Abstract
Objective: To investigate the predictive factors for new fractures in adjacent vertebrae after percutaneous vertebroplasty for osteoporotic vertebral compression fractures, thus providing new insights for clinical practice. Methods: A total of 124 patients were retrospectively included in this study. Based on the presence of new vertebral compression fractures in adjacent vertebrae postoperatively, patients were divided into a non-fracture group and a new-fracture group. Data collected included the amount of bone cement injected into a single vertebral body, postoperative bone cement leakage into the intervertebral disc, the recovery rate of anterior vertebral height, and non-surgical factors such as age, gender, duration of postoperative chest and waist circumference, bone mineral density, the number of preoperative vertebral fractures, and the presence of fissure-like changes in the vertebral body. One-way ANOVA and chi-square tests were used to analyze the correlation between these factors and secondary fractures in adjacent vertebrae. Logistic regression analysis was performed to identify the main risk factors. Results: Univariate analysis found that the amount of bone cement injected, the recovery rate of anterior vertebral height, the duration of chest and waist circumference, and bone mineral density were associated with new fractures in adjacent vertebrae (all P < 0.05). Logistic regression analysis showed that the recovery rate of anterior vertebral height and bone mineral density were the main risk factors for new fractures in adjacent vertebrae. Conclusion: Several factors are associated with new fractures in adjacent vertebrae after percutaneous vertebroplasty for osteoporotic vertebral compression fractures. The recovery rate of anterior vertebral height and bone mineral density are the primary risk factors.
Keywords: New fracture adjacent vertebral, osteoporotic vertebral compression fracture, percutaneous vertebroplasty, related factors
Introduction
In recent years, health issues associated with the aging global population have become increasingly prominent. Osteoporotic vertebral compression fracture (OVCF), a severe complication of senile osteoporosis, has gradually emerged as a public health concern. Percutaneous vertebroplasty (PVP) was first used to treat vertebral hemangiomas by the French physician Galibert in 1984 [1,2]. After years of development, the technique has become more refined and is now widely used in the clinical treatment of OVCF. PVP offers several advantages in treating OVCF, such as a short operative time, minimal intraoperative trauma, rapid pain relief, effective restoration of vertebral height, quick patient rehabilitation, early post-operative mobilization, reduced bed rest complications, and significant improvements in patients’ quality of life [3-5].
The minimally invasive treatment of OVCF via PVP has been widely adopted in most provincial and municipal hospitals across China. However, with the growing number of cases and extended follow-up, it has been observed that some patients experience secondary fractures in adjacent vertebrae and recurrent low back pain. These patients often require additional medical consultations and treatments for the same condition, leading to increased economic and psychological burdens for elderly patients in a short period [6]. Consequently, the occurrence of secondary fractures in adjacent vertebrae after PVP for OVCF has gained increasing attention and research interest from orthopedic specialists and experts in recent years. However, current research findings are often controversial, with no consensus, and some results are even contradictory. In this study, we aim to explore the factors associated with new fractures in adjacent vertebrae following PVP for OVCF.
Materials and methods
Study design
A total of 124 patients with OVCF were treated at Linhai Hospital of Traditional Chinese Medicine from January 2022 to January 2024. The patients were divided into two groups: the new fracture group (n = 61) and the no-fracture group (n = 63). The purpose, process, and role of the study were systematically explained to the patients’ families. The study was approved by the ethics committee of Linhai Hospital of Traditional Chinese Medicine.
Inclusion and exclusion standards
Inclusion criteria: ① Patients were clinically diagnosed with osteoporotic compression fractures of the thoracic/lumbar spine, presenting with kyphosis, severe low back pain, and loss of vertebral height; ② Bone mineral density T-score according to measurements at our hospital before surgery; ③ Preoperative MRI of the thoracolumbar spine showing a recent compression fracture; ④ All patients underwent PVP with a unilateral puncture approach.
Exclusion criteria: ① Compression fractures caused by significant trauma or falls; ② Pathological fractures due to bone tumors or metastatic cancer; ③ Patients with neurological symptoms or spinal cord involvement; ④ History of prior spinal surgery or patients not undergoing their first PVP; ⑤ Patients with severe complications or contraindications; ⑥ Patients who had taken aspirin, statins, or other anticoagulants before surgery; ⑦ Patients unwilling to participate in the study.
Surgical method
The patient was placed in the prone position during the procedure, and the operating table and silicone pads were adjusted for proper positioning. “C-arm” fluoroscopy was used to confirm the location of the vertebral body and its pedicle, which was marked at the surface projection point. Routine disinfection and draping were performed. After successful local anesthesia, a unilateral puncture through the pedicle was performed with “C-arm” fluoroscopy assistance. Once frontal and lateral fluoroscopy confirmed the puncture needle’s position in close to the junction of the anterior one-third and the middle one-third of the vertebral body, toothpaste-like bone cement was injected under fluoroscopic monitoring. The puncture needle was removed after confirming the solidification of the cement, and the skin puncture site was sutured and dressed with a sterile bandage.
During the operation, the patient’s vital signs were closely monitored, and the procedure was paused if any discomfort or inability to continue was reported. After the operation, the patient was monitored in the ward for 24 hours with ECG, and symptoms of the lower limb nervous system were assessed. Routine thoracolumbar X-rays were performed one day postoperatively, and patients were encouraged to mobilize with chest or waist bracing. Postoperative instructions included wearing the brace for at least one month, receiving standardized anti-osteoporosis treatment, and performing appropriate lumbar and back muscle exercises. Follow-up evaluations were scheduled at 3, 6, 12, and 16 months postoperatively.
Primary outcomes
This study analyzed factors that may contribute to recurrent fractures, including gender, age, bone mineral density, changes in the kyphosis angle before and after surgery, the Oswestry Disability Index (ODI), vertebral height restoration rate, preoperative and postoperative Cobb angles, Visual Analog Scale (VAS) scores, and bone cement leakage into the intervertebral disc. Bone mineral density was measured using a dual-energy X-ray absorptiometry system (GK Medical, L’ACN), with the T-score serving as the comparison standard.
The recovery rate of vertebral height was calculated using changes in lateral kyphosis on X-ray films. The measurement method involved identifying the kyphotic region and the transitional vertebrae, drawing vertical lines along the lower and upper edges of these vertebrae, and measuring the acute angle between the two vertical lines. The formula for the kyphosis angle change rate is: (preoperative kyphosis angle - postoperative kyphosis angle)/preoperative kyphosis angle × 100%.
The ODI is commonly used to assess the degree of dysfunction in patients with low back pain [7]. It evaluates how daily activities such as walking, sitting, standing, and sleeping are affected, providing a quantitative measure of disability and functional limitation. Higher ODI scores indicate more severe functional impairment. Pain was assessed using the Visual Analog Scale (VAS) [8], with higher scores representing greater pain severity.
The vertebral height restoration rate was calculated as follows: on lateral lumbar X-rays, the anterior height of the injured vertebra was measured preoperatively (a) and postoperatively (b). The heights of the adjacent vertebrae above and below were recorded as (c) and (d), respectively. The formula for height restoration rate is: (b - a)/[(c + d)/2] × 100%.
Cobb angle measurements were taken preoperatively and postoperatively. The Cobb angle was calculated by drawing parallel lines along the lower edge of the vertebra above the injured vertebra and the upper edge of the vertebra below it. The intersection of the perpendicular lines formed by these parallels was measured as the Cobb angle, with changes before and after surgery used as an evaluation metric.
Sample size estimation
The sample size was calculated using power analysis and estimated as follows: corrected sample size = sample size/(1 - [% attrition/100]) [9]. Ultimately, we determined a sample size of approximately 124. The number of patients in the two groups was obtained through careful screening and statistical analysis of the medical database we used. Specifically, based on the inclusion and exclusion criteria, we selected and classified eligible cases from the database, resulting in 61 cases in the new fracture group and 63 cases in the no fracture group.
Statistical analysis
All data were analyzed using SPSS 25.0 and GraphPad InStat. Numerical data such as age, bone mineral density, and changes in the kyphosis angle were analyzed using an independent sample t-test, with results expressed as mean ± standard deviation (Mean ± SD). Binary logistic regression was applied to analyze categorical data such as bone cement leakage and gender. The statistical significance was set as a bilateral P < 0.05.
Results
Clinical data
Between January 2022 and January 2024, 124 patients were included in this study. There were no statistically significant differences between the two groups in terms of age, sex, body mass index (BMI), smoking status, diabetes, hypertension, hyperlipidemia, coronary heart disease, exercise habits, and chronic obstructive pulmonary disease before intervention (all P > 0.05) (Table 1).
Table 1.
Comparison of clinical data between the two groups
| New fracture group (n = 61) | No fracture group (n = 63) | t/χ2 | P | |
|---|---|---|---|---|
| Age (years) | 74.66±3.75 | 74.62±6.89 | 0.036 | 0.971 |
| Sex | 0.342 | 0.559 | ||
| Male (n%) | 38 (62.3%) | 36 (57.1%) | ||
| Female (n%) | 23 (37.7%) | 27 (42.9%) | ||
| BMI | 22.28±1.40 | 22.10±1.45 | 0.833 | 0.407 |
| Smoking | 37 (60.7%) | 39 (61.9%) | 0.020 | 0.886 |
| Diabetes | 23 (37.7%) | 20 (31.7%) | 0.486 | 0.486 |
| Hypertension | 18 (29.5%) | 17 (27%) | 0.097 | 0.755 |
| Hyperlipidemia | 8 (13.1%) | 9 (14.3%) | 0.036 | 0.850 |
| Coronary heart disease | 6 (9.8%) | 7 (11.1%) | 0.054 | 0.817 |
| Chronic obstructive pulmonary disease | 7 (11.5%) | 7 (11.1%) | 0.004 | 0.949 |
| Exercise habits | 18 (29.5%) | 16 (25.40%) | 0.263 | 0.608 |
Note: BMI: body mass index.
Comparison of biochemical indicators between the two groups
The hemoglobin level in the new fracture group was (11.71±0.73) g/dl, while in the no fracture group it was (11.98±0.96) g/dl (P = 0.085). The hematocrit in the new fracture group was (35.37±1.98)%, and in the no fracture group it was (35.52±2.97)%, showing no significant difference (P = 0.084). The albumin level in the new fracture group was (3.71±0.18) g/dl, and in the no fracture group, it was (3.65±0.18) g/dl, (P = 0.057). Cholesterol levels in the new fracture group were (158.64±26.58) mg/dl, while in the no fracture group they were (162.87±22.07) mg/dl (P = 0.340) (Table 2).
Table 2.
Comparison of biochemical indicators between the two groups
| New fracture group (n = 61) | No fracture group (n = 63) | t | P | |
|---|---|---|---|---|
| Hemoglobin (g/dl) | 11.71±0.73 | 11.98±0.96 | -1.734 | 0.085 |
| Hematocrit (%) | 35.37±1.98 | 35.52±2.97 | -1.742 | 0.084 |
| Albumin (g/dl) | 3.71±0.18 | 3.65±0.18 | 1.924 | 0.057 |
| Cholesterol (mg/dl) | 158.64±26.58 | 162.87±22.07 | -0.957 | 0.340 |
Comparison of surgery factors between the two groups
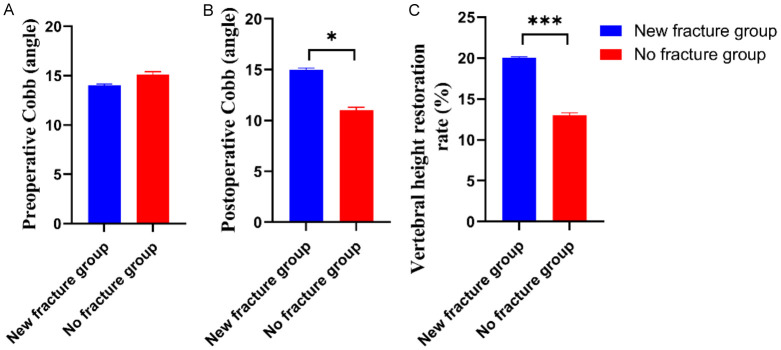
Compared with the no fracture group, the new fracture group showed a significant increase in the postoperative Cobb angle and the recovery rate of vertebral body height, with statistically significant differences (all P < 0.05) (Figure 1).
Figure 1.
Comparison of surgery factors between the two groups. A: Preoperative Cobb; B: Postoperative Cobb; C: Vertebral height restoration rate. Compared to control group, *P < 0.05, ***P < 0.001.
Comparison of VAS score an ODI function index between the two groups
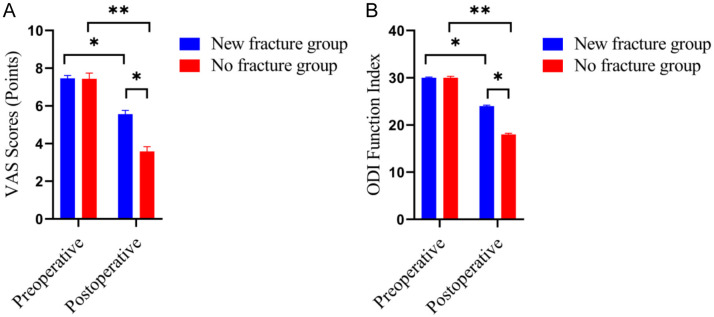
Compared with the no fracture group, the VAS score and ODI Function Index were significantly higher in the new fracture group, with statistically significant differences (both P < 0.05) (Figure 2).
Figure 2.
Comparison of VAS score an ODI Function Index between the two groups. A: VAS score; B: ODI Function Index. Compared to control group, *P < 0.05, **P < 0.01.
Binary analysis of factors associated with new fractures of adjacent vertebrae
Compared with the no fracture group, there were significant differences in the amount of bone cement injected into a single vertebral body (P = 0.042), recovery rate of anterior vertebral height (P = 0.021), postoperative chest and waist brace wearing time (P = 0.001), and bone mineral density (BMD) (P = 0.042). However, there were no significant differences between the two groups in terms of the number of preoperative vertebral fractures (P = 0.332) or the presence of fissure-like degeneration in the vertebral body (P = 0.591) (Table 3).
Table 3.
Binary logistic regression analysis
| New fracture group (n = 61) | No fracture group (n = 63) | t/χ2 | P | |
|---|---|---|---|---|
| Amount of bone cement injected into a single vertebral body (ml) | 5.66±0.85 | 8.95±0.89 | 3.251 | 0.042 |
| Recovery rate of anterior vertebral height (%) | 81.77±9.22 | 85.2±9.98 | 4.282 | 0.021 |
| Time of wearing chest waist circumference after operation (days) | 38.97±9.96 | 22.66±8.32 | 8.472 | 0.001 |
| Bone density (SD) | -3.32±0.66 | -3.83±0.69 | 2.431 | 0.042 |
| Number of preoperative vertebral fractures | 1.45±0.28 | 1.99±0.76 | 1.291 | 0 .332 |
| With fissure like degeneration in vertebral body | 37 (60.7%) | 39 (61.9%) | 2.962 | 0.591 |
Multivariate analysis of factors associated with new fractures of adjacent vertebrae
As shown in Table 4, multivariate logistic regression analysis revealed that the recovery rate of anterior vertebral height (P = 0.0001) and bone density (P < 0.001) are independent prognostic factors for new fractures of adjacent vertebral bodies.
Table 4.
Multivariate analysis of related factors of new fractures of adjacent vertebral bodies
| Dependent variables | Independent variables | B | SE | β | P Value |
|---|---|---|---|---|---|
| New fractures of adjacent vertebral bodies | Recovery rate of anterior vertebral height | 0.203 | 0.053 | 0.333 | 0.001 |
| Bone density | 1.288 | 0.394 | 0.284 | < 0.001 |
Note: B: nonstandard regression coefficient; SE: standard error; b: standardized regression coefficient; β: multiple correlation coefficient adjusted for the degrees of freedom.
Discussion
OVCF predominantly occurs in elderly patients. Given the growing elderly population in China, the related issues of OVCF are increasingly important and merit further study. The current treatment options for OVCF include conservative bed rest, open surgery, and minimally invasive procedures such as PVP [10,11]. Elderly patients with OVCF often have multiple comorbidities, and inadequate family care can lead to secondary complications from prolonged bed rest. This limits the effectiveness of conservative treatments, potentially harming the patients’ health. In open surgery, due to the severe osteoporosis present in OVCF patients, complications such as implant loosening, fractures, and slippage frequently occur, resulting in suboptimal surgical outcomes [12,13].
PVP is a minimally invasive procedure that involves the injection of polymethylmethacrylate into the compressed vertebrae under C-arm guidance, helping to restore vertebral height, alleviate pain, and enable elderly patients to recover quickly and become mobile sooner, thus reducing the risk of bed rest complications [14,15]. PVP also offers the advantages of minimal trauma and a short operative time, which effectively reduces the surgical risk for elderly patients [16]. Due to these benefits, PVP has gained widespread clinical acceptance both domestically and internationally over the past 20 years. However, with the increasing use of PVP, there have been more reports of secondary fractures in adjacent vertebrae after surgery, prompting many researchers to investigate this issue [17].
After analyzing the factors associated with PVP in the treatment of new fractures of adjacent vertebrae following OVCF, we classified the related risk factors into surgical and non-surgical factors.
In PVP surgery, the amount of bone cement injected has always been a critical concern. One study found that a higher percentage of bone cement injected during surgery increased the likelihood of postoperative adjacent vertebral fractures by comparing patients who experienced secondary fractures after PVP with those who did not [18]. Another retrospective study similarly concluded that the more bone cement injected into the injured vertebra, the higher the risk of adjacent vertebral fractures [19]. In our study, the average amount of bone cement injected into a single vertebra in the non-fracture group was 5.67 mL, while in the secondary fracture group, it was 5.97 mL. There was a significant difference between the two groups in the univariate analysis. Thus, we believe that the amount of bone cement injected into a single vertebra can influence the likelihood of adjacent vertebral fractures after PVP.
Cement leakage into the intervertebral disc is a common complication after PVP. Some studies suggest that such leakage increases the risk of adjacent vertebral fractures [20]. The intervertebral disc plays an essential role in vertebral structure by buffering stress. Leakage of bone cement into the disc alters the surrounding environment, accelerating disc degeneration, weakening its stress-buffering capacity, and increasing the load on adjacent vertebrae, thus raising the risk of secondary fractures [21]. However, other researchers argue that disc leakage is not a significant factor in adjacent vertebral fractures after PVP [22-24]. We believe that discrepancies in these findings may be due to small sample sizes, measurement errors, or other factors. Further studies are needed to determine whether bone cement leakage into the intervertebral disc affects the occurrence of secondary fractures and to what extent.
One of the primary therapeutic effects of PVP in treating OVCF is restoring the height of the injured vertebrae [25]. In our study, both univariate and logistic regression analyses identified the recovery rate of anterior vertebral height as a major risk factor for secondary fractures of adjacent vertebrae after PVP, which should not be overlooked. Therefore, we believe that moderate restoration of vertebral height during PVP can achieve the desired clinical outcome, consistent with the findings of Bo et al. [26]. There is no need to fully restore the vertebral height to its original state or aim for optimal imaging results.
The use of chest and waist braces after surgery is an important protective and supportive measure in spinal procedures [27,28]. Our results also indicate that the duration of postoperative chest and waist brace use is a factor influencing secondary fractures of adjacent vertebrae. However, due to the sample size, differences in individual living habits, and regional variability, there is no universally accepted and reliable conclusion regarding the impact of brace duration on adjacent vertebral fractures after PVP [29].
OVCF is the most common complication of osteoporosis [30]. While PVP treatment can significantly alleviate the clinical symptoms of OVCF, it does not imply a cure for osteoporosis. Surgery cannot improve BMD or halt the progression of osteoporosis. Our study confirmed this point [31]. The recorded data showed that patients with new fractures in adjacent vertebrae had significantly lower BMD than those in the non-fracture group. Both univariate and logistic regression analyses identified low BMD as a major risk factor for new fractures in adjacent vertebrae after PVP. Therefore, BMD is closely related to the occurrence of postoperative adjacent vertebral fractures. This finding underscores the importance of standard anti-osteoporosis treatment alongside surgical intervention for patients with OVCF.
This study has a few limitations. First, it is a retrospective case analysis, with certain shortcomings such as a small sample size, limited follow-up period, and incomplete follow-up data. Additionally, the researchers’ time and resources were limited, which prevented the study from including and analyzing other potentially relevant factors, such as patient lifestyle choices that might influence the results. In the future, prospective randomized controlled trials or biomechanical models should be designed to explore potential risk factors affecting secondary fractures of adjacent vertebrae after PVP, providing more practical guidance for clinical practice and postoperative fracture prevention.
In conclusion, factors influencing new adjacent vertebral fractures after PVP for OVCF include the amount of bone cement injected into a single vertebra, recovery rate of anterior vertebral height, duration of postoperative chest and waist brace use, and BMD. The recovery rate of anterior vertebral height and BMD are the primary risk factors.
Disclosure of conflict of interest
None.
References
- 1.Ji C, Rong Y, Wang J, Yu S, Yin G, Fan J, Tang P, Jiang D, Liu W, Gong F, Ge X, Cai W. Risk factors for refracture following primary osteoporotic vertebral compression fractures. Pain Physician. 2021;24:E335–E340. [PubMed] [Google Scholar]
- 2.Ko S, Jun C, Nam J. Effects of vitamin D supplementation on the functional outcome in patients with osteoporotic vertebral compression fracture and vitamin D deficiency. J Orthop Surg Res. 2021;16:571. doi: 10.1186/s13018-021-02717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao QL, Hou KP, Wu ZX, Xiao L, Xu HG. Full-endoscopic spine surgery treatment of lumbar foraminal stenosis after osteoporotic vertebral compression fractures: a case report. World J Clin Cases. 2022;10:656–662. doi: 10.12998/wjcc.v10.i2.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee BJ, Koo HW, Yoon SW, Sohn MJ. Usefulness of trabecular CT attenuation measurement of lumbar spine in predicting osteoporotic compression fracture: is the l4 trabecular region of interest most relevant? Spine (Phila Pa 1976) 2021;46:175–183. doi: 10.1097/BRS.0000000000003756. [DOI] [PubMed] [Google Scholar]
- 5.Krüger A, Bäumlein M, Knauf T, Pascal-Moussellard H, Ruchholtz S, Oberkircher L. Height and volume restoration in osteoporotic vertebral compression fractures: a biomechanical comparison of standard balloon kyphoplasty versus Tektona® in a cadaveric fracture model. BMC Musculoskelet Disord. 2021;22:76. doi: 10.1186/s12891-020-03899-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Che M, Wang Y, Zhao Y, Zhang S, Yu J, Gong W, Zhang D, Liu M. Finite element analysis of a new type of spinal protection device for the prevention and treatment of osteoporotic vertebral compression fractures. Orthop Surg. 2022;14:577–586. doi: 10.1111/os.13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo J, Zhai W, Wei L, Zhang J, Jin L, Yan H, Huang Z, Jia Y. Radiological and clinical outcomes of balloon kyphoplasty for osteoporotic vertebral compression fracture in patients with rheumatoid arthritis. J Orthop Surg Res. 2021;16:435. doi: 10.1186/s13018-021-02573-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JS, Park YS. Survival analysis and risk factors of new vertebral fracture after vertebroplasty for osteoporotic vertebral compression fracture. Spine J. 2021;21:1355–1361. doi: 10.1016/j.spinee.2021.04.022. [DOI] [PubMed] [Google Scholar]
- 9.Pan C, Liu X, Li T, Wang G, Sun J. Kinetic of bone turnover markers after osteoporotic vertebral compression fractures in postmenopausal female. J Orthop Surg Res. 2018;13:314. doi: 10.1186/s13018-018-1025-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfeifle C, Kohut P, Jarvers JS, Spiegl UJ, Heyde CE, Osterhoff G. Does time-to-surgery affect mortality in patients with acute osteoporotic vertebral compression fractures? BMC Geriatr. 2021;21:714. doi: 10.1186/s12877-021-02682-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu K, Li YL, Xiao SH. Vesselplasty versus vertebroplasty in the treatment of osteoporotic vertebral compression fractures with posterior wall rupture. J Int Med Res. 2021;49:3000605211066303. doi: 10.1177/03000605211066303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eguchi Y, Toyoguchi T, Orita S, Shimazu K, Inage K, Fujimoto K, Suzuki M, Norimoto M, Umimura T, Shiga Y, Inoue M, Koda M, Furuya T, Maki S, Hirosawa N, Aoki Y, Nakamura J, Hagiwara S, Akazawa T, Takahashi H, Takahashi K, Shiko Y, Kawasaki Y, Ohtori S. Reduced leg muscle mass and lower grip strength in women are associated with osteoporotic vertebral compression fractures. Arch Osteoporos. 2019;14:112. doi: 10.1007/s11657-019-0668-0. [DOI] [PubMed] [Google Scholar]
- 13.Zuo XH, Chen YB, Xie P, Zhang WD, Xue XY, Zhang QX, Shan B, Zhang XB, Bao HG, Si YN. Finite element analysis of wedge and biconcave deformity in four different height restoration after augmentation of osteoporotic vertebral compression fractures. J Orthop Surg Res. 2021;16:138. doi: 10.1186/s13018-021-02225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Q, Long X, Wang Y, Fang X, Guo D, Lv J, Hu X, Cai L. Development and validation of a nomogram for predicting the probability of new vertebral compression fractures after vertebral augmentation of osteoporotic vertebral compression fractures. BMC Musculoskelet Disord. 2021;22:957. doi: 10.1186/s12891-021-04845-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mao W, Dong F, Huang G, He P, Chen H, Qin S, Li A. Risk factors for secondary fractures to percutaneous vertebroplasty for osteoporotic vertebral compression fractures: a systematic review. J Orthop Surg Res. 2021;16:644. doi: 10.1186/s13018-021-02722-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakazawa F. Subcutaneous edema on back detected by MRI in hospitalized patients with osteoporotic vertebral compression fracture. J Orthop. 2021;28:67–69. doi: 10.1016/j.jor.2021.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang B, Xu S, Chen X, Cui L, Wang Y, Yan X, Liu Y. The impact of intravertebral cleft on cement leakage in percutaneous vertebroplasty for osteoporotic vertebral compression fractures: a case-control study. BMC Musculoskelet Disord. 2021;22:805. doi: 10.1186/s12891-021-04685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai C, Liang G, Zhang Y, Dong Y, Zhou X. Risk factors of vertebral re-fracture after PVP or PKP for osteoporotic vertebral compression fractures, especially in Eastern Asia: a systematic review and meta-analysis. J Orthop Surg Res. 2022;17:161. doi: 10.1186/s13018-022-03038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Wang Y, Wang R, Yue L, Chen S, Li C. Effects of rosuvastatin and zoledronic acid in combination on the recovery of senile osteoporotic vertebral compression fracture following percutaneous vertebroplasty. J Int Med Res. 2020;48:300060520925390. doi: 10.1177/0300060520925390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang ZL, Yang JS, Hao DJ, Liu TJ, Jing QM. Risk factors for new vertebral fracture after percutaneous vertebroplasty for osteoporotic vertebral compression fractures. Clin Interv Aging. 2021;16:1193–1200. doi: 10.2147/CIA.S312623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tucci M, Wilson GA, McGuire R, Benghuzzi HA. The effects of NPY1 receptor antagonism on intervertebral disc and bone changes in ovariectomized rats. Global Spine J. 2021;11:1166–1175. doi: 10.1177/2192568220939908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang M, Li B, Wang Y, Jiang S, Wen G, Jiang L, Zheng X. The effects of bone cement volume in percutaneous vertebroplasty for thoracolumbar junction vertebral compression fractures: a clinical comparative study. Mediators Inflamm. 2022;2022:4230065. doi: 10.1155/2022/4230065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou C, Huang S, Liao Y, Chen H, Zhang Y, Li H, Zhu Z, Wang Y. Correlation analysis of larger side bone cement volume/vertebral body volume ratio with adjacent vertebral compression fractures during vertebroplasty. Front Endocrinol (Lausanne) 2023;14:1072087. doi: 10.3389/fendo.2023.1072087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu D, Zhang H, Fan X. Robot-assisted percutaneous vertebroplasty for osteoporotic vertebral compression fracture treatment and risk factor screening for postoperative refracture. J Robot Surg. 2024;18:23. doi: 10.1007/s11701-023-01776-8. [DOI] [PubMed] [Google Scholar]
- 25.Zhou C, Liao Y, Huang S, Li H, Zhu Z, Zheng L, Wang B, Wang Y. Effect of cement distribution type on clinical outcome after percutaneous vertebroplasty for osteoporotic vertebral compression fractures in the aging population. Front Surg. 2022;9:975832. doi: 10.3389/fsurg.2022.975832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bo J, Zhao X, Hua Z, Li J, Qi X, Shen Y. Impact of sarcopenia and sagittal parameters on the residual back pain after percutaneous vertebroplasty in patients with osteoporotic vertebral compression fracture. J Orthop Surg Res. 2022;17:111. doi: 10.1186/s13018-022-03009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xuan J, Ke B, Ma W, Liang Y, Hu W. Spinal disease diagnosis assistant based on MRI images using deep transfer learning methods. Front Public Health. 2023;11:1044525. doi: 10.3389/fpubh.2023.1044525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Catz A, Watts Y, Amir H, Front L, Gelernter I, Michaeli D, Bluvshtein V, Aidinoff E. The role of comprehensive rehabilitation in the care of degenerative cervical myelopathy. Spinal Cord. 2024;62:200–206. doi: 10.1038/s41393-024-00965-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xue Y, Zhang J, Zhang Z, Dai W, Ma C. Clinical outcomes with second injection after insufficient bone cement distribution in unilateral kyphoplasty for osteoporotic vertebral compressive fracture: a cohort retrospective study. J Orthop Surg Res. 2023;18:530. doi: 10.1186/s13018-023-03968-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Huang X, Zhong G. Evaluation of efficacy and safety of vertebroplasty in the treatment of osteoporotic vertebral compression fracture: a protocol for systematic review and meta-analysis. Medicine (Baltimore) 2021;100:e27408. doi: 10.1097/MD.0000000000027408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu C, Gillis A, Sidani M, Mcleod C, Fazendin J, Chen H, Ramonell K, Lindeman B. Thresholds for surgical referral in primary hyperparathyroidism: a conjoint analysis. Am J Surg. 2023;226:640–645. doi: 10.1016/j.amjsurg.2023.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]