Abstract
Objective: To investigate the effects of magnesium sulfate combined with labetalol treatment on vascular endothelial function and pregnancy outcomes in pregnant women with preeclampsia (PE). Methods: In this retrospective study, clinical data was analyzed from 262 PE parturients who were treated in Xi’an High-Tech Hospital from January 2022 to February 2024. They were divided into the experimental group (138 cases) and the control group (124 cases) based on the treatment plan. The control group received magnesium sulfate monotherapy, while the experimental group received a combination of magnesium sulfate and labetalol. The therapeutic effect was evaluated by measuring changes in vascular endothelial function indicators such as nitric oxide (NO) and endothelin-1 (ET-1) levels, systolic blood pressure (SBP), and diastolic blood pressure (DBP). Adverse reactions and pregnancy outcomes were statistically analyzed. Results: After treatment, the NO level was significantly higher while the ET-1 level was significantly lower in the experiment group than those in the control group (all P<0.001). The DBP and SBP in the experimental group were significantly lower than those in the control group (P=0.002 and P<0.001). Although the incidence of adverse reactions didn’t differ significantly between the two groups (P=0.440), the incidence of adverse pregnancy outcomes in the experimental group was significantly lower than that in the control group (P=0.028). Multivariate logistic analysis showed that fasting blood sugar, gestational age, 24-hour urine protein, post-treatment NO, ET-1, DBP, and SBP are independent factors affecting adverse pregnancy outcomes, with higher gestational age and post-treatment NO levels being protective factors. The areas under the curve for fasting blood sugar, gestational age, urine protein, post-treatment NO, post-treatment ET-1, post-treatment DBP, and post-treatment SBP in predicting adverse pregnancy outcomes were 0.645, 0.598, 0.615, 0.654, 0.685, 0.669, and 0.673, respectively. Post-treatment NO was negatively correlated with fasting blood sugar, urine protein, post-treatment DBP, and post-treatment SBP (r=-0.713, r=-0.605, r=-0.650, r=-0.676, all P<0.001), and positively correlated with gestational age (r=0.626, P<0.001). Conversely, post-treatment ET-1 was positively correlated with fasting blood sugar, urine protein, post-treatment DBP, and post-treatment SBP (r=0.746, r=0.710, r=0.729, r=0.720, all P<0.001), and negatively correlated with gestational age (r=-0.753, P<0.001). Conclusions: The combined treatment of magnesium sulfate and labetalol significantly improves vascular endothelial function and pregnancy outcomes in preeclamptic pregnant women, effectively controlling pregnancy hypertension and reducing the incidence of adverse pregnancy outcomes. This combined treatment is as safe as magnesium sulfate monotherapy and can be considered a preferred treatment plan for pregnant women with preeclampsia.
Keywords: Magnesium sulfate, labetalol, preeclampsia, vascular endothelial function, pregnancy outcomes
Introduction
Preeclampsia (PE) is a multisystem disorder exclusive to pregnancy, characterized by hypertension that typically develops after the 20th week of gestation [1]. As a serious pregnancy complication, PE is a leading cause of maternal and perinatal mortality and morbidity, affecting approximately 2%-8% of pregnant women [2,3]. Notably, the incidence of PE is significantly higher in developing countries, being about seven times higher than in developed countries [4]. While the exact pathogenesis of PE has not been fully elucidated, existing research indicates that placental dysfunction and inadequate perfusion of the uterine placental bed may play an important role in the development of PE [5]. Risk factors for PE include advanced maternal age, obesity, nulliparity, a family or personal history of PE, chronic kidney disease or autoimmune disease, and multiple pregnancies. As the condition worsens [6], PE can lead to maternal organ dysfunction and, in severe cases, may cause convulsions or even coma, severely endangering the health of both mother and child [7].
Magnesium sulfate is a commonly used drug in clinical practice for the treatment of eclampsia. It improves endothelial dysfunction associated with cardiovascular diseases and exerts multiple effects such as diuresis, spasmolysis, and lowering blood pressure [8]. However, some studies have indicated that some patients may not achieve the desired therapeutic effect with magnesium sulfate alone [9]. In addition, excessively high blood concentrations of magnesium sulfate can lead to side effects such as dizziness, nausea and vomiting, abdominal pain, and diarrhea, posing risks to both maternal and fetal safety [10]. Therefore, magnesium sulfate is often used in combination with other drugs to improve therapeutic efficacy. Labetalol, a salicylic acid derivative, is commonly prescribed for hypertension. It antagonizes both α and β adrenergic receptors, promotes peripheral vasodilation, improves local microcirculation, thus effectively lowering blood pressure [11,12].
Preeclamptic patients often experience endothelial dysfunction after delivery, which may persist in some cases and eventually progress to chronic hypertension [13]. The disorder of vascular endothelial function and arterial elasticity plays a key role in the pathogenesis of preeclampsia [14,15]. Therefore, accurately assessing these dysfunctions is of great significance for disease monitoring and treatment. Correcting endothelial dysfunction may help improve the clinical prognosis of patients with preeclampsia and improve pregnancy outcomes. This study evaluates the impact of magnesium sulfate combined with labetalol therapy on vascular endothelial function and pregnancy outcomes in patients with preeclampsia.
Methods and data
Study subjects
We collected the medical records of parturients with PE treated at Xi’an High-Tech Hospital from January 2022 to February 2024. Inclusion criteria: (1) diagnosis of PE meeting the WHO criteria, defined as blood pressure reaching or exceeding 140/90 mmHg at least twice (measured at least 4 hours apart) after the 20th week of gestation, accompanied by proteinuria greater than 3 grams/24 hours [16]; (2) gestational age greater than 20 weeks; (3) patients with normal mental and conscious status; and (4) complete medical record information. Exclusion criteria: (1) history of primary hypertension; (2) severe functional insufficiency of organs such as heart, liver, kidney, or lungs; (3) allergies to the drugs used; (4) other pregnancy-related diseases; and (5) history of unexplained miscarriage. Ultimately, 262 cases were selected according to these criteria.
Ethical statement
The study received approval from the Medical Ethics Committee of Xi’an High-Tech Hospital (Ethical approval number: 191128).
Treatment plan
The control group received monotherapy with magnesium sulfate. Specifically, 20 milliliters of magnesium sulfate (Manufacturer: Nanchang Baiyun Pharmaceutical Company, Batch No.: 20210811) were mixed with 100 milliliters of 5% glucose solution and administered via intravenous drip, at a controlled rate of 1 to 2 grams per hour, once daily.
The experimental group received a combined treatment plan with labetalol (Manufacturer: Jiangsu Desano Pharmaceutical Co., Ltd., batch number: 20210604) and magnesium sulfate. Labetalol hydrochloride tablets were administered at a dose of 100 milligrams each, 2 to 3 times a day. Throughout the treatment period, which lasted one week, blood pressure changes in both groups of patients were closely monitored.
Observation results
Baseline data, including age, pre-pregnancy BMI, fasting blood sugar, gestational age, history of miscarriage, number of fetuses, number of deliveries, family history of hypertension, family history of diabetes, smoking history, and 24-hour urine protein levels of the two groups of research subjects were collected. The levels of vascular endothelial function indicators nitric oxide (NO) and endothelin-1 (ET-1), as well as diastolic blood pressure (DBP) and systolic blood pressure (SBP) before and after treatment in the two groups of patients were recorded. Adverse reactions during treatment in the two groups, including gastrointestinal reactions, dizziness, headache, and hypoalbuminemia, were compared. Adverse pregnancy outcomes after treatment in the two groups of patients, including placental abruption, fetal distress, neonatal asphyxia, and postpartum hemorrhage, were compared. Univariate and multivariate analyses were performed to identify the independent factors influencing adverse pregnancy outcomes. The predictive value of the independent influencing factors was detected through the ROC curve.
Statistical methods
Statistical analysis was performed using SPSS 25.0 software (IBM, NY, USA) and Microsoft Excel (Microsoft, NY, USA). Quantitative data were expressed as mean ± standard deviation, and group comparisons were made using independent sample t-tests. Categorical data were expressed as percentage and group comparisons were made using chi-square tests. P<0.05 was used to indicate significant differences. Univariate logistic regression analysis was performed to estimate the unadjusted risk of adverse pregnancy outcomes in preeclamptic patients by independent variables. Variables with P<0.05 in this preliminary step were included in the subsequent multivariate logistic regression analysis. In multivariate logistic regression, odds ratios, 95% confidence intervals (CI), and P values were calculated to estimate the independent impact of risk factors on the occurrence of adverse pregnancy outcomes. The receiver operating characteristic (ROC) curve was used to determine the predictive value of influencing factors for adverse pregnancy outcomes. Pearson correlation was used to detect the correlation between post-treatment NO and ET-1 levels and the independent influencing factors of adverse pregnancy outcomes.
Results
Baseline data of the two groups
The baseline data of the two groups are shown in Table 1. There were no significant differences in age, pre-pregnancy body mass index (BMI), fasting blood sugar, gestational age, history of miscarriage, number of fetuses, number of deliveries, family history of hypertension, family history of diabetes, smoking history, or 24-hour urine protein between the two groups (all P>0.05).
Table 1.
Comparison of baseline data between the two groups
| Experimental group | Control group | χ2/t | P | |
|---|---|---|---|---|
| Number of cases | 138 | 124 | ||
| Age (<35 years/≥35 years) | 105/33 | 89/35 | 0.632 | 0.427 |
| Pre-pregnancy BMI (<24 kg/m2/≥24 kg/m2) | 81/57 | 78/46 | 0.485 | 0.486 |
| Fasting blood sugar (mmol/L) | 5.07±1.07 | 5.18±0.95 | 0.901 | 0.368 |
| Gestational age (weeks) | 34.54±2.17 | 34.10±2.56 | 1.488 | 0.138 |
| History of miscarriage (yes/no) | 39/99 | 41/83 | 0.711 | 0.399 |
| Number of fetuses (single/multiple) | 127/11 | 110/14 | 0.834 | 0.361 |
| Number of deliveries (primiparous/multiparous) | 29/109 | 33/91 | 1.133 | 0.287 |
| Family history of hypertension (yes/no) | 22/116 | 29/95 | 2.309 | 0.129 |
| Family history of diabetes (yes/no) | 21/117 | 14/110 | 0.870 | 0.351 |
| Family history of diabetes (yes/no) | 17/121 | 10/114 | 1.279 | 0.258 |
| 24-hour urine protein (g) | 3.77±0.36 | 3.76±0.33 | 0.256 | 0.798 |
BMI: Body Mass Index.
Comparison of vascular endothelial function before and after treatment between the two groups
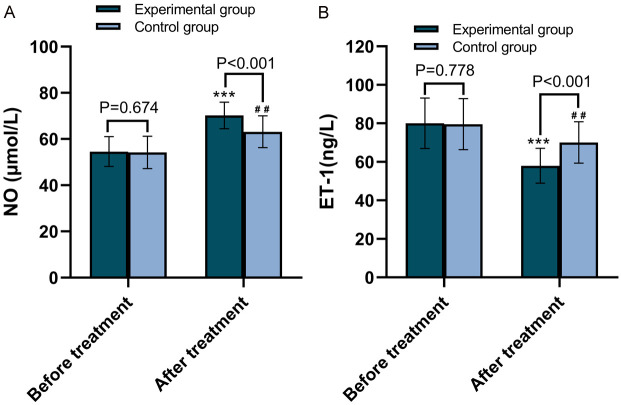
The changes in levels of vascular endothelial function indicators NO and ET-1 before and after treatment in the two groups are shown in Figure 1. The NO level in the experimental group was not statistically different from that in the control group before treatment (P=0.674); however, it was significantly higher after treatment (P<0.001). Conversely, the ET-1 level in the experimental group was not statistically different from that in the control group before treatment (P=0.778), but it was significantly lower after treatment (P<0.001).
Figure 1.
Comparison of vascular endothelial function between the two groups before and after treatment. A. Comparison of NO levels between the two groups of patients before and after treatment. B. Comparison of ET-1 levels between the two groups of patients before and after treatment. NO: Nitric Oxide; ET-1: Endothelin-1. ***P<0.001, compared with pre-treatment; ##P<0.01, compared with pre-treatment.
Comparison of blood pressure control before and after treatment between the two groups
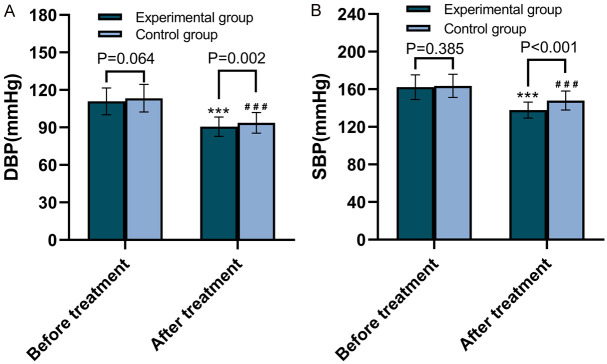
Changes in DBP and SBP before and after treatment in both groups are shown in Figure 2. The DBP and SBP in the experimental group were not statistically different from those in the control group before treatment (P=0.064 and 0.385); however, both were significantly lower after treatment (P=0.002 and P<0.001, respectively).
Figure 2.
Changes in blood pressures between the two groups before and after treatment. A. Comparison of DBP between the two groups of patients before and after treatment. B. Comparison of SBP between the two groups of patients before and after treatment. DBP: Diastolic Blood Pressure; SBP: Systolic Blood Pressure. ***P<0.001, compared with pre-treatment; ###P<0.001, compared with pre-treatment.
Adverse reactions in the two groups
The adverse reactions in the two groups are shown in Table 2. The total incidence of adverse reactions in the experimental group was 10.87%, and in the control group, it was 8.06%. There was no significant difference between the two groups (P=0.440).
Table 2.
Comparison of adverse reactions between the two groups
| Number of cases | Gastrointestinal Reactions | Dizziness | Headache | Hypoalbuminemia | Total number of adverse reactions | Overall incidence of adverse reactions | |
|---|---|---|---|---|---|---|---|
| Experimental Group | 138 | 6 | 3 | 1 | 5 | 15 | 10.87% |
| Control Group | 124 | 3 | 4 | 2 | 1 | 10 | 8.06% |
| χ2 | 0.595 | ||||||
| P | 0.440 |
Adverse pregnancy outcomes in the two groups
The adverse pregnancy outcomes for both groups are shown in Table 3. The total incidence of adverse pregnancy outcomes in the experimental group was 21.74%, and in the control group, it was 33.87%. The incidence in the experimental group was significantly lower than that in the control group (P=0.028).
Table 3.
Comparison of adverse pregnancy outcomes between the two groups
| Number of cases | Placental Abruption | Fetal Distress | Neonatal Asphyxia | Postpartum Hemorrhage | Total number of adverse pregnancy outcomes | The overall incidence of adverse pregnancy outcomes | |
|---|---|---|---|---|---|---|---|
| Experimental group | 138 | 7 | 12 | 8 | 3 | 30 | 21.74% |
| Control group | 124 | 12 | 18 | 8 | 4 | 42 | 33.87% |
| χ2 | 4.824 | ||||||
| P | 0.028 |
Univariate analysis of factors affecting adverse pregnancy outcomes
All patients were divided into two groups: 82 cases with adverse pregnancy outcomes and 180 cases without. The relevant factors for both groups are shown in Table 4. Univariate analysis found that fasting blood sugar, gestational age, 24-hour urine protein, post-treatment NO, post-treatment ET-1, post-treatment DBP, and post-treatment SBP were significantly related to adverse pregnancy outcomes (all P<0.05), while age, pre-pregnancy BMI, history of miscarriage, number of fetuses, number of deliveries, family history of hypertension, family history of diabetes, and smoking history were not (all P>0.05).
Table 4.
Univariate analysis table
| Adverse pregnancy outcomes | Non-adverse pregnancy outcomes | OR (95% CI) | P | |
|---|---|---|---|---|
| Number of cases | 82 | 180 | ||
| Age (<35 years/≥35 years) | 58/24 | 136/44 | 0.782 (0.438-1.416) | 0.409 |
| Pre-pregnancy BMI (<24 kg/m2/≥24 kg/m2) | 53/29 | 106/74 | 1.276 (0.746-2.206) | 0.378 |
| Fasting blood sugar (mmol/L) | 5.53±1.07 | 4.94±0.94 | 1.805 (1.375-2.408) | <0.001 |
| Gestational age (weeks) | 33.67±2.62 | 34.62±2.18 | 0.844 (0.753-0.943) | 0.003 |
| History of miscarriage (yes/no) | 28/54 | 52/128 | 1.276 (0.725-2.224) | 0.392 |
| Number of fetuses (single/multiple) | 71/11 | 166/14 | 0.544 (0.236-1.282) | 0.155 |
| Number of deliveries (primiparous/multiparous) | 16/66 | 46/134 | 0.706 (0.364-1.318) | 0.287 |
| Family history of hypertension (yes/no) | 19/63 | 32/148 | 1.395 (0.726-2.627) | 0.308 |
| Family history of diabetes (yes/no) | 10/72 | 25/155 | 0.861 (0.377-1.839) | 0.709 |
| Family history of diabetes (yes/no) | 9/73 | 18/162 | 1.110 (0.456-2.530) | 0.810 |
| 24-hour urine protein (g) | 3.86±0.30 | 3.72±0.35 | 3.414 (1.566-7.711) | 0.002 |
| After treatment NO (μmol/L) | 64.38±7.50 | 67.97±6.78 | 0.931 (0.894-0.966) | <0.001 |
| After treatment ET-1 (ng/L) | 69.07±11.82 | 61.26±10.61 | 1.066 (1.040-1.095) | <0.001 |
| After treatment DBP (mmHg) | 95.52±7.67 | 90.48±7.85 | 1.087 (1.049-1.129) | <0.001 |
| After treatment SBP (mmHg) | 146.67±9.75 | 140.79±10.43 | 1.056 (1.029-1.085) | <0.001 |
| Treatment options (combination of labetalol/magnesium sulfate alone) | 32/50 | 106/74 | 0.447 (0.260-0.758) | 0.003 |
BMI: Body Mass index; NO: Nitric Oxide; ET-1: Endothelin-1; DBP: Diastolic Blood Pressure; SBP: Systolic Blood Pressure.
Multivariate analysis
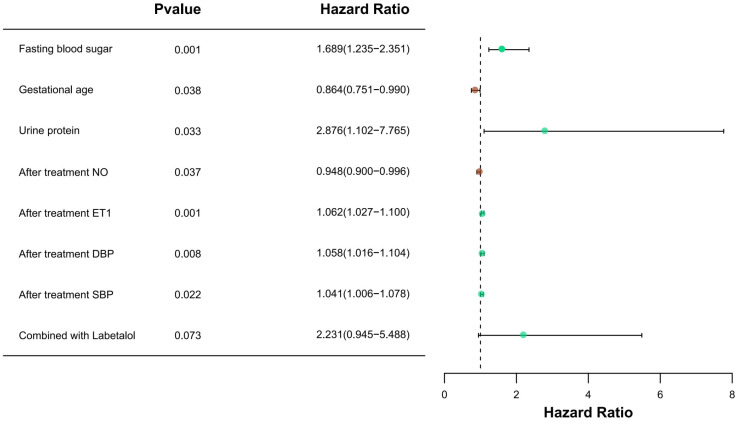
Factors significantly related to adverse pregnancy outcomes in univariate analysis were entered into the Logistic regression equation for multivariate analysis. The results of multivariate analysis (Figure 3) showed that higher fasting blood sugar, urine protein, post-treatment ET-1, post-treatment DBP, and post-treatment SBP were independent risk factors for adverse pregnancy outcomes, while higher gestational age and post-treatment NO were independent protective factors. The treatment plan is not an independent influencing factor for adverse pregnancy outcomes.
Figure 3.
Forest plot of multivariate analysis. NO: Nitric Oxide; ET-1: Endothelin-1; DBP: Diastolic Blood Pressure; SBP: Systolic Blood Pressure.
Predictive value of independent influencing factors for adverse pregnancy outcomes
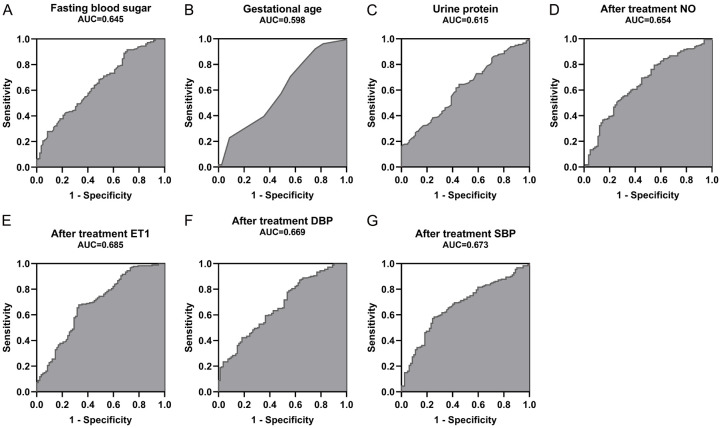
The ROC curve was used to explore the predictive value of independent influencing factors such as fasting blood sugar, gestational age, urine protein, post-treatment NO, post-treatment ET-1, post-treatment DBP, and post-treatment SBP for adverse pregnancy outcomes, as shown in Figure 4. The areas under the curve (AUCs) for fasting blood sugar, gestational age, urine protein, post-treatment NO, post-treatment ET-1, post-treatment DBP, and post-treatment SBP in predicting adverse pregnancy outcomes were 0.645, 0.598, 0.615, 0.654, 0.685, 0.669, and 0.673, respectively.
Figure 4.
ROC curves for independent influencing factors in predicting adverse pregnancy outcomes. A. ROC curve for fasting blood sugar in predicting adverse pregnancy outcomes. B. ROC curve for gestational age in predicting adverse pregnancy outcomes. C. ROC curve for urine protein in predicting adverse pregnancy outcomes. D. ROC curve for post-treatment NO levels in predicting adverse pregnancy outcomes. E. ROC curve for post-treatment ET1 levels in predicting adverse pregnancy outcomes. F. ROC curve for post-treatment DBP levels in predicting adverse pregnancy outcomes. G. ROC curve for post-treatment SBP levels in predicting adverse pregnancy outcomes. NO: Nitric Oxide; ET-1: Endothelin-1; DBP: Diastolic Blood Pressure; SBP: Systolic Blood Pressure.
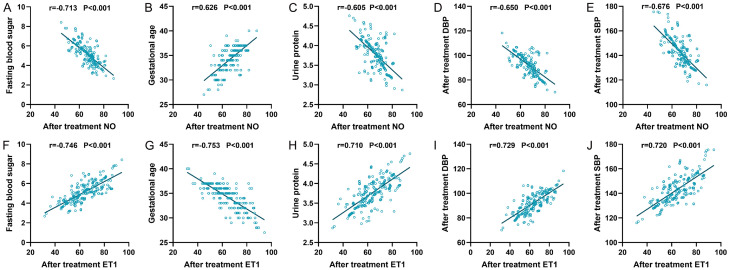
Correlation between post-treatment NO and ET-1 and independent influencing factors
The Pearson correlation analysis was utilized to investigate the relationship between post-treatment NO and ET-1 with independent influencing factors such as fasting blood sugar, gestational age, urine protein, post-treatment diastolic blood pressure (DBP), and post-treatment systolic blood pressure (SBP), as shown in Figure 5. The results indicated that post-treatment NO was negatively correlated with fasting blood sugar, urine protein, post-treatment DBP, and post-treatment SBP (r=-0.713, r=-0.605, r=-0.650, r=-0.676, all P<0.001), and positively correlated with gestational age (r=0.626, P<0.001). In contrast, post-treatment ET-1 was positively correlated with fasting blood sugar, urine protein, post-treatment DBP, and post-treatment SBP (r=0.746, r=0.710, r=0.729, r=0.720, all P<0.001), but negatively correlated with gestational age (r=-0.753, P<0.001).
Figure 5.
Correlation between post-treatment NO and ET-1 and independent influencing factors. A. Post-treatment NO was negatively correlated with fasting blood sugar. B. Post-treatment NO was positively correlated with gestational age. C. Post-treatment NO was negatively correlated with urine protein. D. Post-treatment NO was negatively correlated with post-treatment DBP. E. Post-treatment NO was negatively correlated with post-treatment SBP. F. Post-treatment ET1 was positively correlated with fasting blood sugar. G. Post-treatment ET1 was negatively correlated with gestational age. H. Post-treatment ET1 was positively correlated with urine protein. I. Post-treatment ET1 was positively correlated with post-treatment DBP. J. Post-treatment ET1 was positively correlated with post-treatment SBP. NO: Nitric Oxide; ET-1: Endothelin-1; DBP: Diastolic Blood Pressure; SBP: Systolic Blood Pressure.
Discussion
Low doses of magnesium sulfate can prevent the prolongation of PE and extend gestational weeks to certain extend; however, it is ineffective in controlling blood pressure indicators in patients, while high doses are prone to cause magnesium ion poisoning [17]. Labetalol, an antihypertensive drug, can effectively blocks α and β receptors, achieving the effect of peripheral vasodilation, reducing peripheral vascular resistance, effectively blocking adrenergic receptors, ensuring a gentle sinus rhythm, and controlling blood pressure levels [18,19]. In addition, labetalol can suppress myocardium and smooth muscle contractions, prolong the conduction time of the atrioventricular node, thereby reducing blood pressure, preventing arrhythmias, and reducing peripheral vascular resistance. These effects help minimize risks to both mother and child, ensuring smooth delivery [20].
NO is a vasodilator that can lower blood pressure, while ET-1 is a potent vasoconstrictor [21]. In the treatment of preeclampsia, an increase in NO levels and a decrease in ET-1 levels may indicate an improved balance between vascular dilation and constriction, which helps to lower blood pressure and enhance blood circulation [22]. The recovery of vascular endothelial function is crucial for improving pregnancy outcomes, as endothelial dysfunction is one of the main pathological mechanisms of preeclampsia [23]. This study found that after treatment, the NO levels in the experimental group increased significantly, and the ET-1 levels decreased significantly, indicating that the combined treatment plan is more effective in improving vascular endothelial function. This may help to improve the prognosis of patients with preeclampsia and reduce the risk of complications. In addition, the experimental group showed a significant advantage in controlling SBP and DBP, indicating that the combined use of labetalol can more effectively control hypertension, which is an important factor in improving pregnancy outcomes. Effective blood pressure control can mitigate the risks associated with preeclampsia and reduce the incidence of adverse pregnancy outcomes [24].
There was no significant difference in the incidence of adverse reactions between the two groups, indicating that the safety profile of magnesium sulfate combined with labetalol is comparable to that of magnesium sulfate alone. Notably, the incidence of adverse pregnancy outcomes in the experimental group was significantly lower than that in the control group, further proving the superiority of the combined treatment plan. This suggests that in clinical practice, the combined treatment of labetalol and magnesium sulfate for preeclamptic pregnant women can be prioritized to improve therapeutic effects and reduce the risk of adverse pregnancy outcomes. Wang et al. [25] also showed that magnesium sulfate combined with labetalol effectively improved outcomes in cases of early-onset severe preeclampsia. Additionally, Xie et al. [26] also showed that labetalol can inhibit platelet aggregation, promote fetal lung maturation, and does not cause a rapid drop in blood pressure and palpitations.
Multivariate analysis revealed that higher NO levels and longer gestational age after treatment are protective factors for adverse pregnancy outcomes. NO, as an endogenous vasodilator, can effectively reduce vascular resistance and improve placental blood flow, thereby reducing the incidence of adverse pregnancy outcomes [27]. Conversely, higher ET-1 levels, fasting blood sugar, urine protein, and high systolic and diastolic pressures after treatment were identified as risk factors. ET-1 is a potent vasoconstrictor, and its increased levels are often accompanied by vasoconstriction and endothelial dysfunction [28]. The multivariate results also showed that higher fasting blood glucose and urine protein were independent risk factors for adverse pregnancy outcomes. Zeng et al. [29] mentioned that even in the absence of gestational diabetes, blood sugar levels at the threshold for gestational diabetes were associated with an increased risk of macrosomia. Lei et al. [30] found that the severity of 24-hour urinary protein can predict the outcomes of stillbirth, premature delivery, and fetal distress in PE patients, with higher 24-hour urinary protein correlating with increased rates of induced labor and stillbirth.
Through ROC curve analysis, we assessed the predictive ability of each independent influencing factor for adverse pregnancy outcomes. The AUCs of fasting blood sugar, gestational age, urine protein, post-treatment NO, post-treatment ET-1, post-treatment DBP, and post-treatment SBP were all greater than 0.5. Specifically, the AUCs for post-treatment ET-1 level, SBP, and DBP in predicting adverse pregnancy outcomes were 0.685, 0.669, and 0.673, respectively, indicating that these factors have moderate predictive ability. In particular, the post-treatment ET-1 level, as a potent vasoconstrictor, was significantly related to adverse pregnancy outcomes. The highest AUC of ET-1 indicates that it has the strongest predictive ability for adverse pregnancy outcomes among all influencing factors. Therefore, ET-1 can serve as an important biomarker for the early identification and intervention of high-risk preeclamptic pregnant women in clinical practice.
At the end of the study, it was found that the NO level after treatment was negatively correlated with fasting blood sugar, urine protein, DBP, and SBP, and positively correlated with gestational age. This indicates that increased NO levels are helpful in lowering blood pressure and urine protein levels, and are related to a longer gestational period, all of which are protective factors for favorable pregnancy outcomes. NO, as a vasodilator, can alleviate vascular tension, improve placental perfusion, thereby positively impacting pregnancy outcomes. On the contrary, the post-treatment ET-1 level was positively correlated with the above indicators and negatively correlated with gestational age, further confirming that ET-1, as a vasoconstrictor, is often accompanied by endothelial dysfunction, increased blood pressure, and increased urine protein, thereby increasing the risk of adverse pregnancy outcomes.
This study has a few limitations, such as the study sample only coming from a single medical institution, which may limit the external validity of the results. Future studies should consider multicenter, large sample size, randomized controlled trials to further verify the efficacy and safety of magnesium sulfate combined with labetalol treatment for preeclamptic pregnant women. In addition, future studies can consider combining other medications or non-drug interventions to comprehensively enhance the management of preeclampsia.
In summary, the combined treatment of magnesium sulfate and labetalol significantly improves vascular endothelial function and pregnancy outcomes in preeclamptic pregnant women and more effectively controls pregnancy hypertension and reduces the incidence of adverse pregnancy outcomes. This combined treatment plan is as safe as using magnesium sulfate alone and can be considered a preferred treatment option for pregnant women with preeclampsia.
Disclosure of conflict of interest
None.
References
- 1.Giannakou K. Prediction of pre-eclampsia. Obstet Med. 2021;14:220–224. doi: 10.1177/1753495X20984015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Das S, Das R, Bajracharya R, Baral G, Jabegu B, Odland JO, Odland ML. Incidence and risk factors of pre-eclampsia in the paropakar maternity and women’s hospital, nepal: a retrospective study. Int J Environ Res Public Health. 2019;16:3571. doi: 10.3390/ijerph16193571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armaly Z, Jadaon JE, Jabbour A, Abassi ZA. Preeclampsia: novel mechanisms and potential therapeutic approaches. Front Physiol. 2018;9:973. doi: 10.3389/fphys.2018.00973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osungbade KO, Ige OK. Public health perspectives of preeclampsia in developing countries: implication for health system strengthening. J Pregnancy. 2011;2011:481095. doi: 10.1155/2011/481095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jahan F, Vasam G, Green AE, Bainbridge SA, Menzies KJ. Placental mitochondrial function and dysfunction in preeclampsia. Int J Mol Sci. 2023;24:4177. doi: 10.3390/ijms24044177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sahai K, Saraswathy S, Yadav TP, Arora D, Krishnan M. Pre-eclampsia: molecular events to biomarkers. Med J Armed Forces India. 2017;73:167–174. doi: 10.1016/j.mjafi.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aplin JD, Myers JE, Timms K, Westwood M. Tracking placental development in health and disease. Nat Rev Endocrinol. 2020;16:479–494. doi: 10.1038/s41574-020-0372-6. [DOI] [PubMed] [Google Scholar]
- 8.Okonkwo M, Nash CM. Duration of postpartum magnesium sulphate for the prevention of eclampsia: a systematic review and meta-analysis. Obstet Gynecol. 2022;139:521–528. doi: 10.1097/AOG.0000000000004720. [DOI] [PubMed] [Google Scholar]
- 9.Yalcin SE, Sezik M, Yavuz A, Savran M, Asci H, Ozmen O. Combined use of magnesium sulfate and fingolimod for antenatal neuroprotection against inflammation-mediated experimental preterm brain injury in a rat model. Fetal Pediatr Pathol. 2022;41:603–615. doi: 10.1080/15513815.2021.1945174. [DOI] [PubMed] [Google Scholar]
- 10.Elasy AN, Nafea OE. Critical hypermagnesemia in preeclamptic women under a magnesium sulfate regimen: incidence and associated risk factors. Biol Trace Elem Res. 2023;201:3670–3678. doi: 10.1007/s12011-022-03479-x. [DOI] [PubMed] [Google Scholar]
- 11.Magee LA, Namouz-Haddad S, Cao V, Koren G, von Dadelszen P. Labetalol for hypertension in pregnancy. Expert Opin Drug Saf. 2015;14:453–461. doi: 10.1517/14740338.2015.998197. [DOI] [PubMed] [Google Scholar]
- 12.Bithi N, Merrigan SD, McMillin GA. Does labetalol trigger false positive drug testing results? J Addict Med. 2023;17:e209–e210. doi: 10.1097/ADM.0000000000001117. [DOI] [PubMed] [Google Scholar]
- 13.Erez O, Romero R, Jung E, Chaemsaithong P, Bosco M, Suksai M, Gallo DM, Gotsch F. Preeclampsia and eclampsia: the conceptual evolution of a syndrome. Am J Obstet Gynecol. 2022;226:S786–S803. doi: 10.1016/j.ajog.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vangrieken P, Remels AHV, Al-Nasiry S, Bast A, Janssen GMJ, von Rango U, Vroomans D, Pinckers YCW, van Schooten FJ, Schiffers PMH. Placental hypoxia-induced alterations in vascular function, morphology, and endothelial barrier integrity. Hypertens Res. 2020;43:1361–1374. doi: 10.1038/s41440-020-0528-8. [DOI] [PubMed] [Google Scholar]
- 15.Mishra JS, Kumar S. Activation of angiotensin type 2 receptor attenuates testosterone-induced hypertension and uterine vascular resistance in pregnant ratsdagger. Biol Reprod. 2021;105:192–203. doi: 10.1093/biolre/ioab051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Omotayo MO, Martin SL, Stoltzfus RJ, Ortolano SE, Mwanga E, Dickin KL. With adaptation, the WHO guidelines on calcium supplementation for prevention of pre-eclampsia are adopted by pregnant women. Matern Child Nutr. 2018;14:e12521. doi: 10.1111/mcn.12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mittendorf R, Dammann O, Lee KS. Brain lesions in newborns exposed to high-dose magnesium sulfate during preterm labor. J Perinatol. 2006;26:57–63. doi: 10.1038/sj.jp.7211419. [DOI] [PubMed] [Google Scholar]
- 18.Hafsa H, Zamir A, Rasool MF, Imran I, Saeed H, Ahmad T, Alsanea S, Alshamrani AA, Alruwaili AH, Alqahtani F. Development and evaluation of a physiologically based pharmacokinetic model of labetalol in healthy and diseased populations. Pharmaceutics. 2022;14:2362. doi: 10.3390/pharmaceutics14112362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frishman WH. Beta-adrenergic receptor blockers in hypertension: alive and well. Prog Cardiovasc Dis. 2016;59:247–252. doi: 10.1016/j.pcad.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Kaye AB, Bhakta A, Moseley AD, Rao AK, Arif S, Lichtenstein SJ, Aggarwal NT, Volgman AS, Sanghani RM. Review of cardiovascular drugs in pregnancy. J Womens Health (Larchmt) 2019;28:686–697. doi: 10.1089/jwh.2018.7145. [DOI] [PubMed] [Google Scholar]
- 21.Fu J, Peng J, Tu G. ZNF300 enhances temozolomide resistance in gliomas by regulating lncRNA SNHG12. Gen Physiol Biophys. 2022;41:349–355. doi: 10.4149/gpb_2022019. [DOI] [PubMed] [Google Scholar]
- 22.Tielemans B, Wagenaar A, Belge C, Delcroix M, Quarck R. Pulmonary arterial hypertension drugs can partially restore altered angiogenic capacities in bmpr2-silenced human lung microvascular endothelial cells. Pulm Circ. 2023;13:e12293. doi: 10.1002/pul2.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brislane A, Jones H, Holder SM, Low DA, Hopkins ND. The effect of exercise during pregnancy on maternal and offspring vascular outcomes: a pilot study. Reprod Sci. 2021;28:510–523. doi: 10.1007/s43032-020-00302-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poon LC, Shennan A, Hyett JA, Kapur A, Hadar E, Divakar H, McAuliffe F, da Silva Costa F, von Dadelszen P, McIntyre HD, Kihara AB, Di Renzo GC, Romero R, D’Alton M, Berghella V, Nicolaides KH, Hod M. The international federation of gynecology and obstetrics (FIGO) initiative on pre-eclampsia: a pragmatic guide for first-trimester screening and prevention. Int J Gynaecol Obstet. 2019;145(Suppl 1):1–33. doi: 10.1002/ijgo.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Bao J, Peng M. Effect of magnesium sulfate combined with labetalol on serum sFlt-1/PlGF ratio in patients with early-onset severe pre-eclampsia. Exp Ther Med. 2020;20:276. doi: 10.3892/etm.2020.9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie RH, Guo Y, Krewski D, Mattison D, Walker MC, Nerenberg K, Wen SW. Association between labetalol use for hypertension in pregnancy and adverse infant outcomes. Eur J Obstet Gynecol Reprod Biol. 2014;175:124–128. doi: 10.1016/j.ejogrb.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 27.Khalaf D, Kruger M, Wehland M, Infanger M, Grimm D. The effects of oral l-arginine and l-citrulline supplementation on blood pressure. Nutrients. 2019;11:1679. doi: 10.3390/nu11071679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen YL, Rosa RH Jr, Kuo L, Hein TW. Hyperglycemia augments endothelin-1-induced constriction of human retinal venules. Transl Vis Sci Technol. 2020;9:1. doi: 10.1167/tvst.9.9.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng M, He Y, Li M, Yang L, Zhu Q, Liu J, Mao Y, Chen Q, Du J, Zhou W. Association between maternal pregestational glucose level and adverse pregnancy outcomes: a population-based retrospective cohort study. BMJ Open. 2021;11:e048530. doi: 10.1136/bmjopen-2020-048530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lei T, Qiu T, Liao W, Li K, Lai X, Huang H, Yuan R, Chen L. Proteinuria may be an indicator of adverse pregnancy outcomes in patients with preeclampsia: a retrospective study. Reprod Biol Endocrinol. 2021;19:71. doi: 10.1186/s12958-021-00751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]