Abstract
Objective: To analyze the effect of interventional surgery on treatment efficacy and inflammatory cytokine (ICK) levels in diabetic patients with peripheral vascular disease (PVD). Methods: A total of 116 diabetic patients with PVD admitted to the Second Affiliated Hospital of Guangzhou Medical University from August 2020 to March 2022 were selected for this retrospective analysis. Patients were divided into two groups: 58 patients receiving conventional medication were assigned to the control group, while 58 patients receiving interventional surgery in addition to conventional medication were assigned to the research group. Comparative analyses were conducted based on clinical efficacy, symptom relief (limb pain, numbness, intermittent claudication, and coldness), ankle-brachial index (ABI) scores, vascular endothelial growth factor (VEGF), transforming growth factor-β (TGF-β), arterial inner diameter, and ICK levels. Results: The research group demonstrated a significantly higher overall effective rate compared to the control group, with markedly higher ABI scores, faster symptom resolution, increased VEGF and TGF-β levels, greater arterial diameters, and lower ICK levels. Conclusion: Interventional surgery provides superior outcomes compared to conventional medication for treating diabetic patients with PVD, offering greater benefits in terms of ABI improvement, symptom relief, vascular factors, arterial diameter expansion, and ICK reduction.
Keywords: Interventional surgery, diabetics with peripheral vascular diseases, clinical effectiveness, inflammatory cytokines
Introduction
Diabetes mellitus (DM), a prevalent chronic metabolic disease with a high incidence [1], is characterized by persistently elevated blood glucose levels, which may result from various factors such as genetics and dietary habits [2]. As the disease progresses, DM often leads to complications, among which peripheral vascular disease (PVD) is common [3]. In PVD, the vascular walls lose elasticity and thicken, causing arterial hardening and thrombosis, which may lead to vascular occlusion, peripheral sensory dysfunction, and severe outcomes [4]. The disability rate in diabetics with PVD is high, significantly affecting their quality of life [5]. Common symptoms include pain and numbness in the lower extremities, with some patients developing intermittent claudication or deep tissue damage, such as ulcers [6]. Thus, exploring effective treatment strategies for DM is crucial.
Currently, neurotrophic drugs and local dressing changes are commonly used to treat PVD in diabetics, though their efficacy is limited [7]. Recently, interventional surgery has gained attention as a promising treatment option. It offers advantages such as minimal trauma, rapid recovery, and repeatability [8]. Through interventional procedures, doctors can accurately target stenosed or occluded arteries under imaging guidance and effectively treat the affected areas [9]. This method improves blood supply to the feet, reducing the risk of amputation and enhancing patient prognosis [10]. Given that many diabetic patients suffer from significant lower extremity arteriosclerosis obliterans, interventional surgery can be vital for limb preservation [11], since it reopens affected vessels, restoring blood supply and alleviating ischemic symptoms [12]. Due to the widespread and staged nature of vascular lesions in diabetic feet, traditional surgery often struggles to address the extent of these issues. In contrast, interventional therapy is more flexible [13]. In cases of segmental stenosis, balloon catheters may be used to dilate the vessels, while stents can be implanted for severe stenosis to maintain patency [14]. Furthermore, the combination of glycemic control, anti-infection measures, anticoagulation, microcirculation improvement, and local dressing changes, both before and after interventional treatment, significantly promotes healing of diabetic foot ulcers and reduces disability rates [15]. Interventional therapy has been shown to alleviate ischemic symptoms, reduce pain, and improve prognosis by restoring or increasing blood flow to proximal vessels [16].
In this study, we included 116 diabetic patients with PVD to assess the clinical efficacy of surgical interventional therapy.
Materials and methods
Case selection
This retrospective study was approved by the Ethics Committee of the Second Affiliated Hospital of Guangzhou Medical University. A total of 116 diabetic patients with PVD, admitted from August 2020 to March 2022, were selected. Based on the treatment methods, 58 patients who received conventional medication were assigned to the control group, while 58 patients who received interventional surgery in addition to conventional medication were included in the research group. The specific inclusion and exclusion criteria were as follows:
Inclusion criteria: history of claudication or lower extremity pain at rest; absence of posterior tibial artery pulse or reduced extremity temperature in the lower limbs; arteriosclerosis, stenosis, and intimal-medial thickening confirmed by ultrasound of the lower extremities, or an ankle-brachial index (ABI) less than 0.9 or greater than 1.3; complete case data; initial treatment.
Exclusion criteria: history of cerebrovascular accident or acute myocardial infarction within the past 6 months; presence of malignant tumor, abnormal coagulation function, or other vascular disease; severe organ dysfunction; pregnant or lactating women; psychiatric disorder.
Intervention methods
The control group received conventional treatment, which included neurotrophic drugs, local dressing changes, and long-term subcutaneous insulin therapy. Additionally, a scientific diet and exercise plan was implemented.
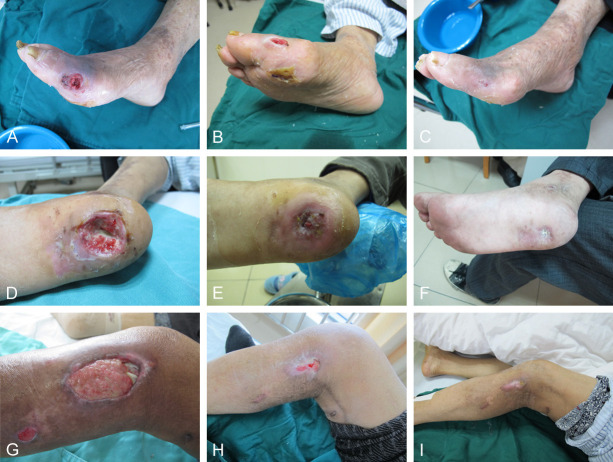
In the research group, patients underwent interventional surgery in addition to the aforementioned treatments. Preoperative screenings and imaging examinations were conducted to ensure the accuracy and safety of the surgery. ABI was assessed, followed by CT imaging of the lower limb arteries to evaluate the degree and location of arterial stenosis. Under local anesthesia, an anterograde or retrograde femoral artery puncture was performed using the Seldinger technique, and a 5F-6F catheter sheath was inserted for heparinization. Digital subtraction angiography was then used to identify the lesion site. Using a guide wire assistance, appropriately sized balloons were delivered to gradually dilate the narrowed arteries. For more severe lesions, percutaneous transluminal angioplasty and, if necessary, stenting were performed to reinforce the vessels. Postoperatively, patients received subcutaneous injections of low molecular weight heparin at 4100 U every 12 hours for one week. Additionally, clopidogrel was administered at 75 mg/day for 6 months, along with long-term aspirin use (100 mg/day). Figure 1 shows the recovery of the ulcer area before and after surgery.
Figure 1.
Recovery of the ulcer site before and after the operation. A-C: Recovery of right arteriosclerosis occlusion and foot ulcer. D-F: Recovery of sole ulcer. G-I: Recovery of left calf ulcer and diabetic foot.
Data collection
The following factors were compared between the two groups: clinical effectiveness, symptom resolution (limb pain, numbness, intermittent claudication, and coldness), vascular endothelial growth factor (VEGF), transforming growth factor-β (TGF-β), arterial inner diameters, adverse reactions, and inflammatory cytokines (ICKs) such as tumor necrosis factor (TNF)-α and interleukin (IL)-6. Primary outcome measures included clinical effectiveness, VEGF, TGF-β, adverse reactions, TNF-α, and IL-6, while secondary outcome measures were symptom resolution and arterial inner diameters.
Clinical Effectiveness: Treatment effectiveness was evaluated using the following criteria. “Markedly effective” was defined as a near-complete resolution of symptoms (e.g., coldness, resting pain, intermittent claudication) and a significant increase or normalization in skin temperature. “Effective” indicated symptom improvement and increased skin temperature compared to before treatment. “Ineffective” was defined as failure to meet these criteria. The total effective rate was calculated as the sum of “markedly effective” and “effective” cases, expressed as a percentage of total cases.
Symptom Resolution Time: The time required for resolution of limb pain, numbness, intermittent claudication, and coldness was recorded and compared between the two groups.
ABI Score: A Doppler ultrasound blood flow detector (RD2, Shanghai Jumu Medical Equipment Co., Ltd., ccc0002) was used to measure the ABI. After positioning the patient in a supine position, upper arm blood pressure was measured, followed by the systolic pressure of the dorsalis pedis and bilateral posterior radial arteries to calculate the ABI. ABI is the ratio of ankle to brachial arterial pressure, with values of ABI<0.9 considered abnormal and 0.9≤ABI≤1.3 considered normal.
Vascular Factors: Before and after treatment, 5 ml of venous blood was collected from the patient’s elbow in a fasting state to measure VEGF and TGF-β levels. Enzyme-linked immunosorbent assays were used to quantify these growth factors following the corresponding kit instructions (Hangzhou MultiSciences Biotech Co., Ltd., 70-EK183-96, EK981).
Arterial Inner Diameters: The inner diameters of the femoral, popliteal, and dorsalis pedis arteries were measured before and after treatment in both groups.
ICKs: Fasting blood samples were collected from both groups before and after the intervention. After allowing the samples to rest at room temperature for 2 hours, they were centrifuged at 3000 r/min for 20 minutes. Plasma was then extracted, sealed in EP tubes, and stored at -30°C. TNF-α and IL-6 levels were determined using chemiluminescent immunoassays (Shanghai Yuduo Biotech Co., Ltd., H356MR) following the instructions of the corresponding kits (Shanghai ExCell Biology Co., Ltd., EH009, EH004).
Statistical methods
Continuous variables were expressed as mean ± SEM and compared between groups using independent t-tests. Categorical variables, expressed as percentages, were compared using the χ2 test. Data were analyzed using SPSS 22.0, with P<0.05 considered statistically significant.
Results
Comparative analysis of general data
There were no significant differences between the research and control groups in terms of age, DM duration, body mass index (BMI), sex, vascular disease type, or comorbidities (all P>0.05) (Table 1).
Table 1.
Comparative analysis of general data
| Indicator | Research group (n=58) | Control group (n=58) | χ2/t | P |
|---|---|---|---|---|
| Age (years) | 57.34±5.21 | 59.04±5.37 | 1.730 | 0.086 |
| Course of diabetes mellitus (year) | 6.21±1.77 | 6.38±1.46 | 0.564 | 0.574 |
| Body mass index (kg/m2) | 24.65±3.21 | 24.57±3.18 | 0.135 | 0.893 |
| Gender | 1.694 | 0.193 | ||
| Male | 24 (41.38) | 31 (53.45) | ||
| Female | 34 (58.62) | 27 (46.55) | ||
| Type of vascular diseases | 1.561 | 0.668 | ||
| Cardiovascular disease | 12 (20.69) | 14 (24.14) | ||
| Cerebrovascular disease | 10 (17.24) | 8 (13.79) | ||
| Retinopathy | 25 (43.10) | 29 (50.00) | ||
| Others | 11 (18.97) | 7 (12.07) | ||
| Comorbidities | 1.429 | 0.490 | ||
| Hypertension | 18 (31.03) | 16 (27.59) | ||
| Coronary heart disease | 24 (41.38) | 20 (34.48) | ||
| Hyperlipidaemia | 16 (27.59) | 22 (37.93) |
Comparative analysis of clinical effectiveness
The total effective rate was 94.83% in the research group and 70.69% in the control group, indicating significantly higher clinical effectiveness in the research group (P<0.05) (Table 2).
Table 2.
Comparative analysis of clinical effectiveness
| Indicator | Research group (n=58) | Control group (n=58) | χ2 | P |
|---|---|---|---|---|
| Marked effectiveness | 42 (72.41) | 15 (25.86) | ||
| Effectiveness | 13 (22.41) | 26 (44.83) | ||
| Ineffectiveness | 3 (5.17) | 17 (29.31) | ||
| Total | 55 (94.83) | 41 (70.69) | 11.842 | 0.001 |
Comparative analysis of symptom resolution
The resolution times for symptoms such as limb pain, numbness, intermittent claudication, and coldness were shorter in the research group compared to the control group (P<0.05) (Table 3).
Table 3.
Comparative analysis of symptom resolution time
| Group | n | Resolution time of limb pain (months) | Resolution time of limb numbness (months) | Resolution time of intermittent claudication (months) | Resolution time of limb coldness (months) |
|---|---|---|---|---|---|
| Research group | 58 | 3.21±0.42 | 3.13±0.38 | 3.15±0.41 | 3.52±0.45 |
| Control group | 58 | 4.45±0.98 | 4.36±0.89 | 4.10±0.82 | 4.61±0.97 |
| t | - | 8.857 | 9.680 | 7.892 | 7.763 |
| P | - | <0.001 | <0.001 | 0.001 | <0.001 |
Comparative analysis of ABI scores before and after intervention
No significant difference in ABI scores was observed between the groups before intervention ((0.68±0.13) vs. (0.70±0.15), P>0.05). After treatment, both groups showed significant increases in ABI scores, with a greater improvement in the research group ((0.98±0.27) vs. (0.83±0.21), P<0.05) (Figure 2).
Figure 2.
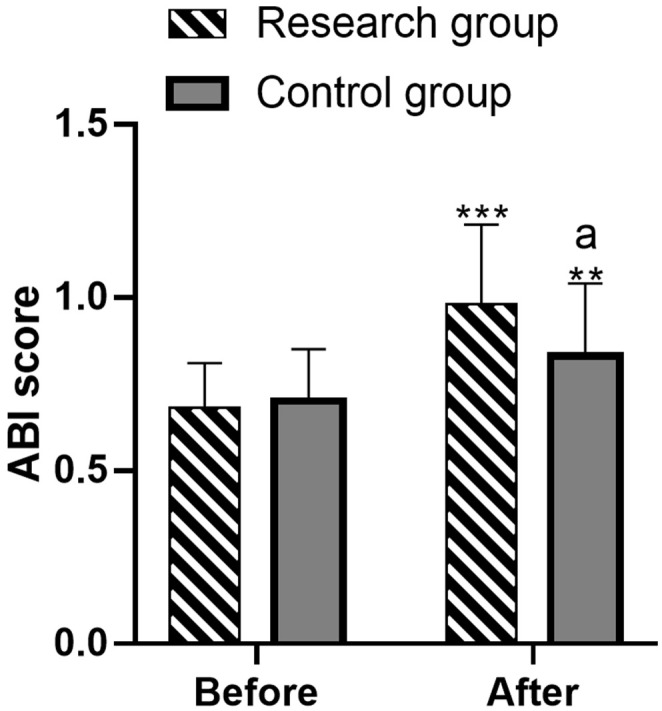
Evaluation of the ankle-brachial index (ABI) scores before and after intervention in two groups. Note: ** denotes P<0.01 versus before treatment, *** denotes P<0.001 versus before treatment, and (a) represents P<0.05 versus the control group.
Comparative analysis of VEGF and TGF-β before and after intervention
Before the intervention, there were no significant differences in VEGF ((112.75±15.34) ng/L vs. (111.38±15.27) ng/L) and TGF-β ((41.45±4.97) ng/mL vs. (41.58±4.89) ng/mL) levels between the two groups (both P>0.05). After intervention, both VEGF ((143.75±20.32) ng/L vs. (122.46±19.74) ng/L) and TGF-β ((56.78±5.09) ng/mL vs. (45.65±5.03) ng/mL) levels increased in both groups, with significantly higher levels in the research group (both P<0.05) (Figure 3).
Figure 3.
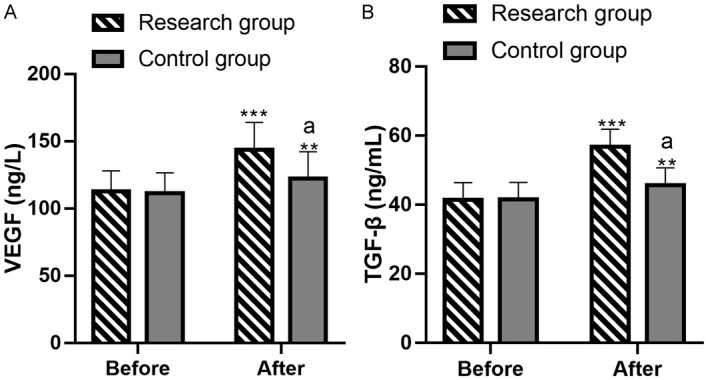
Evaluation of VEGF and TGF-β levels before and after intervention. A: VEGF levels before and after intervention. B: The levels of TGF-β before and after intervention. Note: **P<0.01 vs. before treatment, *** denotes P<0.001 versus before treatment; aP<0.05 vs. Control. VEGF, vascular endothelial growth factor; TGF-β, transforming growth factor-β.
Comparative analysis of arterial inner diameters
Before intervention, the inner diameters of the femoral ((6.94±0.63) mm vs. (7.02±0.68) mm), popliteal ((5.11±0.24) mm vs. (5.08±0.21) mm), and dorsalis pedis arteries ((1.20±0.14) mm vs. (1.19±0.17) mm) were not significantly different between the groups (all P>0.05). After intervention, the inner diameters of the femoral ((7.75±0.72) mm vs. (7.32±0.64) mm), popliteal ((5.73±0.31) mm vs. (5.31±0.28) mm), and dorsalis pedis arteries ((1.68±0.22) mm vs. (1.27±0.19) mm) increased significantly, with greater enlargement in the research group (all P<0.05) (Figure 4).
Figure 4.
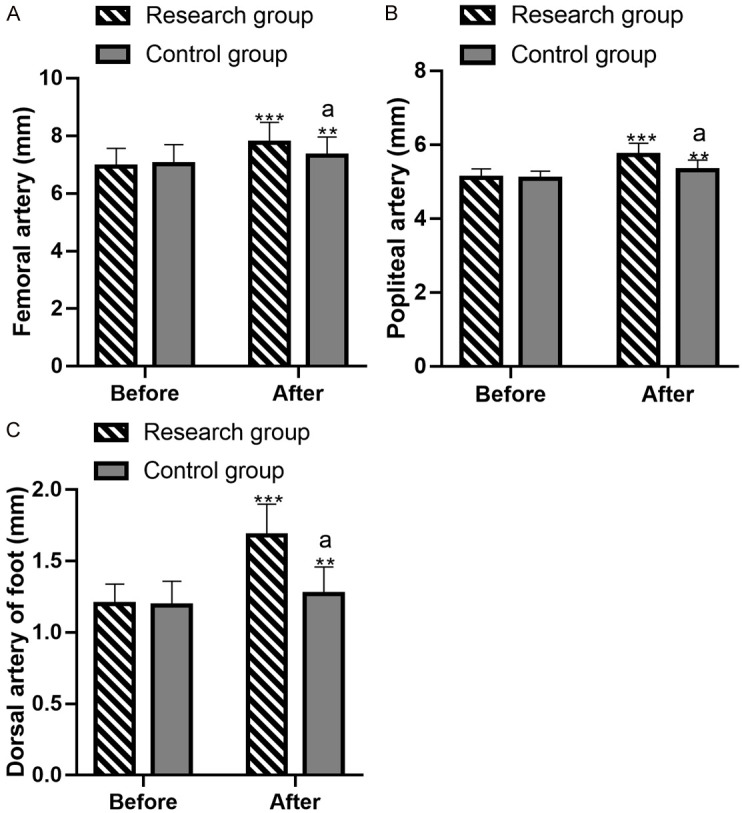
Assessment of the inner diameters of arteries. A: Inner diameter of the femoral artery before and after intervention. B: Inner diameter of the popliteal artery before and after intervention. C: Inner diameter of the dorsalis pedis artery before and after intervention. Note: **P<0.01 vs. before treatment, *** denotes P<0.001 versus before treatment; aP<0.05 vs. control.
Comparative analysis of ICKs
Before the intervention, there were no significant differences in TNF-α ((12.33±1.56) pg/mL vs. (13.08±1.71) pg/mL) and IL-6 ((7.34±0.62) pg/mL vs. (8.11±0.64) pg/mL) levels between the two groups (both P>0.05). After intervention, both TNF-α ((3.54±0.21) pg/mL vs. (5.57±0.42) pg/mL) and IL-6 ((2.07±0.21) pg/mL vs. (5.24±0.32) pg/mL) levels decreased significantly, with lower levels in the research group (both P<0.05) (Figure 5).
Figure 5.
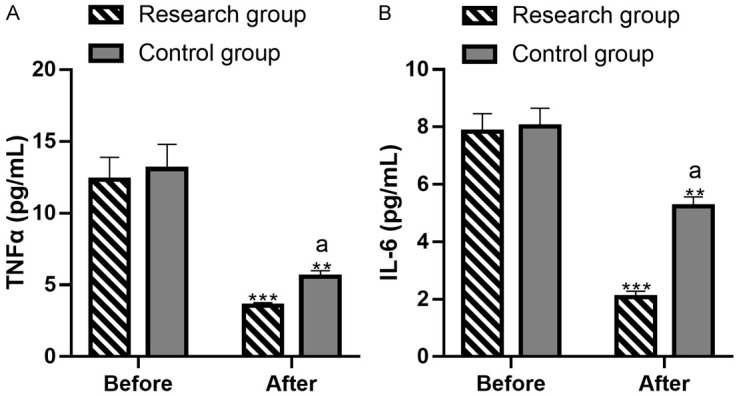
Evaluation of levels of inflammatory cytokines before and after intervention. A: TNFα levels before and after intervention. B: IL-6 levels before and after intervention. Note: **P<0.01 vs. before treatment, *** denotes P<0.001 versus before treatment; aP<0.05 vs. Control. TNF-α, tumor necrosis factor α; IL-6, interleukin 6.
Discussion
Peripheral vascular disease (PVD) is a common and severe complication of DM, particularly affecting the arteries of the lower limbs [17]. Systemic atherosclerosis is a key manifestation of PVD in diabetic patients. When systemic atherosclerosis occurs, thrombosis, plaque formation, and other lesions may develop within the arteries, leading to lumen stenosis and, in severe cases, occlusion, resulting in ischemia in the distal extremities [18,19]. Atherosclerosis-induced vascular disease severely compromises blood flow in the lower limb arteries. When vascular stenosis or occlusion occurs, blood supply to the feet and surrounding tissues is restricted, causing ischemic symptoms [20]. These symptoms may include pain, weakness, numbness in the lower limbs, and in more severe cases, ulcers or gangrene [21]. In this study, we proposed interventional surgery as an intervention method to evaluate its benefits for diabetic patients with PVD and to provide additional clinical evidence.
The primary advantage of intravascular interventional surgery is its minimally invasive nature. Unlike traditional open surgery, it requires only a small catheter or puncture site to access the blood vessels and directly reach the lesion [22]. This minimally invasive approach reduces intraoperative bleeding and infection risk, while also allowing for quicker postoperative recovery [23]. The precision of this technique enables targeted treatment of the lesion, enhancing the therapeutic effect [24]. Patients can often return to normal activities sooner. Studies have shown that intravascular interventional surgery is effective for DM and peripheral arterial diseases, causing less trauma and promoting faster recovery [25]. In our study, the research group had a total effective rate of 94.83%, significantly higher than the control group (70.69%), suggesting that interventional surgery offers substantial advantages in improving clinical efficacy. Many DM patients experience lower limb pain and, in severe cases, face the risk of amputation due to vascular occlusion [26]. The resolution times for limb pain, numbness, intermittent claudication, and coldness were significantly faster in the research group compared to the control group, indicating that interventional surgery effectively identifies narrowed or occluded arteries, improves blood supply to the affected areas, alleviates disease-related symptoms, and reduces the risk of amputation.
ABI is an important medical marker with high diagnostic value in various diseases [27]. Historically, ABI has been primarily used to detect peripheral arterial disease (PAD) in the lower limbs. By measuring the blood pressure ratio between the ankle and brachial arteries, physicians can assess blood flow insufficiency in the lower extremities, enabling timely detection and treatment of vascular diseases [28]. As medical research has advanced, the application of ABI has broadened, and it is now an essential criterion for diagnosing PVD in diabetic patients [29]. In diabetic patients, ABI measurement helps assess PVD risk, allowing clinicians to develop personalized treatment plans, thereby improving treatment outcome and quality of life [30].
In this study, ABI scores were evaluated before and after intervention. Both groups showed significant increases in ABI scores after treatment, with a more pronounced improvement in the research group. The analysis of vascular factors indicated a marked increase in VEGF and TGF-β levels after intervention in both groups, particularly in the research group, suggesting that interventional surgery promotes vascular regeneration and protects the vascular endothelium. Measurements of arterial inner diameters showed enlargement of the femoral, popliteal, and dorsalis pedis arteries post-intervention, with larger increases observed in the research group. This indicates that interventional therapy effectively dilates blood vessels and restores vascular function. Additionally, ICK levels, including TNF-α and IL-6, significantly decreased after intervention, especially in the research group, indicating that interventional surgery reduces inflammation and enhances therapeutic efficacy.
The mechanism of interventional surgery for treating PVD in diabetics likely involves the following: First, the use of imaging-guided technology allows for precise localization and treatment of affected areas, significantly improving ABI and alleviating symptoms [31]. Second, this surgical approach protects vascular endothelium, restores vascular function, reduces inflammatory responses, and delivers significant clinical benefits.
This study has certain limitations. First, as a retrospective, single-center study, there may be biases in data collection. Future multicenter prospective studies are needed to mitigate such biases and improve the accuracy of the results. Second, the study lacks a multivariate regression analysis, which would provide valuable insight into additional positive intervention strategies to enhance efficacy. Third, no long-term prognostic analysis was conducted. Follow-up studies should assess the long-term prognosis to better understand the effects and advantages of this treatment. Future research will address these limitations.
In conclusion, interventional surgery for diabetic patients with PVD demonstrates significant clinical advantages, improving treatment efficacy, reducing ICK levels, expanding arterial inner diameters, and enhancing vascular factor levels.
Acknowledgements
This study was supported by the grants from the National Natural Science Foundation of China (Nos. 82160577, 82073762); Key Program for Regional Joint Funds of Natural Science Foundation of Guangdong Province (Nos. 2020B1515120094, 2021B1515120053); the Program for Science and Technology Plan of Guangzhou (Nos. 202201020197, 2023A03J0405, 202102010071); and Guangdong Medical Science and Technology Research Fund (No. A2023205).
Disclosure of conflict of interest
None.
References
- 1.Poznyak A, Grechko AV, Poggio P, Myasoedova VA, Alfieri V, Orekhov AN. The diabetes mellitus-atherosclerosis connection: the role of lipid and glucose metabolism and chronic inflammation. Int J Mol Sci. 2020;21:1835. doi: 10.3390/ijms21051835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan MT, Chao CT, Lin SH. Chronic kidney disease: strategies to retard progression. Int J Mol Sci. 2021;22:10084. doi: 10.3390/ijms221810084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossing P, Baeres FMM, Bakris G, Bosch-Traberg H, Gislum M, Gough SCL, Idorn T, Lawson J, Mahaffey KW, Mann JFE, Mersebach H, Perkovic V, Tuttle K, Pratley R. The rationale, design and baseline data of FLOW, a kidney outcomes trial with once-weekly semaglutide in people with type 2 diabetes and chronic kidney disease. Nephrol Dial Transplant. 2023;38:2041–2051. doi: 10.1093/ndt/gfad009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altalhi W, Sun X, Sivak JM, Husain M, Nunes SS. Diabetes impairs arterio-venous specification in engineered vascular tissues in a perivascular cell recruitment-dependent manner. Biomaterials. 2017;119:23–32. doi: 10.1016/j.biomaterials.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Yin Z, Wang X, Yang X, Chen Y, Duan Y, Han J. Salvia miltiorrhiza in anti-diabetic angiopathy. Curr Mol Pharmacol. 2021;14:960–974. doi: 10.2174/1874467214999210111222918. [DOI] [PubMed] [Google Scholar]
- 6.Hicks CW, Selvin E. Epidemiology of peripheral neuropathy and lower extremity disease in diabetes. Curr Diab Rep. 2019;19:86. doi: 10.1007/s11892-019-1212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sloan G, Selvarajah D, Tesfaye S. Pathogenesis, diagnosis and clinical management of diabetic sensorimotor peripheral neuropathy. Nat Rev Endocrinol. 2021;17:400–420. doi: 10.1038/s41574-021-00496-z. [DOI] [PubMed] [Google Scholar]
- 8.Stone PH, Libby P, Boden WE. Fundamental pathobiology of coronary atherosclerosis and clinical implications for chronic ischemic heart disease management-the plaque hypothesis: a narrative review. JAMA Cardiol. 2023;8:192–201. doi: 10.1001/jamacardio.2022.3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zembala MO, Suwalski P. Minimally invasive surgery for atrial fibrillation. J Thorac Dis. 2013;5(Suppl 6):S704–712. doi: 10.3978/j.issn.2072-1439.2013.10.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varma P, Stineman MG, Dillingham TR. Epidemiology of limb loss. Phys Med Rehabil Clin N Am. 2014;25:1–8. doi: 10.1016/j.pmr.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hussain MA, Al-Omran M, Salata K, Sivaswamy A, Forbes TL, Sattar N, Aljabri B, Kayssi A, Verma S, de Mestral C. Population-based secular trends in lower-extremity amputation for diabetes and peripheral artery disease. CMAJ. 2019;191:E955–E961. doi: 10.1503/cmaj.190134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manickum P, Ramklass SS, Madiba TE. Diabetes and lower extremity amputation - rehabilitation pathways and outcomes at a regional hospital. S Afr J Surg. 2021;59:128a–128g. [PubMed] [Google Scholar]
- 13.Boulton AJ. Why bother educating the multi-disciplinary team and the patient--the example of prevention of lower extremity amputation in diabetes. Patient Educ Couns. 1995;26:183–188. doi: 10.1016/0738-3991(95)00746-m. [DOI] [PubMed] [Google Scholar]
- 14.Percutaneous transluminal angioplasty. Ann Intern Med. 1983;99:864–869. doi: 10.7326/0003-4819-99-6-864. [DOI] [PubMed] [Google Scholar]
- 15.Galyfos G, Liakopoulos D, Sigala F, Filis K. New paradigms in minimally-invasive vascular surgery. Expert Rev Cardiovasc Ther. 2022;20:207–214. doi: 10.1080/14779072.2022.2058492. [DOI] [PubMed] [Google Scholar]
- 16.Zhao SR, Huang R, Liu F, Li Y, Gong Y, Xing J. Study on correlation between type 2 diabetes and no-reflow after PCI. Dis Markers. 2022;2022:7319277. doi: 10.1155/2022/7319277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pikula A, Howard BV, Seshadri S. Stroke and diabetes. In: Cowie CC, Casagrande SS, Menke A, Cissell MA, Eberhardt MS, Meigs JB, Gregg EW, Knowler WC, Barrett-Connor E, Becker DJ, Brancati FL, Boyko EJ, Herman WH, Howard BV, Narayan KMV, Rewers M, Fradkin JE, editors. Diabetes in America. 3rd edition. Bethesda (MD) interest; 2018. [Google Scholar]
- 18.Mohseni-Moghaddam P, Ghobadian R, Khaleghzadeh-Ahangar H. Dementia in diabetes mellitus and atherosclerosis: two interrelated systemic diseases. doi: 10.1016/j.brainresbull.2022.01.018. [DOI] [PubMed] [Google Scholar]
- 19.Sudar-Milovanovic E, Gluvic Z, Obradovic M, Zaric B, Isenovic ER. Tryptophan metabolism in atherosclerosis and diabetes. Curr Med Chem. 2022;29:99–113. doi: 10.2174/0929867328666210714153649. [DOI] [PubMed] [Google Scholar]
- 20.Menegaut L, Laubriet A, Crespy V, Leleu D, Pilot T, Van Dongen K, de Barros JP, Gautier T, Petit JM, Thomas C, Nguyen M, Steinmetz E, Masson D. Inflammation and oxidative stress markers in type 2 diabetes patients with advanced carotid atherosclerosis. Cardiovasc Diabetol. 2023;22:248. doi: 10.1186/s12933-023-01979-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mills JL Sr, Conte MS, Armstrong DG, Pomposelli FB, Schanzer A, Sidawy AN, Andros G Society for Vascular Surgery Lower Extremity Guidelines Committee. The society for vascular surgery lower extremity threatened limb classification system: risk stratification based on wound, ischemia, and foot infection (WIfI) J Vasc Surg. 2014;59:220–234. e221–222. doi: 10.1016/j.jvs.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Taeymans K, Goverde P, Lauwers K, Verbruggen P. The CERAB technique: tips, tricks and results. J Cardiovasc Surg (Torino) 2016;57:343–349. [PubMed] [Google Scholar]
- 23.Coselli JS, Spiliotopoulos K, Preventza O, de la Cruz KI, Amarasekara H, Green SY. Open aortic surgery after thoracic endovascular aortic repair. Gen Thorac Cardiovasc Surg. 2016;64:441–449. doi: 10.1007/s11748-016-0658-8. [DOI] [PubMed] [Google Scholar]
- 24.Baker B. Anaesthesia and endovascular surgery. Best Pract Res Clin Anaesthesiol. 2002;16:95–113. doi: 10.1053/bean.2001.0210. [DOI] [PubMed] [Google Scholar]
- 25.Chin JA, Sumpio BE. Diabetes mellitus and peripheral vascular disease: diagnosis and management. Clin Podiatr Med Surg. 2014;31:11–26. doi: 10.1016/j.cpm.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Crane H, Boam G, Carradice D, Vanicek N, Twiddy M, Smith GE. Through-knee versus above-knee amputation for vascular and non-vascular major lower limb amputations. Cochrane Database Syst Rev. 2021;12:CD013839. doi: 10.1002/14651858.CD013839.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woo JS. Ankle brachial index: a simple path to the future. Korean J Intern Med. 2023;38:277–279. doi: 10.3904/kjim.2023.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casey S, Lanting S, Oldmeadow C, Chuter V. The reliability of the ankle brachial index: a systematic review. J Foot Ankle Res. 2019;12:39. doi: 10.1186/s13047-019-0350-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nie F, He J, Cao H, Hu X. Predictive value of abnormal ankle-brachial index in patients with diabetes: a meta-analysis. Diabetes Res Clin Pract. 2021;174:108723. doi: 10.1016/j.diabres.2021.108723. [DOI] [PubMed] [Google Scholar]
- 30.Lima ACGB, Goncalves MF, Rocha EV, D’Avila LBO, Mascarenhas AN. Ankle-brachial index and subclinical atherosclerosis in type 1 diabetes. Rev Assoc Med Bras (1992) 2021;67:505–510. doi: 10.1590/1806-9282.20200695. [DOI] [PubMed] [Google Scholar]
- 31.Mohan IV, Stephen MS. Peripheral arterial aneurysms: open or endovascular surgery? Prog Cardiovasc Dis. 2013;56:36–56. doi: 10.1016/j.pcad.2013.06.001. [DOI] [PubMed] [Google Scholar]