Abstract
Elizabethkingia anophelis is an obligate aerobic, Gram-negative bacillus belonging to the family of Weeksellacease. In recent years, there has been a gradual increase in E. anophelis related infections, especially in chronically hospitalized, immunocompromised populations that often develop severe pneumonia. Severe pneumonia is one of the common critical illnesses. Next-generation sequencing technologies developed based on PCR and gene chip technologies play an important role in the identification of pathogenic microorganisms and in molecular diagnosis. In this case, we report an elderly male patient diagnosed with severe pneumonia during the COVID-19 pandemic. The causative organisms were clearly identified as Elizabethkingia anophelis combined with Acinetobacter baumannii by next-generation sequencing. He was discharged from the hospital after being given cefoperazone sulbactam sodium and minocycline, as well as nebulized inhalation of chymotrypsin, salbutamol, and budesonide to assist ventilation. This article summarizes the diagnosis and treatment of this patient in order to share the experience of the subsequent treatments.
Keywords: Elizabethkingia anophelis, severe pneumonia, metagenomic sequencing
Introduction
E. anophelis was first isolated from the midgut of Anopheles gambiae in 2008, and proved in 2011 as a new species in Elizabethkingia genus [1]. To date, the genus Elisabethella comprises eight species: E. meningitidis, E. miltiorrhiza, E. anopheles, E. bruniana, E. ursingii, E. occulta, E. tumeracha sp. nov., and E. argenteiflava sp. nov. [2-5]. Since it was discovered, other studies have shown that Anopheles elizabethiensis is the main endemic causative agent of the genus, and there has been a gradual increase in the incidence of Anopheles infections and occasional epidemic outbreaks [6-9]. In recent years, cases of Anopheles infections have been reported in the Central African Republic, Singapore, Hong Kong (China), Taiwan (China), the United States, and France. Because of its high resistance to most antibiotics, the treatment is very difficult resulting in high mortality rates [10-13]. Patients with lung infections due to Anopheles mosquitoes tend to be immunocompromised, which often causes exacerbation of the infection and severe pneumonia, leading to high risk of death [6,7].
For the treatment of severe pneumonia, next generation sequencing (NGS) can rapidly and accurately identify rare or emerging pathogens, thus establishing a pathogenic diagnosis and timely personal anti-infection treatment. There are two main ways to identify microorganisms based on NGS: metagenomic next generation sequencing (mNGS) and targeted next generation sequencing (tNGS). A study has reported that mNGS could improve the diagnostic accuracy and survival rate of severe pneumonia patients in the ICU [14]. With the rapid development of NGS technology, the throughput of analysis has increased and the cost decreased, promoting its important role in the identification of infectious disease pathogens.
In this case report, we reported an elderly male with severe pneumonia due to infection with Anopheles stephensi and Acinetobacter baumannii during a pandemic of novel coronavirus infections. He was admitted to the hospital with the chief complaints of shortness of breath and progressive decrease in oxygen saturation, and was eventually discharged from the hospital through timely diagnosis and effective treatment. In view of the unique drug resistance of Anopheles mosquitoes and the special infected population, we share the experience of the diagnosis and treatment of this case.
Case presentation
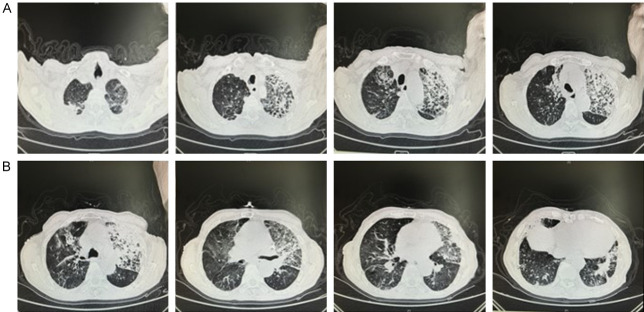
An 88-year-old man was admitted to the hospital with cough and sputum with dyspnea for 2 weeks. 2 weeks prior, the patient developed cough and sputum with dyspnea, and was treated in the local community hospital, but the result was unsatisfactory. 3 days prior, the patient’s cough and dyspnea worsened, and his daughter found his oxygen saturation level was 85% using a portable pulse oximeter. Then he was admitted to the emergency department of our hospital, Changhai Hospital Affiliated to Naval Medical University. He received chest CT and the images showed inflammation of both lungs and a small amount of pleural effusion bilaterally (Figure 1A). The patient was treated with nasal cannula oxygen, plus anti-infection, cough and sputum treatment. After 2 days, the patient’s dyspnea worsened, and the oxygen saturation was 50% under nasal cannula oxygen, so he was intubated, and ventilator-assisted ventilation was performed. Ventilator mode: pressure control (PC), fraction of inspired oxygen (FiO2): 40%, positive end expiratory pressure (PEEP): 8 cmH2O, respiratory rate (RR): 20 breaths/min, pressure control above PEEP (PC above PEEP): 14 cmH2O. Vital signs: Temperature: 36.8°C, Pulse: 68 beats/min, Respiration: 20 breaths/min, Blood pressure: 148/89 mmHg, Oxygen saturation: 98%. Arterial blood gas analysis: pH: 7.43, PCO2: 62.6 mmHg, PO2: 91.6 mmHg, Oxygen concentration: 50%, Oxygenation index: 183.2. At this point, we have been able to diagnose the patient as type II respiratory failure. Given that the patient’s condition already required mechanical ventilation, we diagnosed the patient with severe pneumonia according to the 2019 Infectious Diseases Society of America/American Thoracic Society guidelines [15]. However, we were unsure of what pathogen caused the lung infection, and considering the patient’s previous history of tuberculosis and the pandemic situation of novel coronavirus at that time, novel coronavirus nucleic acid, T-cell enzyme-linked immunospot test (T-SPOT) and fungal G test were performed. At the same time, the patient was given cefoperazone sulbactam sodium for anti-infective treatment based on previous empirical medication, and nutritional support for symptomatic treatment to enhance the patient’s immunity and improve nutritional status.
Figure 1.
Chest CT of patient. A. The CT images of the patient when he was admitted to the emergency department of our hospital showed inflammation of both lungs and a small amount of pleural effusion bilaterally. B. The patient received chest CT again on the 8th day of admission and images suggested that the inflammation in both lungs was better than before.
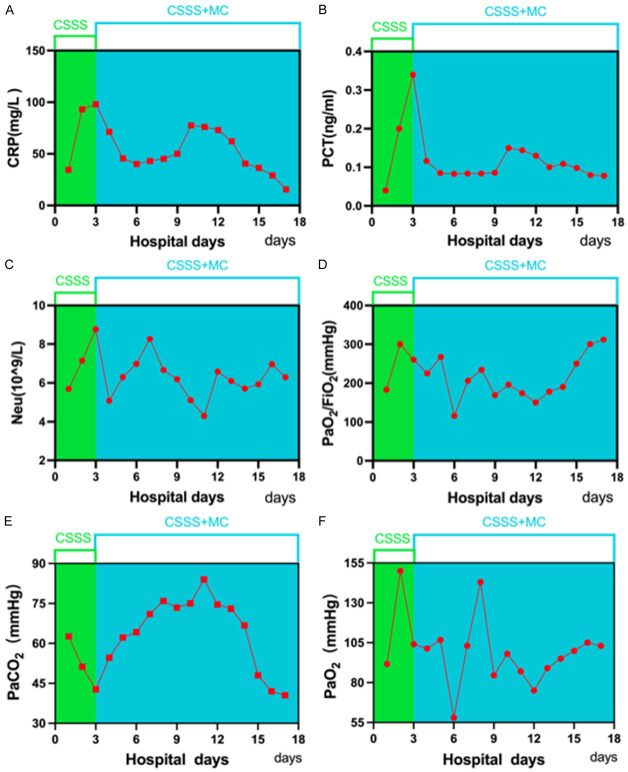
On the 3rd day after admission, the patient’s sputum volume was still high, neutrophil count and inflammatory indexes were still elevated, and the arterial blood gas under ventilator-assisted ventilation indicated that the partial pressure of oxygen and carbon dioxide were fair (Figure 2). Although the patient’s oxygen saturation was maintained above 95%, the inflammatory indexes continued to increase. After obtaining the consent of the patient and his family, we obtained bronchoalveolar lavage fluid (BALF) through bedside bronchoscopy for bacterial culture and a drug sensitivity test as well as mNGS examination to clarify the etiologic agent. We provided targeted anti-infective treatment according to the pathogen detected.
Figure 2.
The patient’s neutrophil count, inflammatory index level and the arterial blood gas level under ventilator-assisted ventilation. A. CRP (C-reactive protein); B. PCT (thrombocytocrit); C. Neu (Neutrophil); D. PaO2/FiO2; E. PaCO2; F. PaO2.
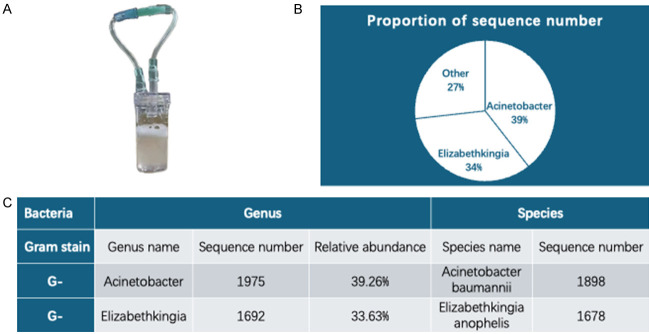
On the 4th day of admission, the mNGS results showed (Figure 3): the sequence number of A. baumannii was 1975, with a relative abundance of 39.26%; the sequence number of A. anopheles was 1,692, with a relative abundance of 33.63%; among the bacteria, there were also Pseudomonas aeruginosa, Staphylococcus aureus, and E. erysipelas, etc., but the sequence numbers were all less than 250 and relative abundance was 0.46%, and there were no viruses and other microorganisms. Based on the mNGS results, we considered A. baumannii and A. anopheles to be the main causative organisms of this infection. For A. baumannii, cefoperazone sulbactam sodium used on admission was sensitive. However, since Anopheles mosquitoes are rare in the clinic, we had little experience with the medication. We searched for previously reported cases as well as studies on S. anopheles and found that S. anopheles is universally resistant to most antibiotics, but is highly susceptible to minocycline, at approximately 97.5%-100% [16]. Therefore, we added minocycline because of the high lethality of Elizabethella anopheles mosquitoes.
Figure 3.
mNGS results. A. Bronchoalveolar lavage fluid of patient was obtained on the 3rd day after admission; B, C. mNGS results.
On the 5th day of admission, the patient was clear, general vital signs were normal, oxygen saturation was 100%, arterial blood gases were stable, and oxygenation index was continuously maintained above 200. The ventilator mode was adjusted to continuous positive airway pressure (CPAP), and ventilator deconditioning exercise was gradually carried out. After 2 days of off-exercise, the patient was able to adapt well to the current ventilator mode and completed the daily off-exercise training well.
On the 7th day of admission, through comprehensive evaluation, we stopped ventilator ventilation for the patient in the afternoon and removed the tracheal tube, and high flow nasal cannula (HFNC) was given for supportive ventilation, with the parameters set to the concentration of inhaled oxygen: 40%, flow rate: 40 L/min, and temperature: 34°C. After the tracheal tube was removed, the patient had no discomfort symptoms such as dyspnea.
On the 8th day of admission, bacterial culture of the sputum specimen reconfirmed the infection with Anopheles elizabethii, but the drug sensitivity test was resistant to all routinely tested antibiotics and lacked minocycline-related tests. The patient received chest CT again to assess the efficacy of the treatment of the lung infection, and images suggested that the inflammation in both lungs was better than before (Figure 1B). The white blood cell count and inflammatory index levels were also significantly lower than before (Figure 2A-C). And the oxygenation index was substantially upgraded compared with the time of admission (Figure 2D). All of the above examinations and tests suggested that the lung infection was under control. However, PaCO2 rose to 62.6 mmHg in the blood gas analysis on the same day (Figure 2E). Considering the patient’s past history of chronic obstructive pulmonary disease, we gave nebulized inhalation of chymotrypsin, salbutamol, and budesonide to assist ventilation. After the above treatments for 2 days, the patient’s PaCO2 gradually decreased to 40.5 mmHg, and PaO2 basically returned to normal (Figure 2F), and his spirit and consciousness improved significantly and he was finally discharged.
Discussion
Anopheles is a rare opportunistic pathogen, which can be transmitted by contact, such as contaminated hands, instruments, clothing, handkerchiefs, and medical equipment. The bite of mosquitoes carrying the bacterium can also cause infection, but there is no evidence that mosquito bites are the main mode of transmission of the disease. A study has shown that vertical transmission is also an important mode of transmission [17]. Neonates and immunocompromised patients are the main susceptible groups, and the infection is usually nosocomial, often causing meningitis, sepsis, pneumonia, and myocarditis. Although, Anopheles stephensi has been recently discovered and is not common in clinical practice, its infection rate in ICU of hospitals is increasing and its mortality rate is high, fluctuating from 23.5% to 60% [6,11]. Anopheles stephensi infection has become one of the life-threatening infections in many countries. An important reason for the high mortality rate of Anopheles elizabethii infection is the generalized resistance to antibiotics, including to most penicillins, cephalosporins, and carbapenems, and polymyxins. Its sensitivity to β-lactamase inhibitor-containing composites, quinolones varies greatly. However, its sensitivity to minocycline in vitro reaches 90% [18]. Even if the diagnosis can be confirmed clinically, treatment is still a great challenge. In 2012, a total of five patients were diagnosed with Anopheles stephensi infection in ICU of a hospital in Singapore, and even after sensitive antibiotics were given, three patients died during treatment, with a mortality rate as high as 60% [11].
In this case, the patient was diagnosed with Anopheles stephensi infection by mNGS and reconfirmed by bacterial culture after admission to the hospital, and the patient’s lung inflammation gradually improved at the beginning of treatment. However, as the condition improved, the patient became irritable and did not cooperate with the routine treatment, which in turn caused an elevation of PaCO2, leading to another exacerbation of the condition. Eventually, the patient’s condition improved again by various methods such as shock expectorator, respiratory stimulant, nebulized inhalation, and antidelirium. Through this case, the anti-infective treatment for Anopheles elizabethiensis still deserves further investigation. According to statistics, the proportion of reasonable use of antimicrobial drugs in the treatment of patients with Elizabethella infections only accounts for 15.8% [19], and another has shown that inappropriate empirical antimicrobial drug therapy is an independent risk factor for death from Anopheles stephensi infection [9]. Early and effective antibiotic use is therefore critical for successful treatment, and identifying the pathogen as soon as possible is critical for early use of sensitive antibiotics.
A study has shown that the VITEK 2 automated bacterial identification system may incorrectly detect Anopheles elizabethiae as Elizabethia meningitidis, which is differentially resistant to antibiotics [6]. Therefore, accurate and timely identification of Anopheles mosquitoes using molecular diagnostic tools is necessary to avoid delays in effective anti-infective therapy. mNGS is capable of accurately identifying Anopheles mosquitoes and confirming the presence of the gene encoding mannose-binding lectin [20]. For the detection of pathogenic microorganisms, mNGS is more advantageous than bacterial culture in terms of both time and accuracy. In our case, we identified the infection of Anopheles mosquitoes in only 1 day by mNGS, which laid the foundation for our proper pre-treatment.
Combined treatment with multiple sensitive antibiotics has been reported to improve therapeutic efficacy [21]. Minocycline is first choice for the treatment of Anopheles stephensi infection. Minocycline mainly exerts its bacteriostatic effect by specifically binding to the A position of the 30S subunit of the ribosome of the pathogen and preventing the synthesis of pathogenic microbial proteins. Yang et al. investigated the effects of minocycline on Anopheles stephensi in vivo and in vitro, and found that the spontaneous mutation of the Anopheles stephensi bacterium reduced minocycline sensitivity and affected its therapeutic efficacy, while the combination with rifampicin could prevent the appearance of reduced sensitivity in vitro [22]. In addition, drug susceptibility test also play a crucial role in treatment. Some researchers reported that antibiotic susceptibility of Elizabethella spp. determined by disc diffusion test, E-test assay (E-test), and agar dilution test are unreliable and inaccurate, who recommended the use of a microbroth dilution test for determining drug susceptibility [16]. This needs for clinical data for validation.
Disinfectant isolation of the ward environment and hand washing by medical staff are also essential to prevent infection by Anopheles spp. Further studies on the mechanisms of drug resistance in Anopheles mosquitoes and effective treatment regimens are essential to improve the prognosis of patients. Biofilm formation, multiple β-lactamase-resistant genes, and plasmid-mediated resistance proteins are collectively involved in the generalized resistance to Anopheles [9,23]. With the widespread and irrational use of antibiotics, drug-resistant bacteria are becoming more and more prevalent. Phage therapy as an alternative therapy becomes a potential therapeutic avenue for multidrug-resistant bacteria, and will inevitably become the future treatment for Anopheles lisbananii [24].
In conclusion, we have reported an elderly male with severe pneumonia due to infection with Anopheles stephensi and Acinetobacter baumannii during a pandemic of the novel coronavirus infection. He was eventually discharged from the hospital through timely diagnosis by mNGS and effective treatment with cefoperazone sulbactam sodium and minocycline. We hope our experience provides a reference for the diagnosis and treatment of Anopheles stephensi infection.
Disclosure of conflict of interest
None.
References
- 1.Kämpfer P, Matthews H, Glaeser SP, Martin K, Lodders N, Faye I. Elizabethkingia anophelis sp. nov., isolated from the midgut of the mosquito Anopheles gambiae. Int J Syst Evol Microbiol. 2011;61:2670–2675. doi: 10.1099/ijs.0.026393-0. [DOI] [PubMed] [Google Scholar]
- 2.Kämpfer P, Busse HJ, McInroy JA, Glaeser SP. Elizabethkingia endophytica sp. nov., isolated from Zea mays and emended description of Elizabethkingia anophelisKampfer et al. 2011. Int J Syst Evol Microbiol. 2015;65:2187–2193. doi: 10.1099/ijs.0.000236. [DOI] [PubMed] [Google Scholar]
- 3.Nicholson AC, Gulvik CA, Whitney AM, Humrighouse BW, Graziano J, Emery B, Bell M, Loparev V, Juieng P, Gartin J, Bizet C, Clermont D, Criscuolo A, Brisse S, McQuiston JR. Revisiting the taxonomy of the genus Elizabethkingia using whole-genome sequencing, optical mapping, and MALDI-TOF, along with proposal of three novel Elizabethkingia species: Elizabethkingia bruuniana sp. nov., Elizabethkingia ursingii sp. nov., and Elizabethkingia occulta sp. nov. Antonie Van Leeuwenhoek. 2018;111:55–72. doi: 10.1007/s10482-017-0926-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hem S, Jarocki VM, Baker DJ, Charles IG, Drigo B, Aucote S, Donner E, Burnard D, Bauer MJ, Harris PNA, Wyrsch ER, Djordjevic SP. Genomic analysis of Elizabethkingia species from aquatic environments: evidence for potential clinical transmission. Curr Res Microb Sci. 2021;3:100083. doi: 10.1016/j.crmicr.2021.100083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hwang JH, Kim J, Kim JH, Mo S. Elizabethkingia argenteiflava sp. nov., isolated from the pod of soybean, Glycine max. Int J Syst Evol Microbiol. 2021;71 doi: 10.1099/ijsem.0.004767. [DOI] [PubMed] [Google Scholar]
- 6.Lau SK, Chow WN, Foo CH, Curreem SO, Lo GC, Teng JL, Chen JH, Ng RH, Wu AK, Cheung IY, Chau SK, Lung DC, Lee RA, Tse CW, Fung KS, Que TL, Woo PC. Elizabethkingia anophelis bacteremia is associated with clinically significant infections and high mortality. Sci Rep. 2016;6:26045. doi: 10.1038/srep26045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi MH, Kim M, Jeong SJ, Choi JY, Lee IY, Yong TS, Yong D, Jeong SH, Lee K. Risk factors for Elizabethkingia acquisition and clinical characteristics of patients, South Korea. Emerg Infect Dis. 2019;25:42–51. doi: 10.3201/eid2501.171985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han MS, Kim H, Lee Y, Kim M, Ku NS, Choi JY, Yong D, Jeong SH, Lee K, Chong Y. Relative prevalence and antimicrobial susceptibility of clinical isolates of Elizabethkingia species based on 16S rRNA gene sequencing. J Clin Microbiol. 2016;55:274–280. doi: 10.1128/JCM.01637-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin JN, Lai CH, Yang CH, Huang YH, Lin HH. Clinical manifestations, molecular characteristics, antimicrobial susceptibility patterns and contributions of target gene mutation to fluoroquinolone resistance in Elizabethkingia anophelis. J Antimicrob Chemother. 2018;73:2497–2502. doi: 10.1093/jac/dky197. [DOI] [PubMed] [Google Scholar]
- 10.Frank T, Gody JC, Nguyen LB, Berthet N, Le Fleche-Mateos A, Bata P, Rafaï C, Kazanji M, Breurec S. First case of Elizabethkingia anophelis meningitis in the Central African Republic. Lancet. 2013;381:1876. doi: 10.1016/S0140-6736(13)60318-9. [DOI] [PubMed] [Google Scholar]
- 11.Teo J, Tan SY, Tay M, Ding Y, Kjelleberg S, Givskov M, Lin RT, Yang L. First case of E anophelis outbreak in an intensive-care unit. Lancet. 2013;382:855–856. doi: 10.1016/S0140-6736(13)61858-9. [DOI] [PubMed] [Google Scholar]
- 12.Perrin A, Larsonneur E, Nicholson AC, Edwards DJ, Gundlach KM, Whitney AM, Gulvik CA, Bell ME, Rendueles O, Cury J, Hugon P, Clermont D, Enouf V, Loparev V, Juieng P, Monson T, Warshauer D, Elbadawi LI, Walters MS, Crist MB, Noble-Wang J, Borlaug G, Rocha EPC, Criscuolo A, Touchon M, Davis JP, Holt KE, McQuiston JR, Brisse S. Evolutionary dynamics and genomic features of the Elizabethkingia anophelis 2015 to 2016 Wisconsin outbreak strain. Nat Commun. 2017;8:15483. doi: 10.1038/ncomms15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guerpillon B, Fangous MS, Le Breton E, Artus M, le Gall F, Khatchatourian L, Talarmin JP, Plesiat P, Jeannot K, Saidani N, Rolland-Jacob G. Elizabethkingia anophelis outbreak in France. Infect Dis Now. 2022;52:299–303. doi: 10.1016/j.idnow.2022.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Xie Y, Du J, Jin W, Teng X, Cheng R, Huang P, Xie H, Zhou Z, Tian R, Wang R, Feng T. Next generation sequencing for diagnosis of severe pneumonia: China, 2010-2018. J Infect. 2019;78:158–169. doi: 10.1016/j.jinf.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, Cooley LA, Dean NC, Fine MJ, Flanders SA, Griffin MR, Metersky ML, Musher DM, Restrepo MI, Whitney CG. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin JN, Lai CH, Yang CH, Huang YH. Elizabethkingia infections in humans: from genomics to clinics. Microorganisms. 2019;7:295. doi: 10.3390/microorganisms7090295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lau SK, Wu AK, Teng JL, Tse H, Curreem SO, Tsui SK, Huang Y, Chen JH, Lee RA, Yuen KY, Woo PC. Evidence for Elizabethkingia anophelis transmission from mother to infant, Hong Kong. Emerg Infect Dis. 2015;21:232–241. doi: 10.3201/eid2102.140623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng YH, Perng CL, Jian MJ, Cheng YH, Lee SY, Sun JR, Shang HS. Multicentre study evaluating matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of clinically isolated Elizabethkingia species and analysis of antimicrobial susceptibility. Clin Microbiol Infect. 2019;25:340–345. doi: 10.1016/j.cmi.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 19.Chen WC, Chen YW, Ko HK, Yu WK, Yang KY. Comparisons of clinical features and outcomes between Elizabethkingia meningoseptica and other glucose non-fermenting Gram-negative bacilli bacteremia in adult ICU patients. J Microbiol Immunol Infect. 2020;53:344–350. doi: 10.1016/j.jmii.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Yasmin M, Rojas LJ, Marshall SH, Hujer AM, Cmolik A, Marshall E, Boucher HW, Vila AJ, Soldevila M, Diene SM, Rolain JM, Bonomo RA. Characterization of a novel pathogen in immunocompromised patients: Elizabethkingia anophelis-exploring the scope of resistance to contemporary antimicrobial agents and beta-lactamase inhibitors. Open Forum Infect Dis. 2023;10:ofad014. doi: 10.1093/ofid/ofad014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee CC, Lai CH, Yang CH, Huang YH, Lin JN. Antibiotic combination to effectively postpone or inhibit the in vitro induction and selection of levofloxacin-resistant mutants in Elizabethkingia anophelis. Int J Mol Sci. 2024;25:2215. doi: 10.3390/ijms25042215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang YS, Huang TW, Huang YC, Huang WC, Hsu SY, Wu HC, Chen FJ, Shang HS, Sytwu HK, Kuo SC. In vitro and in vivo efficacy of minocycline-based therapy for Elizabethkingia anophelis and the impact of reduced minocycline susceptibility. Int J Antimicrob Agents. 2022;60:106678. doi: 10.1016/j.ijantimicag.2022.106678. [DOI] [PubMed] [Google Scholar]
- 23.Hu S, Lv Y, Xu H, Zheng B, Xiao Y. Biofilm formation and antibiotic sensitivity in Elizabethkingia anophelis. Front Cell Infect Microbiol. 2022;12:953780. doi: 10.3389/fcimb.2022.953780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pekkle Lam HY, Peng SY, Paramita P, Wu WJ, Chen LK, Chao HJ, Lai MJ, Chang KC. Biological and genomic characterization of two newly isolated Elizabethkingia anophelis bacteriophages. J Microbiol Immunol Infect. 2022;55:634–642. doi: 10.1016/j.jmii.2022.05.004. [DOI] [PubMed] [Google Scholar]