response rate was significantly higher in the OG than in the CG. Factors such as sex, age, disease duration, lesion location, ICRS (International Cartilage Repair Society) grade, and treatment modality did not significantly affect treatment efficacy. The OG showed elevated HSS, TAS, and BBS scores after treatment, compared to baseline and the CG. Additionally, VAS scores and levels of TNF-α, IL-1β, and MMP-13 were notably lower compared with the baseline and the CG. In terms of life quality, patients in the OG scored markedly higher in dimensions of vitality, physiological function and social function, compared to both the pre-treatment values and CG. Conclusions: PRP combined with MF outperformed MF alone in treating knee cartilage injury, which greatly improves patients’ knee joint function and balance function, reduces pain, inhibits inflammation in the joint cavity, and enhances overall quality of life, with a favorable safety profile.
Keywords: Knee cartilage injury, platelet-rich plasma, arthroscopic microfracture, clinical efficacy
Introduction
Knee cartilage plays a crucial role in transferring load and reducing friction between bones [1]. Knee cartilage injuries are common orthopedic conditions, frequently affecting the medial femoral condyle and the retropatellar cartilage surface. These injuries can alter joint mechanics and cause knee pain [2,3]. According to statistics, knee cartilage injury accounts for 63% of knee joint surgeries, and as a risk factor for knee osteoarthritis (OA), it can increase the risk of developing knee OA in younger patients [4-6]. Previous studies have demonstrated that such cartilage lesions gradually worsen over time, potentially causing long-term sequelae [7]. Currently, surgical treatments, including autologous stroma-induced cartilage formation, bone marrow stimulation, and autologous cartilage transplantation, remain the common methods for repairing knee cartilage injury [8]. Although these procedures are effective in alleviating the clinical symptoms of patients with knee cartilage injury, they also carry the risk of damaging healthy articular cartilage. Hence, optimizing treatment strategies for knee cartilage injuries has huge clinical implications for improving patients’ postoperative recovery and life quality.
Arthroscopic microfracture (MF) is one of the preferred treatments for knee cartilage defects worldwide [9]. In a comparative study by Huang et al. [10] on autologous osteochondral grafting versus MF, it was confirmed that MF offers advantages in controlling complications. However, MF still has some limitations, such as poor postoperative chondrocyte regeneration ability and suboptimal quality of regenerated cartilage [11]. To facilitate cartilage healing, this study integrated platelet-rich plasma (PRP) and MF. PRP, an autologous blood product rich in platelets, is often used to treat ligament, tendon and bone injuries [12]. Many studies suggest that PRP can induce stromal stem cells to differentiate into chondrocytes by releasing growth factors and other active substances, thus promoting chondrocyte regeneration [13]. Gao et al. [14] also highlighted the clinical potential of PRP in articular cartilage repair.
While regenerative medicine has made significant strides in recent years, research on the clinical effect of PRP plus MF (PRP+MF) on patients with knee cartilage injury remains limited. This study primarily analyzes the effectiveness of the combination therapy in treating knee cartilage injuries by evaluating treatment outcomes, knee joint function, joint balance function, pain relief, serum inflammatory indicators, quality of life, and incidence of complications. This research addresses gaps in the field and provides new insights for the clinical treatment optimization of knee cartilage injury, which is exactly the innovation of this study.
Data and methods
Clinical data collection
This retrospective study included 80 cases of knee cartilage injury treated at Lu’an Hospital of PKU HealthCare from July 2019 to July 2021. The patients were divided into a control group (CG, treated with MF) with 36 cases and an observation group (OG, treated with PRP+MF) with 44 cases, based on their treatment regimen. The CG consisted of 20 males and 16 females aged between 34 and 64 (average: 50.94±7.43 years) with a disease course of 5-9 months (mean: 7.17±1.03 months); left and right knee cartilage injuries were observed in 19 and 17 cases, respectively. In the OG, the male-to-female ratio was 26:18, the age range was 34-69 (mean: 53.86±8.23 years), and the disease course was 5-10 months (mean: 7.41±1.13 months); left and right knee lesions were found in 20 and 24 cases, respectively. This study was ethically ratified by Lu’an Hospital of PKU HealthCare.
Patient selection
All enrolled cases (age range: 18-70) were diagnosed with knee cartilage injuries by imaging [15] and received treatment for the first time. The degree of cartilage damage was classified according to the International Cartilage Repair Society (ICRS) classification system [16]; X-rays indicated normal knee joint varus angle, and clinical physical examinations showed varying degrees of joint space tenderness. All patient records were complete.
Exclusion criteria: obvious varus deformity or flexion contracture deformity of the lower limbs; vital organ diseases, coagulation disorders, inability to tolerate surgery, or severe mental diseases; incomplete clinical data that affect the judgment of curative effect.
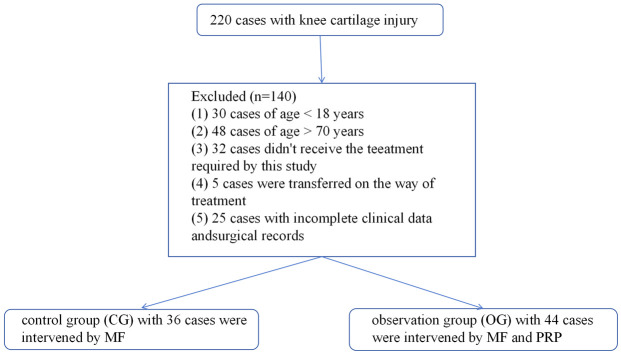
The flowchart for patient selection is shown in Figure 1.
Figure 1.
Patient selection flowchart. PRP, platelet-rich plasma; MF, arthroscopic microfracture.
Data extraction
Patient information, including treatment outcomes, Hospital for Special Surgery (HSS) score, Tegner Activity Scale (TAS) score, Berg Balance Scale (BBS) score (joint balance function), Visual Analogue Scale (VAS) score, and Short-Form 36 Item Health Survey (SF-36) score, as well as levels of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and matrix metalloproteinase (MMP)-13, was extracted from the hospital’s medical record system. We aimed to determine, through a series of validations, which treatment regimen has a more significant clinical effect.
Treatment methods
CG (MF group): Patients in this group received arthroscopic MF operated on by the same group of physicians. After successful epidural anesthesia, the patient, placed in a supine position, underwent routine skin preparation and draping. A routine arthroscopic examination was performed, and the areas of cartilage loss or loose cartilages were cleaned accordingly. The arthroscope was removed upon completion. Postoperative functional exercise was arranged, with patients advised to avoid weight-bearing and strenuous exercise of the affected limb.
OG (PRP+MF group): PRP+MF was implemented in this group. The surgical posture, anesthesia, disinfection and postoperative care were the same as the CG. Ten ml of venous blood was drawn from each patient and centrifuged at 2000 r/min at 4°C. After 10 minutes, the supernatant and the intermediate leukocyte and platelet layer were collected into separate tubes. A second centrifugation was performed under the same conditions for 10 min, after which 2 ml of the supernatant, white blood cells, and platelet layer were retained and mixed to make a suspension. Calcium chloride was added to this suspension to obtain PRP. Two ml PRP was injected into the joint cavity every 7-10 d, for a total of 6 injections.
Response evaluation
Marked response: symptoms like local joint pain and limitation of motion (LOM) disappeared, with basically recovered joint function and no complications.
Response: local joint pain and LOM improved to a certain extent, with partial recovery of joint function.
Non-response: no improvement or worsening of symptoms such as joint pain and LOM, possibly accompanied by complications.
Outcome measures
(1) Treatment outcomes. Treatment outcomes were assessed using the criteria mentioned in the response evaluation section. The overall response rate (ORR) = (cases with marked response + cases with response)/total cases * 100% [17].
(2) Knee function score. HSS and TAS scores [18], both positively associated with the knee function, were recorded before and two weeks after surgery to evaluate the improvements in knee joint function.
(3) Joint balance function. Patients’ joint balance function was assessed using BBS [19] before and after surgery. The BBS has a maximum score of 100 points, with higher score indicating better joint balance function.
(4) Pain relief. Patient levels were assessed using VAS [20] before and after treatment. The scale is a 10-point instrument with the score in direct proportion to pain severity.
(5) Serum inflammatory indices. Serum samples were collected and analyzed using enzyme-linked immunosorbent assay (ELISA, Wuhan Fine Biotech) to measure levels of inflammatory factors like TNF-α, IL-1β and MMP-13 before and after treatment. The operation steps were strictly in compliance with manufacture’s protocols.
(6) Life quality score. Patients’ quality of life was assessed using SF-36 survey, focusing on vitality, physiological function and social function [21]. Higher scores indicate better quality of life.
(7) Complication rate. Both groups were observed for treatment-related complications, including infections and hematomas.
Among them, the primary outcome measures were treatment outcomes, HSS score, TAS score, TNF-α, IL-1β, MMP-13 levels, and incidence of complications. The secondary outcome measures included joint balance function, pain relief, and quality of life score.
Statistical analysis
SPSS 21.0 (SPSS, Inc., Chicago, IL, USA) was used for data analysis, and GraphPad Prism 6 (GraphPad Software, San Diego, USA) was used for figure generation. Enumeration data were presented as the number of cases/percentage (n/%), and comparisons were made using the χ2 test. Quantitative data, expressed as mean ± SEM, were analyzed using independent sample t-tests for between-group comparisons and paired t-tests for within-group (pre- and post-treatment) comparisons. Additionally, a binary logistic regression analysis was performed to identify factors affecting treatment efficacy in patients with knee cartilage injuries. The minimum sample size requirement of 22 cases was estimated through a formula, and the sample sizes of both groups in this study met the minimum sample size requirement. Specific formula:
A P<0.05 was considered as a statistically significant difference.
Results
Comparison of general data between the two groups of patients
The two groups were not statistically different in sex, age, disease course, lesion location, or ICRS grading (all P>0.05, Table 1).
Table 1.
Comparison of general data between the two groups of patients
| Data | Control group (n=36) | Observation group (n=44) | t/χ2 value | P value |
|---|---|---|---|---|
| Sex | 0.101 | 0.750 | ||
| Male | 20 (55.56) | 26 (59.09) | ||
| Female | 16 (44.44) | 18 (40.91) | ||
| Average age (years) | 50.94±7.43 | 53.86±8.23 | 1.649 | 0.103 |
| Course of disease (months) | 7.17±1.03 | 7.41±1.13 | 0.983 | 0.329 |
| Lesion location | 0.425 | 0.514 | ||
| Left knee | 19 (52.78) | 20 (45.45) | ||
| Right knee | 17 (47.22) | 24 (54.55) | ||
| ICRS grading | 4.310 | 0.116 | ||
| I+II | 10 (27.78) | 6 (13.64) | ||
| III | 20 (55.56) | 23 (52.27) | ||
| IV | 6 (16.67) | 15 (34.09) |
Note: ICRS, International Cartilage Repair Society.
Comparison of treatment outcomes between the two groups of patients
Patients’ treatment outcomes were compared to evaluate the effects of the two treatments on patients with knee cartilage injury. The results (Table 2) showed an ORR of 97.73% in the OG, which was notably higher than the 83.33% observed in the CG (P<0.05).
Table 2.
Comparison of treatment outcomes between the two groups of patients [n (%)]
| Groups | n | Marked response | Response | Non-response | Overall response (%) |
|---|---|---|---|---|---|
| Control group | 36 | 16 (44.44) | 14 (38.89) | 6 (16.67) | 30 (83.33) |
| Observation group | 44 | 25 (56.82) | 18 (40.91) | 1 (2.27) | 43 (97.73) |
| χ2 value | - | - | - | - | 5.138 |
| P value | - | - | - | - | 0.023 |
Multivariate analysis of factors affecting treatment efficacy in patients with knee cartilage injury
Binary logistic regression analysis revealed that sex, age, disease duration, lesion location, ICRS grading, and treatment modality were not independent factors affecting the efficacy of patients with knee cartilage injury (all P>0.05, Table 3).
Table 3.
Multivariate analysis of factors affecting treatment efficacy in patients with knee cartilage injury
| Factor | β | S.E. | Wald | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| Sex | -1.824 | 1.154 | 2.497 | 0.114 | 0.161 | 0.017-1.550 |
| Age (years) | -0.855 | 1.173 | 0.531 | 0.466 | 0.425 | 0.043-4.237 |
| Course of disease (months) | 0.115 | 0.888 | 0.017 | 0.897 | 1.122 | 0.197-6.390 |
| Lesion location | -0.156 | 0.878 | 0.032 | 0.859 | 0.855 | 0.153-4.777 |
| ICRS grading | -0.673 | 0.697 | 0.931 | 0.334 | 0.510 | 0.130-2.000 |
| Treatment modality | 2.061 | 1.140 | 3.267 | 0.071 | 7.856 | 0.840-73.444 |
Note: ICRS, International Cartilage Repair Society.
Comparison of knee joint function scores between the two groups of patients before and after the treatment
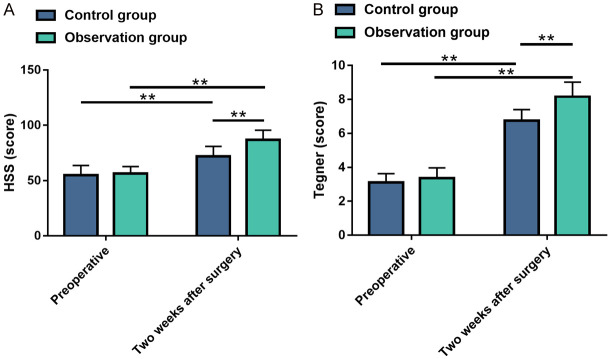
HSS and Tegner scores were compared between the two groups before and two weeks after surgery. As shown in Figure 2, no significant difference was observed in pre-treatment scores between groups (all P>0.05). The post-treatment HSS and Tegner scores elevated remarkably (all P<0.01), with notably greater improvement in knee joint function in the OG compared to the CG (all P<0.01).
Figure 2.
Comparisons of knee function scores between the two groups before and after treatment. A: Comparison of HSS scores between the two groups before and after treatment; B: Comparison of Tegner scores between the two groups before and after treatment. Note: **P<0.01. HSS, Hospital for Special Surgery.
Comparison of joint balance function between the two groups of patients before and after the treatment
BBS was used to compare the improvement in joint balance function before and after intervention to evaluate the impact of the two therapies on patients with knee cartilage injuries. As shown in Figure 3, there was no significant inter-group difference in BBS scores before intervention (P>0.05). However, both groups showed significantly elevated BBS scores after intervention (P<0.01), with the OG demonstrating a greater improvement compared to the CG (P<0.01).
Figure 3.
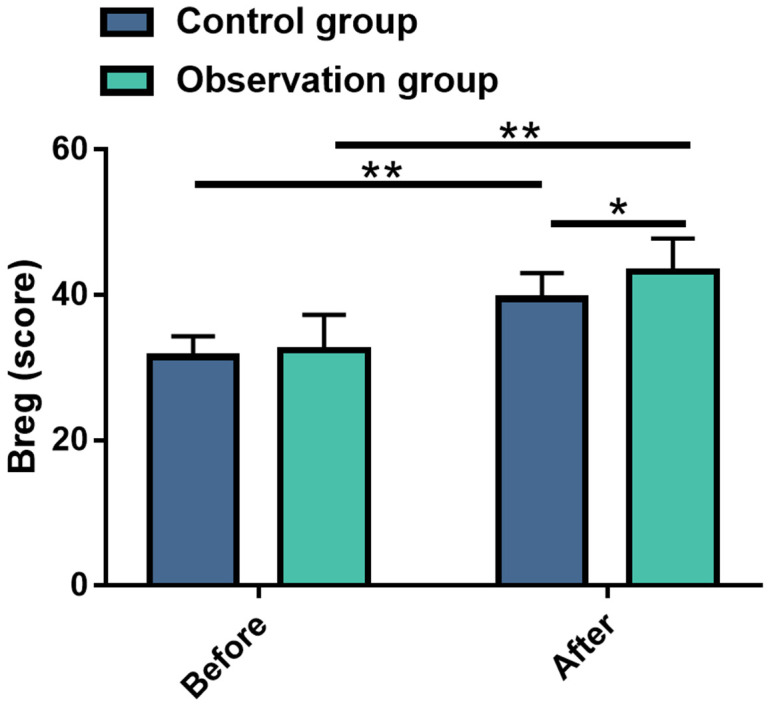
Comparisons of Berg Balance Scale scores between the two groups before and after treatment. Note: *P<0.05; **P<0.01.
Comparison of pain levels between the two groups of patients before and after the treatment
Patients’ pain levels were assessed using the VAS to evaluate the effect of the two therapies on the relief of pain symptoms in patients with knee cartilage injury. Likewise, the pre-treatment VAS scores were similar between the two cohorts (P>0.05). After the intervention, the pain was greatly reduced (P<0.01), with the OG showing a more pronounced decrease in pain levels (P<0.01) (Figure 4).
Figure 4.
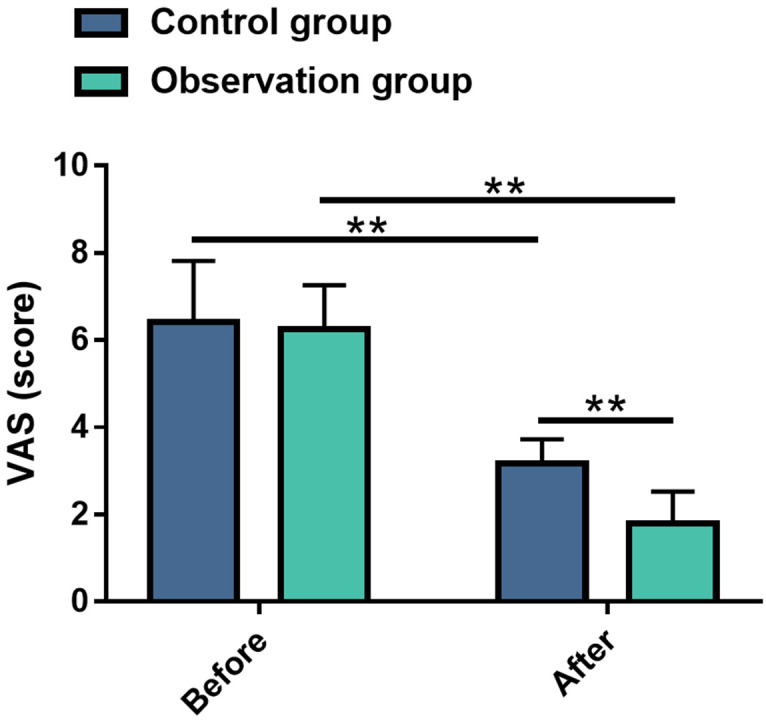
Comparisons of pain score between the two groups before and after treatment. Note: **P<0.01. VAS, Visual Analogue Scale.
Comparison of serum inflammatory indexes between the two groups of patients before and after treatment
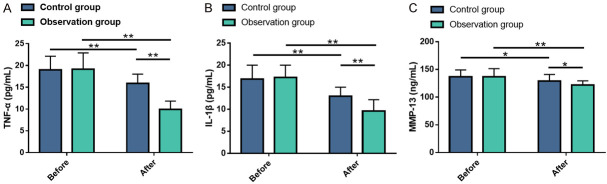
Serum levels of TNF-α, IL-1β and MMP-13 were assessed using ELISA, with the results presented in Figure 5. No distinct differences were found in TNF-α, IL-1β and MMP-13 levels between the two groups prior to treatment (all P>0.05). However, post-treatment levels of TNF-α, IL-1β and MMP-13 were significantly decreased in both cohorts (all P<0.05), with the OG showing significant lower levels compared to the CG (all P<0.05).
Figure 5.
Comparison of serum inflammation markers between the two groups before and after treatment. A: Comparison of TNF-α levels between the two groups before and after treatment; B: Comparison of IL-1β levels between the two groups before and after treatment; C: Comparison of MMP-13 levels between the two groups before and after treatment. Note: *P<0.05; **P<0.01. TNF-α, tumor necrosis factor (TNF)-α; IL-1β, interleukin (IL)-1β; MMP-13, matrix metalloproteinase (MMP)-13.
Comparison of patients’ life quality between the two groups of patients after treatment
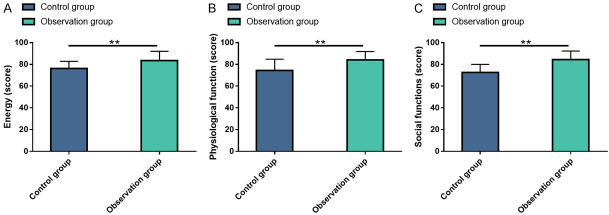
Postoperative quality of life was assessed using SF-36 survey. The data revealed statistically higher energy, physiological function and social function scores in the OG compared to the CG (all P<0.05, Figure 6).
Figure 6.
Comparison of quality of life in patients. A: Energy score; B: Physiological function score; C: Social function score. Note: **P<0.01.
Comparison of complications between the two groups of patients after treatment
In the CG, one patient (2.78%) developed a postoperative infection, while no complications were reported in the OG. The difference in complication rates between the two groups was not statistically significant (P>0.05).
Discussion
Knee cartilage injury remains a major challenge in orthopedic treatment, causing obvious clinical symptoms even with mild injury [22]. Although cartilage has a certain ability of spontaneous repair following injury, the ensuing cartilage terminal differentiation leads to irreversible structural and functional damage to the knee joint [23]. Given the difficulty in ensuring the quality of regenerated cartilage, even with surgical intervention, it is of great significance to optimize the treatment of such injury. This study analyzed the clinical effects and knee function recovery in patients with knee cartilage injuries, aiming to improve the quality of regenerated cartilage in patients.
This study enrolled 80 patients with knee cartilage injury and assigned them into two groups according to the treatment methods: CG treated with MF, and OG treated with MF+PRP. Our findings showed that the ORR was notably higher in the OG compared to the CG (97.73% vs. 83.33%), indicating that PRP+MF can significantly improve the clinical efficacy in such patients. This is consistent with the findings of Lee GW et al. [24], who reported that MF combined with PRP led to more favorable clinical outcomes two years post-surgery compared to MF alone. Our multivariate analysis indicated that sex, age, disease course, lesion location, ICRS grading, and treatment modality were not independent factors significantly affecting treatment efficacy in patients with knee cartilage injury. This may be associated with the information collection bias or the relatively small sample size in this study. In addition, the complication rate was slightly lower in the OG than in the CG (0% vs. 2.78%) without significant difference between the two groups, suggesting that the combined treatment did not affect the safety. In the study of Yang Z et al. [25], MF combined with PRP in patients with knee cartilage injuries significantly reduced the risk of complications while improving knee range of motion and function, supporting our findings.
In terms of clinical symptom improvement, we measured patients’ pain levels before and after treatment using VAS. The data showed that the pain symptoms were more significantly resolved in the OG after treatment compared with the CG, demonstrating that the combined therapy has obvious advantages over single therapy in reducing patients’ pain, similar to the findings in the double-blind randomized study conducted by Louis et al. [26]. Notably, Dubey et al. [27] mentioned that inflammatory mediators such as IL-1β and MMP-13 can affect synovial joints, aggravating joint pain. Our study suggests that the significant reduction in pain in the OG may be related to the ability of MF+PRP to inhibit serum inflammatory factors. To investigate this further, we detected the serum levels of TNF-α, IL-1β and MMP-13 in patients. The results indicated that these inflammatory factors decreased after treatment in both groups, with a more pronounced improvement in the OG. This suggests that the combined treatment has greater anti-inflammatory effect on serum inflammatory markers. TNF-α and IL-1β, known as a major inflammatory mediator and pro-inflammatory cytokine, respectively, are typical inflammatory markers after knee cartilage trauma and are also known to induce knee complications [28-30]. MMP-13, which degrades the cartilage matrix, negatively impacts cartilage repair [31]. Previous study [32] has shown that PRP can promote tissue repair while reducing pain and inflammation, which is consistent with our research results. In addition, Kennedy MI et al. [33] reported that PRP acts on the hyper-inflammatory and catabolic environment in articular cartilage injury through the application of platelets, leukocyte, and growth factor concentrates, further supporting our results. Finally, we evaluated patients’ knee joint function and life quality. Unsurprisingly, the knee joint function and balance function were more significantly improved in the OG, as well as the life quality, manifested as notably higher scores in terms of energy, physiological function and social function, corroborating with the results associated with efficacy and clinical symptoms indicators. This is consistent with the study by Gu Y et al. [34], which reported that PRP combined with MF effectively reduced pain and improved knee function and cartilage repair in patients with knee cartilage injuries, supporting the findings of our study.
Although this study confirmed that the application of PRP combined with MF in patients with knee cartilage injuries contributes to cartilage regeneration, knee function and joint balance function recovery, improvement of serum inflammatory indicators, pain relief, and enhancement of postoperative life quality, there is still room for improvement. First, the sample size was limited to 80 participants, and increasing the sample size would enhance the accuracy and reliability of the results. Second, although this study introduced PRP for combined therapy, it did not explore the platelet concentration in PRP, as different platelet concentrations could affect the chondrocyte proliferation rate [35]. Future research will address these limitations by incorporating a larger sample size and considering the effects of different platelet concentrations.
Conclusion
Conclusively, PRP combined with MF demonstrated superior treatment outcomes over MF treatment alone for knee cartilage injuries, showing significant potential for clinical application and warranting further clinical promotion.
Disclosure of conflict of interest
None.
References
- 1.Komarraju A, Goldberg-Stein S, Pederson R, McCrum C, Chhabra A. Spectrum of common and uncommon causes of knee joint hyaline cartilage degeneration and their key imaging features. Eur J Radiol. 2020;129:109097. doi: 10.1016/j.ejrad.2020.109097. [DOI] [PubMed] [Google Scholar]
- 2.Rath B, Eschweiler J, Betsch M, Gruber G. Cartilage repair of the knee joint. Orthopade. 2017;46:919–927. doi: 10.1007/s00132-017-3463-x. [DOI] [PubMed] [Google Scholar]
- 3.Ono Y, Akagi R, Mikami Y, Shinohara M, Hosokawa H, Horii M, Watanabe S, Ogawa Y, Sadamasu A, Kimura S, Yamaguchi S, Ohtori S, Sasho T. Effect of systemic administration of granulocyte colony-stimulating factor on a chronic partial-thickness cartilage defect in a rabbit knee joint. Cartilage. 2021;13:175S–184S. doi: 10.1177/19476035211021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin R, Laurent A, Applegate LA, Philippe V. Treatment of extensive chondral and osteochondral knee defects with autologous chondrocytes implantation. Rev Med Suisse. 2022;18:2384–2390. doi: 10.53738/REVMED.2022.18.808.2384. [DOI] [PubMed] [Google Scholar]
- 5.Snoeker B, Turkiewicz A, Magnusson K, Frobell R, Yu D, Peat G, Englund M. Risk of knee osteoarthritis after different types of knee injuries in young adults: a population-based cohort study. Br J Sports Med. 2020;54:725–730. doi: 10.1136/bjsports-2019-100959. [DOI] [PubMed] [Google Scholar]
- 6.Muthuri SG, McWilliams DF, Doherty M, Zhang W. History of knee injuries and knee osteoarthritis: a meta-analysis of observational studies. Osteoarthritis Cartilage. 2011;19:1286–1293. doi: 10.1016/j.joca.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Salonen EE, Magga T, Sillanpaa PJ, Kiekara T, Maenpaa H, Mattila VM. Traumatic patellar dislocation and cartilage injury: a follow-up study of long-term cartilage deterioration. Am J Sports Med. 2017;45:1376–1382. doi: 10.1177/0363546516687549. [DOI] [PubMed] [Google Scholar]
- 8.Marom N, Warner T, Williams RJ 3rd. Differences in the demographics and preferred management of knee cartilage injuries in soccer players across FIFA centers of excellence. Cartilage. 2021;13:873S–885S. doi: 10.1177/19476035211018857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aae TF, Randsborg PH, Breen AB, Visnes H, Vindfeld S, Sivertsen EA, Loken S, Brinchmann J, Hanvold HA, Aroen A. Norwegican Cartilage Project - a study protocol for a double-blinded randomized controlled trial comparing arthroscopic microfracture with arthroscopic debridement in focal cartilage defects in the knee. BMC Musculoskelet Disord. 2016;17:292. doi: 10.1186/s12891-016-1156-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C, Lu ZK, Huang C, Wang F, Miao S, Zeng L, Dai SJ, Li L, Li CZ. Comparison of curative effect between osteochondral mosaic transplantation and micro-fracture in the treatment of knee joint articular cartilage injury. Zhongguo Gu Shang. 2019;32:539–543. doi: 10.3969/j.issn.1003-0034.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Kim J, Cho H, Young K, Park J, Lee J, Suh D. In vivo animal study and clinical outcomes of autologous atelocollagen-induced chondrogenesis for osteochondral lesion treatment. J Orthop Surg Res. 2015;10:82. doi: 10.1186/s13018-015-0212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sampson S, Gerhardt M, Mandelbaum B. Platelet rich plasma injection grafts for musculoskeletal injuries: a review. Curr Rev Musculoskelet Med. 2008;1:165–174. doi: 10.1007/s12178-008-9032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39:2135–2140. doi: 10.1177/0363546511417792. [DOI] [PubMed] [Google Scholar]
- 14.Gao L, Goebel LKH, Orth P, Cucchiarini M, Madry H. Subchondral drilling for articular cartilage repair: a systematic review of translational research. Dis Model Mech. 2018;11:dmm034280. doi: 10.1242/dmm.034280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karpinski R, Krakowski P, Jonak J, Machrowska A, Maciejewski M, Nogalski A. Diagnostics of articular cartilage damage based on generated acoustic signals using ANN-part II: patellofemoral joint. Sensors (Basel) 2022;22:3765. doi: 10.3390/s22103765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rimkunas A, Gudas R, Mickevicius T, Maciulaitis J, Malinauskas M, Smailys A, Staskunas M, Usas A. Arthroscopic electromechanical assessment of human articular cartilage injury correlates with ICRS scores. Cartilage. 2024;15:250–258. doi: 10.1177/19476035231216439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Y, Li H, Cao S, Han Y, Shao J, Fu Q, Wang B, Wu J, Xiang D, Liu Z, Wang H, Zhu J, Qian Q, Yang X, Wang S. Clinical efficacy of intra-articular injection with P-PRP versus that of L-PRP in treating knee cartilage lesion: a randomized controlled trial. Orthop Surg. 2023;15:740–749. doi: 10.1111/os.13643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andriolo L, Marin Fermin T, Chiari Gaggia GMM, Serner A, Kon E, Papakostas E, Massey A, Verdonk P, Filardo G. Knee cartilage injuries in football players: clinical outcomes and return to sport after surgical treatment: a systematic review of the literature. Cartilage. 2024:19476035231224951. doi: 10.1177/19476035231224951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sargin S, Guler NS, Sahin N, Aslan A. Effects of total knee arthroplasty on balance and fall risk in elderly patients with severe gonarthrosis: an age- and sex-matched comparative study. Niger J Clin Pract. 2022;25:1445–1451. doi: 10.4103/njcp.njcp_1856_21. [DOI] [PubMed] [Google Scholar]
- 20.Gille J, Schuseil E, Wimmer J, Gellissen J, Schulz AP, Behrens P. Mid-term results of Autologous Matrix-Induced Chondrogenesis for treatment of focal cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc. 2010;18:1456–1464. doi: 10.1007/s00167-010-1042-3. [DOI] [PubMed] [Google Scholar]
- 21.Oak SR, Spindler KP. Measuring outcomes in knee articular cartilage pathology. J Knee Surg. 2021;34:11–19. doi: 10.1055/s-0040-1716362. [DOI] [PubMed] [Google Scholar]
- 22.Medina J, Garcia-Mansilla I, Fabricant PD, Kremen TJ, Sherman SL, Jones K. Microfracture for the treatment of symptomatic cartilage lesions of the knee: a survey of International Cartilage Regeneration & Joint Preservation Society. Cartilage. 2021;13:1148S–1155S. doi: 10.1177/1947603520954503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng M. Stem cells promote the regeneration of knee joint degenerative bone and articular cartilage. J Healthc Eng. 2022;2022:9533211. doi: 10.1155/2022/9533211. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Lee GW, Son JH, Kim JD, Jung GH. Is platelet-rich plasma able to enhance the results of arthroscopic microfracture in early osteoarthritis and cartilage lesion over 40 years of age? Eur J Orthop Surg Traumatol. 2013;23:581–587. doi: 10.1007/s00590-012-1038-4. [DOI] [PubMed] [Google Scholar]
- 25.Yang Z, Wu Y, Yin K, Xiang J, Liu C, Chen W, Dai Z. The therapeutic value of arthroscopic microfracture technique in combination with platelet-rich plasma injection for knee cartilage injury. Am J Transl Res. 2021;13:2694–2701. [PMC free article] [PubMed] [Google Scholar]
- 26.Louis ML, Dumonceau RG, Jouve E, Cohen M, Djouri R, Richardet N, Jourdan E, Giraudo L, Dumoulin C, Grimaud F, George FD, Veran J, Sabatier F, Magalon J. Intra-articular injection of autologous microfat and platelet-rich plasma in the treatment of knee osteoarthritis: a double-blind randomized comparative study. Arthroscopy. 2021;37:3125–3137. e3123. doi: 10.1016/j.arthro.2021.03.074. [DOI] [PubMed] [Google Scholar]
- 27.Dubey NK, Mishra VK, Dubey R, Syed-Abdul S, Wang JR, Wang PD, Deng WP. Combating osteoarthritis through stem cell therapies by rejuvenating cartilage: a review. Stem Cells Int. 2018;2018:5421019. doi: 10.1155/2018/5421019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muhammad W, Khan MM, Zafar S, Alqutub MN, AlMubarak AM, Mokeem S, Khan ZA, Usman MK, Ahmed N, Aldahiyan N, Vohra F, Abduljabbar T. Assessment of unstimulated whole salivary tumor necrosis factor alpha (TNF-alpha) and cellular micronuclei levels in Snuff (Naswar) users and non-users for early diagnosis of oral squamous cell carcinoma. Int J Environ Res Public Health. 2021;18:7230. doi: 10.3390/ijerph18147230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiao Z, Xu Y. Salvianolic acid B alleviating myocardium injury in ischemia reperfusion rats. Afr J Tradit Complement Altern Med. 2016;13:157–161. doi: 10.21010/ajtcam.v13i4.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khella CM, Asgarian R, Horvath JM, Rolauffs B, Hart ML. An evidence-based systematic review of human knee post-traumatic osteoarthritis (PTOA): timeline of clinical presentation and disease markers, comparison of knee joint PTOA models and early disease implications. Int J Mol Sci. 2021;22:1996. doi: 10.3390/ijms22041996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen R, Zhang Y, Xu H, Hu H, Chen M, Shuai Z. Val109Asp polymorphism of the omentin-1 gene and incidence of knee osteoarthritis in a Chinese Han population: a correlation analysis. Drug Des Devel Ther. 2021;15:5075–5086. doi: 10.2147/DDDT.S340410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Vries-van Melle ML, Narcisi R, Kops N, Koevoet WJ, Bos PK, Murphy JM, Verhaar JA, van der Kraan PM, van Osch GJ. Chondrogenesis of mesenchymal stem cells in an osteochondral environment is mediated by the subchondral bone. Tissue Eng Part A. 2014;20:23–33. doi: 10.1089/ten.tea.2013.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kennedy MI, Whitney K, Evans T, LaPrade RF. Platelet-rich plasma and cartilage repair. Curr Rev Musculoskelet Med. 2018;11:573–582. doi: 10.1007/s12178-018-9516-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gu Y, Wang G, Chen P. Platelet rich plasma combined with arthroscopic microfracture versus arthroscopic microfracture alone for the treatment of knee cartilage injury. Am J Transl Res. 2023;15:3705–3713. [PMC free article] [PubMed] [Google Scholar]
- 35.Redondo ML, Naveen NB, Liu JN, Tauro TM, Southworth TM, Cole BJ. Preservation of knee articular cartilage. Sports Med Arthrosc Rev. 2018;26:e23–e30. doi: 10.1097/JSA.0000000000000226. [DOI] [PubMed] [Google Scholar]