Abstract
While methyl-tertiary butyl ether (MTBE) remains the sole clinical topical agent for gallstone dissolution, its utility is limited due to side effects, largely stemming from its relatively low boiling point (55°C). In this study, we introduced 2-methoxy-6-methylpyridine (MMP), a novel gallstone-dissolving compound featuring an aromatic moiety and a substantially higher boiling point (156°C), designed to mitigate these side effects. We conducted a comprehensive evaluation of the efficacy and potential toxicities of MMP compared to MTBE using both in vitro and in vivo models. In the in vitro setting, MMP demonstrated significantly higher solubility than MTBE, achieving dissolution rates of 75% vs. 56%, 95% vs. 69%, and 100% vs. 82% at 60, 120, and 240 minutes, respectively (P < 0.05). In a porcine model with cholesterol gallstones, solubility assessments via direct injection of each solvent into the gallbladder showed that MMP exhibited approximately 1.8 times higher solubility compared to MTBE (P < 0.05). Further pharmacokinetic analysis in SD rats revealed that MMP is rapidly absorbed and efficiently cleared from the bloodstream, with dose-dependent variations in half-life, indicating favorable excretion profiles. Toxicological assessments in both rodents and pigs showed that MMP induces significantly less tissue damage than MTBE, with lower levels of apoptosis and inflammation in vital organs, as confirmed by molecular analyses including real-time PCR, Western blotting, and immunohistochemistry. Our findings indicate that MMP offers superior efficacy in cholesterol gallstone dissolution and presents a significantly lower toxicological profile, suggesting its potential as a safer and more effective alternative to MTBE.
Keywords: Cholesterol gallstone, laparoscopic cholecystectomy, 2-methoxy-6-methylpyridine (MMP), gallstones, methyl-tert-butyl ether (MTBE), topical gallstone-dissolving agent
Introduction
Gallstones have a prevalent occurrence, affecting approximately 10-20% of the adult population worldwide [1]. It is noteworthy that over 20% of individuals with gallstones will experience symptoms during their lifetime, typically manifesting as conditions such as biliary colic or infections, often occurring in adulthood [1]. Currently, the most appropriate standard treatment for symptomatic gallstones is laparoscopic cholecystectomy. Laparoscopic cholecystectomy has brought about minimal invasiveness, operability feasibility, and rapid postoperative recovery in the treatment of gallstone diseases [2-4]. However, there is a potential risk of substantial and fatal complications, such as CBD injury during surgery, occurring in approximately 0.5-1% of cases [5,6]. Additionally, depending on the definition and country, postcholecystectomy syndrome, characterized by symptoms like abdominal pain, indigestion, gas, vomiting, jaundice, and diarrhea, can be observed in a range of 19.8% to 54% of cases [7].
The first publication on percutaneous transhepatic contact litholysis of chole sterol stones in the gallbladder with methyl tert-butyl ether (MTBE) appeared in 1985 [8]. In a study involving 803 patients from 21 European hospitals [9], MTBE successfully dissolved gallstones in 95.1% of cases; however, sludge persisted in 43.5% of the gallbladders. The most serious complication observed was bile leakage, necessitating elective cholecystectomy in 1.6% of patients, with no documented instances of toxic injuries attributable to MTBE. Nonetheless, the extensive utilization of MTBE faces constraints due to the emergence of adverse reactions, including symptoms like nausea, upper abdominal discomfort, duodenitis, mild-to-moderate sedation, and hemolysis [10-19]. A substantial portion of these adverse effects can be attributed to MTBE’s relatively low boiling point (55°C), leading to heightened evaporation. MTBE’s evaporation rate stands at 8.0, signifying that it evaporates at a rate eight times faster than the standard n-butyl acetate, categorizing it as a “rapidly evaporating” substance [20-22].
We previously uncovered a novel gallstone-dissolving compound, named 2-methoxy-6-methylpyridine (MMP) [23]. Within the structure of MMP, the substantial tert-butyl moiety of MTBE is replaced by an aromatic pyridine group, resulting in a significantly higher boiling point (156°C) and reduced vaporization rate compared to MTBE. This alteration offers the potential for MMP to serve as a gallstone-dissolving agent with enhanced safety, as it may mitigate the adverse effects associated with MTBE. Our previous investigation using the Hamster gallstone model validated that MMP demonstrated superior solubility for cholesterol gallstones compared to MTBE (59.0% vs. 50.0%, P < 0.05), while concurrently exhibiting lower levels of associated toxicities than MTBE [23]. This study serves as the preliminary investigation into the efficacy and toxicity of MMP, incorporating both rodent pharmacokinetics and toxicity assessments, as well as evaluations in a large animal model. The promising results from these investigations are expected to provide a crucial foundation for the potential clinical application of MMP.
Methods
Materials
MTBE was sourced from Sigma-Aldrich (St. Louis, MO), while MMP was manufactured at the Korea Research Institute of Chemical Technology (KRICT, Daejeon, Republic of Korea). Gallstones were collected subsequent to cholecystectomy from patients diagnosed with gallstones, and the study received approval from the Ethics Committee of Seoul St. Mary’s Hospital, the Catholic University of Korea (IRB code: KC18TESI0103).
Design of pharmacokinetics and toxicity tests of MMP using SD rats
The pharmacokinetics analysis and toxicity comparison of MMP and MTBE were conducted using Sprague-Dawley (SD) rats. The experimental design included: 1) a pharmacokinetics study of MMP assessing blood concentration (n = 3) and urinary excretion (n = 3), 2) a comparative organ distribution study following MMP administration (n = 3), 3) single (n = 12) and repeated dose (n = 15) toxicity tests, and 4) a comparative blood concentration study following oral (n = 6) and intraperitoneal (IP) (n = 6) administration. The anesthesia for SD rats was conducted by administering an intraperitoneal injection of Zoletil at a dose of 0.006 cc/10 g (30 mg/kg) and Rompun at a dose of 0.004 cc/10 g (10 mg/kg).
For the plasma level assessment following oral administration of MMP, male SD rats (7 weeks old, n = 3) were administered 400 mg/kg of MMP orally. Blood samples were collected via the jugular vein at 0, 0.5, 2, 4, 8, and 24 hours post-administration. After collection, the blood samples were centrifuged at 13,000 rpm and 4°C to obtain plasma. In the urinary excretion study, male SD rats (7 weeks old, n = 6) were administered 400 mg/kg of MMP orally. Urine was collected using a metabolic cage at the following time intervals: 0-2 h, 2-4 h, 4-8 h, and 8-24 h.
In the acute toxicity test, male SD rats (7 weeks old, n = 16) were selected, and MMP was administered at doses of 0, 2000, 4000, and 8000 mg/kg via oral gavage as a single dose. Clinical signs were observed daily for 14 days following the administration. Observations included lethargy, gait disturbance, response to stimuli, abnormal behavior, paralysis, diarrhea, vomiting, edema, and respiratory distress. Any deaths or clinical abnormalities, including lethargy and gait disturbance, were noted during the study period. In the repeated-dose toxicity test, male SD rats (7 weeks old, n = 20) were selected, and MMP was administered at doses of 0, 2000, 4000, and 8000 mg/kg orally once daily for 2 weeks. Clinical signs were monitored daily for 14 days. Observations included lethargy, gait disturbance, response to stimuli, abnormal behavior, paralysis, diarrhea, vomiting, edema, and respiratory distress. Any deaths or clinical abnormalities, including lethargy and gait disturbance, were noted during the study period.
In the pharmacokinetics study comparing blood concentrations following PO and IP administration of MMP, male SD rats (7 weeks old, n = 12) were selected. For PO administration, the dose groups consisted of 6 rats, and for IP administration, 5 rats were used. MMP was administered at doses of 200, 400, and 800 mg/kg. Blood samples were collected via the jugular vein at 0, 0.25, 0.5, 1, 2, 4, 8, and 24 hours post-administration. The samples were then centrifuged at 13,000 rpm and 4°C to obtain plasma. After the experiment, the rats were euthanized by inhaling carbon dioxide in a specially designed chamber for five minutes. To confirm death, the heart area was palpated for 30 seconds using the index and middle fingers for verification. Once death was confirmed, a thoracolaparotomy was performed to harvest the targeted organs.
Histological assessment
Tissues from each group were preserved in 10% buffered formalin, embedded in paraffin, and sectioned into 3 µm-thick slices. The sections were then stained with hematoxylin and eosin (H&E). The tissue injury score (TIS) was calculated based on histopathological evaluations, considering parameters such as necrosis, inflammation, and structural disruption, with each parameter graded on a scale of 0 to 4. The TIS was determined using the formula: TIS = (necrosis score + inflammation score + structural disruption Score)/number of parameters assessed. The mean score for each animal was calculated by summing the individual scores across different tissue sections.
Serological test
Blood samples were collected, centrifuged at 1000 g for 10 minutes (for ELISA) and 13,000 rpm for 10 minutes at 4°C (for serological tests), and stored at -20°C until analysis. The concentrations of mouse IL-6 and TNF-α were quantified using a sandwich ELISA kit (Mybiosource, San Diego, CA), while parameters indicative of liver and kidney injury (AST, ALT, creatinine, and total bilirubin) were measured using a Fujifilm-Dri-Chem Analyzer (Stanford, CT, USA).
High-performance liquid chromatography (HPLC)
HPLC analysis was conducted using the Waters Arc HPLC core system (Waters Corporation. Milford, MA) with an XBridge C18 column (4.6 × 150 mm, 5 μm particle size). Chromatographic separation was achieved using a mobile phase of acetonitrile and water (75:25, v/v) at a flow rate of 0.6 mL/min. UV detection was performed at 270 nm with an injection volume of 10 µL.
Determination of in vitro solubility of gallstone-dissolving solvents
Following air-drying, gallstones were meticulously matched for size, weight, and shape. Subsequently, these matched gallstones were individually placed into separate glass containers. In each container, 10 mL aliquots of either MTBE or MMP were introduced. The aliquots were aspirated and replenished on an hourly basis. To facilitate dissolution, the glass containers were gently stirred at 50 rpm within a VS-8480SF reactor (Vision Co., Daejeon, Republic of Korea) operating at a temperature of 37°C for a total duration of 24 hours. Evaluation of gallstone solubility was performed by measuring the dry weights of the gallstones at defined intervals of 30, 60, 90, and 120 min.
Determination of in vivo solubility using pig animal model
Three-month-old pigs (Cronex Co., Hwaseong, Korea) were utilized as experimental subjects. Animal experimentation procedures adhered to the guidelines established by the Institute for Laboratory Animal Research, Korea (IRB No: CRONEX-IACUC-202102001). The pigs underwent a seven-day acclimatization period upon introduction to their new environment. They were subsequently housed in conditions featuring a controlled temperature of 24 ± 2°C, humidity levels maintained at 50 ± 10%, a 12-hour light-dark cycle with an illuminance of 200 lux, and ventilation occurring at a rate of 10-15 times per hour. For the experimental procedure, five uniform 5-mm cholesterol gallstones were implanted into each pig’s gallbladder through an incision, which was subsequently closed using sutures. Following this, a catheter was inserted through the liver into the gallbladder, through which MTBE and MMP were separately injected at a rate of 10 ml/15 min, four times each. Finally, the pigs were euthanized, and the solubility of cholesterol gallstones within the gallbladder was assessed by measuring their weight. The experimental group was stratified into three subgroups based on the treatment modality: control (n = 3), MTBE (n = 5), and MMP (n = 5). The anesthesia for pigs was conducted by administering an intramuscular injection of Zoletil at a dose of 0.1 mL/kg and Rompun at a dose of 0.1 mL/kg. After achieving deep anesthesia, the state was maintained using a respirator with an oxygen:isoflurane (2:1) mixture for inhalation anesthesia. After the experiment, the animals were euthanized by rapidly administering a high dose of potassium chloride (KCl) solution (2 mmol/kg) intravenously or intracardially to induce cardiac arrest. Cardiac arrest was confirmed via electrocardiography or auscultation.
TUNEL assay
TUNEL analysis was conducted to detect apoptosis in gallbladder tissues, employing an in situ apoptosis detection kit (Takara Bio, Inc., Japan) in accordance with the manufacturer’s instructions. In summary, slides containing tissue samples were subjected to a 1-hour incubation at 37°C in the absence of light with 50 μl of the TUNEL reaction mixture and TdT labeling reaction mix. Following this incubation, the samples underwent three PBS rinses and were subsequently visualized using a fluorescence imaging system (EVOS U5000; Invitrogen, CA, USA).
Real-time PCR
Total RNA was extracted from gallbladder tissues using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Reverse transcription was carried out with 1 µg of RNA, employing an RT-premix kit (TOYOBO, Osaka, Japan), following the manufacturer’s instructions. SYBR Green-based real-time quantitative polymerase chain reaction (PCR) was conducted utilizing the following primers: For pig MCL-1, the forward primer was 5’-TTC TCG GAT GAT CCA TGT TTT C-3’ and the reverse primer was 5’-CCA GCA GCA CAT TTC AGA TGC CGC-3’. For pig Bax, the forward primer was 5’-CCG AAA TGT TTG CTG ACG-3’ and the reverse primer was 5’-AGC CGA TCT CGA AGG AAG T-3’. Additionally, for GAPDH, the forward primer was 5’-ACA CTC ACT CTT CTA CCT TTG-3’ and the reverse primer was 5’-CAA ATT CAT TGT CGT ACC AG-3’. PCR reactions were conducted using the Applied Biosystems StepOnePlus Real-Time PCR System (Thermo, Carlsbad, CA, USA).
Immunohistochemical analysis
For immunohistochemical analysis, formalin-fixed, paraffin-embedded tissue sections were subjected to deparaffinization and subsequent rehydration in an ethanol series, following standard procedures. Epitope retrieval was performed as per established protocols. Immunohistochemical staining was conducted using antibodies against c-Caspase 3 (Cell Signaling Technology) and Bcl-xL (Abcam). Subsequently, the samples were examined for antibody expression using a laser-scanning microscope (Eclipse TE300; Nikon, Tokyo, Japan).
Western blot analysis
Gallbladder tissues were lysed using the EzRIPA lysis kit (ATTO Corporation; Tokyo, Japan) and subsequently quantified using Bradford reagent (Bio-Rad, Hercules, CA, USA). Protein visualization was achieved through western analysis employing primary antibodies (dilution 1:1000) sourced from Cell Signaling Technology (Beverly, MA), followed by HRP-conjugated secondary antibodies (dilution 1:2000) obtained from Vector Laboratories (Burlingame, CA, USA). Specific immune complexes were detected utilizing the Western blotting plus chemiluminescence reagent (Millipore, Bedford, MA). The primary antibody against c-Caspase 3 was procured from Cell Signaling Technology (Beverly, MA, USA), while the primary antibody against MCL-1 was sourced from Abcam (Cambridge, UK). Additionally, primary antibodies against β-actin were obtained from Sigma Aldrich (St. Louis, MO, USA), and HRP-conjugated secondary antibodies were acquired from Vector Laboratories (Burlingame, CA, USA).
Statistical analysis
The data analysis was conducted using SPSS 11.0 software (SPSS Inc., Chicago, IL), and the results are presented as mean ± standard deviation. Statistical comparisons among groups were assessed using the Kruskal-Wallis test. Statistically significant differences were defined as those with probability values (P) less than 0.05.
Results
Pharmacokinetics of MMP in SD rats
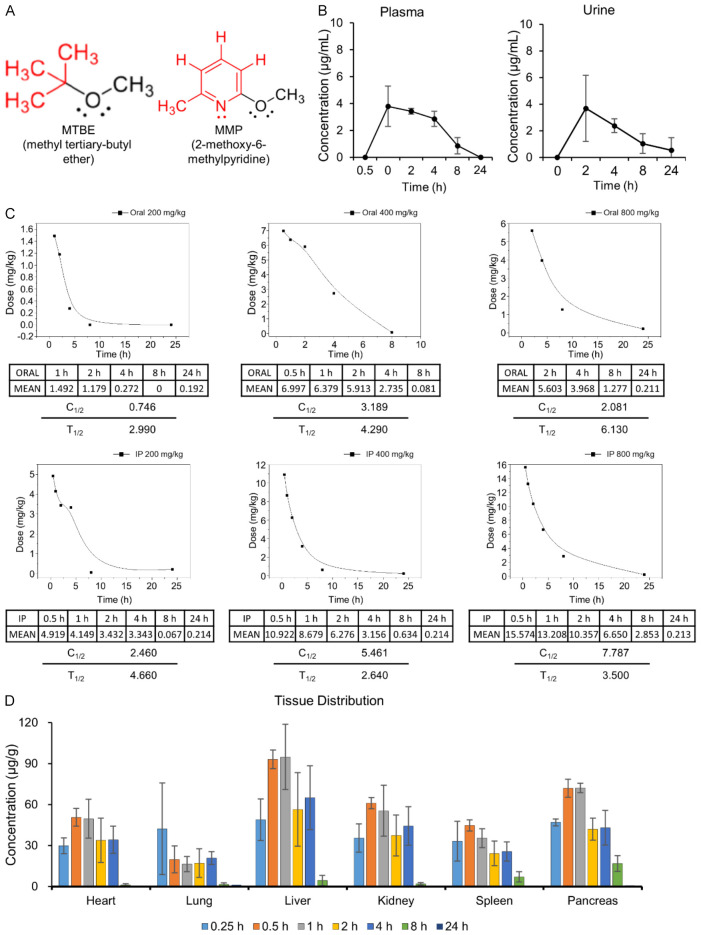
Among the various compounds used for dissolving gallstones, MTBE stands out as a representative solvent and is presently the only drug of its kind in use. MTBE is an alkyl ether compound akin to diethyl ether, an anesthetic agent (Figure 1A, left). In MMP, the substitution of MTBE involves replacing its bulky aliphatic tert-butyl group with a pyridine moiety, an aromatic group (Figure 1A, right). Compounds containing an aromatic ring typically exhibit higher boiling points and lower vapor pressures. Consequently, MMP possesses a relatively higher boiling point (156°C) and exhibits lower volatility, potentially resulting in reduced toxicity while maintaining comparable dissolving properties to MTBE.
Figure 1.
Pharmacokinetics of MMP in SD rats. A. Chemical structures of MTBE and MMP. MMP is a structural derivative of MTBE, where the isobutyl ether group of MTBE is replaced with a pyridine ring, resulting in a benzene-like ring structure. In MMP, the pyridine ring has a methoxy (-OCH3) group at the 2-position and a methyl (-CH3) group at the 6-position, contrasting with the simple alkyl ether structure of MTBE. B. Plasma and urine concentrations of MMP following oral administration of 400 mg/kg in SD rats. Plasma concentration peaked at 0.5 hour post-administration, and urine concentration peaked at 2 hours, with significant elimination observed by 24 hours. Values are presented as mean ± SD (n = 3). C. Half-life of MMP in the blood was determined following oral and intraperitoneal administration. MMP was administered at doses of 200 mg/kg, 400 mg/kg, and 800 mg/kg via both oral and intraperitoneal routes. For oral administration, the half-life increased with dose, ranging from approximately 2.99 hours at 200 mg/kg to 6.13 hours at 800 mg/kg. In contrast, for intraperitoneal administration, the half-life was shorter and showed a non-linear relationship with dose, varying between approximately 2.64 hours at 400 mg/kg and 4.66 hours at 200 mg/kg. D. Time-dependent distribution of MMP in various organs was assessed using HPLC. MMP was widely distributed across different organs shortly after administration, with the highest concentrations typically observed within the first hour. By 24 hours, MMP was nearly undetectable in all tissues, indicating effective clearance of the drug from the body. Values are presented as mean ± SD (n = 5).
The pharmacokinetics of MMP were evaluated in SD rats following oral administration at a dose of 400 mg/kg. Time-dependent plasma and urine concentrations of MMP were measured (Figure 1B). The plasma concentration of MMP peaked at 0.5 hours post-administration and was undetectable at 24 hours. The urinary concentration of MMP showed a peak at 2 hours, with a significant decrease observed by 24 hours, indicating extensive excretion.
To determine the half-life of MMP, blood concentrations were measured in SD rats following oral and intraperitoneal administration of MMP at doses of 200 mg/kg, 400 mg/kg, and 800 mg/kg (Figure 1C). The half-life of MMP in the blood varied based on the dose and administration route. For oral administration at a dose of 200 mg/kg, the half-life (T1/2) was approximately 2.99 hours. At a higher oral dose of 400 mg/kg, the half-life extended to approximately 4.29 hours, and at 800 mg/kg, it further increased to around 6.13 hours. For intraperitoneal administration, the half-life also varied: at a dose of 200 mg/kg, the half-life was approximately 4.66 hours, while at 400 mg/kg, it was shorter, around 2.64 hours. These results suggest that the half-life of MMP in the bloodstream is dose-dependent and varies significantly with the method of administration.
To assess the time-dependent distribution of MMP across different tissues, residual amounts of MMP were measured using HPLC in various organs following the administration (Figure 1D). When MMP was administered at a dose of 4000 mg/kg, the residual concentration was approximately 100 µg/kg at 1 hour post-administration, but it was undetectable at 24 hours, indicating complete clearance from the body.
Toxicity test of MMP in SD rats
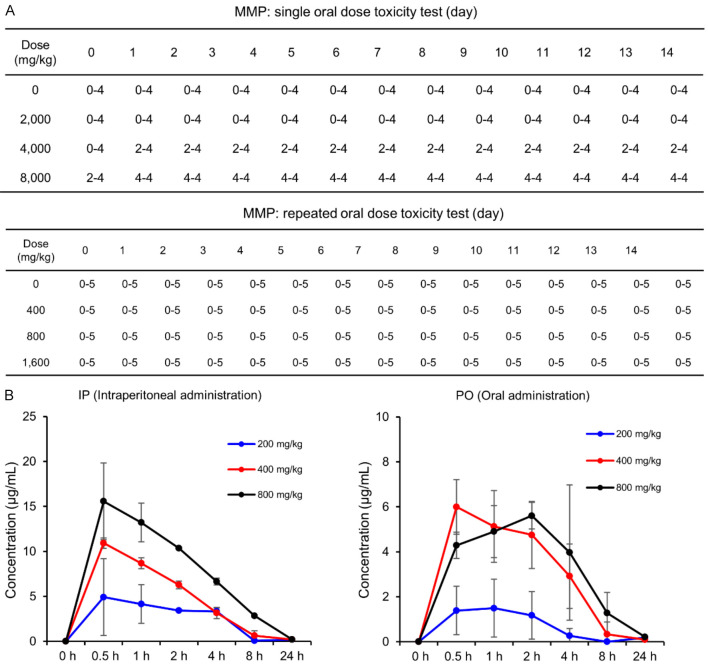
Acute toxicity testing was performed to assess the safety profile of MMP at doses of 2000 mg/kg, 4000 mg/kg, and 8000 mg/kg (Figure 2A, top) in SD rats. The median lethal dose (LD50) for oral administration was determined to be 4000 mg/kg. This conclusion was based on the observation that 50% of the rats (2 out of 4) administered 4000 mg/kg of MMP died, while no deaths occurred at 2000 mg/kg, and all rats (4 out of 4) died at the 8000 mg/kg dose. Mortality was observed within the first two days post-administration, but no deaths occurred beyond this period. During the 14-day observation period following a single oral dose, no clinical signs such as lethargy, gait disturbance, abnormal behavior, paralysis, diarrhea, vomiting, edema, or respiratory distress were observed. A subsequent repeated dose toxicity test was conducted with MMP administered at concentrations of 400 mg/kg, 800 mg/kg, and 1600 mg/kg, and the animals were monitored for 14 days (Figure 2A, bottom). Similar to the single-dose study, no adverse clinical signs were noted.
Figure 2.
Toxicity tests of MMP in SD rats. A. Acute and repeated dose toxicity tests in SD rats. The acute toxicity test involved single oral doses of MMP at 2000 mg/kg, 4000 mg/kg, and 8000 mg/kg, with an LD50 determined at 4000 mg/kg (top). The repeated dose toxicity test involved administration of MMP at 400 mg/kg, 800 mg/kg, and 1600 mg/kg over 14 days, with no adverse clinical signs observed (bottom). In the table, the first number represents the number of animals that died at each time point, while the second number indicates the total number of animals tested. B. Pharmacokinetic analysis of MMP in SD rats following oral (PO) and intraperitoneal (IP) administration at doses of 200 mg/kg, 400 mg/kg, and 800 mg/kg. Blood concentrations of MMP were measured over time, revealing peak concentrations at 0.5 hour for both administration routes, followed by substantial elimination by 8 hours and near-complete clearance by 24 hours. Values are presented as mean ± SD (n = 5).
Additionally, the absorption rates of MMP via different administration routes-oral (PO) and intraperitoneal-were compared using distilled water as the solvent (Figure 2B). Blood concentration of MMP was measured over time for both administration routes. For oral administration, the peak concentration was observed at 0.5 hour, but the absorption rates were generally lower compared to the intraperitoneal route, with substantial elimination by 8 hours and near-complete clearance by 24 hours. Intraperitoneal administration also showed peak concentrations at 0.5 hour for all tested doses (200 mg/kg, 400 mg/kg, 800 mg/kg), followed by a rapid decline, with MMP becoming negligible by 8 hours. These findings suggest that MMP is rapidly absorbed and eliminated from the bloodstream, with peak concentrations occurring at 0.5 hour and substantial clearance by 8 hours, consistent across both oral and intraperitoneal administration routes.
Determination of solubility of MMP
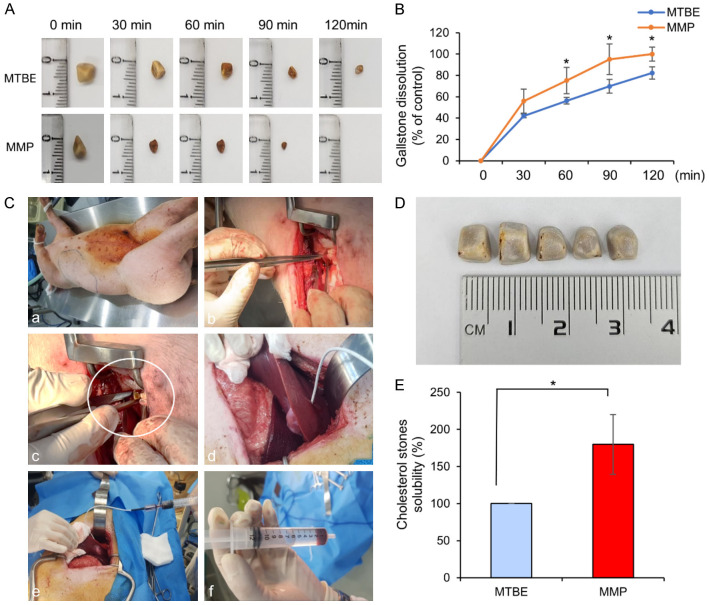
Gallstones were collected from patients who underwent cholecystectomy for gallstone-related issues at Seoul St. Mary’s Hospital, the Catholic University of Korea. Among these, gallstones with an outer appearance indicative of cholesterol content were selected for the study. Analysis confirmed that cholesterol constituted 79.6% of these gallstones. Figure 3A shows representative images of gallstones after treatment with MTBE and MMP at 30, 60, 90, and 120 minutes. After exposure to either MTBE or MMP, gallstones of similar size (approximately 6 mm) showed a greater reduction in size with MMP compared to MTBE at all time points. By 120 minutes, the size of the gallstones in the MTBE-treated group had decreased to approximately 3 mm, whereas in the MMP-treated group, the gallstones were completely dissolved and no residual stones were visible. The solubility of each solvent was further assessed by comparing the weights of residual gallstones in an in vitro environment. Briefly, individual gallstones were placed into separate glass containers, and 10 mL of either MTBE or MMP was added. The containers were gently stirred at 37°C to facilitate dissolution, and the dry weights of the gallstones were measured at 30, 60, 90, and 120 minutes (Figure 3B). After 30 minutes, MMP exhibited a dissolution rate of 55%, compared to 42% with MTBE, although this difference was not statistically significant. However, at 60, 120, and 240 minutes, MMP demonstrated significantly higher dissolution rates than MTBE, with rates of 75% vs. 56%, 95% vs. 69%, and 100% vs. 82%, respectively (P < 0.05). These results suggest that MMP has a significantly greater solubility than MTBE in in vitro models of cholesterol gallstones.
Figure 3.
Evaluation of MMP’s solubility compared to MTBE in both in vitro and in vivo models. A. Representative images of cholesterol gallstones treated with MTBE or MMP at 30, 60, 90, and 120 minutes. MMP-treated gallstones showed a greater reduction in size compared to MTBE-treated gallstones at all time points, with complete dissolution observed in the MMP group by 120 minutes. B. In vitro dissolution rates of gallstones treated with MTBE or MMP, measured by the dry weight of residual stones at various time intervals. MMP demonstrated significantly higher dissolution rates compared to MTBE at 60, 120, and 240 minutes (P < 0.05). Values are presented as mean ± standard deviation of three independent experiments. *P < 0.05. C. Overview of the surgical procedure used in the in vivo pig model to evaluate gallstone solubility. D. A representative image depicting the five cholesterol gallstones that were surgically inserted into the gallbladder of a pig. E. Comparison of the solubility between MTBE and MMP in the in vivo pig model. The MMP-treated group (n = 5) exhibited a solubility 1.8 times higher than that of the MTBE-treated group (n = 5). *P < 0.05.
The solubility of the gallstone-dissolving agents was further compared in an in vivo pig model. The surgical procedure is briefly outlined as follows (Figure 3C): After inducing respiratory anesthesia, a laparotomy was performed to access the gallbladder. Five cholesterol stones, each measuring 5 mm in diameter (Figure 3D), were inserted into the gallbladder, which was then sutured. A catheter (Uresil REF MPL2: Indianapolis, IL, USA) was subsequently inserted into the gallbladder through the liver, and 10 mL of either MTBE or MMP (100%) was administered to each group, with five pigs per group (MTBE and MMP groups), and a total of 13 pigs in the study, including the control group (n = 3). The gallstone-dissolving agents were administered at 15-minute intervals, totaling eight administrations over two hours, with aspiration performed before each injection to evacuate the gallbladder’s contents. The solubility between the MTBE and MMP groups was compared, revealing that the MMP-treated group exhibited a solubility that was 1.8 times higher than that of the MTBE-treated group (P < 0.05) (Figure 3E).
Comparison of in vivo pharmacokinetics and toxicity of MTBE and MMP
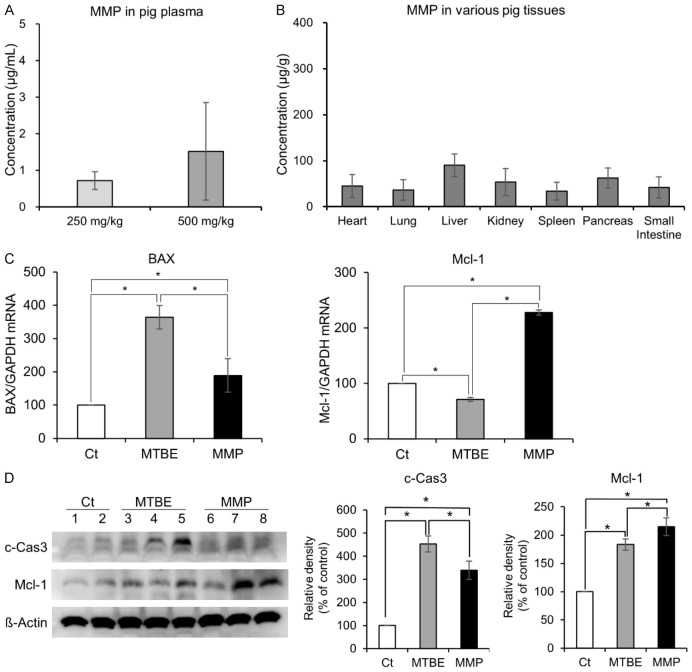
The pharmacokinetics of MMP were evaluated following the administration for gallstone dissolution. Our study confirmed that MMP, when administered into the gallbladder, can enter the bloodstream. HPLC analysis showed that 1 hour after administration, MMP concentrations in the blood were 0.7 µg/mL following a 5 mL (250 mg/kg) dose and 1.52 µg/mL following a 10 mL (500 mg/kg) dose (Figure 4A). Subsequently, the concentrations of MMP in various organs were measured using HPLC analysis. The liver exhibited the highest concentration of MMP, followed by the pancreas, though these differences were not statistically significant (Figure 4B).
Figure 4.
In vivo pharmacokinetics and toxicity of MMP compared to MTBE. A. Plasma concentrations of MMP measured 1 hour after administration of 5 mL (250 mg/kg) and 10 mL (500 mg/kg) doses into the gallbladder. B. Tissue distribution of MMP in various organs, including the heart, lungs, liver, kidneys, spleen, pancreas, and small intestine, measured by HPLC analysis, showing the highest concentration in the liver, followed by the pancreas. C. Expression levels of apoptosis-related markers Bax (pro-apoptotic) and Mcl-1 (anti-apoptotic) in gallbladder tissues measured by real-time PCR. MMP treatment significantly decreased Bax mRNA levels and increased Mcl-1 mRNA levels compared to MTBE (P < 0.05). D. Western blot analysis of c-Caspase 3 (pro-apoptotic) and Mcl-1 (anti-apoptotic) expression in gallbladder tissues. MMP treatment led to a significant reduction in c-Caspase 3 expression and an increase in Mcl-1 expression compared to MTBE (P < 0.05). Values are presented as mean ± standard deviation of three independent experiments. *P < 0.05.
To assess the toxicity of gallstone-dissolving solvents on gallbladder epithelial cells, gallbladder tissues were collected, and real-time PCR was conducted to examine apoptosis-related markers. Compared to MTBE, MMP resulted in a significant reduction in the expression of the pro-apoptotic marker Bax mRNA and a significant increase in the expression of the anti-apoptotic marker Mcl-1 mRNA in gallbladder tissues (P < 0.05) (Figure 4C). Additionally, Western blot analysis was performed to compare the expression of apoptotic markers (c-caspase 3 and Mcl-1) in gallbladder tissues following each treatment. MMP significantly reduced the expression of the pro-apoptotic marker c-Caspase 3 and significantly increased the expression of the anti-apoptotic marker Mcl-1 compared to MTBE in gallbladder tissues (P < 0.05) (Figure 4D).
Comparative analysis of hematological and biochemical parameters following MTBE and MMP administration
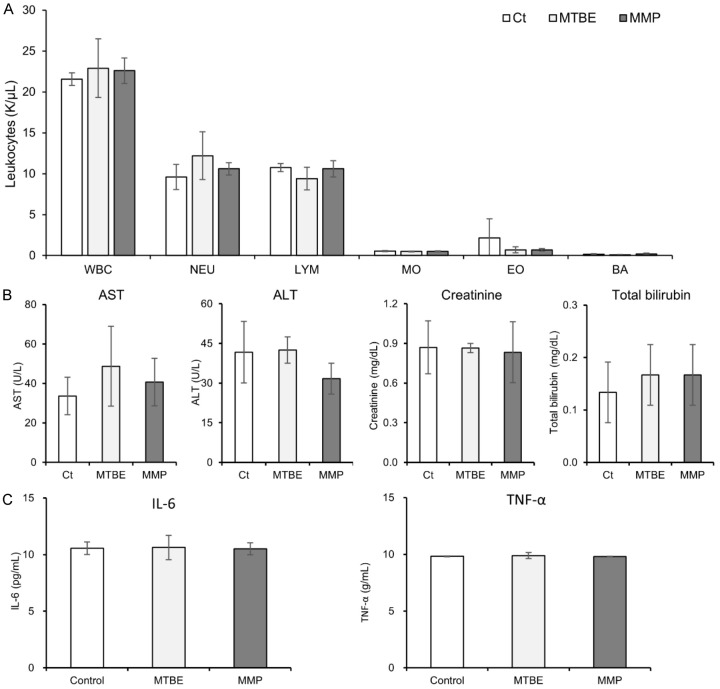
The effects of MTBE and MMP administration on blood leukocytes and biochemical parameters were evaluated. Using an automated hematology analyzer, we compared the total WBC count and WBC fractions post-administration of MTBE and MMP with the control group. The analysis revealed no significant increase in total WBC count or WBC fractions between the treatment and control groups (Figure 5A). Additionally, biochemical tests were performed to monitor liver and kidney function, including AST, ALT, creatinine, and total bilirubin levels. The results showed no significant differences in these parameters between the MMP-treated group, the MTBE-treated group, and the control group (Figure 5B). To assess the potential inflammatory response induced by MMP, levels of the inflammatory cytokines IL-6 and TNF-α were measured using ELISA. The findings indicate that there were no significant increases in IL-6 or TNF-α levels in the MMP-treated group compared to the control and MTBE-treated groups (Figure 5C).
Figure 5.
Comparative analysis of hematological and biochemical parameters following MTBE and MMP administration. A. Total WBC count and WBC fractions (neutrophils, lymphocytes, monocytes, eosinophils and basophils) measured using an automated hematology analyzer post-administration of MTBE and MMP. No significant increases were observed compared to the control group. B. Biochemical analysis of liver and kidney function markers (AST, ALT, creatinine, total bilirubin) across different treatment groups, showing no significant differences. C. Measurement of inflammatory cytokines IL-6 and TNF-α levels using ELISA to evaluate potential inflammatory responses. No significant increase in cytokine levels was observed in the MMP-treated group compared to the control and MTBE-treated groups.
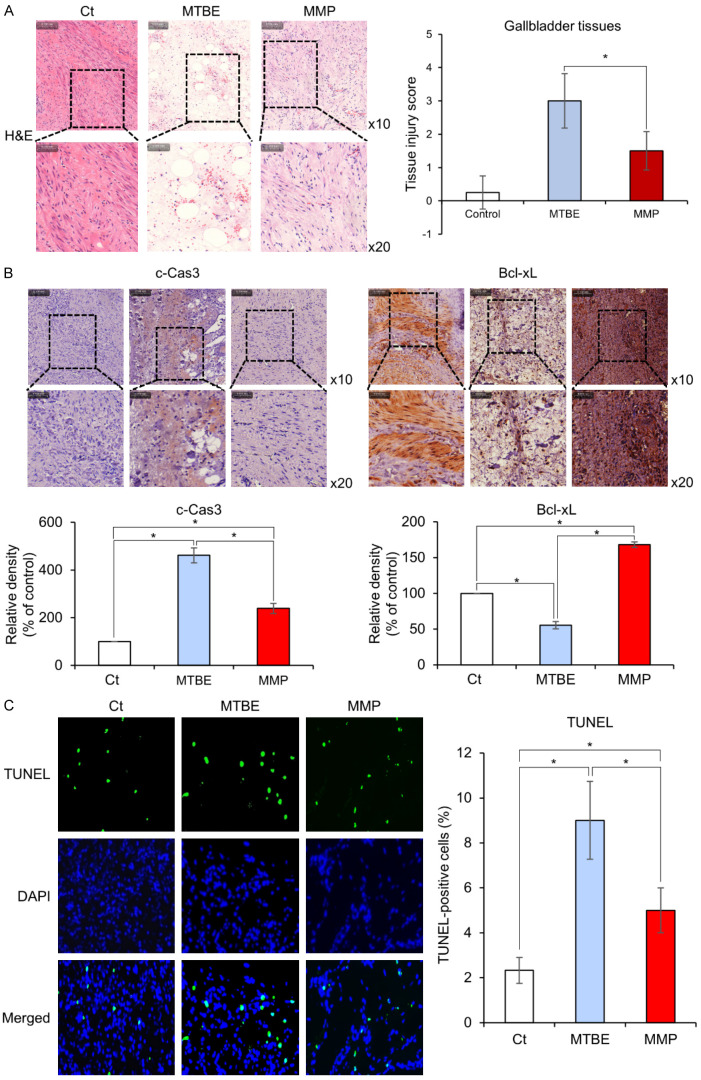
Histological comparison of gallbladder tissues after treatment
Histological changes in gallbladder tissue following each treatment were compared using H&E staining. The MTBE-treated group displayed considerable disorganization of the tissue architecture and increased inflammatory cell infiltration, whereas these changes were less severe in the MMP-treated group (Figure 6A). Subsequently, immunohistochemical stains utilizing pro-apoptotic marker c-Caspase 3 and anti-apoptotic marker Bcl-xL were compared in each group. In comparison to the MTBE-treated group, the MMP-treated group significantly reduced the expression of c-Caspase 3 and significantly increased the expression of Mcl-1 (P < 0.05) (Figure 6B). Finally, the degree of apoptosis was compared in gallbladder tissues obtained from each group through TUNEL assay comparisons (Figure 6C). The MMP-treated group significantly reduced TUNEL immunofluorescence compared to the MTBE-treated group (P < 0.05). These results indicate that MMP treatment induces significantly lower levels of apoptosis in gallbladder tissues compared to MTBE treatment.
Figure 6.
Histological comparison of gallbladder tissues after MTBE or MMP treatment. A. H&E staining of gallbladder tissues showing tissue disorganization and inflammatory cell infiltration following MTBE treatment, with less severe changes observed in the MMP-treated group. Images are shown at 10× and 20× magnifications. B. Immunohistochemical staining for the pro-apoptotic marker c-Caspase 3 and the anti-apoptotic marker Bcl-xL. The MTBE-treated group shows significantly higher c-Caspase 3 expression, while the MMP-treated group displays significantly higher Bcl-xL expression (P < 0.05). Percentages of immunoreactive areas were measured using NIH image J and expressed as relative values to those in control tissues. *P < 0.05. C. TUNEL assay for the determination of apoptosis levels in gallbladder tissues. The MMP-treated group exhibited a significant reduction in TUNEL immunofluorescence when compared to the MTBE-treated group (P < 0.05), demonstrating that MMP treatment induces significantly lower levels of apoptosis in gallbladder tissues in comparison to MTBE treatment. Percentages of immunoreactive areas were measured using NIH image J and expressed as relative values to those in control tissues. *P < 0.05.
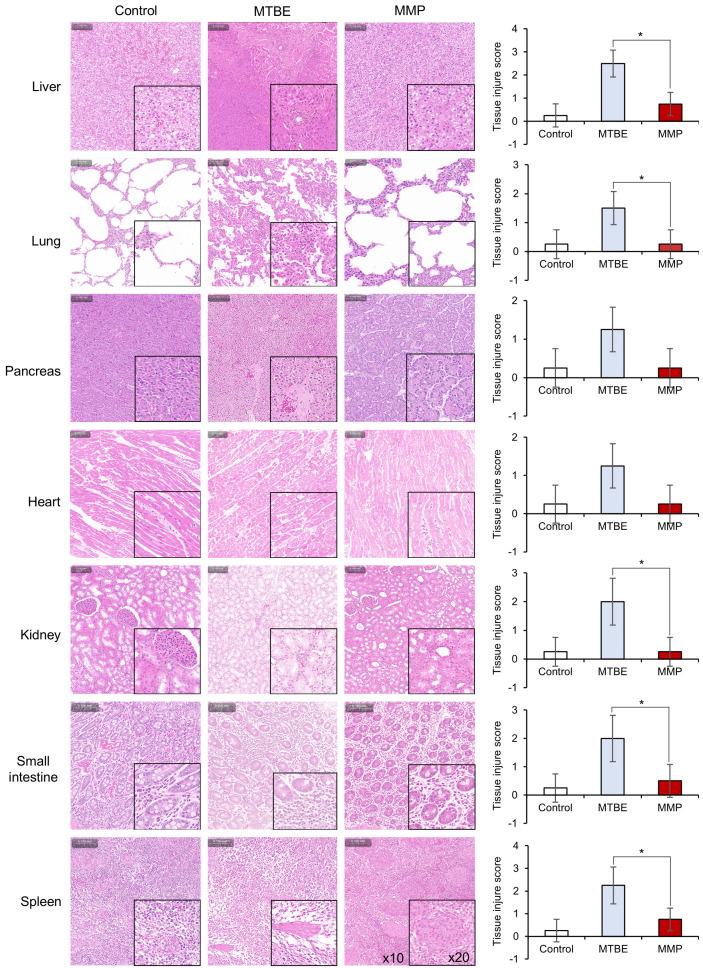
The potential toxicological effects of MMP on various organs, including the liver, lungs, kidneys, heart, pancreas, small intestine, and spleen, were investigated in pig. After administering MMP for one hour, organ morphology was assessed using H&E staining. The results indicated that MMP exhibited significantly less toxicity in the liver, lungs, and kidneys compared to MTBE (P < 0.05) (Figure 7). The histological images and corresponding TIS clearly demonstrate that tissue morphology in the MMP-treated group remained well-preserved with minimal signs of damage or inflammation, whereas the MTBE-treated group exhibited more pronounced histological alterations, indicating a higher degree of tissue injury.
Figure 7.
Histopathological analysis of various organs, including the liver, lungs, kidneys, heart, pancreas, small intestine, and spleen, following MMP administration compared to MTBE and control groups. H&E staining was performed to assess tissue morphology. The images reveal that MMP-treated groups exhibited significantly less tissue damage compared to MTBE-treated groups across all examined organs. Tissue injury scores (TIS) were calculated based on the assessment of necrosis, inflammation, and structural disruption, with each parameter graded on a scale from 0 to 4. The TIS results show that MMP treatment resulted in well-preserved tissue morphology with minimal signs of damage or inflammation, whereas MTBE treatment led to more pronounced histological alterations, indicating higher degrees of tissue injury. Magnification levels are indicated in the spleen images (×10 and ×20). Statistical significance is denoted by *P < 0.05.
Discussion
In spite of its relatively high solubility as a contact gallstone-dissolving solvent, MTBE is currently not widely employed due to its low boiling point, resulting in adverse effects caused by evaporation within the human body. To address this limitation, we have identified MMP, a derivative of MTBE with an elevated boiling point, and this study represents the inaugural large animal investigation conducted on MMP. Pharmacokinetic analysis in SD rats revealed that MMP exhibits favorable absorption and clearance profiles, with dose-dependent variability in half-life across different administration routes. Toxicological assessments indicated that MMP induces significantly less tissue damage in vital organs compared to MTBE, with a higher therapeutic index. In vitro analysis demonstrated that MMP significantly outperformed MTBE in dissolving cholesterol gallstones, achieving 95% dissolution at 120 minutes (versus 69% for MTBE) and reaching complete dissolution (100%) by 240 minutes, while MTBE reached only 82% dissolution. Furthermore, in a porcine model, MMP exhibited a solubility 1.8 times greater than that of MTBE over a 2-hour administration period (P < 0.05). Histological examinations of gallbladder tissues post-treatment confirmed that MMP leads to reduced apoptosis and tissue disruption compared to MTBE. These findings underscore the potential of MMP as a promising topical agent for gallstone dissolution, warranting further investigation and development.
In this study, the pharmacokinetics and toxicity of MMP were evaluated in both rodent and pig models, demonstrating its favorable safety profile compared to MTBE. Pharmacokinetic analysis in SD rats showed that MMP is rapidly absorbed and cleared from the bloodstream, with dose-dependent variability in half-life, indicating efficient excretion. In the pig model, MMP was also found to enter the bloodstream following gallbladder administration, but it was efficiently cleared, with minimal accumulation in tissues. Toxicity assessments in both models revealed that MMP induces significantly less tissue damage than MTBE, with lower levels of apoptosis and inflammation observed in vital organs such as the liver, lungs, and kidneys. These findings suggest that MMP is not only more efficiently excreted but also exhibits a lower toxicity profile, making it a safer alternative to MTBE for clinical use in gallstone dissolution.
MMP demonstrated approximately 1.8 times higher solubility compared to MTBE in the gallstone pig model. Furthermore, various investigative approaches, encompassing real-time PCR, Western blot analysis, and immunohistochemistry, consistently revealed a noteworthy reduction in apoptotic markers such as Bax and c-Caspase-3, coupled with an enhanced expression of the anti-apoptotic marker Bcl-xL in the MMP treatment group relative to the MTBE group. H&E staining demonstrated a well-preserved and organized structure with reduced inflammatory cell infiltration in the MMP-treated group. The TUNEL assay provided corroborative evidence of reduced apoptosis in the MMP group compared to the MTBE group. Given the validation through clinical trials, these findings propose that contact litholysis employing MMP may have the capacity to significantly substitute conventional MTBE treatments and present an attractive alternative to laparoscopic cholecystectomy for specific gallstone patients.
Over 80% of cholesterol gallstones consist of cholesterol, making cholesterol solvents effective in dissolving them. Cholesterol solvents include ether, chloroform, saline solution, and heparin [24]. Among these, ether is an excellent cholesterol solvent, but it has a critical drawback in that it evaporates at body temperature. Whereas ether exhibits a boiling point of 34.6°C, its derivative, MTBE, possesses a higher boiling point of 55°C [21,22]. This is attributed to the structure of MTBE. MTBE possesses an asymmetric molecular structure consisting of a methyl group and a tert-butyl group bonded to an oxygen atom [23]. This structural asymmetry leads to increased molecular complexity and non-uniform electron distribution, resulting in stronger intermolecular forces, particularly dipole-dipole interactions and London dispersion forces, between MTBE molecules. As a result, MTBE exhibits a higher boiling temperature than ether, necessitating more energy to disrupt the intermolecular bonds within its molecules. Therefore, MTBE has been recognized as one of the very few ethers that can be used as a gallstone-dissolving compound.
Despite the higher boiling point of MTBE, it remains insufficiently high to prevent evaporation within the body, leading to the occurrence of various adverse effects, which poses a significant concern. Upon administered into gallbladder, MTBE not only gets absorbed in the gallbladder or duodenum, leading to side effects like drowsiness, nausea, or vomiting [9-14,17], but it can also be systemically absorbed, resulting in hemolysis and reversible kidney injury [25]. Inhalation of MTBE in rats has been associated with a statistically significant increase in kidney and liver tumors [26]. Additionally, MTBE exposure has been linked to the development of cancers in various organs and tissues, resembling those induced by exposure to equivalent doses of known carcinogens like benzene, vinyl chloride, and 1,3-butadiene [27]. In rats, oral exposure to MTBE has shown dose-dependent, statistically significant increases in carcinomas such as lymphoma, leukemia, and Leydig cell carcinoma of the testes [28].
Our quest to mitigate the adverse effects of MTBE and enhance the safety of gallstone-dissolving compounds for potential medical use has led to the discovery of MMP, a derivative of MTBE. MMP primarily functions as an MTBE analogue distinguished by the inclusion of an aromatic moiety. MTBE itself is characterized as an asymmetric chemical compound housing an ether functional group. One facet of MTBE consists of a substantial tert-butyl group, while the opposing facet comprises a more straightforward methyl group. In the case of MMP, its structure involves the substitution of MTBE’s bulky aliphatic tert-butyl group with an aromatic functional group, while retaining the methyl group on the opposite side. A noteworthy attribute of compounds containing an aromatic ring is their relatively higher boiling point and reduced vapor pressure. Consequently, MMP exhibits a notably higher boiling point at 156°C, leading to diminished evaporation at room temperature and ultimately resulting in reduced toxicities while upholding the dissolving capabilities of MTBE. In our previous publication, we have demonstrated the heightened dissolving capacity and reduced toxicity of MMP when compared to MTBE, through investigations involving the dissolution mechanisms and the use of a hamster gallstone model [23].
In summary, this study represents the first validation report of MMP, a gallstone-dissolving compound, applied to the large animal model. To assess the solubility and toxicological profile of MMP, a comprehensive series of experiments were conducted, encompassing both in vitro investigations employing human gallstones and in vivo assessments employing a pig model of gallstones. Across these experiments, MMP consistently exhibited superior solubility compared to MTBE for cholesterol gallstones, while maintaining a lower level of associated toxicity. Furthermore, it should be noted that MMP possesses a higher boiling point relative to MTBE (55°C vs. 156°C). Consequently, MMP is anticipated to exhibit reduced in vivo evaporation-induced adverse effects, including nausea, vomiting, abdominal pain, diarrhea, and hemolytic anemia, which are often associated with MTBE’s lower boiling point. In light of these findings, MMP holds promise as a compelling alternative capable of replacing MTBE effectively.
Acknowledgements
We express our gratitude to Jeong-Yeon Seo for manuscript processing and to Jennifer Lee and Jeong-Hun Kwak for the contributions in illustration work. This work was supported by the Catholic Medical Center Research Foundation made in the program year of 2020, and by Korea Drug Development Fund funded by Ministry of Science and ICT, Ministry of Trade, Industry, and Energy, and Ministry of Health and Welfare (RS-2022-00167125, Republic of Korea).
Disclosure of conflict of interest
None.
References
- 1.Lammert F, Gurusamy K, Ko CW, Miquel JF, Mendez-Sanchez N, Portincasa P, van Erpecum KJ, van Laarhoven CJ, Wang DQ. Gallstones. Nat Rev Dis Primers. 2016;2:16024. doi: 10.1038/nrdp.2016.24. [DOI] [PubMed] [Google Scholar]
- 2.Pavlidis ET, Pavlidis TE. Current management of concomitant cholelithiasis and common bile duct stones. World J Gastrointest Surg. 2023;15:169–176. doi: 10.4240/wjgs.v15.i2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurihara H, Binda C, Cimino MM, Manta R, Manfredi G, Anderloni A. Acute cholecystitis: which flow-chart for the most appropriate management? Dig Liver Dis. 2023;55:1169–1177. doi: 10.1016/j.dld.2023.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Shabanzadeh DM. The symptomatic outcomes of cholecystectomy for gallstones. J Clin Med. 2023;12:1897. doi: 10.3390/jcm12051897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Worth PJ, Kaur T, Diggs BS, Sheppard BC, Hunter JG, Dolan JP. Major bile duct injury requiring operative reconstruction after laparoscopic cholecystectomy: a follow-on study. Surg Endosc. 2016;30:1839–1846. doi: 10.1007/s00464-015-4469-2. [DOI] [PubMed] [Google Scholar]
- 6.Tornqvist B, Stromberg C, Persson G, Nilsson M. Effect of intended intraoperative cholangiography and early detection of bile duct injury on survival after cholecystectomy: population based cohort study. BMJ. 2012;345:e6457. doi: 10.1136/bmj.e6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alotaibi AM. Post-cholecystectomy syndrome: a cohort study from a single private tertiary center. J Taibah Univ Med Sci. 2022;18:383–389. doi: 10.1016/j.jtumed.2022.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen MJ, Borody TJ, Bugliosi TF, May GR, LaRusso NF, Thistle JL. Rapid dissolution of gallstones by methyl tert-butyl ether. Preliminary observations. N Engl J Med. 1985;312:217–220. doi: 10.1056/NEJM198501243120406. [DOI] [PubMed] [Google Scholar]
- 9.Hellstern A, Leuschner U, Benjaminov A, Ackermann H, Heine T, Festi D, Orsini M, Roda E, Northfield TC, Jazrawi R, Kurtz W, Schmeck-Lindenau HJ, Stumpf J, Eidsvoll BE, Aadland E, Lux G, Boehnke E, Wurbs D, Delhaye M, Cremer M, Sinn I, Horing E, v Gaisberg U, Neubrand M, Paul F, et al. Dissolution of gallbladder stones with methyl tert-butyl ether and stone recurrence: a European survey. Dig Dis Sci. 1998;43:911–920. doi: 10.1023/a:1018811409538. [DOI] [PubMed] [Google Scholar]
- 10.Allen MJ, Borody TJ, Bugliosi TF, May GR, LaRusso NF, Thistle JL. Cholelitholysis using methyl tertiary butyl ether. Gastroenterology. 1985;88:122–125. doi: 10.1016/s0016-5085(85)80143-8. [DOI] [PubMed] [Google Scholar]
- 11.Brandon JC, Teplick SK, Haskin PH, Sammon JK, Muhr WF, Hofmann AF, Gambescia RA, Zitomer N. Common bile duct calculi: updated experience with dissolution with methyl tertiary butyl ether. Radiology. 1988;166:665–667. doi: 10.1148/radiology.166.3.3340760. [DOI] [PubMed] [Google Scholar]
- 12.Di Padova C, Di Padova F, Montorsi W, Tritapepe R. Methyl tert-butyl ether fails to dissolve retained radiolucent common bile duct stones. Gastroenterology. 1986;91:1296–1300. doi: 10.1016/s0016-5085(86)80030-0. [DOI] [PubMed] [Google Scholar]
- 13.Diaz D, Bories P, Ampelas M, Larrey D, Michel H. Methyl tert-butyl ether in the endoscopic treatment of common bile duct radiolucent stones in elderly patients with nasobiliary tube. Dig Dis Sci. 1992;37:97–100. doi: 10.1007/BF01308349. [DOI] [PubMed] [Google Scholar]
- 14.Foerster EC, Matek W, Domschke W. Endoscopic retrograde cannulation of the gallbladder: direct dissolution of gallstones. Gastrointest Endosc. 1990;36:444–450. doi: 10.1016/s0016-5107(90)71112-1. [DOI] [PubMed] [Google Scholar]
- 15.Kelly E, Williams JD, Organ CH. A history of the dissolution of retained choledocholithiasis. Am J Surg. 2000;180:86–98. doi: 10.1016/s0002-9610(00)00428-1. [DOI] [PubMed] [Google Scholar]
- 16.Neoptolemos JP, Hall C, O’Connor HJ, Murray WR, Carr-Locke DL. Methyl-tert-butyl-ether for treating bile duct stones: the British experience. Br J Surg. 1990;77:32–35. doi: 10.1002/bjs.1800770111. [DOI] [PubMed] [Google Scholar]
- 17.Saraya A, Rai RR, Tandon RK. Experience with MTBE as a solvent for common bile duct stones in patients with T-tube in situ. J Gastroenterol Hepatol. 1990;5:130–134. doi: 10.1111/j.1440-1746.1990.tb01817.x. [DOI] [PubMed] [Google Scholar]
- 18.vanSonnenberg E, Hofmann AF, Neoptolemus J, Wittich GR, Princenthal RA, Willson SW. Gallstone dissolution with methyl-tert-butyl ether via percutaneous cholecystostomy: success and caveats. AJR Am J Roentgenol. 1986;146:865–867. doi: 10.2214/ajr.146.4.865. [DOI] [PubMed] [Google Scholar]
- 19.vanSonnenberg E, Casola G, Zakko SF, Varney RR, Cox J, Wittich GR, Hofmann AF. Gallbladder and bile duct stones: percutaneous therapy with primary MTBE dissolution and mechanical methods. Radiology. 1988;169:505–509. doi: 10.1148/radiology.169.2.3174999. [DOI] [PubMed] [Google Scholar]
- 20.Thistle JL, May GR, Bender CE, Williams HJ, LeRoy AJ, Nelson PE, Peine CJ, Petersen BT, McCullough JE. Dissolution of cholesterol gallbladder stones by methyl tert-butyl ether administered by percutaneous transhepatic catheter. N Engl J Med. 1989;320:633–639. doi: 10.1056/NEJM198903093201004. [DOI] [PubMed] [Google Scholar]
- 21.Leuschner U, Hellstern A, Schmidt K, Fischer H, Guldutuna S, Hubner K, Leuschner M. Gallstone dissolution with methyl tert-butyl ether in 120 patients--efficacy and safety. Dig Dis Sci. 1991;36:193–199. doi: 10.1007/BF01300756. [DOI] [PubMed] [Google Scholar]
- 22.Esch O, Spinosa JC, Hamilton RL, Crombie DL, Schteingart CD, Rondinone JF, D’Agostino HB, Lillienau J, Hofmann AF. Acute effects of topical methyl tert-butyl ether or ethyl propionate on gallbladder histology in animals: a comparison of two solvents for contact dissolution of cholesterol gallstones. Hepatology. 1992;16:984–991. doi: 10.1002/hep.1840160422. [DOI] [PubMed] [Google Scholar]
- 23.Choi HJ, Cho SJ, Kim OH, Song JS, Hong HE, Lee SC, Kim KH, Lee SK, You YK, Hong TH, Kim EY, Park JH, Na GH, Do You D, Han JH, Park JW, Kwak BJ, Lee TY, Ahn J, Lee HH, Kang SK, Hwang KS, Jung JK, Jung KY, Kim SJ. Efficacy and safety of a novel topical agent for gallstone dissolution: 2-methoxy-6-methylpyridine. J Transl Med. 2019;17:195. doi: 10.1186/s12967-019-1943-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pitt HA, McFadden DW, Gadacz TR. Agents for gallstone dissolution. Am J Surg. 1987;153:233–246. doi: 10.1016/0002-9610(87)90822-1. [DOI] [PubMed] [Google Scholar]
- 25.Ponchon T, Baroud J, Pujol B, Valette PJ, Perrot D. Renal failure during dissolution of gallstones by methyl-tert-butyl ether. Lancet. 1988;2:276–277. doi: 10.1016/s0140-6736(88)92562-7. [DOI] [PubMed] [Google Scholar]
- 26.Mehlman MA. Methyl-tertiary-butyl-ether (MTBE) misclassified. Am J Ind Med. 2001;39:505–508. doi: 10.1002/ajim.1044. [DOI] [PubMed] [Google Scholar]
- 27.Bird MG, Burleigh-Flayer HD, Chun JS, Douglas JF, Kneiss JJ, Andrews LS. Oncogenicity studies of inhaled methyl tertiary-butyl ether (MTBE) in CD-1 mice and F-344 rats. J Appl Toxicol. 1997;17(Suppl 1):S45–55. doi: 10.1002/(sici)1099-1263(199705)17:1+<s45::aid-jat410>3.3.co;2-b. [DOI] [PubMed] [Google Scholar]
- 28.Mehlman MA. Dangerous and cancer-causing properties of products and chemicals in the oil-refining and petrochemical industry--Part XXII: health hazards from exposure to gasoline containing methyl tertiary butyl ether: study of New Jersey residents. Toxicol Ind Health. 1996;12:613–627. doi: 10.1177/074823379601200502. [DOI] [PubMed] [Google Scholar]