Abstract
Objective: To evaluate the clinical efficacy of hysteroscopy in the treatment of molar pregnancy and postoperative residual tissue. Methods: This retrospective study involved 68 patients who underwent treatment for molar pregnancy in Shanxi Children’s Hospital Shanxi Maternal and Child Health Hospital from April 2020 to May 2022. Based on intervention methods, patients were divided into a conventional group (n=33) and a hysteroscopy group (n=35). The effects of different treatment methods on perioperative outcomes, prognosis, serum factor levels and complication rates were compared between the two groups. Results: The comparison of general data, including age, gestational times, the number of patients with a cleared uterus, and the time interval from the last menstruation to hysteroscopic surgery, showed no significant differences between the two groups (all P > 0.05). The operation duration and menstrual recovery time of the hysteroscopy group were significantly shorter than those of the conventional group, and the intraoperative blood loss was significantly less than that in the conventional group (all P < 0.05). After surgery, endometrial thickness in hysteroscopy group was significantly thinner than that in conventional group (P < 0.05), though there was no significant difference in uterine adhesion rates (P > 0.05). Before surgery, there was no significant difference in serum factor levels between the two groups (P > 0.05). After surgery, the levels of β-human chorionic gonadotropin (β-HCG), creatine kinase (CK), trend factor interleukin-10 (CXCL10), and placental growth factor (PlGF) in the hysteroscopic group were all lower than those in the conventional group (all P < 0.05), while pregnancy-associated plasma protein-A (PAPP-A) was higher. The cure rate in the hysteroscopy group was significantly higher, while the total incidence of complications was significantly lower than those in the conventional group (all P < 0.05). Conclusion: Hysteroscopic treatment for molar pregnancy effectively removes lesions, reduces the treatment load, and provides the potential to preserve fertility function.
Keywords: Mole, postoperative residual, hysteroscopy
Introduction
A hydatidiform mole, also known as molar pregnancy, is a benign lesion that originates from placental villi and is the most common gestational trophoblastic disease [1]. It is characterized by the hyperplasia of placental villous trophoblasts and interstitial edema during pregnancy, which leads to the formation of clusters of fluid-filled vesicles resembling grapes, connected by pedicles. This condition is also known as a vesicular mole [2]. Statistics show that the risk of having hydatidiform moles in Europe is about 1.0-2.2 cases per 1,000 pregnancies, with higher rates reported in other regions [3,4]. From a molecular perspective, the hydatidiform mole may be associated with a truncated homozygous variant of the KHDC3L gene, which can increase the risk of recurrent miscarriage and impair fertility [5]. Despite advancements, there remains room for improvement in the diagnosis and treatment of hydatidiform moles. Continued research in this area could optimize patient management and help preserve fertility, which holds significant clinical value.
Once the diagnosis of hydatidiform mole is established, uterine aspiration is the preferred treatment. However, compared with non-trophoblastic diseases, hydatidiform moles, while benign, are characterized by the trophoblast’s strong reproductive capacity, active growth, aggressive pathology, and inherent potential for malignancy [6,7]. Therefore, patients with hydatidiform moles often require multiple curettage. Traditional methods for removing intrauterine residual tissues after curettage, while effective to some extent, are performed without direct visualization, making it difficult to identify atypical cases promptly, thus leading to missed diagnosis or excessive treatment [8-10]. Therefore, identifying an optimal treatment approach is crucial for improving the therapeutic outcome and prognosis of these patients.
Hysteroscopy is widely used for removing residual tissues in gynecology [11]. However, concerns regarding the potential of hysteroscopy to promote cell metastasis remain [12]. There are few reports on the application of hysteroscopy for the curettage of hydatidiform moles and the treatment of intrauterine residuals. This study aims to address this gap by analyzing the clinical data of patients who underwent hysteroscopic treatment for hydatidiform moles, to evaluate the value of hysteroscopy in removing residual molar tissue. This investigation seeks to provide an optimized approach for the treatment of hydatidiform moles, representing the innovative aspect of the study. The following report presents the findings of this analysis.
General material and methods
General material
This retrospective study involved 68 patients who underwent curettage of hydatidiform moles at Shanxi Children’s Hospital Shanxi Maternal and Child Health Hospital from April 2020 to May 2022. Based on the intervention method received, the patients were divided into a conventional group (n=33) and a hysteroscopy group (n=35). This study was approved by the Ethics Committee of Shanxi Children’s Hospital Shanxi Maternal and Child Health Hospital. Patient information was collected anonymously, and a flowchart of patient selection is shown in Figure 1.
Figure 1.
Flowchart of patient selection.
Criteria of inclusion and exclusion
Inclusion criteria: (1) Diagnosis of postoperative embryonic tissue residue with a localized lesion in the uterine cavity that was confirmed by pathology or clinical examination; (2) Clear history of intrauterine pregnancy and abortion; (3) Postpartum or post-abortion vaginal bleeding lasting ≥ 15 d; (4) Presence of increased serum human chorionic gonadotropin (HCG) level following an initial decrease, with persistent abnormality (> 5 IU/L), or space-occupying lesion in the uterine cavity indicated by imaging despite normal serum HCG level; (5) Consistent with surgical indications; (6) Complete Medical records.
Exclusion criteria: (1) Patients with other uterine cavity diseases or a history of other uterine cavity operations; (2) Presence of acute or chronic infections of the reproductive system or other infectious diseases; (3) Presence of hysteromyoma or malignant tumor; (4) Abnormal coagulation function; (5) Severe dysfunction or insufficiency of the heart, pancreas, liver and kidney and other important organs; (6) Cognitive impairment or abnormal mental behavior.
Methods
Routine intervention
Patients in both groups underwent preoperative fasting, abstained from drinking, and received electrocardiogram (ECG). Serum β-chorionic gonadotropin (β-HCG) level was measured one day before surgery and reexamined 24 hours after surgery. Postoperative treatment included oral Chinese medicine to promote uterine contraction. A pelvic ultrasound was performed 1-2 weeks after surgery to assess the uterine condition and rule out any recurrence of intrauterine residue.
Conventional group
In the conventional group, painless curettage was performed under general anesthesia, with routine oxygen inhalation and ECG monitoring. The patients were placed in bladder lithotomy position, and the orifice of the uterus was dilated using the orifice dilator No.5-7.5 to align the uterus horizontally. A negative pressure drainage probe was slowly inserted to remove the residual tissue in the uterine cavity guided by ultrasound with the probe frequency set at 3.5 MHz. If the cavity was not fully cleared, a small curette was used until a B-scan confirmed that no residual tissue remained. Postoperatively, gauze was used to filter and remove tissue, which was then observed for decidual remnants.
Hysteroscopic group
In the hysteroscopy group, patients received an intravenous injection of 80 mg phloroglucinol 0.5 h before surgery to relax the cervix. An intravenous channel was established, and hysteroscopy was performed using both a diagnostic and electrosurgical hysteroscope. The entire procedure was monitored continuously using bedside B ultrasonography. Anesthesia was administer intravenously with patients placed in a bladder lithotomy position, along with routine oxygen inhalation and ECG monitoring. Once anesthesia was effective, the surgical site was disinfected. Uterine distension was achieved using 5% glucose or normal saline at a flow rate of 200 mL/min, with intrauterine pressure maintained at or below 9.31 kPa. The drainage pipe was opened, and air bubbles were removed from the irrigation system before the endoscope was inserted. The cervical canal was sterilized with cotton swabs, and the hysteroscopy was guided by B ultrasound monitoring.
1. First curettage: The duration of hysteroscopic curettage was controlled within 30 s, and the output and input volumes were maintained below 1500 ml. The hysteroscope was used to observe the morphology of uterine cavity, endometrial thickness, bilateral fallopian tube openings and uterine mucosa, and any lesions. After dilatation of the uterus, the location of residual embryo tissue was determined by B-ultrasound monitoring, which was subsequently excised from the base using a ring-shaped electrode, and electrocoagulation was performed to stop bleeding. Alternatively, the intrauterine pregnancy remnants were suctioned out by negative pressure, and the uterine walls were palpated for roughness. A detailed observation under the microscope was conducted to assess the morphology, texture and peripheral blood supply of uterus to confirm the absence of residual tissue. The surgical area was sutured routinely, and the procedure was completed.
2. Re-curettage: The uterine cavity was explored locate any residual embryonic tissue. If residual tissue was found in the cavity or adhered to the uterine wall, an oval forceps or a spatula was used to scrape the tissue. If it was difficult to remove, electrosurgical techniques were employed, and the uterine cavity was inspected repeatedly until the inner wall was confirmed clean. If the lesion was confined to the muscular layer with a smooth intima and abundant blood flow, it was left treated. If the lesion involved the muscular layer, any protruding tissue was excised and sent for pathological examination. The operator strictly followed the hysteroscopy procedures and technical specifications.
Venous blood collection
The venous blood (1.8 mL) was drawn from the antecubital vein and stored in a test tube. The samples were centrifuged at 3000 r/min for 10 min. Serum from the upper layer was collected and stored at -20°C for subsequent examination.
Serum factor levels
Enzyme-linked immunosorbent assay (ELISA) was used to detect the levels of β-HCG, serum creatine kinase (CK), trend factor interleukin-10 (CXCL10), pregnancy-associated plasma protein-A (PAPP-A), and placental growth factor (PLGF) (SenBeiJia Biological Technology Co., Ltd., SBJ-H0993, SBJ-H1356, SBJ-H1608; AmyJet Scientific Inc., CEK1124, 900-K307). After allowing the serum samples to reach room temperature, standard substances were prepared, and the samples were diluted at a ratio of 1:2. A volume of 100 μL of both the serum samples and standard substances was added to the respective wells. Following sample addition, the enzyme-labeled plate was covered with a membrane and incubated at 37°C for 2 hours. The reaction plate was washed three times. An antibody working solution (1:100 times dilution) was added in at 100 μL/well and placed in a 37°C incubator for 30 min. The reaction plate was washed four times. TMB solution was added to the reaction wells at a volume of 100 μL/well and incubated at 37°C for 45 min. The stop solution was added into the reaction well at 100 μL/well. The absorbance (A) value was measured at the wavelength of 450 nm. The intensity of the color reaction was proportional to the serum factor level, which was calculated by drawing the standard curve.
Observational index
(1) The perioperative indicators compared between the two groups included operation time, intraoperative bleeding volume and menstrual recovery time.
(2) The endometrial thickness and intrauterine adhesions were compared between the two groups at 1, 2, and 3 months after surgery.
(3) Serum indexes, including β-HCG, CK, CXCL10, PAPP-A, and PLGF, were compared between the two groups before operation and 1 and 3 months after operation.
(4) The one-time cure rate was compared between the two groups, defined as no residual tissue observed on B ultrasound 14 days post-surgery, menstruation resuming within 35-42 days after operation, and cessation of vaginal bleeding within 10 days after operation.
(5) The incidence of complications, including embryo aspiration, intrauterine residue, intrauterine effusion, genital and urinary tract infection was compared between the two groups.
The primary outcome measures were operation time, intraoperative blood loss, menstrual recovery time, endometrial thickness, intrauterine adhesions, one-time cure rate, and the postoperative complication rate. The secondary outcome measures were serum levels of β-HCG, CK, CXCL10, PAPP-A, and PLGF.
Statistical analysis
SPSS 23.0 statistical software was used for data analysis. The measurement data conforming to a normal distribution were expressed as mean standard ± deviation (x̅±s), and t test was used for comparison between groups. Enumeration data were expressed as number (N) or percentage (%) and were compared using χ2 test. The difference was statistically significant when P < 0.05.
Results
Comparison of general information between the two groups
General data such as age, number of pregnancies, number of previous curettage, and the time interval from the last menstrual period to hysteroscopic surgery were compared between the two groups, and the results showed no statistical differences (all P > 0.05), indicating that the two groups were comparable, as shown in Table 1.
Table 1.
Comparison of baseline data between the two groups (x̅±s)
| Group | Conventional group (n=33) | Hysteroscopy group (n=35) | t | P |
|---|---|---|---|---|
| Age (years) | 31.16±3.02 | 31.35±2.96 | 0.262 | 0.794 |
| Number of pregnancies | 2.01±0.24 | 2.05±0.37 | 0.525 | 0.601 |
| Number of previous curettages | 1.65±0.72 | 1.47±0.66 | 0.286 | 1.076 |
| Time interval from the last menstrual period to hysteroscopic surgery (d) | 94.61±23.58 | 93.34±23.19 | 0.824 | 0.224 |
Comparison of perioperative indexes between the two groups
The operation time and menstrual recovery time of the hysteroscopy group were significantly shorter than those of the conventional group, and the intraoperative blood loss was significantly lower (all P < 0.05), as shown in Table 2.
Table 2.
Comparison of perioperative indexes between the two groups (x̅±s)
| Group | Conventional group (n=33) | Hysteroscopy group (n=35) | t | P |
|---|---|---|---|---|
| Operation time (min) | 38.24±4.37 | 23.50±4.16 | 14.250 | < 0.001 |
| Intraoperative bleeding volume (mL) | 79.15±6.71 | 20.99±2.83 | 47.048 | < 0.001 |
| Menstrual recovery time (d) | 34.02±3.43 | 25.61±3.27 | 10.351 | < 0.001 |
Comparison of postoperative endometrial thickness and intrauterine adhesion rate between the two groups
After the operation, the endometrial thickness of patients in the hysteroscopy group was significantly thinner than that of the conventional group (P < 0.05), while the difference in the intrauterine adhesion rate between the two groups was not statistically significant (P > 0.05), as shown in Table 3.
Table 3.
Comparison of postoperative endometrial thickness and intrauterine adhesion rate (x̅±s, %)
| Group | Conventional group (n=33) | Hysteroscopy group (n=35) | t | P | |
|---|---|---|---|---|---|
| Endometrial thickness (mm) | 1 month after operation | 5.24±0.37 | 4.35±0.16 | 13.004 | < 0.001 |
| 2 months after operation | 4.38±0.25a | 3.12±0.18a | 23.954 | < 0.001 | |
| 3 months after operation | 3.62±0.14a,b | 2.15±0.07a,b | 55.242 | < 0.001 | |
| Intrauterine adhesion rate (%) | 1 month after operation | 2 (6.06) | 1 (2.86) | 0.413 | 0.520 |
| 2 months after operation | 4 (12.12)a | 2 (5.71)a | 0.867 | 0.352 | |
| 3 months after operation | 5 (15.15)a,b | 3 (8.57)a,b | 0.708 | 0.400 | |
Note: intra-group comparison with the 1 month after operation;
P < 0.05;
intra-group comparison with 2 month after operation;
P < 0.05.
Comparison of serum factors between two groups before and after operation
Before the operation, there was no significant difference in serum levels of β-HCG, CK, CXCL10, PLGF, and PAPP-A between the two groups (all P > 0.05). After operation, the levels of β-HCG, CK, CXCL10, and PLGF in the hysteroscope group were significantly lower, while the PAPP-A level was significantly higher than those in the conventional group (all P < 0.05), as shown in Table 4.
Table 4.
Comparison of serum factor levels between the two groups before and after operation (x̅±s)
| Group | Conventional group (n=33) | Hysteroscopy group (n=35) | t | P | ||
|---|---|---|---|---|---|---|
| β-HCG (IU/L) | Before operation | 205.34±11.43 | 207.35±14.10 | 0.643 | 0.522 | |
| After operation | 1 month | 96.09±20.74a | 55.13±18.69a | 8.564 | 0.000 | |
| 3 months | 5.71±0.24a,b | 3.29±0.13a,b | 52.109 | 0.000 | ||
| CK (μg/mL) | Before operation | 172.84±9.46 | 172.56±9.34 | 0.123 | 0.903 | |
| After operation | 1 month | 60.41±2.87a | 49.37±2.92a | 15.712 | 0.000 | |
| 3 months | 38.71±1.43a,b | 26.37±1.35a,b | 36.605 | 0.000 | ||
| CXCL10 (ng/mL) | Before operation | 98.56±6.27 | 97.90±6.15 | 0.438 | 0.663 | |
| After operation | 1 month | 76.61±4.23a | 63.70±3.70a | 13.416 | 0.000 | |
| 3 months | 49.78±1.68a,b | 40.05±1.46a,b | 25.533 | 0.000 | ||
| PAPP-A (ng/mL) | Before operation | 20.15±2.93 | 19.60±3.71 | 0.676 | 0.502 | |
| After operation | 1 month | 23.30±4.36a | 25.17±2.61a | 2.160 | 0.034 | |
| 3 months | 24.85±3.73a,b | 26.80±2.84a,b | 2.434 | 0.018 | ||
| PLGF (ng/L) | Before operation | 198.12±16.75 | 203.91±20.98 | 1.253 | 0.215 | |
| After operation | 1 month | 167.39±16.29a | 152.80±15.04a | 3.840 | < 0.001 | |
| 3 months | 147.52±17.40a,b | 133.31±14.81a,b | 3.633 | < 0.001 | ||
Note: intra-group comparison with preoperative level;
P < 0.05.
intra-group comparison with postoperative 1 month;
P < 0.05.
HCG, Human chorionic gonadotropin; CK, creatine kinase; CXCL10, trend factor interleukin-10; PAPP-A, pregnancy-associated plasma protein-A; PLGF, placental growth factor.
Comparison of primary cure rate between the two groups
The primary cure rate of the hysteroscopy group was significantly higher than that of the conventional group (P < 0.05), as shown in Figure 2.
Figure 2.
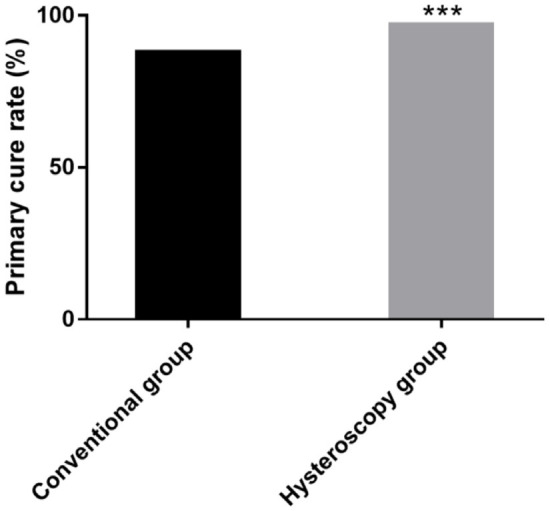
Comparison of the primary cure rate between the two groups. ***P < 0.001.
Comparison of complication rates between the two groups
The total incidence of complications in the hysteroscopy group was significantly lower than that in the conventional group (2.86% vs. 18.18%, P < 0.05), as shown in Table 5.
Table 5.
Comparison of complication rate between the two groups (%)
| Group | Conventional group (n=33) | Hysteroscopy group (n=35) | X2 | P |
|---|---|---|---|---|
| Intrauterine fluid | 1 (3.03) | 1 (2.86) | - | - |
| Embryo aspiration | 1 (3.03) | 0 (0.00) | ||
| Intrauterine residue | 2 (6.06) | 0 (0.00) | ||
| Genital and urinary tract infection | 2 (6.06) | 0 (0.00) | ||
| Total incidence of complications | 6 (18.18) | 1 (2.86) | 4.320 | 0.038 |
Discussion
Hydatidiform moles originate from placental villi and are characterized by irregular hyperplasia of the villous trophoblast layer, edema and degeneration of villous stroma, resulting in the formation of fluid-filled blisters of varying sizes, connected in strings resembling grapes. If not cleared in time, it can lead to vaginal bleeding, increase the risk of secondary infection, infertility, irregular menstruation and intrauterine adhesions, all of which can seriously affect the reproductive health of patients [13,14]. In clinic, curettage and other methods are primarily employed to promote the repair of uterus and avoid infection by removing residual pregnancy tissues [15]. Historically, blind curettage was commonly used, where doctors used a negative pressure aspirator for curettage based on experience and tactile feedback. While this approach has a certain curative effect, it requires multiple procedures and carries risks such as incomplete removal, missed curettage, endometrium damage to, and mechanical perforation of the uterine wall. In serious cases, it can cause amenorrhea and infertility [16-18], greatly affecting the physical and mental health of patients. Therefore, finding a more scientific and effective treatment that enhances surgical effect and safety, improves the prognosis of patients, and promotes long-term benefits has become a key focus in clinical research.
With the continuous development of medical technology, hysteroscopy technology has become increasingly refined, offering unique advantages in the diagnosis and treatment of uterine cavity lesions [19]. Therefore, in this study, hysteroscopy was used for the removal of residual embryos. During the first curettage, the results showed grayish-white or yellowish-white blisters with different sizes connected by thin pedicles, resembling real blisters. Close observation of the uterine wall revealed decidua-like tissues, bleeding areas, and floating endometrium between the grape-like blisters, with microvascular hemorrhage intermittently oozing into the liquid medium. In some cases, significant hematocele in the uterine cavity was observed. Upon re-curettage, yellow decidua-like substance, occasional vesicular remnants, membranous and fibrous adhesion bands, and a rich blood supply in certain lesions were visible.
Postoperative results showed that the operation time and menstrual recovery time of the hysteroscope group were significantly shorter than those of the conventional group. The intraoperative bleeding volume and endometrial thickness were significantly lower than those of the conventional group. However, the difference in the intrauterine adhesion rate between the two groups was not statistically significant. These findings suggest that hysteroscopic removal of residual embryos in patients with hydatidiform moles not only shortens the operation time and menstrual recovery time, but also minimizes bleeding and intrauterine adhesion. This may be attributed to the lower risk of complications such as tubal obstruction and adhesions associated with traditional curettage [20].
Although uterine ultrasound-guided curettage can monitor uterine changes to remove residual tissue, its accuracy remains limited. Multiple operations may be required to remove residual lesions, increasing uterine trauma, complications, and adversely affecting prognosis [21,22]. Hysteroscopy, by transmitting visual information through fiber optics, allows the operator to view the anatomical morphology of uterine cavity in real-time on a monitor, ensuring precise identification and removal of residual tissue. This technique is particularly advantageous in cases where residual tissue is located in the uterine cornua or when uterine deformities complicate the procedure. Hysteroscopy also enables histopathological examination, guiding further treatment while avoiding excessive curettage and reducing trauma to the endometrium [23-27]. This method preserves normal endometrial function, prevents complications, and promotes rapid postoperative recovery.
Serum β-HCG, a glycoprotein synthesized and secreted by trophoblasts, increases during pregnancy and serves as a key biochemical indicator for auxiliary diagnosis [28,29]. CK is critical for muscle cell function, and its concentration is of great significance for the assessment of myometrial injury [30]. CXCL10 is an inflammatory marker associated with myometrial infection [5]. This study analyzed changes in the these indicators, revealing no significant difference between the two groups before surgery. However, after surgery, the levels of β-HCG, CK, CXCL10, and PLGF in the hysteroscope group were significantly lower than those in the conventional group, while PAPP-A level was notably higher. These findings indicate that hysteroscopic removal of residual embryos can effectively improve the biochemical indicators of patients with hydatidiform mole.
Clinically, hydatidiform moles are prone to local invasion and distant metastasis, often requiring multiple curettage procedures. Due to the enlargement and soft nature of the uterus in cases of hydatidiform moles, complications such as bleeding and perforation are common [31,32]. This study showed that the one-time cure rate in the hysteroscope group was significantly higher, while the total complication rate was significantly lower in the hysteroscope group than that in the conventional group, suggesting that hysteroscopy is both safe and effective for removing intrauterine pregnancy residues. Studies in China have reported that traditional curettage often fails to completely remove residual hydatidiform moles, while hysteroscopy can achieve complete removal in one procedure. Research comparing hysteroscopic curettage with conventional methods has shown that hysteroscopy leads to fewer complications and better outcomes, including a higher rate of successful re-pregnancy [33-35]. However, while hysteroscopy causes minimal trauma, it is still an invasive procedure [36]. Operators should undergo professional training on hysteroscopy, conduct comprehensive preoperative assessments, and strictly adhere to surgical indications. During the operation, imaging must be carefully interpreted, intrauterine pressure should be minimized to avoid gas embolism, and operation time should be kept short. To reduce complications, predictive interventions should be in place, and surgery should be immediately halted if issues such as suspected uterine perforation or fluid overload syndrome arise.
As mentioned above, hysteroscopy has a unique value in the diagnosis and treatment of hydatidiform moles, as it effectively improves the primary cure rate and prevents excessive damage and residual tissue associated with blind operations. However, the limited sample size in this study and short observation time may affect the reliability and accuracy of the findings. Future studies should include larger sample sizes and evidence-based medicine to thoroughly analyze the long-term effects of hysteroscopy on the prognosis of patients with hydatidiform moles. This will provide more objective and reliable guidance for clinical practice.
Disclosure of conflict of interest
None.
References
- 1.Chiofalo B, Palmara V, Lagana AS, Triolo O, Vitale SG, Conway F, Santoro G. Fertility sparing strategies in patients affected by placental site trophoblastic tumor. Curr Treat Options Oncol. 2017;18:58. doi: 10.1007/s11864-017-0502-0. [DOI] [PubMed] [Google Scholar]
- 2.Horowitz NS, Goldstein DP, Berkowitz RS. Placental site trophoblastic tumors and epithelioid trophoblastic tumors: biology, natural history, and treatment modalities. Gynecol Oncol. 2017;144:208–214. doi: 10.1016/j.ygyno.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 3.Loukovaara M, Pukkala E, Lehtovirta P, Leminen A. Epidemiology of hydatidiform mole in Finland, 1975 to 2001. Eur J Gynaecol Oncol. 2005;26:207–208. [PubMed] [Google Scholar]
- 4.Altieri A, Franceschi S, Ferlay J, Smith J, La Vecchia C. Epidemiology and aetiology of gestational trophoblastic diseases. Lancet Oncol. 2003;4:670–678. doi: 10.1016/s1470-2045(03)01245-2. [DOI] [PubMed] [Google Scholar]
- 5.Fatemi N, Ray PF, Ramezanali F, Shahani T, Amiri-Yekta A, Kherraf ZE, Cazin C, Almadani N, Varkiani M, Sarmadi S, Sodeifi N, Gourabi H, Biglari A, Totonchi M. KH domain containing 3 like (KHDC3L) frame-shift mutation causes both recurrent pregnancy loss and hydatidiform mole. Eur J Obstet Gynecol Reprod Biol. 2021;259:100–104. doi: 10.1016/j.ejogrb.2021.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Eysbouts YK, Massuger L, IntHout J, Lok CAR, Sweep F, Ottevanger PB. The added value of hysterectomy in the management of gestational trophoblastic neoplasia. Gynecol Oncol. 2017;145:536–542. doi: 10.1016/j.ygyno.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Kayraklioglu N, Gasper C, Cho SJ, Lage J, Rabban JT. Intact chorionic vesicle in very early products of conception specimens: clinicopathologic features of 26 cases that may mimic complete hydatidiform mole. Am J Surg Pathol. 2023;47:397–408. doi: 10.1097/PAS.0000000000002006. [DOI] [PubMed] [Google Scholar]
- 8.Cetin F, Kayar I, Serin AN, Birge O. Efficacy of suction curettage as the first-line treatment of cesarean scar pregnancy: a retrospective study. J Gynecol Obstet Hum Reprod. 2023;52:102531. doi: 10.1016/j.jogoh.2022.102531. [DOI] [PubMed] [Google Scholar]
- 9.Ragab A, Goda H, Raghib M, Barakat R, El-Samanoudy A, Badawy A. Retraction note: does immediate postpartum curettage of the endometrium accelerate recovery from preeclampsia-eclampsia? A randomized controlled trial. Arch Gynecol Obstet. 2023;307:655. doi: 10.1007/s00404-022-06829-1. [DOI] [PubMed] [Google Scholar]
- 10.Yu K, Zhou H. Clinical curative effects and influencing factors of uterine artery chemoembolization combined with uterine curettage treating with cesarean scar pregnancy patients. Evid Based Complement Alternat Med. 2022;2022:7785573. doi: 10.1155/2022/7785573. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Huang CC, Chiu SC, Pan CM, Huang CC, Chang CY, Chao SC, Cho DY, Lin WC. New efficient method for hysteroscopic isthmoplasty: four simple steps lead to a significant improvement in bleeding status. J Clin Med. 2022;11:6541. doi: 10.3390/jcm11216541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez A, Alonso L, Nieto L, Carugno J. Hysteroscopic management of partial hydatidiform mole: a novel approach to an old disease. J Minim Invasive Gynecol. 2019;26:21–22. doi: 10.1016/j.jmig.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Braga A, Andrade T, do Carmo Borges de Souza M, Campos V, Freitas F, Maesta I, Sun SY, Pedrotti LG, Bessel M, Junior JA, Filho JR, Elias KM, Horowitz NS, Berkowitz RS. Presentation, medical complications and development of gestational trophoblastic neoplasia of hydatidiform mole after intracytoplasmic sperm injection as compared to hydatidiform mole after spontaneous conception - a retrospective cohort study and literature review. Gynecol Oncol. 2023;170:179–185. doi: 10.1016/j.ygyno.2023.01.016. [DOI] [PubMed] [Google Scholar]
- 14.Zhong L, Song L, Yin R, Li Q, Wang D. Risk factors for gestational trophoblastic neoplasia development of singleton normal fetus with partial hydatidiform mole pregnancy: a retrospective cohort and literature review. J Obstet Gynaecol Res. 2023;49:479–486. doi: 10.1111/jog.15488. [DOI] [PubMed] [Google Scholar]
- 15.Huang J, He P, Li D, Zhou J. Predictive factors analysis of cesarean scar pregnancy treated by local injection of Lauromacrogol combined with curettage. Medicine (Baltimore) 2023;102:e32783. doi: 10.1097/MD.0000000000032783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Margaliot Kalifa T, Lang Ben Nun E, Sela HY, Khatib F, Grisaru-Granovsky S, Rottenstreich M. Maternal and neonatal outcomes of women conceived less than 6 months after first trimester dilation and curettage. J Clin Med. 2022;11:2767. doi: 10.3390/jcm11102767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Velipasaoglu M, Arslan S. Management of caesarean scar pregnancy with ultrasound guided suction curettage followed by foley balloon catheter placement. J Gynecol Obstet Hum Reprod. 2022;51:102471. doi: 10.1016/j.jogoh.2022.102471. [DOI] [PubMed] [Google Scholar]
- 18.Gu Z, Jia P, Gao Z, Gu W, Zhao H, Zhao S. Uterine artery embolization combined with ultrasound-guided dilation and curettage for the treatment of cesarean scar pregnancy: efficacy and 5-8-year follow-up study. J Interv Med. 2022;5:148–152. doi: 10.1016/j.jimed.2022.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cespedes Martinez MA, Rovira Pampalona J, Degollada Bastos M, Izquierdo Argelich R, Bou Tapias J, Flores Laura MD, Bresco Torras P, Carugno Jose MD. Effectiveness and patient satisfaction with office hysteroscopic polypectomy in patients with symptomatic endometrial polyps. Facts Views Vis Obgyn. 2022;14:325–329. doi: 10.52054/FVVO.14.4.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Yang B, Chen W, Chen J. Clinical efficacy and re-pregnancy outcomes of patients with previous cesarean scar pregnancy treated with either high-intensity focused ultrasound or uterine artery embolization before ultrasound-guided dilatation and curettage: a retrospective cohort study. BMC Pregnancy Childbirth. 2023;23:85. doi: 10.1186/s12884-023-05376-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rein DT, Schmidt T, Hess AP, Volkmer A, Schondorf T, Breidenbach M. Hysteroscopic management of residual trophoblastic tissue is superior to ultrasound-guided curettage. J Minim Invasive Gynecol. 2011;18:774–778. doi: 10.1016/j.jmig.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Zheng YJ, Chen Q, Li S, Yan XT, Zhu T, He Z. Cesarean scar pregnancies treated by uterine artery chemotherapy embolization combined with ultrasound-guided dilation and curettage: a retrospective study. J Ultrasound Med. 2023;42:27–33. doi: 10.1002/jum.16050. [DOI] [PubMed] [Google Scholar]
- 23.Takasaki K, Henmi H, Ikeda U, Endo T, Azumaguchi A, Nagasaka K. Intrauterine adhesion after hysteroscopic myomectomy of submucous myomas. J Obstet Gynaecol Res. 2023;49:675–681. doi: 10.1111/jog.15499. [DOI] [PubMed] [Google Scholar]
- 24.Cheng X, Huang C, Xie G. Letter to the editor: Prevalence and risk factors for malignancy in hysteroscopy-resected endometrial polyps. Int J Gynaecol Obstet. 2023;160:353–354. doi: 10.1002/ijgo.14546. [DOI] [PubMed] [Google Scholar]
- 25.Henry Wong CL, So PL. Response: prevalence and risk factors for malignancy in hysteroscopy-resected endometrial polyps. Int J Gynaecol Obstet. 2023;160:355. doi: 10.1002/ijgo.14548. [DOI] [PubMed] [Google Scholar]
- 26.Ben-Ami I, Melcer Y, Smorgick N, Schneider D, Pansky M, Halperin R. A comparison of reproductive outcomes following hysteroscopic management versus dilatation and curettage of retained products of conception. Int J Gynaecol Obstet. 2014;127:86–89. doi: 10.1016/j.ijgo.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Smorgick N, Barel O, Fuchs N, Ben-Ami I, Pansky M, Vaknin Z. Hysteroscopic management of retained products of conception: meta-analysis and literature review. Eur J Obstet Gynecol Reprod Biol. 2014;173:19–22. doi: 10.1016/j.ejogrb.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 28.Eysbouts Y, Brouwer R, Ottevanger P, Massuger L, Sweep F, Thomas C, van Herwaarden A. Serum human chorionic gonadotropin normogram for the detection of gestational trophoblastic neoplasia. Int J Gynecol Cancer. 2017;27:1035–1041. doi: 10.1097/IGC.0000000000000966. [DOI] [PubMed] [Google Scholar]
- 29.Rakprasit C, Ruengkhachorn I, Therasakvichya S, Inthasorn P, Achariyapota V, Kuljarasnont S, Khemworapong K, Jareemit N. Combined analysis of clinical features, human chorionic gonadotropin (hCG) value, and hCG ratios for early prediction of postmolar gestational trophoblastic neoplasia. Arch Gynecol Obstet. 2023;307:1145–1154. doi: 10.1007/s00404-022-06785-w. [DOI] [PubMed] [Google Scholar]
- 30.Wray S, Prendergast C. The myometrium: from excitation to contractions and labour. Adv Exp Med Biol. 2019;1124:233–263. doi: 10.1007/978-981-13-5895-1_10. [DOI] [PubMed] [Google Scholar]
- 31.Gotsch F, Romero R, Friel L, Kusanovic JP, Espinoza J, Erez O, Than NG, Mittal P, Edwin S, Yoon BH, Kim CJ, Mazaki-Tovi S, Chaiworapongsa T, Hassan SS. CXCL10/IP-10: a missing link between inflammation and anti-angiogenesis in preeclampsia? J Matern Fetal Neonatal Med. 2007;20:777–792. doi: 10.1080/14767050701483298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subbaiah M, Raj A, Durairaj J, Keepanasseril A. Role of hysteroscopy and ultrasound in early identification of post-molar gestational trophoblastic neoplasia. Eur J Obstet Gynecol Reprod Biol. 2020;254:33–37. doi: 10.1016/j.ejogrb.2020.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Carrera M, Alonso L, Dominguez JA, Alcazar JL, Carugno J, Moratalla E, Perez Milan F, Caballero M. Hysteroscopic metroplasty for the treatment of the dysmorphic uterus: a SWOT analysis. Front Surg. 2022;9:1097248. doi: 10.3389/fsurg.2022.1097248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoeijmakers YM, Gorris MAJ, Sweep F, Bulten J, Eysbouts YK, Massuger L, Ottevanger PB, de Vries J. Immune cell composition in the endometrium of patients with a complete molar pregnancy: effects on outcome. Gynecol Oncol. 2021;160:450–456. doi: 10.1016/j.ygyno.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Erdem S, Bagli I, Ege S, Aydin E, Ozgokce C, Kulahcioglu MI. Efficacy of hysteroscopic septum resection in infertile women: a single center experience. Minim Invasive Ther Allied Technol. 2023;32:62–65. doi: 10.1080/13645706.2022.2164467. [DOI] [PubMed] [Google Scholar]
- 36.Abdollahi Fard S, Mostafa Gharabaghi P, Montazeri F, Mashrabi O. Hysteroscopy as a minimally invasive surgery, a good substitute for invasive gynecological procedures. Iran J Reprod Med. 2012;10:377–382. [PMC free article] [PubMed] [Google Scholar]