Abstract
The Ficus L. genus, belonging to the Moraceae family, includes around 850 species that are widely distributed in tropical and subtropical regions around the world; including the Eastern Mediterranean, Asia, Africa, Australia, and a large territory of America. Among the most important species are F. deltoidea, F. exasperata, F. sycomorus, F. religiosa, F. microcarpa, F. hirta Vahl, F. benghalensis, F. racemosa, F. elástica, and F. carica. Different parts of Ficus plants (root, stem bark, latex, leaves, pulp and fruits) contain bioactive compounds [flavonoids (flavanols, flavones, flavonols, isoflavones, chalcones, anthocyanins), phenolic acids (hidroxylcinnamic acids, hidroxylbenzoic acids), phytosterols, terpenes (triterpenes, tetraterpenes, diterpenes, sesquiterpenes, monoterpenes), coumarins, hydroxybenzoates, phenylpropanoids, chlorins, pheophytins, megastigmanes, chitinases, organic acids, fatty acids, amino acids, alkaloids, glycosides] which together, are currently useful to more than 30 traditional ethnomedical uses. The present manuscript is the result of scientific search processed with the main electronic databases (PubMEd, SciELO, Latindex, Redalyc, BiologyBrowser, ScienceResearch, ScienceDirect, Academic Journals, Ethnobotany, and Scopus). This first review (Part 1), compiles information from published research (in vitro, in vivo and clinical studies) on its antimicrobial, antifungal, antiviral, anti-helminthic, hypoglycemic, hypolipidemic, hepatoprotective, anti-inflammatory, analgesic, and antipyretic properties; as well as its possible adverse and/or toxicological effects. Given the amount of evidence described in this review it aims to trigger a more detailed scientific research on the important pharmacological properties of all angiosperm plants of the genus Ficus L.
Keywords: Ficus L., phytochemicals, biological activities, toxicological safety
Introduction
Angiosperm plants of the genus Ficus L. belonging to the Moraceae family include more than 850 species of trees, shrubs, hemiepiphytes (a plant that germinates and begins its development on the branches of a tree and later produces its roots to absorb nutrients from the soil), climbers and vines widely distributed in different tropical and subtropical regions of the world. Most of the species tend to grow wild in the eastern Mediterranean, Asia, Africa, Australia, and a large territory of the American continent. Ficus trees are considered ecologically relevant species as they provide food and shelter for many frugivorous animals (both birds and mammals). They are also an important natural resource for humans due to their high economic, nutritional and medicinal values. This is the reason why some species are considered ornamental, sacred, religious plants and have been domesticated (Table 1) [1-6]. Among the most important species are F. deltoidea, F. exasperata, F. sycomorus, F. religiosa, F. microcarpa, F. hirta Vahl, F. benghalensis, F. racemosa, F. elástica, and F. carica. These last four species being the best known and most significant worldwide [5-7]. Specifically, F. carica is considered a very popular fruit crop and from a historical point of view, in the first part of the Bible, Genesis, it is mentioned that Adam and Eve, realizing that they were naked, covered themselves with Angiosperm leaves. The oldest evidence of its consumption comes from the Neolithic period (ca. 10,000-8,800 BCE) of southeastern Turkey and it is considered one of the oldest food tree crops domesticated and used by humans [8-10]. Some archaeological evidence suggests that the harvest of its fruit is more than 11,000 years old (before cereal grains) [8-11]. F. carica, also known as fig tree or common fig, is a deciduous shrub to 15 to 20 feet, with numerous spreading branches, non-adventitious roots, and bright green, simple, waxy leaves, hairy rough on the upper surface and downy hairy on the bottom. Generally, when the leaves are broken they exude a milky white emulsion with a sticky consistency called latex that contains ficin (protein hydrolytic enzyme). Its fruits (figs) are on the branches organized in pairs or solitary, they are thin-skinned, fleshy, hollow, pear-shaped, with inflorescences inside and can be black (they are the most common), green, purple, and blue. They are consumed by different populations of the world as fresh, dry or dehydrated food, canned, jams, sweets or preserves. Like the fruits, their seeds are edible, of medium size and hollow when not pollinated (Figure 1) [1-6]. Ficus plants are native to southwest Asia and the eastern Mediterranean but due to their easy adaptation and dispersion in different types of soil, their domestication process (especially F. carica) has increased favoring the constant collection of figs [1-6,12]. The Food and Agriculture Organization (FAO) of the United Nations (2017) indicated that the main producing countries of this fruit are Turkey, Egypt, Morocco, Algeria, Iran, Syria, Spain, the United States, Brazil, and Tunisia. In the specific case of Mexico, the plant was introduced by the Spanish Franciscan missionaries by placing it in the atriums of the churches. The territorial surface (approximately 1,200 hectares) of F. carica cultivation is low (compared to corn and beans) and is located in 10 states [Morelos (58%), Hidalgo (14%), Veracruz (10%), Baja California Sur (6.5%), Mexico City (3.5%), Puebla (2.6%), Durango (2.4%), San Luis Potosí (1.5%), Sonora (1.1%) and Baja California (0.4%)], which together generate an annual production of 6 to 7 thousand tons between the months of August and October [13-15].
Table 1.
Common and traditional uses of the most studied and evaluated Ficus species
| Species (Common name) | Uses | Ref |
|---|---|---|
| F. deltoidea (Mistletoe fig) | Alimentary (Consumption of fruits) | [16] |
| Ornamental | ||
| Medicinal (Anti-inflammatory, antinociceptive, antibacterial, anticancer, antioxidant, wound healing, antidiabetic, hypoglycemic, hypocholesterolemic, hypolipidemic, aphrodisiac, antiulcerogenic, uterotonic, antirheumatic, action in disorders of the menstrual cycle, decreases cold and headache) | ||
| F. exasperata (Sandpaper tree, forest sandpaper fig, white fig, or sandpaper leaf tree) | Alimentary (Consumption of fruits, fodder for animals) | [17] |
| Ornamental | ||
| Medicinal (Anti-inflammatory, antinociceptive, antipyretic, antimicrobial, antituberculous, antileprous, antidiabetic, hypolipidic, antioxidant, anticancer, anthelmintic, vermifuge, ascaricide, anti-gouty, antihemorrhoids, antiepileptic, antirheumatic, antihypertensive, antiulcer, antihypertensive, diuretic, antidiarrheal, antidysentery, antitussive, anxiety prevention, wound healing, prevention of weakness and malnutrition, action on ophthalmic infections, respiratory tract and venereal diseases, antiarthritic, anxiolytic, uterotonic, effect on kidney disorders) | ||
| F. sycomorus (Sycamore fig or the fig-mulberry) | Alimentary (Consumption of fruits and leaves) | [18-20] |
| Ornamental | ||
| Medicinal (Anti-inflammatory, antinociceptive, antimicrobial, antifungal, anthelmintic, antiulcer, antiwarts, neuroprotective, antiepileptic, antidiarrheal, prevents respiratory and thoracic diseases, laxative and anticonstipation, antidiabetic, prevents insomnia, healing of wounds and burns, hepatoprotective, effect on infertility and excessive menstrual flow, antihypertensive, antioxidant) | ||
| F. religiosa (Sacred fig) | Alimentary (Consumption of fruits) | [21-23] |
| Ornamental and ceremonial plant | ||
| Medicinal (Anti-inflammatory, antinociceptive, antidiabetic, antidiarrheal, anticancer, antiproliferative, antimutagenic, antiasthmatic, antioxidant, antimicrobial, antiviral, anti-helminthic, hepatoprotective, wound healing, anticoagulant, immunomodulatory, antiepileptic, astringent, laxative, effect on gastric problems, sexual disorders and skin diseases, mosquitocide, stress prevention) | ||
| F. microcarpa (Chinese banyan, Malayan banyan, or curtain fig) | Alimentary (Consumption of fruits) | [10] |
| Ornamental | ||
| Medicinal (Antinoceptive, antipyretic, antibacterial, antileper, antiviral, antifungal, antimalarial, antidiabetic, anticancer, antioxidant, antiulcer, antirheumatic, antidiarrhoeal, hepatoprotective, antitussive, prevention of acute enteritis and bronchitis, expectorant, relieves toothache) | ||
| F. benghalensis (Indian banyan or banyan fig) | Alimentary (consumption of fruits) | [21,24] |
| Ornamental | ||
| Medicinal (Anti-inflammatory, antinociceptive, antimicrobial, antiemetic, antirheumatic, diuretic, antidiabetic, antidiarrheal, antioxidant, anticancer/antitumor, antimutagenic, antiproliferative, hepatoprotective, wound healing, anticoagulant, immunomodulatory, anti-stress potential, mosquitocide, action on respiratory diseases) | ||
| F. racemosa (Cluster fig or red river fig) | Alimentary (Consumption of fruits, fodder for animals) | [25,26] |
| Ceremonial plant | ||
| Medicinal (Anti-inflammatory, antinociceptive, antipyretic, antimicrobial, antifungal, antifilarial, larvicide, hepatoprotective, antidiarrheal, antidiabetic, antioxidant, antihypertensive, gastroprotective, antihemorrhoids, effect on respiratory and urinary diseases, antitussive, antihyperglycemic, wound healing) | ||
| F. elástica (Rubber tree) | Alimentary (Consumption of fruits) | [27] |
| Ornamental (Decorative element for home and office interiors) | ||
| Medicinal (Anti-inflammatory, antinoceptive, antibacterial, antituberculous, antidiarrheal, antihypertensive, antirheumatic, antidiabetic, anticancer, wound healing, antihemorrhoids, action on skin allergies, improves blood circulation, antiulcer, diuretic, effect on digestive, endocrine and metabolic disorders, anti-dysentery, prevention of anemia, action on reproductive, respiratory, and gastrointestinal disorders, astringent, effect on neurodegenerative disorders and liver problems) | ||
| F. carica (Common fig) | Alimentary (Consumption of fruits) | [1,5,6] |
| Ornamental | ||
| Medicinal (Anti-inflammatory, antinociceptive, antipyretic, antimicrobial, antiviral, antifungal, anti-helminthic, antidiabetic, hypoglycemic, hypocholesterolemic, hypolipidimic, antidiarrheal, hepatoprotective, anticancer/antitumor, antimutagenic, antispasmodic, antiplatelet, anticonstipation potential, anticoagulant, antiangiogenic, antioxidant, immunostimulant, haemostatic, erythropoietic stimulant, cardioprotective, antihypertensive, diuretic, anti-warts, action in respiratory and stomach disorders) |
Figure 1.
Tree and fruit (immature fig) belonging to the species F. carica.
Nutritional composition and phytochemistry of the Ficus L. genus
Various research has documented the nutritional value of the genus. In general, it is made up of water (80%), starches, sugars (this proportion depends on the maturation time and the most common are glucose, fructose, sucrose and pectins), amino acids [(especially aspartic acid (Asp) and glutamine Gln)], vitamins (A, C, B1, B2 and B6), organic acids (oxalic, quinic, malic, citric and succinic) minerals [Copper (Cu), Manganese (Mn), Magnesium (Mg), Potassium (K), Calcium (Ca), Sodium (Na), Iron (Fe), Zinc (Zn)] and large amounts of dietary soluble fiber [1-6,28].
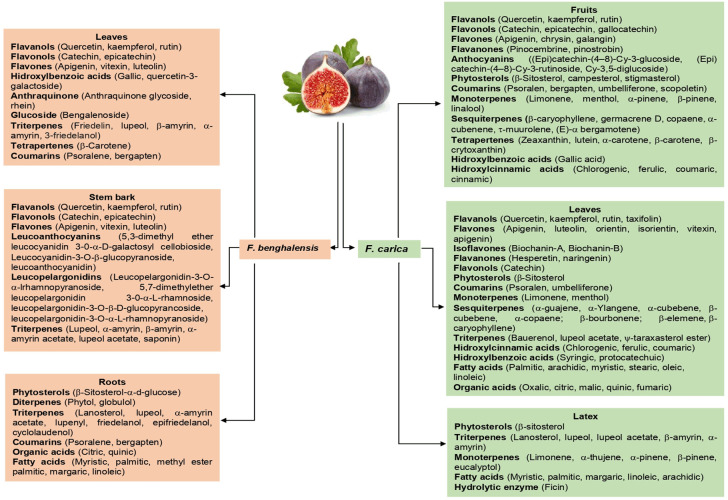
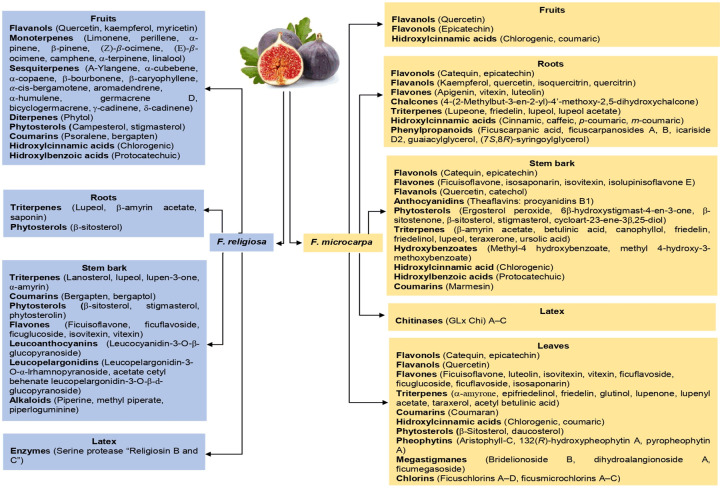
The majority of studies, using different extraction methods, suggest that there are differences in the phytochemical composition of the plant parts (roots, flowers, stems, bark, leaves, seeds, latex, pulp and fruits) between wild and domesticated species; which indisputably modifies its functional and therapeutic properties. These differences can be attributed to environmental conditions (climate, humidity), type of soil of the cultivation sites, harvest time and age of maturity of the bushes [1-6,28]. Figures 2 and 3 show the phytochemical composition of the different anatomical parts of four of the common species of Ficus L. Likewise, some of the phytochemical compounds identified in the aforementioned species are shown in Figure 4.
Figure 2.
Phytochemical composition in different anatomical parts of F. benghalensis and F. carica [1-8,21,24,28-30].
Figure 3.
Phytochemical composition in different anatomical parts of F. religiosa and F. microcarpa [10,18,21,22,24,28,29].
Figure 4.
Flavanols, monoterpenes, hydroxybenzoic acid, and phytosterol identified in Ficus species.
As mentioned above, the genus Ficus L. has the particularity of adapting and growing in different types of soil, which favors its nutritional, medicinal and economic impacts. Although most species have been used as a food source, F. carica continues to be the most representative for the biopharmaceutical and nutritional industries; especially for its fruits, which are considered by some authors as nutraceutical agents. That is, a food supplement that is apparently non-toxic and has been scientifically proven to be beneficial for health both in the treatment and in the prevention of some diseases [1,28]. In this sense, the group and/or combination of bioactive compounds [flavonoids (flavanols, flavones, flavonols, isoflavones, chalcones, anthocyanins), phenolic acids (hydroxylcinnamic acids, hydroxylbenzoic acids), phytosterols, terpenes (triterpenes, tetraterpenes, diterpenes, sesquiterpenes, monoterpenes), coumarins, hydroxybenzoates, phenylpropanoids, chlorins, pheophytins, megastigmanes, chitinases, organic acids, fatty acids, amino acids, alkaloids, glycosides] from different parts of the plant (Figures 2 and 3) have been associated with the control, progression, and prevention of chronic and infectious diseases; mainly its antimicrobial, antifungal, antiviral, anti-helminthic, hypoglycamic, hypolipidemic, hepatoprotective, anti-inflammatory, analgesic, and antipyretic activities. Therefore, when using and combining keywords; as Ficus L. genus, Ficus plants or species, pharmacological and/or therapeutic properties, phytochemical composition and adverse and/or toxic effects, a scientific search was carried out in the main electronic databases (PubMEd, SciELO, Latindex, Redalyc, BiologyBrowser, ScienceResearch, ScienceDirect, World Wide Science, Web of Science, Academic Journals, Ethnobotany, Scopus and Google Scholar). The present review (Part 1) compiles information from published research (in vitro, in vivo and clinical studies) on these therapeutic and/or pharmacological properties, which will be discussed below. Unlike most scientific articles where the individual characteristics and properties of each species are mainly described; this document brings together the largest number of studies on Ficus plants described by different authors.
Biological activities
Antimicrobial effect
A dormant threat when treating bacterial diseases (such as pneumonia, tuberculosis, urinary tract infections, pharyngitis, meningitis) and/or fungal diseases is the resistance that microorganisms develop to avoid being attacked by synthetic anti-infective agents. Thus, natural products are becoming a rich source of these agents that, by working at different target sites, and apparently being safer, can replace synthetic compounds. More than 30,000 compounds with this activity have been extracted from approximately 1,300 plants, including garlic, parsley, oregano, ginger, rosemary, lemongrass, and chili pepper. Among the most relevant anti-infective bioactive compounds are organic acids, thiosulfinates, glucosinolates, saponins, flavonoids and phenols [31].
The genus Ficus L. is not an exception, since the analysis of its antimicrobial capacity has generated a significant number of investigations in recent decades. Mousa et al. (1994) and Valsaraj et al. (1997) initiated this field of study. First, the bioactivity of fruit extracts from four Egyptian species of Ficus was evaluated; finding an antimicrobial effect with F. benghalensis and F. religiosa by inhibiting the growth of Azobacter chroococcum, Bacillus cereus, Bacillus megaterium, Streptococcus faecalis, and Klebsiella pneumonia [32]. This therapeutic activity of 78 medicinal plants from India was confirmed in the second study, in which aqueous and ethanolic extracts from the leaves of F. religiosa developed antibacterial action against Staphylococcus aureus, Escherichia coli, Salmonella paratyphi, Salmonella typhi, Shigella dysenteriae, S. typhimurium, Pseudomonas aeruginosa, and Bacillus subtillis [33]. Farrukh and Iqbal (2003) confirmed these results by inhibiting the growth of these bacteria with an ethanolic (EtOH) extract from the same Ficus species [34]. Considering the therapeutic impact shown by F. religiosa, extracts of methanol (MeOH), chloroform (Chl) and water from its leaves were analyzed, confirming that the greatest antibacterial spectrum corresponded to chloroform (Chl) [35]. On the other hand, when analyzing a methanol (MeOH) extract obtained from the bark of F. microcarpa, the inhibition of Bacillus brevis, Bacillus cereus, Bacillus subtilis, and Escherichia coli was evidenced [36]. Similarly, when using the same type of extract but from F. carica leaves, a strong antibacterial activity was shown against Streptococcus anginosus, Streptococcus gordonii, Prevotella intermedia, Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis (bacteria common in the oral cavity) [37].
To date, different extracts [(aqueous, methanol (MeOH), ethanol (EtOH), hexane (Hx), chloroform (Chl), ethyl acetate (EtOAc), dichloromethane (DCM), petroleum ether and acetone (Ace)] evaluated and obtained from the leaves, fruits, latex, roots, and stem bark of F. elástica, F. benghalensis, F. racemose, F. religiosa, F. sycomorus, and F. carica (mainly these last two species) have demonstrated their potential to inhibit the growth of Gram-negative bacteria (Salmonella typhimurium, Salmonella typhi, Escherichia coli, Pseudomonas aeruginosa, Shigella flexneri, Shigella boydii, Vibrio cholerae, Enterobacter cloacae, Enterobacter aerogenes, Enterobacter sakazakii, Klebsiella pneumoniae, Citrobacter freundii, Proteus mirabilis, Proteus vulgaris, Klebsiella oxytoca, Chromobacterium violaceum, Prevotella intermedia, Aggregatibacter actinomycetemcomitans, and Porphyromonas gingivalis) and Gram-positive bacteria (Staphylococcus aureus, Staphylococcus saprophyticus, Staphylococcus epidermidis, Bacillus subtilis, Bacillus cereus, Bacillus pumilis, Enterococcus faecalis, Mycobacterium tuberculosis, Streptococcus pyogenes, Streptococcus gordonii, Clostridium perfringens, Streptococcus anginosus, Listeria innocua and Listeria monocytogenes) (Table 2).
Table 2.
Studies testing for antimicrobial effects of the most representative species of the genus Ficus L.
| Extract or phytochemical/Plant part | Objective and biological effect | Ref |
|---|---|---|
| F. carica | ||
| Mixture of aqueous and EtOH (80:20)/fruit peel and pulp | After identifying bioactive compounds and the antibacterial effect of the extract mixture. The results showed that the phenolic and nutritional profiles were higher in the fig skin. However, the extracts of both plant parts showed practically the same potential to reduce the growth of S. aureus E. coli and M. morganii (MIC between 2.5 and 5.0 mg/mL) | [4] |
| MeOH/leaves | The extract showed an MIC of 1600 μg/mL against the H37Rv strain of M. tuberculosis | [6] |
| MeOH/leaves | The extract showed a minimum inhibitory concentration (MIC) between 0.156 and 5 mg/ml against bacteria from the oral cavity (S. anginosus, S. gordonii, P. intermedia, A. actinomycetemcomitans and P. gingivalis). It is suggested that figs could be used as natural antibacterial agents in oral care products | [37] |
| EtOH/leaves | The purpose of the study was to determine the MIC and the minimum bactericidal concentration (MBC) of the extract against E. faecalis and its cytotoxicity in fibroblastic cells in vitro. The results showed a reduction in bacterial growth at a concentration of 25% of the extract; while the MBC was 50%. Fibroblasts did not show any cytotoxic effect | [38] |
| Hx/latex | The final result of the study showed a MIC of 19 μg/mL, especially for S. saprophyticus and S. aureus. Apparently, this antibacterial activity could be attributed to the coumarins present in the chemical composition of the extract | [41] |
| MeOH, Chl, Hx and EtOAc/latex | The results indicated that EtOAc acted on the proliferation of 5 bacterial species (E. faecalis, C. freundei, P. aeruginosa, E. coli and P. mirabilis). While MeOH only inhibited P. mirabilis | [43] |
| MeOH/leaves | The microbroth dilution method showed MIC ranges between 31.25 and 250 g/ml against S. aromaticum, S. aureus, S. epidermidis, S. pyogenes, S. typhi and P. aeruginosa. These results suggest that F. carica could provide natural antimicrobial agents to control infections caused by bacteria resistant to conventional antibiotics | [44] |
| MeOH, EtOH, DCM/Whole plant | Quorum sensing (QS), as the basis of bacterial communication, is a promising approach to reduce the incidence of multidrug resistance. Therefore, the anti-QS properties of some extracts against C. violaceum and P. aeruginosa were evaluated. After evaluating this activity by violacein production and swarm motility, the conclusion was that the DCM extract showed the most pronounced inhibition for both bacteria | [45] |
| MeOH/fruits | Initially, the antioxidant and antibacterial activities of MeOH extracts from some edible fruits were evaluated. Subsequently, nanocomposite biofilms based on chitosan and carboxymethylcellulose were characterized, adding the most significant fruit extract. The best antibacterial effects belong to P. granatum and F. carica; especially against S. aureus, B. subtilis, B. cereus, L. monocytogenes, S. typhimurium and E. coli. With this result, 1% of the extract of F. carica was added to the nanocomposite biofilms and its antimicrobial activity was again evaluated; concluding that both the antioxidant activity and the halos of bacterial inhibition increased | [46,47] |
| Aqueous, EtOH, MeOH and Ace/pulp | Using the Folin-Ciocalteu method and HPLC, it was revealed that the extracts contained polyphenols, flavonoids and tannins. On the other hand, the microplate assay and diffusion in agar medium confirmed that EtOH and MeOH extracts were the most effective to inhibit the growth of most of the tested strains (L. innocua, B. subtilis, C. perfringens, E. faecalis and S. aureus, V. cholerae, P. aeruginosa, E. coli, E. sakazakii, E. cloacae, P. mirabilis, C. freundii, K. oxytoca and S. odorifera) | [48] |
| Aqueous and MeOH/fruits | After using matrix-assisted laser desorption ionization time in flight mass spectrometry (MALDI-TOF MS), 3 bacteria belonging to the species E. cloacae were identified. Subsequently, its control was analyzed using the aforementioned extracts. The MIC and MBC results showed that the extracts significantly inhibited the activity of E. cloacae. This seems to provide good guidance for the use of dried figs against infections by this bacterial species | [49] |
| F. benghalensis | ||
| Flavonoids/stem bark | The evaluation showed that flavonoids significantly inhibited P. vulgaris, S. aureus and P. aeruginosa (MIC between 25 and 100 mg/ml) | [50] |
| Flavonoids/stem bark | The relationship between the antibacterial activity of flavonoids (2 polymethoxyflavones and 4 isoflavonoids) and their interaction with the E. coli membrane was analyzed. A positive correlation between the antibacterial capacity and the effect of membrane rigidity was evidenced and a quantitative structure-activity relationship (QSAR) study demonstrated that the effect of flavonoids is attributed to a molecular hydrophobicity and charges on C atom at position3 (C3). Probably, the QSAR model can predict the antibacterial activity of other flavonoids | [51] |
| F. religiose | ||
| EtOH/Whole plant | Phytochemical groups, cytotoxicity (lethality test of brine shrimp) and antibacterial action (agar disk diffusion method) of the extract were determined. The results indicated the presence of alkaloids, glycosides, steroids, gums, reducing sugars, tannins, flavonoids and saponins. Shrimp mortality increased as the extract dose increased, obtaining an LC50 of 2.7 µg/ml. Of the 14 pathogenic bacteria evaluated, E. faecalis was the most inhibited (MIC: 250-500 μg/ml) | [52] |
| EtOH/fruits | At the end of the study, the extract showed an MIC of 15 mg/ml for S. aureus and S. epidermidis. While for K. pneumoniae and P. vulgaris it was 30 mg/ml | [53] |
| F. sycomorus | ||
| Aqueous/leaves and latex | Silver nanoparticles (SNPs) obtained from latex and an aqueous extract were tested against Gram positive and negative pathogenic bacteria. The SNPs showed antibacterial activity in all strains tested (S. typhimurium, S. typhi, S. flexneri, E. faecalis, E. cloacae, E. aerogenes, P. aeruginosa, K. pneumoniae, E. coli, and S. aureus). However, the most significant effectiveness corresponded to the liquid medium (aqueous extract); probably due to the higher silver content and better contact with bacteria | [54] |
| MeOH and Ace/leaves (LE) and stem-bark (SBE) | The results showed that the Ace extract was the one with the highest antimicrobial activity against A. baumannii and S. aureus, recording a MIC of 2.5 mg/ml; for LE and for SBE of 4.9 mg/ml | [55] |
| EtOH and EtOAc/fruits | The evidence suggests that both extracts inhibited the growth of S. aureus, B. cereus, E. coli and S. typhimurium with a MIC between 12.72 and 17.11 mm. In addition, Gas Chromatography coupled to Mass Spectrometry (GC/MS) analysis identified high concentrations of flavonoids, tannins, alkaloids, and steroids; which, together, favor the antimicrobial effectiveness | [56] |
| F. racemose | ||
| Ether/leaves | The antibacterial potency of the extract against E. coli, B. pumilis, B. subtilis, P. aeruginosa and S. aureus was determined. The study confirmed an inhibitory activity comparable to that of chloramphenicol. This property could be attributed to the terpenoids present in its chemical composition | [25] |
| MeOH/leaves (MCELFr) and fruits (MCEFFr) | After identifying the bioactive polyphenols by high performance liquid chromatography with diode array detector (HPLC-DAD) of the extracts. Its antioxidant and antibacterial activity was determined. The HPLC-DAD analysis evidenced the presence of flavonoids and tannins. Both MCELFr and MCEFFr inhibited the growth of E. coli, S. flexneri, S. boydii, and S. epidermidis. In addition, they showed the ability to eliminate superoxide and hydroxyl radicals | [57] |
| F. elastica | ||
| MeOH/aerial roots | The phytochemical analysis of the extract showed Linear aliphatic alkanes with n-hexacosane, β-sitosterol, biochanin A, sitosteril 3-O-β-Dglucopyranoside, elasticamide, elastiquinone and ficusoside B. The latter demonstrated significant inhibition against S. aureus, E. coli, P. vulgaris, P. stuartii, and P. aeruginosa | [58] |
Several authors agree and consider that flavonoids, tannins, terpenoids, sterols, saponins and coumarins are involved in the antimicrobial activity of extracts from the Ficus genus. Flavonoids especially, (such as rutin, quercetin and luteolin) have multiple cellular targets where they form complexes with proteins through hydrogen bonds and/or covalent bonds. These complexes induce an antimicrobial action by inactivating microbial adhesins, enzymes and cell envelope transport proteins. In general, flavonoids inhibit nucleic acid synthesis, reduce energy metabolism and alter the function of the cytoplasmic membrane [38-40].
On the other hand, tannins are considered toxic compounds for bacteria, because they bind to their cell walls and prevent their growth (they form hydrogen bonds with proteins and phospholipids in the bacterial membrane, causing damage, leakage of metabolites and reduction of microbial biochemical activity). Terpenoids are also believed to modify the bacterial membrane through the action and alteration of lipophilic compounds. Finally, coumarins (such as psoralen and bergapten) have shown similar mechanisms of action against Gram-positive and Gram-negative bacteria [41,42].
Antifungal effect
Regarding the antifungal effect, Mavlonov et al. (2008) identified a low molecular weight antifungal protein from the latex of F. carica [59]. This motivated the group of Lazreg Aref (2010) to evaluate the antifungal activity of different extracts (MeOH, Hx, Chl, and EtOAc) from F. carica latex against Aspergillus fumigates, Trichophyton rubrum, Trichophyton soudanense, Microsporum canis, Scopulariopsis brevicaulis, Candida albicans and Cryptococcus neoformans. Their results showed that the MeOH, Hx, and EtOAc fractions were the most effective in inhibiting C. albicans and M. canis [43].
In general, relevant antifungal action has been observed against Candida albicans, Aspergillus fumigatus, Aspergillus niger, Trichophyton rubrum, Trichophyton soudanense, Microsporum canis, Scopulariopsis brevicaulis, Penicillium notatum, Penicillium italicum, Cryptococcus neoformans, Fusarium solani, Saccharomyces cerevisiae, Alternaria alternata, F. chlamydosporum, Rhizoctonia bataticola, and Trichoderma viride. Table 3 shows the main studies that demonstrate the antifungal effects of the genus Ficus L.
Table 3.
Studies testing for antifungal effects of the most representative species of the genus Ficus L.
| Extract or phytochemical/Plant part | Objective and biological effect | Ref |
|---|---|---|
| F. hirta Vahl | ||
| EtOH/fruits | Two carboline alkaloids, five sesquiterpenoids/norsesquiterpenoids, three flavonoids, and one phenylpropane-1,2-diol were identified for the first time from this extract by 1D and 2D NMR and HR-ESI-MS analysis. On the other hand, the antifungal assay revealed that the flavonoid (pinocembrin-7-O-β-d-glucoside) showed an inhibition effect against P. italicum of 13.70% at 25 μg/mL | [61] |
| Aqueous/fruits | The Xinyu mandarin is a perishable citrus vulnerable to P. italicum infections. To reduce economic losses caused by fungal damage, edible coatings based on F. hirta Vahl. fruits extract-incorporated chitosan have been applied. Such coating decreased fungal growth on mandarins stored at 5°C reducing postharvest losses and improving the storage capacity of Xinyu mandarins | [65] |
| EtOAc and Ace/fruits | Through in vitro tests, the antioxidant and antifungal activity of various solvent extracts of its fruits was evaluated. The EtOAc and Ace extracts showed abundant antioxidant components, emphasizing the flavonoid content; to which an important antifungal activity against P. italicum was attributed | [66] |
| Fruits | As mentioned above, P. italicum affects the postharvest of citrus fruits. Therefore, after obtaining a flavonone (pinocembroside) from the fruit of F. hirta Vahl., its antifungal activity against this blue mold was evaluated. Mycelial growth in Newhall navel oranges was inhibited by flavonone in a dose-dependent manner (minimum fungicidal concentration (MFC): 800 mg/L). The conclusion is that flavonone could increase the permeability of the membrane, accelerating lipid peroxidation in the fungus | [67] |
| F. carica | ||
| Latex | Using mass spectroscopy and an amino acid sequencer, a low molecular weight (6481 Da) antifungal protein was isolated. In general, composed of Arginine (Arg), Proline (Pro), Aspartic Acid (Asp), Phenylalanine (Phe), Leucine (Leu) and Glutamine (Glu) | [59] |
| MeOH, Chl, Hx, and EtOAc/latex | The antifungal potential of the extracts against opportunistic pathogenic yeasts was explored. The Chl and EtOAc fractions showed the highest inhibition (100%) in all fungi. In contrast, the MeOH fraction only inhibited C. albicans and M. canis (500 μg/ml concentration); unfortunately, it had no effect against C. neoformans. Finally, the Hx extract showed low inhibitory capacity | [43] |
| Latex | Fig latex contains proteases (mainly ficin) and chitinolytic enzymes that protect the plant from pathogens. For this reason, the changes in its composition of proteins, enzymes and antifungal activity during the ripening of the fruit were studied. The latex samples showed a uniform increase in protein concentration and a decrease in ficin content according to the ripening time of the fruit. Another important observation was that the antifungal activity against S. cerevisiae was more extensive in Spring | [68] |
| F. religiose | ||
| EtOH/Whole plant | 16 extracts from 22 Indian medicinal plants showed antifungal activity against 5 filamentous fungi (A. niger, A. alternata, F. chlamydosporum, R. bataticola and T. viride) and one yeast (C. albicans); highlighting F. religiosa by significantly inhibiting the growth of C. albicans | [34] |
| Aqueous, MeOH and Chl/leaves | The results indicated that all the extracts were active against fungal strains, especially A. niger and P. notatum | [35] |
| F. elastica | ||
| MeOH/aerial roots | Initially, a ficusoside B was isolated from the extract with antimicrobial activity against S. aureus and E. coli. Subsequently, the same methodological process was developed to analyze the antifungal capacity of ficusoside B. It was found that it also inhibits the growth of C. albicans | [58] |
As well as the antimicrobial effect, most studies suggest that the antifungal potential can also be attributed to the bioactive compounds found in the different extracts, especially flavonoids, coumarins, hydroxybenzoic acids, terpenoids and sterols (Figure 4) [2,5,17,18,29]. Specifically, flavonoids inhibit fungal growth through several mechanisms including plasma membrane alteration, induction of mitochondrial dysfunction, inhibition of cell wall formation, modification of cell division and blocking of RNA and protein synthesis [60]. Wan et al. (2017) isolated and evaluated the antifungal activity of pinocembrin-7-O-β-D-glucoside (a flavonoid present in an EtOH extract of F. hirta fruits) against postharvest citrus infections caused by P. italicum (blue mold). The results showed a significant decrease in the phytopathogen during spore germination and mycelium growth when they are exposed to the flavonoid. It was concluded that pinocembrin inhibited mycelial growth by interfering with energy homeostasis and cell membrane damage [61].
In the case of coumarins, Aboody et al. (2020) studied 40 of these phytochemicals against C. albicans, A. fumigates and F. solani. They found that ostenol and 4-acetatecoumarin showed a very significant antifungal activity [60]. Another study also confirmed that 4-methoxycoumarin showed relevant antifungal activity against F. solani [62]. This research suggests that the death of fungal mycelia occurs by affecting the structure and function of peroxisomes and mitochondria and by inhibiting β-oxidation of fatty acids.
When evaluating vanillic acid (hydroxybenzoic acid) on the growth of Sclerotium rolfsii, we observed that concentrations of 0.05 and 0.10% significantly inhibited the growth of the fungus. Its inhibition is attributable to a morphological distortion of hyphae and sclerotia, and to a reduction in fungal enzymatic activity (catalase, peroxidase, polyphenol oxidase and phenylalanine ammonia-lyase) [63]. Finally, Leite-Andrade et al. (2022) demonstrated that D-limonene (natural Monoterpene) showed a relevant antifungal and antivirulent activity against Candida parapsilosis by inhibiting the morphogenesis of its yeasts, adhering to the human epithelium and inducing an apoptotic mechanism [64].
Antiviral effect
In relation to the antiviral effect; again, natural products are a good alternative for the treatment of viral infections that are responsible for various chronic and acute diseases associated with high rates of morbidity and mortality. A wide variety of bioactive and/or phytochemical compounds (such as coumarins, flavonoids, terpenoids, organosulfur compounds, lignans, polyphenols, saponins, proteins, and peptides) have been found to influence cell functions, membrane permeability, and viral replication [31]. To date, there are few studies on the genus Ficus L. where its antiviral potential has been evaluated. The first was developed by Wang et al. (2004) to determine the anti-HSV-1 (herpes simplex type 1) effect of the aqueous extract of F. carica leaves on human epithelial (Hep-2), baby hamster kidney fibroblasts (BHK21) and primary rat kidney (PRK) cells. Ultimately, they confirmed low cell toxicity and direct virus killing at a maximum tolerated concentration (MTC) of 15 mg/ml [69]. Subsequently, they analyzed the antiviral activity of five extracts (MeOH, Hx, EtOAc, Chl, and Hx/EtOAc) from F. carica latex against HSV-1, echovirus type 11 (ECV-11) and adenovirus (ADV). The results showed that the Hx extract and the Hx/EtOAc (V/V) mixture were the best candidates to inhibit viral replication in Vero cells [70]. Finally, considering the above results, F. carica latex was added to Madin Darby bovine kidney (MDBK) cell cultures infected with caprine herpesvirus-1 (CpHV-1). The addition of latex showed significant MTC, reducing CpHV-1 infection and suggesting that latex possibly interferes with viral replication [71].
In this sense, several authors [7,70-73] consider that the combination of caoutchouc, resin, albumin, cerin, malic acid, rennin, proteolytics enzymes (mainly ficin), esterase, lipase, catalase, peroxidase, bioactive compounds of latex, affect viral infection by acting on cellular receptors, and preventing adsorption and/or penetration of the virus into host cells (that is, they can be active on intracellular viral replication). Lazreg-Aref et al. (2012) have also suggested that coumarins have an inhibitory effect on DNA gyrase and that it may be related to anti-HIV (human immunodeficiency virus) activity [41].
Recently, Dell’Annunziata et al. (2022) investigated on VERO CCL-81 cell lines the antiviral potential of three extracts (MeOH, Hx and EtOAc) from F. rubiginosa leaves against Herpes simplex virus-1 (HSV-1), Human coronavirus (HCoV)-229E, and Poliovirus-1 (PV-1). After analyzing different stages of viral infection through viral pretreatment, cotreatment, cell pretreatment, and post-infection assays; their results showed that the extracts exerted their inhibitory early action in the viral infection cycle, mainly during the absorption and/or penetration into the host cell. They were found to be active against HSV-1 and HCoV-229E through direct interaction with viral particles and by blocking the access of the viruses to host cells. This mode of action was similar for both wrapped viruses, but not for naked viruses (such as PV-1), suggesting that MeOH, Hx and EtOAc might act on the outermost viral structure, particularly on the envelope glycoproteins [74].
Anti-helminthic effect
Although antiparasitic drugs (also called anthelmintics) are often effective, there is still a high prevalence of taeniasis (Taenia saginata, Taenia solium, and Taenia asiatica), ascariasis (Ascaris lumbricoides), and threadworm infection (Enterobius vermicularis) in Asia, Africa, and Latin America. Anthelmintic or vermifuge medicinal plants (capacity to kill or expel worms), constitute an alternative to help conventional treatments to avoid discomfort and/or serious damage to the health of the host [75]. Among the most common plants with vermifuge capacity are pumpkin (Cucurbita pepo), papaya (Carica papaya), epazote (Dysphania ambrosioides) and, indisputably, the genus Ficus. This field of research began with Nagaty et al. (1959) using latex extracts of F. carica and C. papaya to control canine ascariasis [76]. Considering that the latex of the genus Ficus has a high content of a proteolytic enzyme (Ficin), the effect of F. glabrata against intestinal helminthiasis in three Amazonian villages was analyzed. The results of the clinical trial demonstrated that a dose of 1.0 cm3 of latex/kg/day for 3 days and repeating this treatment every 3 months reduced the incidence of Strongyloides stercoralis, Trichuris trichiura, Necator americanus, T. asiatica, and A. lumbricoides; especially against this last helminth where a lethal effect was presented, whose effectiveness was related to the action of ficin [77]. Similar data were also obtained with latex extracted from F. insipida and F. carica when administered intragastrically (4 mL/kg/day for 3 days) to NIH mice infected with Syphacia obvelata, Aspiculuris tetraptera, and Vampirolepis nana [78]. On the other hand, Mishra et al. (2005) studied the effect of EtOH and aqueous extracts of the fruits of F. racemosa on the movements of Setaria cervi both of the whole parasite and in preparations of the nervous muscle; as well as in the survival of their microfilariae. Both extracts (concentrations between 50 and 350 μg/mL) inhibited the spontaneous motility of the worm and in the tissue analyzed. In addition, the in vitro assay confirmed an antifilaria effect at a lethal concentration 90 (LC90) between 21 and 42 ng/ml [79]. In order to clarify the mechanism of action and compare the anthelmintic efficacy of cysteine proteinases (CPs) from various fruits, including figs, an in vitro assay was developed using the rodent gastrointestinal nematode Heligmosomoides polygyrus. After a 2-h incubation, cysteine proteinases caused damage to the protective cuticle of the worms; especially those belonging to the raw latex extracted from the fig. The data obtained suggested that these plant enzymes (particularly ficin) may be potential candidates for new anthelmintic agents [80]. F. racemosa has also shown vermifuge capacity. In a first study it was shown that aqueous extracts (50 mg/mL) significantly inhibited the motility of adult earthworms [81]. While at a dose of 10 mg of its latex per gram of soil, the population of the root-knot nematode Meloidogyne incognita was suppressed [82]. Both results suggest that F. racemosa could be considered for farmers as a biocontrol agent against infective management of earthworms. Other parasites, such as Bursaphelenchus xylophilus (pine wood nematode), Panagrellus redivivus (fish microparasite) and Caenorhabditis elegans (soil worm) have also been killed (mortality between 73 and 98%) by methanolic extracts from the leaves of F. carica; suggesting that this nematicidal effect is attributed to a coumarin called psoralen [83].
Nigerian researchers examined the potential of three ethnoveterinary plants (Irvingia gabonensis, F. exasperata and Vernonia amygdalina Delile) to treat worm infestation in eastern Nigeria. After infecting mice with Heligmosomoides bakeri larvae and administering different doses (200, 400, 800 mg/kg) of the leaf extracts, they confirmed that F. exasperata was the one that showed the best anthelmintic effect, causing 100% larval mortality [84]. Subsequently, the antischistosomal activity of aqueous extracts of Calotropis procera (Cp), Zingiber officinale (Zo) and F. elastica (Fe) was compared in male mice infected with Schistosoma mansoni. After oral administration (500 mg/kg for three consecutive days) with aqueous latex from stem and flower, the researchers evaluated the safety, worm recovery, tissue egg load and oogram pattern. The results of the study were that Cp and Fe reduced the number of S. mansoni and affected the egg load in the tissues and the pattern of oograms; unlike Zo, which slightly decreased 7.0% of the worms. In addition, it was established that the Fe extract was safer; since Cp proved to be toxic even in a small dose (250 mg/kg) [85]. Because the data suggest that ficin and psoralen in latex are the bioactive compounds responsible for exerting its vermifuge capacity; Guo et al. (2016) isolated from an EtOH extract of F. carica leaves two linear furocoumarins (bergapten and psoralen), which again had a nematicidal effect against Bursaphelenchus xylophilus by inhibiting its amylase, cellulase and acetylcholinesterase; confirming that bergapten is also involved in the vermifugal potential [86]. Finally, Wanderley et al. (2018) studied the anthelmintic activity of a purified cysteine protease (FbP) from F. benjamina latex against Haemonchus contortus (a gastrointestinal nematode found in ruminant herds). FbP had an approximate molecular weight of 24 kDa and its proteolytic activity was stable at pH ranges between 6 and 10 where it inhibited both larval development and unsheathing [87].
In general, the anthelmintic potential of the different extracts of Ficus plants has been attributed to the synergistic action of their flavonoids, aminoacids, steroids, saponins, coumarins and tannins [88-90]. These phytoconstituents favor an individual or combined effect by killing the parasite (vermicidal and/or nematicidal), or by inducing its paralysis in the gastrointestinal tract of the infected host. As mentioned, furocoumarins (bergapten and psoralen) have demonstrated nematicidal potential by inhibiting enzymatic activities of the parasites [83,86].
In the case of ficin and CPs, their mechanism of action is similar, since they have a high proteolytic activity (digestion and/or elimination) on the protective cuticles (mainly formed by graticuled collagens linked by disulfide bonds) of nematodes. Therefore, if the cuticle is degraded, it turns the parasite into a weak organism, without motility and sensitive to elimination. Some authors consider ficin and CPs an alternative strategy in the treatment of gastrointestinal nematode infections (both in humans and animals); since the risk of developing resistance is presumably low (in comparison to the use of ivermectin, albendazole, and levamisole). This hypothesis is based on the consideration that nematodes would have to alter the structure or components of their cuticle, and/or encode inhibitors of ficin or CPs to prevent their insertion into the cuticle [78,80,87,91]. On the other hand, Athanasiadou et al. (2001) and Ahamat et al. (2021) reported and agreed that gallic and gentisic acids present in the chemical composition of tannins extracted from F. sycomorus are toxic to Caenorhabditis elegans and Onchocerca ochengi. Possibly, death is attributed to the binding of tannins to glycoproteins of the cuticle of the parasites in the gastrointestinal tract of the host [89,92].
Hypoglycemic and hypolipidemic effect
The metabolic syndrome (MetS) is the set of metabolic abnormalities of carbohydrates and lipids that describes insulin resistance and/or type 2 diabetes mellitus (DM2) associated with abdominal obesity (AO) and cardiovascular diseases. These alterations are closely related; when there is a decrease in glucose uptake, stimulated by insulin, and an increase in visceral fat (mainly in the liver, muscles and pancreas) they induce the formation of adipokines that favors proinflammatory and prothrombotic states that contribute to the development of insulin resistance, hyperinsulinemia, endothelial dysfunction, macrovascular (atherosclerosis) and microvascular (retinopathy, nephropathy and neuropathy) complications. In addition, poor clinical control of patients can cause serious problems in the kidneys and heart [31]. It should be remembered that oxidative stress (OXs) is also related to insulin resistance and AO and that a high concentration of reactive oxygen species (ROS) can induce and/or favor the development of MetS [31,93].
After studying the antimicrobial and antifungal effects, the greatest scientific interest in the genus Ficus L. has focused on its ability to treat DM2 and AO. To date, approximately 42 scientific articles have been published since the 1960s; 33 of which belong to in vivo studies (mainly using rodents and rabbits), 5 are in vitro trials and 4 of them are clinical investigations.
Research on hypoglycemic and hypolipidemic activity began with Brahmachari and Augusti (1962) and Ambika and Rao (1967). The first authors found that administering an aqueous extract of F. religiosa root bark (2.5 g/kg) to albino rabbits reduced blood glucose levels [94]. Subsequently, a sitosteryl-d-glucoside (phytosterolin) was isolated from the same Ficus species, which was also administered to rabbits. Intravenous (i.v.) administration of phytosterolin (7.5 mg/kg) decreased glucose concentration, suggesting that this phytochemical could be responsible for the hypoglycemic effect of the cortex [95].
Later, Spanish scientists carried out six studies that were essential to expand this field of research. Their analysis began with the effect of an aqueous extract of F. carica leaves on rats with streptozocin-induced diabetes (STZ). Oral (v.o.) and intraperitoneal (i.p.) administration of the extract prevented loss of body weight in the animals and increased the survival rate that had been affected by elevated plasma insulin levels [96]. In a second study, the decoction of F. carica leaves (aqueous extract) and its supplementation at breakfast in patients with insulin-dependent diabetes mellitus (IDDM) were evaluated on preprandial and postprandial glycemia, glycosylated hemoglobin (HbA1c), and total cholesterol (T-Cho). At the end of the month of treatment, all the parameters had been significantly reduced; in particular, the administration of insulin was reduced and there were no alterations in preprandial glycemia [97]. Using a rat model of hypertriglyceridemia, i.p. administration of the same decoction of leaves was measured. After injection of the extract, plasma triglyceride (TG) levels had significantly decreased [98]. In the fourth study, diabetic rats were changed from regular water consumption to the aqueous extract for three weeks. At the end of the period, the extract reduced plasma glucose in diabetic animals and in non-diabetic rats, insulin levels had also decreased. The combination of both data sets suggested that in addition to hypoglycemic activity, there may be a peripheral insulin-like effect [99]. These results led to the analysis of a Chl extract obtained from the decoction of leaves on the cholesterolemia of diabetic rats. Administration of the organic phase in diabetic animals with STZ improved T-Cho levels and reduced hyperglycemia [100]. Finally, parameters related to the OXs were studied in diabetic rats. Elevated levels of erythrocyte catalase (CAT), saturated fatty acids, and monounsaturated and polyunsaturated fatty acids returned to normal values with the administration of a single dose of a chloroform extract of F. carica [101]. Taken together, these results suggest that F. carica has hypoglycemic activity, influence on lipid catabolism, and antioxidant potential. In this regard, Deepa et al. (2018) reported that crude extracts and active compounds of various species of Ficus; especially F. benghalensis, F. capensis, F. religiosa, F. microcarpa, F. racemosa, F. glumosa, F. deltoidea and F. carica by means of in vitro and in vivo models have demonstrated their antidiabetic properties. In particular, the trials with the use of alloxane (Allox) and STZ showed antidiabetic actions related to the inhibition of glucose absorption and regulation of enzyme activities in the intestinal tract, stimulation of secretion and improvement of insulin sensitivity, increase in hepatic glycogen synthesis and peripheral glucose uptake and favorable stimulation of antioxidant status [102]. Given that the information is very extensive, Table 4 summarizes and analyzes the most significant documents of the genus Ficus L. on DM2 and AO.
Table 4.
Scientific evidence of the most representative species of the genus Ficus L. on diabetes and obesity
| Type of Study | Extract or phytochemical/Plant part | Objective and biological effect | Ref |
|---|---|---|---|
| F. carica | |||
| In vivo | Aqueous/leaves | Since excessive lipid storage affects the net yield of poultry meat, it was questioned whether the dietary supplement of the extract could decrease TG and T-Cho content in livers of 8-week-old roosters. It was concluded that the extract drastically decreased the level of TG and the secretion of Cho depending on the concentration of the extract | [110] |
| In vivo | Flavonoids/fruits (FFc) and leaves (LFc) | This study was developed to identify the flavonoid content in the fruit (FFc) and leaves (LFc) and to investigate its Action on blood sugar level, daily food intake, body weight gain, feed efficiency ratio, serum lipid profile, renal function and liver function in diabetic rats. Phytochemical analysis of FFc and LFc revealed the presence of progalic acid, ferulic acid, coumaric acid, galangin, cinnamic acid, and pinostorbin quercetin. The elevation of sugar in the rats injected with Allox improved when. FFc and LFc were included in their diet. In addition, both hypercholesterolemia and hyperlipidemia, as well as hepatic and renal functions improved remarkably. Presumably, the antioxidant power, the fiber content and the presence of flavonoids in FFc and LFc were the bases for such beneficial reactions | [111] |
| In vivo | MeOH/Leaves (MELFc) | The objective of the research was to determine the antidiabetic effect of the extract (MELFc) in allox-induced diabetic rats. MELFc administration (200 mg/kg p.o. dose) showed a reduction in blood glucose and TG levels; as well as a significant improvement in body weights | [112] |
| In vivo | Aqueous/leaves (AELFc) | Joerin et al. investigated the preventive potential of the extract (AELFc) on hyperlipidemia in obese male Sprague-Dawley rats induced by a high-fat diet (HFD). The benefit of AELFc was more significant than pioglitazone (positive control), since it significantly improved the lipid profile (T-Cho, TG, and LDL) and decreased interleukin-6 (IL-6) levels and adipogenic risk factors; probably attributed to an improvement in HDL levels | [113] |
| In vivo | Leaves | The study evaluated the antioxidant, antilipidemic, and antidiabetic effects of ficusin isolated from F. carica and its action on GLUT4 translocation and PPARγ expression in HFD-STZ-induced type 2 diabetic rats. Ficusin (20 and 40 mg/kg doses) decreased serum antioxidant enzyme levels (SOD, CAT, and GPx), lipids, plasma insulin, and fasting blood glucose concentrations to nearly normal values. It also improved the PPARγ expression and GLUT4 translocation and activation in adipose tissue; suggesting promising interactions of GLUT4 and PPARγ at their active sites | [106] |
| In vitro | EtOH/fruits, leaves, and stem bark | The role of some extracts from the aerial parts of F. carica in the antioxidant, antidiabetic and antiobesogenic effects was studied. In addition, their content of polyphenols and flavonoids was estimated. The EtOH extract of the fruits was the most significant by inhibiting α-amylase and α-glucosidase (antidiabetic capacity), reducing antilipase activity (antiobesogenic potential) and showing a high antioxidant effect (attributed to a high amount of polyphenols and flavonoids) | [114] |
| In vivo | EtOAc/leaves | The action of an EtOAc extract on glucose, lipid and carbohydrate metabolism enzyme levels in HFD-STZ-induced type 2 diabetic Wistar rats was determined. After 28 days of administering the extract (250 and 500 mg/kg), they demonstrated a positive action on oral glucose tolerance (OGTT), intraperitoneal insulin tolerance test (ITT), T-Cho, TG, and body weight. Furthermore, the activity of G6Pase, fructose-1,6-bisphosphatase and hexokinase in liver tissue was enhanced. An immunohistochemical study confirmed the protective effect of pancreatic β cells | [115] |
| In vitro | Aqueous-EtOH/two cultivars (Whole plant) | Using a Chromatography-Diode Array Detection-Electrospray Ionization-Quadrupole Time-of-Flight-Mass Spectrometry (HPLC-DAD-QTOF-MS) analysis, the phenolic components of an aqueous-EtOH extract were studied. Finding mainly dihydroxybenzoic acid, dipentoside, rutin, psoralen and methoxypsoralen. With this result, we proceeded to determine the hypoglycemic, lipid-lowering and antioxidant activities of both cultivars in diabetic rats induced by allox. The extract improved the lipid profile and the blood glucose level. Likewise, the activity of antioxidant enzymes present in liver and heart tissue, increased | [116] |
| In vivo | |||
| In vitro | Aqueous, Hx, EtOAc and EtOH/fruit, leaves and stem bark | Different types of extracts were obtained from which their total content of polyphenols and flavonoids was estimated. Subsequently, its antioxidant, antidiabetic and antiobesogenic effects were demonstrated. The use of GC/MS showed that the EtOH extract of the fruits contained the highest amount of polyphenols and flavonoids (highlighting butyl butyrate, 5-hydroxymethyl furfural, 1-butoxy-1-isobutoxy butane, malic acid, tetradecanoic acid, phytol acetate, trans phytol, n-hexadecanoic acid, stearic acid, sitosterol, and 2,4,5-trimethyl-2,4-dihydro-3H-pyrazol-3-one). This set of phytochemicals is possibly responsible for inhibiting α-amylase, α-glucosidase, and antilipase | [117] |
| In vivo | MeOH/leaves (MELFc) and branches (MEBFc) | Using methanolic extracts, their protective effects were investigated in hyperlipidemic Swiss albino mice induced by Triton WR-1339. Also, their contents of phenols, flavonoids and anthocyanins, and their antioxidant activities were determined by means of DPPH*, (2,2-Diphenyl-1-Picrylhydrazil), ABTS*+ (2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) and iron reducing capacity of plasma (FRAP). After 24 hours of administration to MELFc and MEBFc in doses higher than 5,000 mg/kg, no signs of toxicity were evident. Conversely, blood tests indicated a decrease in TG, T-Cho, and LDL levels, while HDL increased. Likewise, the DPPH*, ABTS*+ and FRAP trials demonstrated its high antioxidant potential. The suggestion is that flavonoids and anthocyanins may have a synergistic effect on hyperlipidemia | [103] |
| Clinical study | Abscisic acid (ABA)/fruits | This investigation examined the protective effect of two fig fruit extracts (FFEs), each administered in two different doses of ABA, on the glycemic index (GI) and insulinemic index (II) of a standard glucose drink ingested by healthy adults. Test beverages contained 200 mg FFE-50× and 1,200 mg FFE-10× and were ingested by subjects with postprandial glucose and insulin assessed at regular intervals for 2 h to determine GI and II responses. After supplementing both doses, the GI and II values were significantly reduced; suggesting that FFEs may be a promising nutritional intervention for the management of insulin homeostasis | [118] |
| In vivo | MeOH/Whole plant | The protective effect of F. carica (CEFc) was evaluated in type 2 diabetic Wistar rats induced by HFD-STZ. CEFc (250 mg/kg/day) controlled DM2 by reducing body weight, serum glucose, T-Cho, TG, LDL, and very-low-density lipoproteins (VLDL); It also increased the beneficial effect of HDL and SOD | [119] |
| In vivo | MeOH/seeds | The purpose of the study was to compare and investigate whether the extracts of F. carica (MESFc) and Lepidium sativum (MESLs) showed any protective effect on blood sugar levels in normal and diabetic rats induced with STZ. For five weeks, MESFc and MESLs (100 and 200 mg/kg) were administered v.o., and at the end of this period glucose levels, lipid profile, and liver enzymes remarkably restored. A significant increase in HbA1c levels was also observed. However, the most significant beneficial effect corresponded to MEFcS | [120] |
| In vitro | Flavonoids/fruit peel | Extracts rich in flavonoids were tested against five enzymes (α-glucosidase, tyrosinase, urease, acetylcholinesterase, and butyrylcholinesterase) and their antioxidant activities were evaluated. The extracts were mainly effective in inhibiting tyrosinase and α-glucosidase. However, all the extracts showed a relevant antioxidant potential. On the other hand, the phytochemical analysis revealed large amounts of flavonoids (mainly flavonols, 81%). Since F. carica has shown hypoglycemic action, its flavonoids could be considered multifunctional bioactive ingredients to be used in pharmaceutical formulations | [121] |
| In vivo | EtOH/leaves | Despite fig leaves have been shown to lower blood glucose levels, their collection and use are not entirely practical or consistent. For this reason, this research determined the antidiabetic efficiency of an extract using dosages formulated in tablets. Three formulations were prepared with doses of 40, 60 and 80 mg of the extract and administered for 14 days to diabetic rats induced with allox. The results proved that all three types of tablets lowered blood glucose levels | [122] |
| F. racemosa | |||
| In vivo | EtOH/stem bark (EEBFr) | The aim of the study was to investigate the antihyperglycemic and hypolipidemic activity of the extract (EEBFr) in alloxan (Allox)-induced diabetic rats. Oral administration (100-500 mg/kg) of EEBFr reduced glucose, T-Cho, TG, and HDL levels in Allox-treated rats in a dose-dependent manner | [123] |
| In vivo | Ace/stem bark | After separating a tannin fraction (TF) from the extract, two doses (100 and 200 mg/kg) were orally administered (v.o.) to hypercholesterolemic and diabetic rats to evaluate their effects on lipid and antioxidant profiles. Administration of TF supplementation reversed the STZ-induced increase in blood glucose and decreased the elevated levels of T-Cho, TG, and LDL caused by the high-fat diet. Additionally, the antioxidant status was improved by restoring the activity of SOD, CAT and decreasing GSH-Px | [124] |
| Clinical study | Aqueous/stem bark | In order to know the hypoglycemic effect of F. racemosa in diabetic patients who were taking sulfonylureas, they were suggested to ingest 5 ml (approximately 100 mg) of an extract of its bark twice a week for 15 days. At the end of the period, blood samples where there was a notable decrease in the sugar level an hour and a half after breakfast were analyzed. Normal values of bilirubin, alanine aminotransferase (ALT), alkaline phosphatase (ALP), total protein and urea were presented | [125] |
| In vivo | Flavonoids/stem bark | Using infrared spectroscopy and nuclear magnetic resonance, four flavonoids were isolated and administered once a day for one week to diabetic rats. The results indicated that its flavonoids (100 mg/kg dose) reduced the blood glucose level and the body weight of the animals increased by the STZ. There was a decrease in lipid profile parameters. Besides, oxidative stress biomarkers and liver enzymes were normalized. In addition, coupling studies suggest that the antidiabetic potential of flavonoids is related to PPARγ and GLUT1 receptors | [126] |
| F. religiosa | |||
| In vivo | Aqueous/stem bark (AEBFr) | Evidence indicate that OXs is related to the pathogenesis of DM2. Therefore, the potential of the extract (AEBFr) to lower fasting blood glucose in diabetic Wistar rats induced by STZ was evaluated. Orally administered doses of AEBFr (100 and 200 mg/kg) modulated and restored antioxidant enzymes [CAT, glutathione peroxidase (GSH-Px), and superoxide dismutase (SOD)] to normal values. In addition, the highest dose significantly lowered blood glucose | [127] |
| In vivo | Aqueous/stem bark (AEBFr) | Pandit et al. investigated the effect of oral administration (25, 50 and 100 mg/kg) of the extract in normal, glucose loaded, hyperglycemic and diabetic rats induced by STZ. The three doses caused a reduction in blood glucose in all the experimental models. In addition, higher doses increased serum insulin, body weight, and liver glycogen content. Such phenomena are comparable to glibenclamide. AEBFr also presented an antilipoperoxidative effect by reducing the levels of TG and T-Cho | [128] |
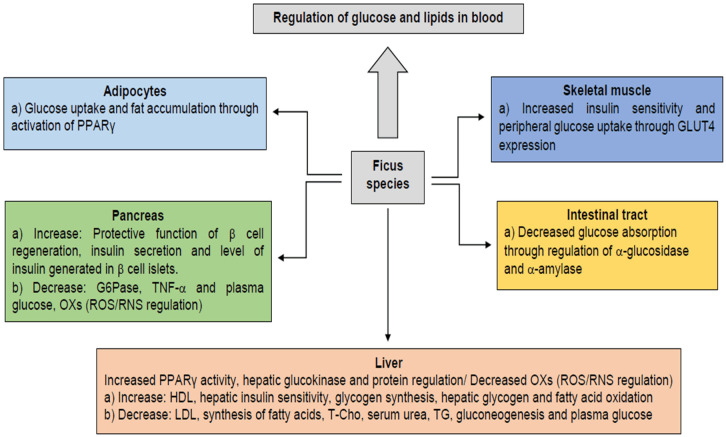
Different authors agree that the mechanisms of action involved in the hypoglycemic and hypolipidemic effect of Ficus species are diverse (Figure 5). In general, these mechanisms are related to: a) inhibition of glucose absorption in the intestinal tract, b) improvement of glucose uptake in skeletal muscle, c) reduction of blood glucose through an antioxidant mechanism, and d) pancreatic and hepatic homeostatic regulation of blood glucose levels. The latter, divided into two forms; either by breaking down glycogen (glycogenesis) or by synthesizing glucose from other metabolites such as amino acids, lactate, pyruvate and glycerol (gluconeogenesis) [102-109]. Bioactive compounds isolated from Ficus species with antidiabetic potential are quercetin, psoralen, kaempferol, ficusin, α-amyrin acetate, vitexin, isovitexin and naringenin [102-106].
Figure 5.
Hypoglycemic and hypolipidemic mechanisms of Ficus species. LDL: Low-density lipoproteins; HDL: High-density lipoproteins; PPARγ: Peroxisome proliferator-activated receptor gamma; G6Pase: Glucose-6-phosphatase; GLUT4: Glucose transporter-4; TNF-α: Tumor necrosis factor alpha; RNS: Reactive nitrogen species; TG: Triglycerides.
Hepatoprotective effect
The liver is the key organ responsible for regulating the body’s metabolism, secretion, storage, and detoxification. Therefore, if it is affected or damaged (by various toxic components acquired through food and/or medication), its functions will be distorted. Although there are many advances in modern medicine, liver diseases continue to be a constant threat to public health; and unfortunately, there are no completely effective drugs that offer a total protection of the organ or help to regenerate its cells [129]. Therefore, the use of some plants and the consumption of different fruits have played an important role due to their hepatoprotective capacity and their extracts and/or constituents, mainly from the leaves, roots, fruits and latex of different species of Ficus L., which have evidenced this property. The studies that promoted the exploration of the investigations were those carried out by Mandal et al. (2000) and Mohan et al. (2007), who, when evaluating MeOH extracts from F. hispida and F. carica leaves, found that both plant species decreased the serum levels of transaminases [Aspartate aminotransferase (AST) and Alanine aminotransferase (ALT)], bilirubin and alkaline phosphatase (ALP) in rats with acetaminophen-induced liver injury (APAP) [130] and carbon tetrachloride (CCl4) [131]. The same type of extract (250 and 500 mg/kg doses), but obtained from the bark of F. glomerata also showed a strong antioxidant and hepatoprotective activity against CCl4. Both the histological study and the significant reversal of biochemical changes in serum, liver and kidney confirmed these properties and were comparable to silymarin (well known hepatoprotective agent). Furthermore, acute toxicity tests demonstrated that the extract was safe up to 5,000 mg/kg of body weight [132]. On the other hand, when studying the effect of F. hirta aqueous extract (FhAE) on liver injury induced by N,N-Dimethylformamide (DMF) in C57BL/6 and ICR mice, it turned out that after orally administering FhAE (100, 200, 300 g/kg) for 5 days, organ damage was attenuated as ALT, AST, and lactate dehydrogenase (LDH) activities were markedly reduced [133]. Not only the species above mentioned have shown antihepatotoxic activities. Table 5 presents a summary of the main studies that confirm this beneficial and promising property, mainly attributed to anti-inflammatory and antioxidant mechanisms basically related to its phenolic components, flavonoids, saponins, steroids and glycosides [134-143].
Table 5.
Main studies examining the hepatoprotective effects of the most representative species of the genus Ficus L.
| Extract or phytochemical/Plant part | Objective and biological effect | Ref |
|---|---|---|
| F. carica | ||
| Petroleum ether/leaves | Gond et al. administered the extract to rats with liver damage induced by rifampicin. They obtained a significant reversal of the histological changes generated by hepatic toxicity; as well as a reduction of dysregulated serum levels of ALT, AST and bilirubin | [134] |
| MeOH/leaves (MELFc) | The absence of reliable liver protective drugs in modern medicine motivated scientists to determine the antioxidant and hepatoprotective activity of MELFc. His administration (50 mg/kg) regulated the activity of GSH (Glutathione), SOD and CAT. In addition, it decreased the elevated levels of AST, ALT, ALP, and bilirubin induced by CCl4. This finding suggests that its phenolic components may be responsible for the protective effect | [135] |
| EtOH/leaves | Various doses (50, 100, 200, 400 and 800 mg/kg) of the extract were administered to male albino mice (Study A) and rats Wistar (Study B) previously intoxicated with CCl4. Both studies confirmed that CCl4 increased the serum levels of ALT, AST, ALP, total bilirubin, total protein (TP), and total albumin (TA), and induced changes in the liver architecture of the animals. These alterations were significantly reversed by the extract in a dose-dependent manner | [136,137] |
| Dried fruit (DFFc) | The objective of this study was to investigate the experimental exposure during 50 days of dried fig against liver damage induced by EtOH consumption in rats. At the end of the evaluation period, the results established that DFFc had antioxidant and hepatoprotective potential, since it improves liver histopathology, decreased the elevation of serum enzymes, and restored the MDA content. The bioactive compounds of DFFc are suggested to inhibit the excessive generation of free radicals induced by EtOH | [138] |
| Aqueous/fruits | The hepatoprotective and antioxidant activity of a mixture of the edible parts of Cynara cardunculus (artichoke), Morus nigra (blackberry) and F. carica was studied and compared in rats and in a culture of HepG2 cells, both experimental designs treated with CCl4. Beneficial activities were more significant in the in vivo study; since the consumption of the mixture decreased from 47 to 37% to the AST and ALT; and increased from 77 to 101% to GSH. Besides, the phytochemical analysis showed that the fig and the artichoke have a greater amount of phenolic compounds, which probably act synergistically to combat the EOx generated by CCl4 | [139] |
| EtOH/branches | After seven days of joint exposure of Tebuconazole and extract to fruitarian bats, a hepatoprotective effect was confirmed by attenuating oxidative stress markers in the liver and testicles of bats. Among the parameters evaluated, the decrease in lipid peroxidation, SOD, CAT, vascular congestion and inflammatory infiltrates stood out | [140] |
| F. religiosa | ||
| MeOH/leaves (MELFr) | The hepatoprotective potential of MELFr against damage induced by the mixture of isoniazid-rifampicin and paracetamol (INH+RIF+APAB) was investigated in Wistar rats. Upon concluding the protocol and administering the substances, the animals were sacrificed and blood and liver samples were obtained for biochemical and histological analyses, respectively. The administration of INH+RIF+APAB caused a significant elevation in the levels of liver enzymes and TBARS. This phenomenon was reversed by MELFr. In addition, by increasing the levels of GSH and TP, the improvement of the histological profile was supported and corroborated | [141] |
| Petroleum ether/latex (PEELFr) | The experimental protocol of the study was established to evaluate the protective effects of PEELFr on cisplatin-induced liver injury in Wistar rats. While cisplatin markedly degenerated liver cells and significantly increased AST, ALT, and ALP; PEELFr administration (300 mg/kg) prevented the inflammatory process, liver necrosis, and reversed the hepatic parameters that were analyzed | [142] |
| F. microcarpa | ||
| Petroleum ether, EtOH and EtOAc/stem bark | The antioxidant and hepatoprotective activity of the extracts was analyzed against the toxicity induced by CCl4 and APAP in rats. The EtOAc extract revealed the highest protective activity by reducing the changes induced by toxic agents. Phytochemical studies confirmed the presence of catechin, a phenolic compound, which possibly interferes with the formation of free radicals and may explain its hepatoprotective effects | [143] |
In this regard, Parameswari et al. (2013) and Mujeeb et al. (2011) reported the presence of flavonoids and phenolic compounds in Ficus plant extracts, which suggests that the hepatoprotective activity of the genus can be attributed to these phytoconstituents. Both groups of researchers agree that the antioxidant effect is the main mechanism of action against liver toxicity [136,141]. On the other hand, El-Hawary et al. (2019) identified four phenolic compounds (gallic, chlorogenic, caffeic acid and rutin) in different Ficus extracts (F. mysorensis Roth ex Roem. & Schult, F. pyriformis Hook. & Arn., F. auriculata Lour., F. trigonata L., and F. spragueana Mildbr. & Burret). Subsequently, in rats with intrahepatic cholestasis induced by 17a-Ethinylestradiol (EE) and treated simultaneously with these extracts, they confirmed an improvement of liver regeneration and suppression of proinflammatory cytokines. Taken together, their results suggested that the extracts present a promising hepatoprotective potential against EE by modulating the signaling pathways of nuclear factor kappa β (NF-κB) and tumor necrosis factor alpha (TNF-α). Furthermore, a molecular coupling revealed that rutin and chlorogenic acid act effectively as Farnesoid X receptor (FXR) agonists [144].
Anti-inflammatory, analgesic and antipyretic activity
Inflammation, pain and fever are the body’s biological responses to potential threats such as trauma (blows), infections (caused by pathogens) and/or chronic degenerative diseases (activation of non-infectious endogenous molecules). Inflammation can be classified as acute and chronic. Other symptoms might be flushing or redness (produced by increased blood flow), edema or swelling (accumulation of fluid in the tissue), increased temperature (heat in the affected area) and pain. In general, acute inflammation is a rapid protective action of the immune system where leukocytes and plasma proteins accumulate. In this process, proinflammatory cytokines (including TNF-α, IL-1β, IL-6), mediators such as NO and PGE2 are increased in the body producing ROS. The transcription of the IL-1, IL-6 and TNF-α genes is induced by the nuclear factor kappa β (NF-κβ). Stimulatory agents lead to the expression of NF-κβ and then to the transcription of genes related to proinflammatory proteins. Several regulatory enzymes involving phospholipase A2 (PLA2), cyclooxygenase (COX), and lipoxygenase (LOX) play critical roles in the production of proinflammatory mediators. Therefore, any failure in these stages can lead to prolonged or chronic periods of inflammation. Also, the same inflammatory process stimulates nerve terminals in the area and induces activation of prostaglandins, which is why pain is produced [145,146]. On the other hand, fever (elevation of body temperature above 37°C) is a characteristic sign of infectious diseases; where apparently, pathogens are responsible of temperature increase. The reality is that endogenous pyrogens favor the elevation of body temperature. The process begins when bacterial lipopolysaccharides (LPS) are detected and an immunological protein binds to form the LPS-LBS complex. This complex binds to the CD14 receptor of macrophages, favoring the synthesis of IL-1, IL-6 and TNF-α; which reach the brain where they bind to microglial cells to activate the arachidonic acid pathway and induce PLA2 and COX that synthesize and release PGE2 (eicosanoid responsible of raising the temperature) [147].
The treatment of inflammatory diseases (acute and chronic) and the control of pain and fever are a great threat to public health. Therefore, the World Health Organization (WHO) considers them as fundamental issues that require great importance. Once again, natural remedies are a good strategy, and in the case of the genus Ficus L., a considerable amount of research has been carried out on its anti-inflammatory, analgesic and antipyretic role, which can favor the prevention of unwanted side affects generated by conventional medications [145].
Finding that oral administration (2 and 10 mg/kg doses) of an aqueous extract of F. elastica was effective against inflammation induced by Carrageenin (CA) and pain caused by an experimental arthritis process (both test models carried out in rats) began these fields of study [148]. Subsequently, it was observed that an aqueous extract of the bark of F. religiosa had a significant anti-inflammatory effect both in an acute test (CA-induced hind paw edema) and in a chronic model (Cotton pellet implantation) in male albine rats. Additionally, it protected mast cells from degranulation in vitro induced by propranolol and carbachol [149]. Mandal et al. (2000) induced rat hind paw edema using various substances (including CA, serotonin, histamine and dextran) and found that an extract of F. racemosa (200 and 400 mg/kg) also decreased the inflammatory process in all experimental models [150]. These results motivated another investigation to determine the anti-inflammatory and antinociceptive effect of an aqueous extract (AEFb) and methanolic extract of F. benghalensis (MEFb). After oral pretreatment (v.o.) with each extract, it was shown that MEFb had the most relevant potential for both properties; since the edema of the hind paw produced by CA, the granuloma induced by cotton granules, and the contortions induced by acetic acid (AcOH) all decreased. In addition, it prevented increased malondialdehyde (MDA) formation and increased AST, ALT, and ALP enzyme activity in inflammatory conditions. This evidence suggested that the benefits of MEFb could be attributed to its antioxidant and stabilizing effects on the lysosomal membrane [151]. F. pumila is another species that has also shown significant therapeutic properties. Histopathologically, it was verified that a methanolic extract (MEFp) decreased the level of swelling of the edematous paws, the levels of inflammatory mediators such as NO, IL-1β, TNF-α and cyclooxygenase-2 (COX-2). In addition to reducing the writhing response in the AcOH test and the licking time in the formalin test. Rutin, luteolin and apigenin were the three bioactive compounds directly related to their antinociceptive and anti-inflammatory activities [152].
Interestingly, Rao et al. (2002) and Patil et al. (2010) have provided the only evidence on the antipyretic potential of the genus Ficus L. In both studies, they induced an increase in rectal temperature by subcutaneous injection of a yeast suspension in albino rats. Pyrexia was decreased; in the first case, by a MeOH extract of F. racemosa stem bark (MEFrSB) and in the second study, by an EtOH extract of F. carica leaves (EEFcL). The protective effect of the three doses (100, 200 and 300 mg/kg) tested of MEFrSB and EEFcL were better than the standard antipyretic agent APAB; as the benefit extended for 5 hours after treatment of the extracts [153,154].
To date, 50 investigations have been carried out, of which 32 correspond to in vivo tests. Table 6 shows the most representative results of the analyzes of different extracts (aqueous, MeOH, EtOH, Chl, petroleum ether), oils, and some chemical mixtures (hydroethanolic, methanol/methylene chloride) from their stem bark, leaves, roots, fruits, branches, and seeds from different Ficus species (F. iteophylla, F. exasperata, F. hirta Vahl, F. racemosa, F. benghalensis, F. deltoidea, F. religiosa, and F. carica). Again, the last 3 species are the most studied. In summary, the results agree that the three activities (anti-inflammatory, analgesic and antipyretic) present a mechanism of action related to the ability to decrease ROS (antioxidant potential), which could be attributed to phytochemicals (phenolic compounds, polyphenols, flavonoids, terpenoids, alkaloids, steroids, tannins and saponins) predominant in the chemical composition of each extract. Likewise, the evidence suggests that these three pharmacological virtues inhibit and/or regulate signaling pathways such as inducible nitric oxide synthase (iNOS), c-Jun N-terminal kinase (JNK), p38 mitogen-activated protein kinase (MAPK), LOX and COX (1 and 2); which reduce the production of inflammatory mediators (NO, TNF-α, PGE2, NF-κβ, IL-1β and IL-6, IL-8) [145,155-182].
Table 6.
Scientific evidence of the anti-inflammatory, analgesic and antipyretic effects of the most representative species of the genus Ficus L.
| Extract or phytochemical/Plant part | Objective and biological effect | Ref |
|---|---|---|
| F. carica | ||
| Hydroalcoholic (50% EtOH/50% distilled water)/leaves | The aim of the study was to validate the anti-inflammatory and antioxidant activities of a hydroalcoholic extract. TBARS assay and superoxide scavenging activity confirmed its antioxidant capacity. Subsequently, the CA test demonstrated the anti-inflammatory effect of the extract. The anti-inflammatory activity of the extract could be attributed to its antioxidant potential, generated by the steroids and flavonoids present in its chemical composition | [155] |
| EtOH/branches (EEBFc) | In this research, an extract of branches and their fractions of water, butanol (BuOH), EtOAc, Hx were prepared to examine their ability to eliminate ROS and inhibit inflammatory reactions. The data showed that the EtOAc fraction contained the highest amount of phenolic compounds and showed the highest ROS removal activity. Particularly, EEBFc and its Hx and EtOAc fractions reduced NO production and TNF-α level in RAW264.7 cells | [156] |
| MeOH/leaves (MELFc) | After inducing an inflammatory process in rats by injection of CA, the antiangiogenic effect of MELFc was verified. Angiogenesis of granulation tissues was determined by measuring the hemoglobin content. The DPPH* assay showed a significant antioxidant activity related to its total phenolic content. MELFc inhibited leukocyte accumulation, exudate volume, as well as TNF-α and PGE2 production. Possibly, its antiangiogenic effects are related to its antioxidant and inflammatory potential | [157] |
| Aqueous/leaves (AELFc) | Vascular endothelial growth factor (VEGF), TNF-α, IL-1a and 5 alpha-reductase type II (SRD5A2) gene expression levels were tested in AELFc-treated human keratinocyte (HaCaT) cells. The cells treated with AELFc regulated the gene expression of all the markers evaluated. These results partially explain the antioxidant and anti-inflammatory effects of F. carica; which can again be attributed to its polyphenols | [158] |
| Fruits | Four new prenylated isoflavone derivatives [ficucaricones A-D (1-4), along with 12 known analogues (5-16)] were identified and isolated from the fruits of F. carica. Prenylated isoflavone derivatives (1-16) showed remarkable inhibitory effects (comparable to hydrocortisone) against NO production. In addition, all 16 compounds also exhibited antiproliferative activities in various human cancer cell lines | [159] |
| Aqueous and EtOH/leaves | The scant information on the impact of the extraction methods and the types of solvents on the profile of antioxidant and anti-inflammatory compounds in the genus Ficus L., motivated Alcantara et al. to perform an ultrasound-assisted extraction (UAE) and the phenolic analysis of F. carica leaves. The results showed that UAE in comparison to conventional extraction had an impact on obtaining total phenols. The antioxidant capacity of the aqueous extract was three times higher than the extract obtained with ethanol for conventional extraction and four times higher with UAE. While the EtOH extract presented a greater anti-inflammatory activity | [160] |
| Oil/seed (OSFc) | In this study, acute mesenteric ischemia was induced to albino Wistar rats and were administered 3 and 6 ml/kg/day of OSFc. Subsequently, they underwent an ischemia and reperfusion procedure. Both doses of OSFc reduced levels of TNF-α, IL-1β, and the enzymatic activity of SOD and CAT. Oral administration of OSFc reverses ischemia-reperfusion injury in this experimental model, probably due to its antioxidant and anti-inflammatory compounds | [161] |
| Rezagholizadeh et al. performed a systematic review using search sites such as Scopus, PubMed, Science Direct, and Google Scholar with the purpose of discussing and analyzing the anti-inflammatory effects of F. carica emphasizing its impact on key proinflammatory cytokines, including IL-1, IL-6 and TNF-α. The analysis gave as results that its polyphenols and flavonoids are mainly responsible for exerting anti-inflammatory effects by inhibiting or decreasing proinflammatory cytokines | [145] | |
| Morin | Generally, neuropathic pain is triggered through inflammation when vincristine (antineoplastic agent) is used. With that purpose, it was investigated if the morin alleviate this adverse reaction in vivo. Morin was administered via oral and pain behaviors were determined by measuring threshold (PWT) and latency (PWL) of paw withdrawal. Sciatic nerve function deficits were assessed by sciatic functional index and sciatic nerve conduction velocity. Histological abnormalities of their axons were determined and inflammatory mediators were detected by western blotting in dorsal root ganglia. The reduction of PWT and PWL and the increase in body weight of the animals was attenuated by the morin treatment. Histological alterations, neuronal hyperexcitability and increased expression of IL-6 and NF-κB were also abolished | [162] |
| F. carica and F. benghalensis | ||
| Petroleum ether, Chl, and EtOH/stem bark | Initially, the anti-inflammatory activity of three extracts of F. benghalensis from plants with different ages of maturity was compared. Subsequently, they explored the same property of these extracts but obtained from F. carica. Both studies confirmed that all the extracts administered orally (600 mg/kg/day) reduced the acute and chronic inflammatory process. However, the best effect (76%) corresponded to the EtOH extracts from young plants of both species. After concluding a phytochemical analysis, the idea that flavonoids, sterols, triterpene compounds, tannins and saponins were possibly responsible for the anti-inflammatory potential, was supported | [163,164] |
| F. benghalensis | ||
| Aqueous/roots | An experiment was developed with Swiss albino mice to determine the nociceptive potential of two doses (100 and 200 mg/kg) of an extract. Results were comparable to the indomethacin positive control; confirming that only the highest dose presented analgesic effects both in the hot plate and tail flick test (central action) and in the writhing model (peripheral pain) | [165] |
| Aqueous/stem bark (AESBFb) | The purpose of the investigation was to study the antinociceptive effect of three doses (100, 200 and 400 mg/kg) of AESBFb using the formalin-induced pain and rapid movement tests. In the tail flick test, all three tested doses increased the time elapsed for the animals to move the tail away from the source of the thermal stimulus. While in the formalin test, only the highest doses significantly reduced the duration of the lick response | [166] |
| F. religiosa | ||
| MeOH/leaves (MELFr) | Excessive production of inflammatory mediators by activated microglia is known to be related to neurodegeneration in human diseases. Therefore, the effect of MELFr on theproduction of NO and proinflammatory cytokines (TNF-α, IL-1β, and IL-6) induced by LPS in mouse microglial BV-2 cells was demonstrated. MELFr inhibited the production of inflammatory mediators and the expression of mRNA and iNOS in a dose-dependent manner. The molecular mechanism of attenuation is presumed to be related to low activity of the c-Jun N-terminal kinase (JNK) and the p38 mitogen-activated protein kinase (MAPK) pathway, which suppresses NF-κβ activation | [167] |
| EtOH/leaves (EELFr) | The study focuses on the evaluation of the anti-inflammatory (CA-induced paw edema), antioxidant (DPPH* assay), and healing (incision/excision and histopathology) activity of EELFr. The extract evidenced prominent anti-inflammatory and antioxidant activity. Beneficial effects, probably related to its glycosides and tannins | [168] |
| Aqueous/stem bark (AESBFr) | Neonatal diabetic rats were orally treated with AESBFr for 4 weeks to investigate its anti-inflammatory capacity. During the period, fasting glycemia, postprandial glycemia and serum TNF-α were quantified. The administration of 200 mg/kg AESBFr decreased all the evaluation parameters. This fact supports the idea that the antidiabetic and anti-inflammatory activity of the extract can modulate the cytokine TNF-α | [169] |
| MeOH/stem bark (MESBFr) | The extract was evaluated for its anti-inflammatory activity in Wistar albino rats and its analgesic effects in Swiss albino mice. The CA trial demonstrated that three doses of MESBFr had a comparable reduction to indomethacin in the inflammatory process. An inhibition of AcOH-induced writhing was also observed in mice. The analgesic effect was comparable to that caused by aspirin, which is the standard drug | [170] |
| F. religiosa and F. iteophylla | ||
| Petroleum ether-EtOH/leaves | Using known experimental models (AcOH-induced writhing, rapid tail flick, hot plate test, CA-generated paw edema, and granuloma formation), two investigations were carried out to assess the antinociceptive and anti-inflammatory potentials of two species of Ficus. The first focused on the analysis of five fractions (FRI, FRII, FRIII, FRIV and FRV) obtained from a first extract of F. religiosa; while the second study analyzed an extract from F. iteophylla (EELFi). The set of results showed: a) orally administered FRI and FRIII (40 mg/kg) were the most effective in reducing pain and inflammation, b) the intraperitoneal LD50 of EELFi was found to be above 3,800 ml/kg; therefore, doses of 100, 200 and 400 mg/kg were tested by the same route, c) the same beneficial effects were evidenced in the experimental models and d) both therapeutic activities were related to bioactive compounds (mainly, flavonoids, steroids, tannins and saponins) present in the chemical composition of the fractions and EELFi | [171,172] |
| EtOH/leaves (EELFi) | ||
| F. deltoidea | ||
| Aqueous/leaves | By means of acute and chronic inflammatory models (CA test, granuloma test induced by cotton pellets and formalin test), the anti-inflammatory activity of an extract intraperitoneally administered to rats was verified. The results showed that a protective activity was exerted in all tests. The conclusion was, therefore, that the leaves of F. deltoidea have a beneficial action against acute and chronic inflammatory responses | [173] |
| EtOH/leaves | Human coronary artery endothelial cells (HCAEC) were incubated with different concentrations of EtOH extracts obtained from 4 varieties of F. deltoidea [var. trengganuensis (EELFdT), var. kunstleri (EELFdK), var. deltoidea (EELFdD) and var. intermedia (EELFdI)], together with LPS to determine its action on the expression of proteins and genes of the vascular cell adhesion molecule-1 (VCAM-1), intercellular cell adhesion molecule-1 (ICAM-1), Endothelial leukocyte adhesion molecule-1 (selectin E), IL-6, iNOS, and NF-κB (assessed by ELISA and QuantiGene plex). The adhesion of monocytes to HCAEC and the formation of ROS were also detected. EELFdK showed the highest biomarker inhibition in relation to endothelial activation and inflammation and was the most potent (25%) in decreasing ROS production. It also presented a high reduction in binding to monocytes (17%). In conclusion, this Ficus species has significant antiatherogenic effects, possibly mediated through the NF-κB and iNOS pathways | [174] |
| Aqueous/leaves (AELFd) | An extract of F. deltoidea was obtained to determine its possible antinociceptive activity in three models of nociception (acetic acid-induced abdominal writhing, formalin and hot plate test). The results showed that i.p. administration of three doses of AELFd (1, 50, and 100 mg/kg), 30 min before pain induction, produced a dose-dependent analgesic effect in all the models used; which suggest both central and peripheral actions | [175] |
| Aqueous/leaves (AELFd) | Zolkiffly et al. determined the inhibitory properties of AELFd on the proinflammatory mediators involved in LPS-induced microglial cells and quantified isovitexin and vitexin in the extract by HPLC. AELFd did not show cytotoxicity at the concentrations tested (0.1, 1.0, 10, and 100 μg/mL). On the contrary, it showed neuroprotective effects by attenuating the levels of proinflammatory factors (ROS, NO, TNF-α, IL-1β and IL-6 levels) in microglial cells. Possibly, this beneficial property acts on the NF-κB signaling pathway | [176] |
| F. exasperata | ||
| EtOH/stem bark (EESBFe) | Since the increase in ROS is implicated in inflammatory diseases of the joints, the protective potential of EESBFe was investigated in the Freund’s adjuvant-induced arthritis model in rats and the antioxidant effect in vitro. Oral administration of EESBFe (30-300 mg/kg) showed a significant anti-inflammatory effect by reducing arthritic edema in the ipsilateral paw. It also reduced DPPH* and prevented lipid peroxidation in rat brain homogenates. Both activities possibly related to the phenols in the extract | [177] |
| Methylene chloride/MeOH/leaves (MMELFe) and Aqueous/leaves (AELFe) | The authors conducted two studies. The first one evaluated the topical and systemic anti-inflammatory activities of MMELFe in rodents. The second was on the effects of AELFe on the expression of IL-1β, TNF-α and iNOS induced by LPS in a culture of murine bone marrow-derived macrophages. (BMDM). MMELFe (200 and 400 mg/kg) treatment in rats led to an inhibition of agar and formaldehyde-induced acute and chronic inflammation of their hind paws, respectively. In addition, topical application (5 µg/ear) in mice also decreased (approximately 21%) the edema generated by xylene. The in vitro study revealed that concentrations between 5 and 100 µg AELFe/ml significantly reduced inflammatory mediators. In conclusion, the flavonoids, alkaloids and terpenoids, predominant in each extract, may be the basis of the benefits associated with the popular use of the plant | [178] |
| F. hirta Vahl | ||
| Phenylpropanoids and phenolic/roots | After the chemical structures of 4 new phenylpropanoids and 10 phenolic compounds were elucidated by spectroscopic analysis. Their anti-inflammatory activities were evaluated. The results indicated that most of the purified compounds showed inhibitory effects on NO production induced by LPS in RAW264.7 macrophages | [179] |
| EtOH/roots | Seven new undescribed phenolic glycosides (1-7), together with 20 analogues, were isolated from the roots of F. hirta. Their structures were determined by comprehensive spectroscopic methods (UV, IR, and NMR). While the antineuroinflammatories of all the compounds were examined by Western blot. The results indicated that compounds 1 and 11 reduced the occurrence of neuroinflammation in BV2 microglia cells, as they regulated the NF-κB, MAPK, and JNK signaling pathways. In addition, compound 11 inhibited p65 NF-κB phosphorylation. Therefore, 1 and 11 could show anti-neuroinflammatory activities | [180] |
| F. racemose | ||
| EtOH/leaves (EELFr) | A bioassay-guided fractionation of EELFr identified a new compound called racemosic acid “(rel)-4,6-dihydroxy-5-[3-methyl-(E)-propenoic acid-3-yl]-7-beta-glucopyranosyl-[2alpha,3beta-dihydrobenzofuran]-(3,2:b)-[4alpha,5beta-dihydroxy-6alpha-hydroxymethyltetrahydropyran]”. Racemosic acid demonstrated strong antioxidant activity to eliminate ABTS*+ free radical cations and a strong inhibitory activity against COX-1 and 5-LOX in vitro | [181] |
| EtOH/stem bark (EESBFr) and fruits (EEFFr) | Two extracts of F. racemosa (EESBFr and EEFFr) were tested for their possible analysis of antinociceptive activity in the acetic acid-induced writhing method in mice. Both extracts demonstrated analgesic activity in experimental animals. However, the most powerful effect corresponded to EEFFr | [182] |
Toxic evidence of the genus Ficus L.
Despite the fact that herbal medicines are considered relatively safe by the public and some health professionals because they are “natural”; little scientific data exists to support this assumption. According to the U.S. Food and Drug and Administration (FDA), it is essential to evaluate any new molecule to determine its possible therapeutic activity and potential for toxicity using animal models. In particular, plants may contain metabolites with synergistic or antagonistic action, or in other cases, induce severe poisoning, hypersensitivity reactions and/or anaphylactic shocks. Therefore, it is crucial to evaluate the adverse and toxic effects of plant extracts and isolated bioactive compounds that are intended to be used for human therapy [21]. Unquestionably, the evidence shown in this review demonstrates the therapeutic properties of the plants of the genus Ficus L.; however, they are not exempt from possible adverse and toxic effects. Most of the evidence indicates that its toxicity is related to the number and concentration of toxic chemical compounds (ficin, phytosterolin, furocoumarins and psoralens). The amount of toxic bioactive compounds is not similar in all plants and depends on the plant part, origin of the plant, environmental conditions and the type of soil where it grows. Furthermore, all edible parts of the genus Ficus L., like other plants, during cultivation, pre-harvest, post-harvest, processing and subsequent storage are prone to biological, chemical and physical contamination; critical contaminants such as mycotoxins (aflatoxins, ochratoxins, fumonisins), pesticides (Chlorpyrifos, malathion, profenofos, aldrin, dieldrin, chlordane, endosulfan, lindane), heavy metals (Cd, Cr, Pb, As, Hg) and fumigants (Ethylene oxide, phosphine, methylbromide, sulphur, polycyclic aromatic hydrocarbons) which may also have adverse effects on the consumer. Today, the main challenge in the international import/export of medicinal plants (including Ficus and/or its products) is the lack of strict regulations, standard limits, evaluative measures and/or monitoring systems. For this reason, good agricultural practices and certified organic products established by each government must be adopted to increase high-quality products, as well as quality assurance systems to provide the safety demanded by consumers [24]. Table 7 shows the main adverse effects of some species of Ficus L. Unfortunately, although Ficus plants are considered reliable and safe; even more so when the studies (mainly carried out with aqueous extracts, EtOH, and MeOH of the stem bark, fruits, roots, and leaves from species such as F. amplissima, F. arnottiana, F. asperifolia, F. benghalensis, F. benjamina, F. carica, F. cunia, F. dalhousiae, F. exasperata, F. iteophylla, F. macrophylla, F. platyphylla, F. pumila, F. racemosa, F. religiosa, and F. sycomorus) suggest lethal doses 50 (LDs50) above 2,000, 3,000, 4,000, 5,000, 6,000, 10,000 and up to 20,000 mg/kg, in oral and intraperitoneal administrations of one, two, three and for 14 days, some authors have identified and reported certain side effects, such as skin irritation and/or allergies (pigmentation on the arms and face), respiratory disease (rhinitis, asthma), abdominal pain, catharsis (evacuation of liquid stools), fluid balance disorders, muscle weakness, albuminuria, hematuria and discoloration of the urine [2,5,21,183]. Despite these side effects, Ficus plants (especially their fruits) continue to be traditional foods of frequent consumption with high agrotechnological potential, maintining that its safety is favored by presenting very high LDs50. Curiously, to date there is no established dose and/or concentration for its consumption and there are different intervals that depend on the route of administration, and the species (humans/animals) in which they are used, along with the approach to use it either in food (fresh, juice or extract) or for experimental evaluation.
Table 7.
Possible reactions and/or adverse effects of different species of Ficus L.
| Product analyzed | Assay/via/evaluated species | Reaction and/or adverse effect | Evaluated dose and/or concentration | Ref |
|---|---|---|---|---|
| F. amplissima | ||||
| Bark | Oral/rat toxicity | No effect observed | LD50 of 2,000 mg/kg | [184] |
| F. arnottiana | ||||
| Bark Extract | Acute Oral toxicity/Rat | No effect observed | LD50 of 3,000 mg/kg | [17] |
| F. asperifolia | ||||
| Aqueous extract | Acute oral toxicity/rat | No effect observed | LD50 of 4,000 mg/kg | [185] |
| F. benghalensis | ||||
| Aqueous and EtOH extracts | Oral/rat toxicity | No effect observed | LD50 of 2,000 and 5,000 mg/kg | [18] |
| Erial roots | Oral/rat toxicity | No effect observed | LD50 of 300 mg/kg | [186] |
| F. benjamina | ||||
| Leaf EtOH extract | Liver function/rat | No effect observed | LD50 of 800 mg/kg | [187] |
| Latex | Human activity/IgE assay | Oral and/or respiratory allergies (rhinitis, asthma) | [188,189] | |
| Fresh fruits | Consumption and human activity/IgE assay | Occupational allergy. Skin sensitization in plant keepers | [190,191] | |
| F. carica | ||||
| Aqueous leaf extracts | Oral/rat toxicity | Behavioral changes but no mortality | LD50 of 5,000, 5,500, 5,700 and 6,000 mg/kg | [192] |
| EtOH fruit extract (fig syrup) | Electronic Medicines (EMC)/Human Compendium | Allergy, abdominal pain and evacuation of liquid stools (after an individual overdose) | [193] | |
| Disorders in fluid balance, albuminuria, hematuria and discoloration of urine (after chronic use) | ||||
| Fresh fruits, fruit juice, contact with leaves | Human consumption | Pigmentation in the arms when rubbing the juice of the fruit with exposure to the sun | [194,195] | |
| Pigmentation of the face after eating fresh figs (phototoxic reaction), Vesicular dermatitis | ||||
| Fresh fruits | Human consumption/IgE assay | Food allergy (rhinitis) | [196,197] | |
| Aqueous leaf extract | Acute oral toxicity/mice | Mortality | 3,000, 4,000 and 8,000 mg/kg after a 12-hour fast | [198] |
| leaf extract | Brine shrimp bioassay | Mortality | LC50 of 0.158 mg/ml | [199] |
| MeOH extracts from latex | Toxicity in human melanocyte cell line HFB4 | No effect observed | LC50 of 100 μg/ml | [200] |
| Silver nanoparticles using dried fruit extract | Oral administration/rats | No effect observed | [201] | |
| F. cunia | ||||
| Extract of leaves | Brine Shrimp Bioassay | Mortality | LC50 of 55.48 μg/mL | [202] |
| F. dalhousiae | ||||
| Root EtOH Extract | Oral/mice toxicity | Mortality | LD50 of 2,000 mg/kg | [203] |
| F. deltoidea | ||||
| Leaf EtOH Extract | Oral/rat toxicity | No effect observed | LD50 of 6,400 mg/kg | [204] |
| F. exasperate | ||||
| Aqueous leaf extract | Oral and intraperitoneal toxicity/mouse and rat | No effect observed for 24 hours | LD50 of 2,500, 5,000, 10,000 and 20,000 mg/kg administered 24 hours and 14 days | [205] |
| Daily doses for 14 days, increase in body temperature and decrease in red blood cell count and hemoglobin | ||||
| F. iteophylla | ||||
| Leaf EtOH Extract | Intraperitoneal/mouse toxicity | No effect observed | LD50 of 3,807 mg/kg | [172] |
| F. macrophylla and F. pumila | ||||
| Fresh fruits | Food/Human Production | Skin irritation | [206] | |
| F. platyphylla | ||||
| MeOH extract of stem bark | Intraperitoneal toxicity/mouse | No effect observed | LD50 of 5,000 mg/kg | [207] |
| F. racemose | ||||
| MeOH extract of stem bark | Oral/rat toxicity | No effect observed | LD50 of 2,000 mg/kg | [208] |
| F. religiosa | ||||
| Aqueous extract of bark/powdered bark | Oral toxicity/rats | Catharsis/high dose allergy | [209] | |
| Aqueous extract of bark | Oral toxicity/rats | No effect observed | LD50 of 2,000 mg/kg | [128] |
| Leaf EtOH Extract | Oral toxicity/rats | No effect observed | LD50 of 4,000 mg/kg | [210] |
| F. sycomorus | ||||
| EtOAc extract of the fruits | Oral toxicity/rats | No effect observed | LD50 of 26.80 mg/ml | [211] |
| MeOH extracts from latex | Toxicity in HFB4 cell line of human melanocytes | No effect observed | LC50 of 100 μg/ml | [200] |
Median lethal dose (LD50); Median lethal concentration (LC50).
Perspectives
(1) The scientific evidence shown in this manuscript confirms the relevance of the genus Ficus L., in some pharmacological and/or therapeutic activities. However, they are not the only ones; since some authors have recorded more than 40 traditional ethnomedical uses of its plants; among which are the management of neuropsychiatric disorders (antiparkinsonian, anticonvulsant, anti-anxiety, cognitive enhancer, antiamnestic activity), gastric problems (antidiarrheal, antiulcerative, antiflatulent/carminative, indigestion, loss of appetite, gut motility, laxative, anti-constipation, antispasmodic), skin diseases (wound healing, antiwarts), cardiovascular disorders (cardioprotective, antihypertensive, endotheilin receptor antagonistic), blood disorders (antiplatelet, anticoagulant, management and/or prevention of anemia), respiratory problems (antiasthmatic, antitussive, expectorant), urinary diseases (diuretic, reduction of kidney stones), parasympathetic modulation, esterogenic, antistress, sexual disorders, menstrual pain, antimigraine, immunomodulatory, oxidative stress related disorders (antioxidant), as well as cytotoxic, an apoptosis inducer, with antiproliferative, antiangiogenic, antimutagenic, anticancer/antitumor effects [1-3,5,6,174,175,182]. Due to difficulties of analyzing them all in a single manuscript, the rest of the ethnopharmacological virtues of the genus (specifically, neuropsychiatric disorders, gastric problems, skin diseases, blood disor-ders, respiratory problems, antihypertensive and immunomodulatory actions, diuretic effect, antioxidant potential and genoprotective capacity) will be addressed in a second part of this review.
(2) The data shown in this manuscript on the extracts (aqueous, MeOH, EtOH, Hx, Chl, DCM, Ace, BuOH, EtOAc and petroleum ether), oils and latex of leaves, roots, fruits, stem bark, branches and seeds of the different species of Ficus L., confirm their relevant therapeutic effects. However, even though the experiments validated its medicinal uses; unfortunately, most of them come from uncharacterized crude extracts. This makes it difficult to point specifically to the bioactive metabolite. Therefore, it would be essential and necessary to perform a phytochemical standardization and bioactivity-guided identification of the metabolites contained in each type of extract. In some cases, through bioassay-guided fractionation, the phytochemicals responsible for the beneficial effect were identified; such is the case of the proteolytic enzyme ficin and the linear furocoumarins bergapten and psoralen, to which anthelmintic, anti-filarial, vermifuge activity and interference with latex viral replication are attributed. Phytosterolin (sitosteryl-d-glucoside) is considered to be responsible for the hypoglycemic effect of the root bark of F. religiosa. Identification and analysis of seven galactolipids from a MeOH extract of F. microcarpa leaves demonstrated their ability to inhibit TNF-α-induced IL-8 expression in vitro. Chromatography and experimental rodent models with croton oil-induced ear edema and CA testing, confirmed that coumarins (7-methoxycoumarin, 7-hydroxy-6-methoxycoumarin and methoxy-3,4-dihydrocoumarin), steroids (β-sitosterol and β-sitosterol 3-O-β-glucopyranoside), a flavonoid (trans-4-methoxy-2-β-D-glucopyranosyloxycinnamic acid), and quercetin reduced inflammatory processes. Similar results were found with kaempferol, psoralen, and carpachromene (obtained from the dried roots of F. formosana) when placed in a culture of LPS-stimulated RAW264.7 cells. Finally, using another in vitro assay, the inhibitory activity of racemosic acid (extracted from an EtOH extract of F. racemosa) against COX-1 and 5-LOX was demonstrated. Therefore, it is clear that it would be convenient to increase the individual studies of each phytochemical, to determine its protective action, since each individual substrate can activate different biochemical reactions and provide important health benefits. In addition, it would be relevant to extend the research to other species to analyze and confirm their pharmacological capacities. In this sense, it is worth mentioning that the modern pharmaceutical industry is paying more attention to medicinal plants as scientists rediscover that plant life is an almost infinite resource for medicine development. As the case of F. carica that has been included in occidental Pharmacopoeias (i.e. Spanish Pharmacopoeia, British Pharmacopoeia) and in therapeutic guides of herbal medicines, as important as the PDR for Herbal Medicines [212-215].
(3) As previously mentioned, the Ficus species is used as a nutritional source for humans and its different plant parts (roots, stem, bark, leaves, fruits, pulp, seeds) are medicinally important due to their bioactive phytochemical compounds; most of which have strong antioxidant potential in terms of metal chelation, metal reduction, lipid reduction and ROS removal; capabilities that are useful for reducing oxidative stress in biological systems. Despite the fact that the antioxidant effect of the genus Ficus L. has not been explored in detail, a large number of the studies analyzed in this manuscript support this property. Regarding this, some authors consider that the leaves contain more than 120 phytoconstituents, of which polyphenolic compounds predominate; other researchers suggest that phenolic acids and flavonoids are more relevant in fresh and dried figs; however, in both cases their levels (hence their antioxidant capacity) are strongly influenced by factors such as color, part of the fruit, ripeness, and the drying process. In general, dark-colored varieties contain a greater amount of phenolic compounds than light-colored ones. The skin of the fruit contributes more to the amount of phenolic compounds compared to the pulp of the fruit. The stage of maturation affects the concentrations of phenolic compounds in figs, the maximum have been found in ripe fruits. The difference between drying in the air and in the sun also affects the total content of bioactive compounds. For this reason, future research should provide such information and focus on improving the sensitivity and efficiency of the evaluation methods in both fresh products as in by-products since this could offer financial benefits to farmers and solve environmental problems about the sustainable management of these materials. In addition, it would increase the consumption of leaves and fruits (fresh or dried) since the significant content of their phytoconstituents favors them to have a high antioxidant power, perhaps greater than red wine and tea [7,25,26,30,212].
(4) This review leaves no doubt that the genus Ficus L. contains very versatile plants, which may motivate research groups to increase scientific evidence for their therapeutic use. Even more so when observing that most of the results on its biological activities derive mainly from in vitro tests and animal evaluations. That is, few clinical studies have been developed. This fact is striking, considering that many varieties of its plants (domesticated or wild) have been consumed since ancient times and currently continue being essential food for the human population (especially the fruits).
(5) Finally, to establish solid scientific evidence of the genus Ficus L., additional analyzes must be carried out; especially with very little evaluated species, to explore their pharmacological properties, identify the pharmacokinetics and/or pharmacodynamics, synergistic/additive/antagonist interactions between the active components, their doses and administration intervals; as well as its possible toxic effects in the short and long term. In addition, the individual identification of the bioactive compounds extracted from its plants could be used in the preparation of pharmaceutical, cosmetological and nutraceutical products for the promotion of human health.
Conclusions
(a) At the end of the scientific search, it was confirmed that there are more than 2,000 documents (scientific articles, reviews, clinical trials and book chapters) that generally describe the individual characteristics and properties of each species. Therefore, an advantage of the present review is the compilation of a significant number of studies on Ficus L. described by different authors.
(b) The scientific evidence shown in this manuscript confirms the relevance of the genus Ficus L., in 12 pharmacological and/or therapeutic activities (Antimicrobial, antifungal, antiviral, anti-helminthic, anti-filaria, vermicide, hypoglycemic, hypolipidemic, hepatoprotective, anti-inflammatory, analgesic and antipyretic).
(c) In general, most of the results confirm that the different extracts, the oils and latex from the leaves, roots, fruits, bark stem, branches, and seeds of the different species of Ficus L., present therapeutic relevant effects, whose mechanisms of action are related to the inhibition of glucose absorption and regulation of enzymatic activities in the intestinal tract, stimulation of insulin secretion and improvement of insulin sensitivity, increase in hepatic glycogen synthesis and peripheral glucose absorption, influence on lipid catabolism, favorable stimulation of antioxidant status, and activation of various anti-inflammatory mechanisms where signaling pathways such as iNOS, JNK, MAPK, LOX and COX (1 and 2) are apparently inhibited and/or regulated.
(d) It was confirmed that these beneficial properties are possibly attributed to a synergistic and/or combined effect between the different bioactive compounds (phenolic acids, sterols, flavonoids, coumarins, anthocyanins, terpenoids, glycosides, carotenoids, alkaloids, tannins, saponins, proteins, vitamins, organic acids, and soluble fiber).
(e) The most authors consider that the genus Ficus L. is safe (even in doses higher than 6,000 mg/kg, in oral and intraperitoneal administrations).
(f) The genus Ficus L. is considered to have mild/moderate toxicity due to presenting some adverse effects (mainly, skin irritation, allergies, phototoxicity from topical application, rhinitis, asthma, abdominal pain, and muscle weakness).
(g) Only about 30 angiosperm plants among the more than 850 species of the genus Ficus L have been evaluated; the most studied are F. benghalensis, F. hirta Vahl, F. elástica, F. racemosa, F. religiosa, and F. carica. Unquestionably, F. carica continues being the one with the greatest research impact in terms of phytochemicals and biological properties. As a final conclusion, there is still a long way to go in scientific research to understand in more detail the important beneficial properties of genus Ficus L.
Acknowledgements
The authors thank Florencia Ana María Talavera Silva for all her academic support. Her comments and observations in reviewing articles are always valuable and we give her immense recognition for her efforts.
All subjects gave their informed consent for inclusion before participating in the study.
Disclosure of conflict of interest
None.
References
- 1.Hajam TA, H S. Phytochemistry, biological activities, industrial and traditional uses of fig (Ficus carica): a review. Chem Biol Interact. 2022;368:110237. doi: 10.1016/j.cbi.2022.110237. [DOI] [PubMed] [Google Scholar]
- 2.Li Z, Yang Y, Liu M, Zhang C, Shao J, Hou X, Tian J, Cui Q. A comprehensive review on phytochemistry, bioactivities, toxicity studies, and clinical studies on Ficus carica Linn. Leaves. Biomed Pharmacother. 2021;137:111393. doi: 10.1016/j.biopha.2021.111393. [DOI] [PubMed] [Google Scholar]
- 3.Gafoor H, Bhattacharjee A, Hegde K, Shabaraya AR. Ficus carica: a brief review. Int J Pharm Sci Rev Res. 2019;59:40–43. [Google Scholar]
- 4.Palmeira L, Pereira C, Dias MI, Abreu RMV, Corrêa RCG, Pires TCSP, Alves MJ, Barros L, Ferreira ICFR. Nutritional, chemical and bioactive profiles of different parts of a portuguese common fig (Ficus carica L.) variety. Food Res Int. 2019;126:108572. doi: 10.1016/j.foodres.2019.108572. [DOI] [PubMed] [Google Scholar]
- 5.Badgujar SB, Patel VV, Bandivdekar AH, Mahajan RT. Traditional uses, phytochemistry and pharmacology of Ficus carica: a review. Pharm Biol. 2014;52:1487–503. doi: 10.3109/13880209.2014.892515. [DOI] [PubMed] [Google Scholar]
- 6.Mawa S, Husain K, Jantan I. Ficus carica L. (Moraceae): phytochemistry, traditional uses and biological activities. Evid Based Complement Alternat Med. 2013;2013:974256. doi: 10.1155/2013/974256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmad S, Bhatti FR, Khaliq FH, Irshad S, Madn A. A review on the prosperous phytochemical and pharmacological effects of Ficus carica. Int J Bioassays. 2013;2:843–849. [Google Scholar]
- 8.Shi Y, Mon AM, Fu Y, Zhang Y, Wang C, Yang X, Wang Y. The genus Ficus (Moraceae) used in diet: its plant diversity, distribution, traditional uses and ethnopharmacological importance. J Ethnopharmacol. 2018;226:185–196. doi: 10.1016/j.jep.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 9.Fuller DQ, Stevens CJ. Between domestication and civilization: the role of agriculture and arboriculture in the emergence of the first urban societies. Veg Hist Archaeobot. 2019;28:263–282. doi: 10.1007/s00334-019-00727-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiang-Chan EW, Tangah J, Inoue T, Kainuma M, Baba K, Oshiro N, Kezuka M, Kimura N. Botany, uses, chemistry and pharmacology of Ficus microcarpa: a short review. Sys Rev Pharm. 2017;8:103–111. [Google Scholar]
- 11.Kislev ME, Hartmann A, Bar-Yosef O. Early domesticated fig in the jordan valley. Science. 2006;312:1372–4. doi: 10.1126/science.1125910. [DOI] [PubMed] [Google Scholar]
- 12.Rodríguez-Solana R, Romano A. In: Chemical and Biological Characteristics of Ficus carica L. Fruits, Leaves, and Derivatives (Wine, Spirit, and Liqueur). Modern fruit industry. Kahramanoglu I, Kafkas NE, Küden A, Cömlekcioğlu S, editors. Croatia: InTech; 2020. pp. 1–18. [Google Scholar]
- 13.Castañeda-Barrera JO. Autonomous University of the State of Morelos. 2021. Thesis. Impact of the organization of fig (Ficus carica L.) producers in the highlands of Morelos. [Google Scholar]
- 14.Ibarra-Manríquez G, Cornejo-Tenorio G, González-Castañeda N, Piedra-Malagón EM, Luna A. The genus Ficus L. (Moraceae) in Mexico. Bot Sci. 2012;90:389–452. [Google Scholar]
- 15.González-Castañeda N, Cornejo-Tenorio G, Ibarra-Manríquez G. Ficus (Moreceae) in the balsas basin biogeographic province, Mexico. Bol Soc Bot Méx. 2010;87:105–124. [Google Scholar]
- 16.Bunawan H, Amin NM, Bunawan SN, Baharum SN, Mohd Noor N. Ficus deltoidea jack: a review on its phytochemical and pharmacological importance. Evid Based Complement Alternat Med. 2014;2014:902734. doi: 10.1155/2014/902734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmed F, Ahmed KKM, Abedin MZ, Karim AA. Traditional uses and pharmacological potential of Ficus exasperata Vahl. Sys Rev Pharm. 2012;3:15–23. [Google Scholar]
- 18.Singh D, Singh B, Goel RK. Traditional uses, phytochemistry and pharmacology of Ficus religiosa: a review. J Ethnopharmacol. 2011;134:565–83. doi: 10.1016/j.jep.2011.01.046. [DOI] [PubMed] [Google Scholar]
- 19.Erhirhie EO, Ilodigwe EE, Ihekwereme CP. Ficus sycomorus L (Moraceae): a review on its phytopharmacology and toxicity profile. Discov Phytomedicine. 2018;5:64–71. [Google Scholar]
- 20.Hossain MA. A review on Ficus sycomorus: a potential indigenous medicinal plant in Oman. J King Saud Univ Sci. 2018;31:961–965. [Google Scholar]
- 21.Murugesu S, Selamat J, Perumal V. Phytochemistry, pharmacological properties, and recent applications of Ficus benghalensis and Ficus religiosa. Plants (Basel) 2021;10:2749. doi: 10.3390/plants10122749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chandrasekar SB, Bhanumathy M, Pawar AT, Somasundaram T. Phytopharmacology of Ficus religiosa. Pharmacogn Rev. 2010;4:195–199. doi: 10.4103/0973-7847.70918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deshmukh T, Yadav B, Badole S, Bodhankar S, Dhaneshwar S. Antihyperglycaemic activity of alcoholic extract of Ficus religiosa leaves in alloxan induced diabetic mice. J Herb Med Toxicol. 2007;1:80–86. [Google Scholar]
- 24.Salehi B, Prakash Mishra A, Nigam M, Karazhan N, Shukla I, Kiełtyka-Dadasiewicz A, Sawicka B, Głowacka A, Abu-Darwish MS, Hussein Tarawneh A, Gadetskaya AV, Cabral C, Salgueiro L, Victoriano M, Martorell M, Docea AO, Abdolshahi A, Calina D, Sharifi-Rad J. Ficus plants: state of the art from a phytochemical, pharmacological, and toxicological perspective. Phytother Res. 2021;35:1187–1217. doi: 10.1002/ptr.6884. [DOI] [PubMed] [Google Scholar]
- 25.Dubey PK, Yaduwanshi P, Gouttam V. Ficus racemosa Lin-Gooler a review. World J Pharm Res. 2018;7:325–341. [Google Scholar]
- 26.Ahmed F, Urooj A. Traditional uses, medicinal properties, and phytopharmacology of Ficus racemosa: a review. Pharm Biol. 2010;48:672–681. doi: 10.3109/13880200903241861. [DOI] [PubMed] [Google Scholar]
- 27.Arsyad AS, Nurrochmad A, Fakhrudin N. Phytochemistry, traditional uses, and pharmacological activities of Ficus elastica Roxb. ex Hornem: a review. J Herbmed Pharmacol. 2023;12:41–53. [Google Scholar]
- 28.Nawaz H, Waheed R, Nawaz M. In: Phytochemical Composition, Antioxidant Potential, and Medicinal Significance of Ficus. Modern fruit industry. Kahramanoglu I, Kafkas NE, Küden A, Cömlekcioğlu S, editors. Rijeka, Croatia: InTech; 2020. pp. 1–20. [Google Scholar]
- 29.Cruz JMDA, Corrêa RF, Lamarão CV, Kinupp VF, Sanches EA, Campelo PH, Bezerra JA. Ficus spp. fruits: bioactive compounds and chemical, biological and pharmacological properties. Food Res Int. 2022;152:110928. doi: 10.1016/j.foodres.2021.110928. [DOI] [PubMed] [Google Scholar]
- 30.Arvaniti OS, Samaras Y, Gatidou G, Thomaidis NS, Stasinakis AS. Review on fresh and dried figs: chemical analysis and occurrence of phytochemical compounds, antioxidant capacity and health effects. Food Res Int. 2019;119:244–267. doi: 10.1016/j.foodres.2019.01.055. [DOI] [PubMed] [Google Scholar]
- 31.Madrigal-Santillán E, Portillo-Reyes J, Madrigal-Bujaidar E, Sánchez-Gutiérrez M, Mercado-Gonzalez PE, Izquierdo-Vega JA, Vargas-Mendoza N, Álvarez-González I, Fregoso-Aguilar T, Delgado-Olivares L, Morales-González Á, Anguiano-Robledo L, Morales-González JA. Opuntia spp. in human health: a comprehensive summary on its pharmacological, therapeutic and preventive properties. Part 1. Horticulturae. 2022;8:88. doi: 10.3390/plants11182333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mousa O, Vuorela P, Kiviranta J, Wahab SA, Hiltunen R, Vuorela H. Bioactivity of certain Egyptian Ficus species. J Ethnopharmacol. 1994;41:71–6. doi: 10.1016/0378-8741(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 33.Valsaraj R, Pushpangadan P, Smitt UW, Adsersen A, Nyman U. Antimicrobial screening of selected medicinal plants from India. J Ethnopharmacol. 1997;58:75–83. doi: 10.1016/s0378-8741(97)00085-8. [DOI] [PubMed] [Google Scholar]
- 34.Farrukh A, Iqbal A. Broad-spectrum antibacterial and antifungal properties of certain traditionally used Indian medicinal plant. World J Microbiol Biotechnol. 2003;19:653–7. [Google Scholar]
- 35.Hemaiswarya S, Poonkothai M, Raja R, Anbazhagan C. Comparative study on the antimicrobial activities of three Indian medicinal plants. Egypt J Biol. 2009;11:52–4. [Google Scholar]
- 36.Ao C, Li A, Elzaawely AA, Xuan TD, Tawata S. Evaluation of antioxidant and antibacterial activities of Ficus microcarpa L. fil. extract. Food Control. 2008;19:940–948. [Google Scholar]
- 37.Jeong M, Kim H, Cha J. Antimicrobial activity of methanol extract from Ficus carica leaves against oral bacteria. J Bacteriol Virol. 2009;39:97–102. [Google Scholar]
- 38.Nirwana I, Rianti D, Soekartono RH, Listyorini RD, Basuki DP. Antibacterial activity of fig leaf (Ficus carica Linn.) extract against Enterococcus faecalis and its cytotoxicity effects on fibroblast cells. Vet World. 2018;11:342–347. doi: 10.14202/vetworld.2018.342-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cushnie TP, Lamb AJ. Antimicrobial activity of flavonoids. Int J Antimicrob Agents. 2005;26:343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mailoa MN, Mahendradatta M, Laga A, Djide N. Antimicrobial activities of tannins extract from guava leaves (Psidium guajava L) on pathogens microbial. Int J Sci Technol Res. 2014;3:236–239. [Google Scholar]
- 41.Lazreg-Aref H, Mars M, Fekih A, Aouni M, Said K. Chemical composition and antibacterial activity of a hexane extract of Tunisian caprifig latex from the unripe fruit of Ficus carica. Pharm Biol. 2012;50:407–12. doi: 10.3109/13880209.2011.608192. [DOI] [PubMed] [Google Scholar]
- 42.Bisignano G, Sanogo R, Marino A, Aquino R, D’Angelo V, Germanò MP, De Pasquale R, Pizza C. Antimicrobial activity of Mitracarpus scaber extract and isolated constituents. Lett Appl Microbiol. 2000;30:105–108. doi: 10.1046/j.1472-765x.2000.00692.x. [DOI] [PubMed] [Google Scholar]
- 43.Aref HL, Salah KB, Chaumont JP, Fekih A, Aouni M, Said K. In vitro antimicrobial activity of four Ficus carica latex fractions against resistant human pathogens (antimicrobial activity of Ficus carica latex) Pak J Pharm Sci. 2010;23:53–8. [PubMed] [Google Scholar]
- 44.Ali NH, Faizi S, Kazmi SU. Antibacterial activity in spices and local medicinal plants against clinical isolates of Karachi, Pakistan. Pharm Biol. 2011;49:833–839. doi: 10.3109/13880209.2010.551136. [DOI] [PubMed] [Google Scholar]
- 45.Sun S, Li H, Zhou W, Liu A, Zhu H. Bacterial quorum sensing inhibition activity of the traditional Chinese Herbs, Ficus carica L. and Perilla frutescens. Chemotherapy. 2014;60:379–83. doi: 10.1159/000440946. [DOI] [PubMed] [Google Scholar]
- 46.Shahbazi Y. Antibacterial and antioxidant properties of methanolic extracts of apple (Malus pumila), grape (Vitis vinifera), pomegranate (Punica granatum L.) and common fig (Ficus carica L.) fruits. Pharm Sci. 2017;24:308–315. [Google Scholar]
- 47.Shahbazi Y. Characterization of nanocomposite films based on chitosan and carboxymethylcellulose containing Ziziphora clinopodioides essential oil and methanolic Ficus carica extract. J Food Process Preserv. 2018;42:e13444. [Google Scholar]
- 48.Benmaghnia S, Meddah B, Tir-Touil A, Gabaldon-Hernández JA. Phytochemical analysis, antioxidant and antimicrobial activities of three samples of dried figs (Ficus carica L.) from the region of mascara. J Microbiol Biotechnol Food Sci. 2019;9:208–215. [Google Scholar]
- 49.Benmaghnia S, Asma B, Boumediene M, Aicha TT. Antimicrobial activity of dried fig (Ficus carica L.) extracts from the region of Mascara (Western Algeria) on enterobacter cloacae identified by MALDI-TOF/MS. Eur J Biol Res. 2021;11:234–241. [Google Scholar]
- 50.Manimozhi DM, Sankaranarayanan S, Sampathkumar G. Evaluating the antibacterial activity of flavonoids extracted from Ficus benghalensis. Int J Pharma Bio Sci. 2012;3:7–18. [Google Scholar]
- 51.Wu T, He M, Zang X, Zhou Y, Qiu T, Pan S, Xu X. A structure-activity relationship study of flavonoids as inhibitors of E. coli by membrane interaction effect. Biochim Biophys Acta. 2013;1828:2751–2756. doi: 10.1016/j.bbamem.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 52.Rahman M, Khatun A, Khan S, Hossain F, Khan A. Phytochemical, cytotoxic and antibacterial activity of two medicinal plants of Bangladesh. Pharmacology. 2014;1:3–10. [Google Scholar]
- 53.Kaur R, Goyal AK, Kaushik D, Sharma RK. In vitro studies on antibiotic activity of Ficus religiosa fruits extract against human pathogenic Bacteria. J Chem Pharm Res. 2014;6:80–84. [Google Scholar]
- 54.Salema WM, Haridyb M, Sayeda WF, Hassanc NH. Antibacterial activity of silvernanoparticles synthesized from latex and leaf extract of Ficus sycomorus. Ind Crops Prod. 2014;62:228–234. [Google Scholar]
- 55.Saleh B, Hammoud R, Al-mariri A. Antimicrobial activity of Ficus sycomorus L. (Moraceae) leaf and stem-bark extracts against multidrug resistant human pathogens. Herba Pol. 2015;61:39–49. [Google Scholar]
- 56.El-Beltagi HS, Mohamed HI, Abdelazeem AS, Youssef R, Safwat G. GC-MS analysis, antioxidant, antimicrobial and anticancer activities of extracts from Ficus sycomorus fruits and leaves. Not Bot Horti Agrobo. 2019;47:493–505. [Google Scholar]
- 57.Sumi SA, Siraj MA, Hossain A, Mia MS, Afrin S, Rahman MM. Investigation of the key pharmacological activities of Ficus racemosa and analysis of its major bioactive polyphenols by HPLC-DAD. Evid Based Complement Alternat Med. 2016;2016:3874516. doi: 10.1155/2016/3874516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mbosso THE, Noundou XS, Fannang S, Meyer F, Vardamides JC, Mpondo E, Mpondo RWM, Krause AGB, Azebaze JCA. In vitro antimicrobial activity of the methanol extract and compounds from the wood of Ficus elastica Roxb. ex Hornem. aerial roots. S Afr J Bot. 2017;111:302–306. [Google Scholar]
- 59.Mavlonov GT, Ubaidullaeva KA, Rakhmanov MI, Abdurakhmonov IY, Abdukarimov A. Chitin-binding antifungal protein from Ficus carica latex. Chem Nat Compd. 2008;44:216–219. [Google Scholar]
- 60.Aboody MSA, Mickymaray S. Anti-fungal efficacy and mechanisms of flavonoids. Antibiotics (Basel) 2020;9:45. doi: 10.3390/antibiotics9020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wan C, Chen C, Li M, Yang Y, Chen M, Chen J. Chemical constituents and antifungal activity of Ficus hirta Vahl. Fruits. Plants (Basel) 2017;6:44. doi: 10.3390/plants6040044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.He YH, Shang XF, Li HX, Li AP, Tang C, Zhang BQ, Zhang ZJ, Wang R, Ma Y, Du SS, Hu YM, Wu TL, Zhao WB, Yang CJ, Liu YQ. Antifungal activity and action mechanism study of coumarins from Cnidium monnieri Fruit and structurally related compounds. Chem Biodivers. 2021;18:e2100633. doi: 10.1002/cbdv.202100633. [DOI] [PubMed] [Google Scholar]
- 63.Yousaf M, Shoaib A, Fatima Q, Bukhari S, Ali N, Fatima U. In vitro antifungal potential of vanillic acid against sclerotium rolfsii. J Biores Manag. 2023;10:01–08. [Google Scholar]
- 64.Leite-Andrade MC, de Araújo Neto LN, Buonafina-Paz MDS, de Assis Graciano Dos Santos F, da Silva Alves AI, de Castro MCAB, Mori E, de Lacerda BCGV, Araújo IM, Coutinho HDM, Kowalska G, Kowalski R, Baj T, Neves RP. Antifungal effect and inhibition of the virulence mechanism of D-limonene against candida parapsilosis. Molecules. 2022;27:8884. doi: 10.3390/molecules27248884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen C, Nie Z, Wan C, Chen J. Preservation of Xinyu Tangerines with an edible coating using Ficus hirta Vahl. fruits extract-incorporated chitosan. Biomolecules. 2019;9:46. doi: 10.3390/biom9020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen C, Peng X, Chen J, Wan C. Antioxidant, antifungal activities of ethnobotanical Ficus Hirta Vahl. and analysis of main constituents by HPLC-MS. Biomedicines. 2020;8:15. doi: 10.3390/biomedicines8010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen C, Wan C, Peng X, Chen J. A flavonone pinocembroside inhibits Penicillium italicum growth and blue mold development in ‘Newhall’ navel oranges by targeting membrane damage mechanism. Pestic Biochem Physiol. 2020;165:104505. doi: 10.1016/j.pestbp.2019.11.025. [DOI] [PubMed] [Google Scholar]
- 68.Raskovic B, Lazic J, Polovic N. Characterisation of general proteolytic, milk clotting and antifungal activity of Ficus carica latex during fruit ripening. J Sci Food Agric. 2016;96:576–82. doi: 10.1002/jsfa.7126. [DOI] [PubMed] [Google Scholar]
- 69.Wang G, Wang H, Song Y, Jia C, Wang Z, Xu H. Studies on anti-HSV effect of Ficus carica leaves. Zhong Yao Cai. 2004;27:754–6. [PubMed] [Google Scholar]
- 70.Lazreg Aref H, Gaaliche B, Fekih A, Mars M, Aouni M, Pierre Chaumon J, Said K. In vitro cytotoxic and antiviral activities of Ficus carica latex extracts. Nat Prod Res. 2011;25:310–9. doi: 10.1080/14786419.2010.528758. [DOI] [PubMed] [Google Scholar]
- 71.Camero M, Marinaro M, Lovero A, Elia G, Losurdo M, Buonavoglia C, Tempesta M. In vitro antiviral activity of Ficus carica latex against caprine herpesvirus-1. Nat Prod Res. 2014;28:2031–5. doi: 10.1080/14786419.2014.918120. [DOI] [PubMed] [Google Scholar]
- 72.Field HJ. Herpes simplex virus antiviral drug resistance-current tends and future prospects. J Clin Virol. 2001;21:261–269. doi: 10.1016/s1386-6532(00)00169-4. [DOI] [PubMed] [Google Scholar]
- 73.Lansky EP, Paavilainen HM, Pawlus AD, Newman RA. Ficus spp. (fig): ethnobotany and potential as anticancer and anti-inflammatory agents. J Ethnopharmacol. 2008;119:195–213. doi: 10.1016/j.jep.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 74.Dell’Annunziata F, Sellitto C, Franci G, Marcotullio MC, Piovan A, Della Marca R, Folliero V, Galdiero M, Filippelli A, Conti V, Delfino DV. Antiviral activity of Ficus rubiginosa leaf extracts against HSV-1, HCoV-229E and PV-1. Viruses. 2022;14:2257. doi: 10.3390/v14102257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Werner-Apt B. Frequently parasite infections and their management. Rev Med Clin Condes. 2014;25:485–528. [Google Scholar]
- 76.Nagaty HF, Rifaat MA, Morsy TA. Trials of the effect on dog Ascaris in vivo produced by the latex of Ficus carica and Papaya carica growing in Cairo gardens. Ann Trop Med Parasitol. 1959;53:215–9. doi: 10.1080/00034983.1959.11685918. [DOI] [PubMed] [Google Scholar]
- 77.Hansson A, Veliz G, Naquira C, Amren M, Arroyo M, Arevalo G. Preclinical and clinical studies with latex from Ficus glabrata HBK, a traditional intestinal anthelminthic in the Amazonian area. J Ethnopharmacol. 1986;7:105–38. doi: 10.1016/0378-8741(86)90053-x. [DOI] [PubMed] [Google Scholar]
- 78.de Amorin A, Borba HR, Carauta JP, Lopes D, Kaplan MA. Anthelmintic activity of the latex of Ficus species. J Ethnopharmacol. 1999;64:255–8. doi: 10.1016/s0378-8741(98)00139-1. [DOI] [PubMed] [Google Scholar]
- 79.Mishra V, Khan NU, Singhal KC. Potential antifilarial activity of fruit extracts of Ficus racemosa Linn. against Setaria cervi in vitro. Indian J Exp Biol. 2005;43:346–50. [PubMed] [Google Scholar]
- 80.Stepek G, Buttle DJ, Duce IR, Lowe A, Behnke JM. Assessment of the anthelmintic effect of natural plant cysteine proteinases against the gastrointestinal nematode, Heligmosomoides polygyrus, in vitro. Parasitology. 2005;130:203–11. doi: 10.1017/s0031182004006225. [DOI] [PubMed] [Google Scholar]
- 81.Chandrashekhar CH, Latha KP, Vagdevi HM, Vaidya VP. Anthelmintic activity of the crude extracts of Ficus racemosa. Int J Green Pharm. 2008;2:100–103. [Google Scholar]
- 82.Badgujar SB, Mahajan RT. Evaluation of nematicidal properties of some laticiferous plants. Green Farming. 2009;2:680–4. [Google Scholar]
- 83.Liu F, Yang Z, Zheng X, Luo S, Zhang K, Li G. Nematicidal coumarin from F. carica L. J Asia Pac Entomol. 2011;14:79–81. [Google Scholar]
- 84.Nweze NE, Ogidi A, Ngongeh LA. Anthelmintic potential of three plants used in Nigerian ethnoveterinary medicine. Pharm Biol. 2013;51:311–5. doi: 10.3109/13880209.2012.727833. [DOI] [PubMed] [Google Scholar]
- 85.Seif el-Din SH, El-Lakkany NM, Mohamed MA, Hamed MM, Sterner O, Botros SS. Potential effect of the medicinal plants Calotropis procera, Ficus elastica and Zingiber officinale against Schistosoma mansoni in mice. Pharm Biol. 2014;52:144–50. doi: 10.3109/13880209.2013.818041. [DOI] [PubMed] [Google Scholar]
- 86.Guo Q, Du G, He H, Xu H, Guo D, Li R. Two nematicidal furocoumarins from Ficus carica L. leaves and their physiological effects on pine wood nematode (bursaphelenchus xylophilus) Nat Prod Res. 2016;30:1969–73. doi: 10.1080/14786419.2015.1094804. [DOI] [PubMed] [Google Scholar]
- 87.Wanderley LF, Soares AMDS, Silva CRE, Figueiredo IM, Ferreira ATDS, Perales J, Mota HRO, Oliveira JTA, Costa Junior LM. A cysteine protease from the latex of Ficus benjamina has in vitro anthelmintic activity against Haemonchus contortus. Rev Bras Parasitol Vet. 2018;27:473–480. doi: 10.1590/S1984-296120180070. [DOI] [PubMed] [Google Scholar]
- 88.Aswar M, Aswar U, Watkar B, Vyas M, Wagh A, Gujar KN. Anthelmintic activity of Ficus benghalensis. Int J Green Pharm. 2008;2008:170–172. [Google Scholar]
- 89.Ahamat A, Dikti Vildina J, Liebau E, Ndjonka D. Phytochemical composition and anthelmintic activities of Ficus sycomorus L. (Moraceae) on the bovine parasite onchocerca ochengi and drug resistant strains of the free-living nematode caenorhabditis elegans. Int J Herb Med. 2021;9:100–110. [Google Scholar]
- 90.Pahari N, Majumdar S, Karati D, Mazumder R. Exploring the pharmacognostic properties and pharmacological activities of phytocompounds present in Ficus racemosa linn. : a concise review. Modern Chinese Medicine Pharmacol Res. 2022;4:100137. [Google Scholar]
- 91.Stepek G, Behnke JM, Buttle DJ, Duce IR. Natural plant cysteine proteinases as anthelmintics? Trends Parasitol. 2004;20:322–7. doi: 10.1016/j.pt.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 92.Athanasiadou S, Kyriazakis I, Jackson F, Coop RL. Direct anthelmintic effects of condensed tannins towards different gastrointestinal nematodes of sheep: in vitro and in vivo studies. Vet Parasitol. 2001;99:205–219. doi: 10.1016/s0304-4017(01)00467-8. [DOI] [PubMed] [Google Scholar]
- 93.Holvoet P. Relations between metabolic syndrome, oxidative stress and inflammation and cardiovascular disease. Verh K Acad Geneeskd Belg. 2008;70:193–219. [PubMed] [Google Scholar]
- 94.Brahmachari HD, Augusti KT. Orally effective hypoglycemic agents from plants. J Pharm Pharmacol. 1962;14:254–255. doi: 10.1111/j.2042-7158.1962.tb11089.x. [DOI] [PubMed] [Google Scholar]
- 95.Ambika SH, Rao MRR. Studies on a phytosteroin from the bark of Ficus religiosa. Indian J Pharm. 1967;29:91–94. [Google Scholar]
- 96.Perez S, Dominguez E, Ramiro J, Torres DA. A study on the glycaemic balance in streptozotocin-diabetic rats treated with an aqueous extract of Ficus Carica (fig tree) leaves. Phytother Res. 1996;10:82–83. [Google Scholar]
- 97.Serraclara A, Hawkins F, Pérez C, Domínguez E, Campillo JE, Torres MD. Hypoglycemic action of an oral fig-leaf decoction in type-I diabetic patients. Diabetes Res Clin Pract. 1998;39:19–22. doi: 10.1016/s0168-8227(97)00112-5. [DOI] [PubMed] [Google Scholar]
- 98.Pérez C, Canal JR, Campillo JE, Romero A, Torres MD. Hypotriglyceridaemic activity of Ficus carica leaves in experimental hypertriglyceridaemic rats. Phytother Res. 1999;13:188–191. doi: 10.1002/(SICI)1099-1573(199905)13:3<188::AID-PTR411>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 99.Pérez C, Domínguez E, Canal JR, Campillo JE, Torres MD. Hypoglycaemic activity of an aqueous extract from Ficus carica (fig tree) leaves in streptozotocin diabetic rats. Pharm Biol. 2000;38:181–186. doi: 10.1076/1388-0209(200007)3831-SFT181. [DOI] [PubMed] [Google Scholar]
- 100.Canal JR, Torres MD, Romero A, Perez C. A chloroform extract obtained from a decoction of Ficus carica leaves improves the cholesterolaemic status of rats with streptozotocin-induced diabetes. Acta Physiol Hung. 2000;87:71–76. doi: 10.1556/APhysiol.87.2000.1.8. [DOI] [PubMed] [Google Scholar]
- 101.Perez C, Canal JR, Torres MD. Experimental diabetes treated with Ficus carica extract: effect on oxidative stress parameters. Acta Diabetol. 2003;40:3–8. doi: 10.1007/s005920300001. [DOI] [PubMed] [Google Scholar]
- 102.Deepa P, Sowndhararajan K, Kim S, Park SJ. A role of Ficus species in the management of diabetes mellitus: a review. J Ethnopharmacol. 2018;215:210–232. doi: 10.1016/j.jep.2017.12.045. [DOI] [PubMed] [Google Scholar]
- 103.Boukhalfa F, Kadri N, Bouchemel S, Ait Cheikh S, Chebout I, Madani K, Chibane M. Antioxidant activity and hypolipidemic effect of Ficus carica leaf and twig extracts in Triton WR-1339-induced hyperlipidemic mice. Med J Nutrition Metab. 2018;11:37–50. [Google Scholar]
- 104.Zhang YJ, Gan RY, Li S, Zhou Y, Li AN, Xu DP, Li HB. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules. 2015;20:21138–21156. doi: 10.3390/molecules201219753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mahmoudi S, Khali M, Benkhaled A, Benamirouche K, Baiti I. Phenolic and flavonoid contents, antioxidant and antimicrobial activities of leaf extracts from ten Algerian Ficus carica L. varieties. Asian Pac J Trop Biomed. 2016;6:82–83. [Google Scholar]
- 106.Irudayaraj SS, Stalin A, Sunil C, Duraipandiyan V, Al-Dhabi NA, Ignacimuthu S. Antioxidant, antilipidemic and antidiabetic effects of ficusin with their effects on GLUT4 translocation and PPARγ expression in type 2 diabetic rats. Chem Biol Interact. 2016;256:85–93. doi: 10.1016/j.cbi.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 107.Stephen Irudayaraj S, Christudas S, Antony S, Duraipandiyan V, Naif Abdullah AD, Ignacimuthu S. Protective effects of Ficus carica leaves on glucose and lipids levels, carbohydrate metabolism enzymes and beta-cells in type 2 diabetic rats. Pharm Biol. 2017;55:1074–1081. doi: 10.1080/13880209.2017.1279671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Farsi E, Ahmad M, Hor SY, Khadeer Ahamed MB, Yam MF, Asmawi MZ, Khoo BY. Correction to: standardized extract of Ficus deltoidea stimulates insulin secretion and blocks hepatic glucose production by regulating the expression of glucose-metabolic genes in streptozitocin-induced diabetic rats. BMC Complement Altern Med. 2018;18:262. doi: 10.1186/s12906-018-2333-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lin L, Zhang Y. Chemical constituents and antidiabetic activity of dichloromethane extract from Ficus carica Leaves. Diabetes Metab Syndr Obes. 2023;16:979–991. doi: 10.2147/DMSO.S405150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Asadi F, Pourkabir M, Maclaren R, Shahriari A. Alterations to lipid parameters in response to fig tree (Ficus carica) leaf extract in chicken liver slices. Turk J Vet Anim Sci. 2006;30:315–318. [Google Scholar]
- 111.El-Shobaki FA, El-Bahay AM, Esmail RSA, El-Megeid AAA, Esmail NS. Effect of figs fruit (Ficus carica L.) and its leaves on hyperglycemia in alloxan diabetic rats. World J Dairy Food Sci. 2010;5:47–57. [Google Scholar]
- 112.Stalin C, Dineshkumar P, Nithiyananthan K. Evaluation of antidiabetic activity of methanolic leaf extract of Ficus carica in alloxan-induced diabetic rats. Asian J Pharm Clin Res. 2012;5:85–87. [Google Scholar]
- 113.Joerin L, Kauschka M, Bonnländer B, Pischel I, Benedek B, Butterweck V. Ficus carica leaf extract modulates the lipid profile of rats fed with a high-fat diet through an increase of HDL-C. Phytother Res. 2014;28:261–7. doi: 10.1002/ptr.4994. [DOI] [PubMed] [Google Scholar]
- 114.Mopuri R, Islam MS. Antidiabetic and anti-obesity activity of Ficus carica: in vitro experimental studies. Diabetes Metab. 2016;42:300. [Google Scholar]
- 115.Stephen Irudayaraj S, Christudas S, Antony S, Duraipandiyan V, Naif Abdullah AD, Ignacimuthu S. Protective effects of Ficus carica leaves on glucose and lipids levels, carbohydrate metabolism enzymes and β-cells in type 2 diabetic rats. Pharm Biol. 2017;55:1074–1081. doi: 10.1080/13880209.2017.1279671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Belguith-Hadriche O, Ammar S, Contreras MDM, Fetoui H, Segura-Carretero A, El Feki A, Bouaziz M. HPLC-DAD-QTOF-MS profiling of phenolics from leaf extracts of two Tunisian fig cultivars: potential as a functional food. Biomed Pharmacother. 2017;89:185–193. doi: 10.1016/j.biopha.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 117.Mopuri R, Ganjayi M, Meriga B, Koorbanally NA, Islam MS. The effects of Ficus carica on the activity of enzymes related to metabolic syndrome. J Food Drug Anal. 2018;26:201–210. doi: 10.1016/j.jfda.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Atkinson FS, Villar A, Mulà A, Zangara A, Risco E, Smidt CR, Hontecillas R, Leber A, Bassaganya-Riera J. Abscisic acid standardized fig (Ficus carica) extracts ameliorate postprandial glycemic and insulinemic responses in healthy adults. Nutrients. 2019;11:1757. doi: 10.3390/nu11081757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Arafa EA, Hassan W, Murtaza G, Buabeid MA. Ficus carica and Sizigium cumini regulate glucose and lipid parameters in high-fat diet and streptozocin-induced rats. J Diabetes Res. 2020;2020:6745873. doi: 10.1155/2020/6745873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ramadan S, Hegab AM, Al-Awthan YS, Al-Duais MA, Tayel AA, Al-Saman MA. Comparison of the efficiency of lepidium sativum, Ficus carica, and punica granatum methanolic extracts in relieving hyperglycemia and hyperlipidemia of streptozotocin-induced diabetic rats. J Diabetes Res. 2021;2021:6018835. doi: 10.1155/2021/6018835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Meziant L, Bachir-bey M, Bensouici C, Saci F, Boutiche M, Louaileche H. Assessment of inhibitory properties of flavonoid-rich fig (Ficus carica L.) peel extracts against tyrosinase, α-glucosidase, urease and cholinesterases enzymes, and relationship with antioxidant activity. Eur J Integr Med. 2021;43:101272. [Google Scholar]
- 122.Kurniawan MF, Yusuf FA. The possible antidiabetic effect of Ficus carica L. Tablet on lloxan-induced diabetes model in rats. Maced J Med Sci. 2021;9:727–734. [Google Scholar]
- 123.Sophia D, Manoharan S. Hypolipidemic activities of Ficus racemosa Linn. bark in alloxan induced diabetic rats. Afr J Tradit Complement Altern Med. 2007;4:279–288. doi: 10.4314/ajtcam.v4i3.31220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Velayutham R, Sankaradoss N, Ahamed KF. Protective effect of tannins from Ficus racemosa in hypercholesterolemia and diabetes induced vascular tissue damage in rats. Asian Pac J Trop Med. 2012;5:367–73. doi: 10.1016/S1995-7645(12)60061-3. [DOI] [PubMed] [Google Scholar]
- 125.Gul-e-Rana, Karim S, Khurhsid R, Saeed-ul-Hassan S, Tariq I, Sultana M, Rashid AJ, Shah SH, Murtaza G. Hypoglycemic activity of Ficus racemosa bark in combination with oral hypoglycemic drug in diabetic human. Acta Pol Pharm. 2013;70:1045–1049. [PubMed] [Google Scholar]
- 126.Keshari AK, Kumar G, Kushwaha PS, Bhardwaj M, Kumar P, Rawat A, Kumar D, Prakash A, Ghosh B, Saha S. Isolated flavonoids from Ficus racemosa stem bark possess antidiabetic, hypolipidemic and protective effects in albino Wistar rats. J Ethnopharmacol. 2016;181:252–62. doi: 10.1016/j.jep.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 127.Kirana H, Agrawal SS, Srinivasan BP. Aqueous extract of Ficus religiosa linn. reduces oxidative stress in experimentally induced type 2 diabetic rats. Indian J Exp Biol. 2009;47:822–6. [PubMed] [Google Scholar]
- 128.Pandit R, Phadke A, Jagtap A. Antidiabetic effect of Ficus religiosa extract in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2010;128:462–6. doi: 10.1016/j.jep.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 129.Madrigal-Santillán E, Madrigal-Bujaidar E, Álvarez-González I, Sumaya-Martínez MT, Gutiérrez-Salinas J, Bautista M, Morales-González A, García-Luna y González-Rubio M, Aguilar-Faisal JL, Morales-González JA. Review of natural products with hepatoprotective effects. World J Gastroenterol. 2014;20:14787–804. doi: 10.3748/wjg.v20.i40.14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mandal SC, Saraswathi B, Kumar CK, Mohana Lakshmi S, Maiti BC. Protective effect of leaf extract of Ficus hispida Linn. against paracetamol-induced hepatotoxicity in rats. Phytother Res. 2000;14:457–9. doi: 10.1002/1099-1573(200009)14:6<457::aid-ptr610>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 131.Mohan GK, Pallavi E, Ravi KB, Ramesh M, Venkatesh S. Hepatoprotective activity of Ficus Carica Linn. leaf extract against carbon tetrachloride-induced hepatotoxicity in rats. Daru. 2007;15:162–166. [Google Scholar]
- 132.Channabasavaraj KP, Badami S, Bhojraj S. Hepatoprotective and antioxidant activity of methanol extract of Ficus glomerata. J Nat Med. 2008;62:379–83. doi: 10.1007/s11418-008-0245-0. [DOI] [PubMed] [Google Scholar]
- 133.Lv YJ, Jia FL, Ruan M, Zhang BX. The hepatoprotective effect of aqueous extracts from Ficus hirta on N, N-dimethylformamide induced acute liver injury in mice. Zhong Yao Cai. 2008;31:1364–8. [PubMed] [Google Scholar]
- 134.Gond NY, Khadabadi SS. Hepatoprotective activity of Ficus carica leaf extract on rifampicin-induced hepatic damage in rats. Indian J Pharm Sci. 2008;70:364–6. doi: 10.4103/0250-474X.43003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Singab AN, Ayoub NA, Ali EN, Mostafa NM. Antioxidant and hepatoprotective activities of Egyptian moraceous plants against carbon tetrachloride-induced oxidative stress and liver damage in rats. Pharm Biol. 2010;48:1255–64. doi: 10.3109/13880201003730659. [DOI] [PubMed] [Google Scholar]
- 136.Mujeeb M, Alam Khan S, Aeri V, Ali B. Hepatoprotective activity of the ethanolic extract of Ficus carica Linn. leaves in carbon tetrachloride-induced hepatotoxicityin rats. Iran J Pharm Res. 2011;10:301–6. [PMC free article] [PubMed] [Google Scholar]
- 137.Aghel N, Kalantari H, Rezazadeh S. Hepatoprotective effect of Ficus carica leaf extract on mice intoxicated with carbon tetrachloride. Iran J Pharm Res. 2011;10:63–8. [PMC free article] [PubMed] [Google Scholar]
- 138.Turan A, Celik I. Antioxidant and hepatoprotective properties of dried fig against oxidative stress and hepatotoxicity in rats. Int J Biol Macromol. 2016;91:554–9. doi: 10.1016/j.ijbiomac.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 139.Youssef FS, Labib RM, Eldahshan OA, Singab ANB. Synergistic hepatoprotective and antioxidant effect of artichoke, fig, blackberry herbal mixture on HepG2 cells and their metabolic profiling using NMR coupled with chemometrics. Chem Biodivers. 2017:14. doi: 10.1002/cbdv.201700206. [DOI] [PubMed] [Google Scholar]
- 140.Freitas RMP, Linhares BS, Oliveira JM, Leite JPV, da Matta SLP, Gonçalves RV, Freitas MB. Tebuconazole-induced toxicity and the protective effect of Ficus carica extract in Neotropical fruit-eating bats. Chemosphere. 2021;275:129985. doi: 10.1016/j.chemosphere.2021.129985. [DOI] [PubMed] [Google Scholar]
- 141.Parameswari SA, Chetty CM, Chandrasekhar KB. Hepatoprotective activity of Ficus religiosa leaves against isoniazid+rifampicin and paracetamol induced hepatotoxicity. Pharmacognosy Res. 2013;5:271–6. doi: 10.4103/0974-8490.118828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yadav YC. Hepatoprotective effect of Ficus religiosa latex on cisplatin induced liver injury in Wistar rats. Braz J Pharm. 2015;25:278–283. [Google Scholar]
- 143.Kalaskar MG, Surana SJ. Free radical scavenging and hepatoprotective potential of Ficus microcarpa L. fil. bark extracts. J Nat Med. 2011;65:633–40. doi: 10.1007/s11418-011-0532-z. [DOI] [PubMed] [Google Scholar]
- 144.El-Hawary SS, Ali ZY, Younis IY. Hepatoprotective potential of standardized Ficus species in intrahepatic cholestasis rat model: involvement of nuclear factor-κB, and Farnesoid X receptor signaling pathways. J Ethnopharmacol. 2019;231:262–274. doi: 10.1016/j.jep.2018.11.026. [DOI] [PubMed] [Google Scholar]
- 145.Rezagholizadeh L, Aghamohammadian M, Oloumi M, Banaei S, Mazani M, Ojarudi M. Inhibitory effects of Ficus carica and olea europaea on pro-inflammatory cytokines: a review. Iran J Basic Med Sci. 2022;25:268–275. doi: 10.22038/IJBMS.2022.60954.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Crespo-Pardo L, Taboada-Iglesias Y. Inflammatory mediators: its connection with chronic pain and associated problems: review. Rev Soc Esp Dolor. 2021;28:37–46. [Google Scholar]
- 147.Ramón-Romero F, Farías JM. Fever. Rev Fac Med Mex. 2014;57:20–33. [Google Scholar]
- 148.Sackeyfio AC, Lugeleka OM. The anti-inflammatory effect of a crude aqueous extract of the root bark of Ficus elastica in the rat. Arch Int Pharmacodyn Ther. 1986;281:169–76. [PubMed] [Google Scholar]
- 149.Viswanathan S, Thirugnanasambantham P, Reddy MK, Narasimhan S, Subramaniam GA. Anti-inflammatory and mast cell protective effect of ficus religiosa. Anc Sci Life. 1990;10:122–5. [PMC free article] [PubMed] [Google Scholar]
- 150.Mandal SC, Maity TK, Das J, Saba BP, Pal M. Anti-inflammatory evaluation of Ficus racemosa Linn. leaf extract. J Ethnopharmacol. 2000;72:87–92. doi: 10.1016/s0378-8741(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 151.Thakare VN, Suralkar AA, Deshpande AD, Naik SR. Stem bark extraction of Ficus bengalensis Linn for anti-inflammatory and analgesic activity in animal models. Indian J Exp Biol. 2010;48:39–45. [PubMed] [Google Scholar]
- 152.Liao CR, Kao CP, Peng WH, Chang YS, Lai SC, Ho YL. Analgesic and anti-inflammatory activities of methanol extract of Ficus pumila L. in mice. Evid Based Complement Alternat Med. 2012;2012:340141. doi: 10.1155/2012/340141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rao RB, Anupama K, Swaroop KR, Murugesan T, Pal M, Mandal SC. Evaluation of anti-pyretic potential of Ficus racemosa bark. Phytomedicine. 2002;9:731–3. doi: 10.1078/094471102321621340. [DOI] [PubMed] [Google Scholar]
- 154.Patil VV, Bhangale SC, Patil VR. Evaluation of anti-pyretic potential of Ficus carica leaves. Int J Pharm Sci Rev Res. 2010;2:48–50. [Google Scholar]
- 155.Ali B, Mujeeb M, Aeri V, Mir SR, Faiyazuddin M, Shakeel F. Anti-inflammatory and antioxidant activity of Ficus carica Linn. leaves. Nat Prod Res. 2012;26:460–5. doi: 10.1080/14786419.2010.488236. [DOI] [PubMed] [Google Scholar]
- 156.Park S, Han J, Im K, Whang WK, Min H. Antioxidative and anti-inflammatory activities of an ethanol extract from fig (Ficus carica) branches. Food Sci Biotechnol. 2013;22:1071–1075. [Google Scholar]
- 157.Eteraf-Oskouei T, Allahyari S, Akbarzadeh-Atashkhosrow A, Delazar A, Pashaii M, Gan SH, Najafi M. Methanolic extract of Ficus carica Linn. leaves exerts antiangiogenesis effects based on the rat air pouch model of inflammation. Evid Based Complement Alternat Med. 2015;2015:760405. doi: 10.1155/2015/760405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Turkoglu M, Pekmezci E, Kilic S, Dundar C, Sevinc H. Effect of Ficus carica leaf extract on the gene expression of selected factors in HaCaT cells. J Cosmet Dermatol. 2017;16:e54–e58. doi: 10.1111/jocd.12344. [DOI] [PubMed] [Google Scholar]
- 159.Liu YP, Guo JM, Yan G, Zhang MM, Zhang WH, Qiang L, Fu YH. Anti-inflammatory and antiproliferative prenylated isoflavone derivatives from the fruits of Ficus carica. J Agric Food Chem. 2019;67:4817–4823. doi: 10.1021/acs.jafc.9b00865. [DOI] [PubMed] [Google Scholar]
- 160.Alcántara C, Žugčić T, Abdelkebir R, García-Pérez JV, Jambrak AR, Lorenzo JM, Collado MC, Granato D, Barba FJ. Effects of ultrasound-assisted extraction and solvent on the phenolic profile, bacterial growth, and anti-inflammatory/antioxidant activities of mediterranean olive and fig leaves extracts. Molecules. 2020;25:1718. doi: 10.3390/molecules25071718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Orak C, Şirinyıldız F, Gökmen Yılmaz E, Cesur G, Ek RO. Protective effects of Ficus carica seed oil on ischemia and reperfusion injury in a rat model of acute mesenteric ischemia. Ulus Travma Acil Cerrahi Derg. 2021;27:402–409. doi: 10.14744/tjtes.2020.76767. [DOI] [PubMed] [Google Scholar]
- 162.Jiang K, Shi J, Shi J. Morin alleviates vincristine-induced neuropathic pain via nerve protective effect and inhibition of NF-κB pathway in rats. Cell Mol Neurobiol. 2019;39:799–808. doi: 10.1007/s10571-019-00679-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Patil VV, Patil VR. A comparative evaluation of anti-inflammatory activity of the bark of ficus bengalensis in plants of different age. J Basic Clin Pharm. 2010;1:107–13. [PMC free article] [PubMed] [Google Scholar]
- 164.Patil VV, Patil VR. Evaluation of anti-inflammatory activity of Ficus carica linn leaves. Indian J Nat Prod Res. 2011;2:151–155. [Google Scholar]
- 165.Panday DR, Rauniar GP. Effect of root-extracts of Ficus benghalensis (Banyan) in pain in animal models. J Neurosci Rural Pract. 2016;7:210–5. doi: 10.4103/0976-3147.178660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rajdev K, Jain S, C H M, Bhattacharaya SK. Antinociceptive effect of Ficus bengalensis bark extract in experimental models of pain. Cureus. 2018;10:e2259. doi: 10.7759/cureus.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jung HW, Son HY, Minh CV, Kim YH, Park YK. Methanol extract of Ficus leaf inhibits the production of nitric oxide and proinflammatory cytokines in LPS-stimulated microglia via the MAPK pathway. Phytother Res. 2008;22:1064–9. doi: 10.1002/ptr.2442. [DOI] [PubMed] [Google Scholar]
- 168.Charde RM, Dhongade HJ, Charde MS, Kasture AV. Evaluation of antioxidant, wound healing and anti-inflammatory activity of ethanolic extract of leaves of Ficus religiosa. Int J Pharm Sci Res. 2010;1:73–82. [Google Scholar]
- 169.Kirana H, Jali MV, Srinivasan BP. The study of aqueous extract of Ficus religiosa Linn. on cytokine TNF-α in type 2 diabetic rats. Pharmacognosy Res. 2011;3:30–4. doi: 10.4103/0974-8490.79112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sreelekshmi R, Latha P, Arafat M, Sukumaran S, Shine VJ, Anuja GI, Rajasekharan S. Anti-inflammatory, analgesic and anti-lipid peroxidation studies on stem bark of Ficus religiosa Linn. Indian J Nat Prod Res. 2007;6:377–381. [Google Scholar]
- 171.Gulecha V, Sivakumar T, Upaganlawar A, Mahajan M, Upasani C. Screening of Ficus religiosa leaves fractions for analgesic and anti-inflammatory activities. Indian J Pharmacol. 2011;43:662–6. doi: 10.4103/0253-7613.89822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Abdulmalik IA, Sule MI, Musa AM, Yaro AH, Abdullahi MI, Abdulkadir MF, Yusuf H. Evaluation of analgesic and anti-inflammatory effects of ethanol extract of Ficus iteophylla leaves in rodents. Afr J Tradit Complement Altern Med. 2011;8:462–6. doi: 10.4314/ajtcam.v8i4.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zakaria ZA, Hussain MK, Mohamad AS, Abdullah FC, Sulaiman MR. Anti-inflammatory activity of the aqueous extract of ficus deltoidea. Biol Res Nurs. 2012;14:90–7. doi: 10.1177/1099800410395378. [DOI] [PubMed] [Google Scholar]
- 174.Mohd Ariff A, Abu Bakar NA, Abd Muid S, Omar E, Ismail NH, Ali AM, Mohd Kasim NA, Mohd Nawawi H. Ficus deltoidea suppresses endothelial activation, inflammation, monocytes adhesion and oxidative stress via NF-κB and eNOS pathways in stimulated human coronary artery endothelial cells. BMC Complement Med Ther. 2020;20:56. doi: 10.1186/s12906-020-2844-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sulaiman MR, Hussain MK, Zakaria ZA, Somchit MN, Moin S, Mohamad AS, Israf DA. Evaluation of the antinociceptive activity of Ficus deltoidea aqueous extract. Fitoterapia. 2008;79:557–61. doi: 10.1016/j.fitote.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 176.Zolkiffly SZI, Stanslas J, Abdul Hamid H, Mehat MZ. Ficus deltoidea: potential inhibitor of pro-inflammatory mediators in lipopolysaccharide-induced activation of microglial cells. J Ethnopharmacol. 2021;279:114309. doi: 10.1016/j.jep.2021.114309. [DOI] [PubMed] [Google Scholar]
- 177.Abotsi WM, Woode E, Ainooson GK, Amo-Barimah AK, Boakye-Gyasi E. Antiarthritic and antioxidant effects of the leaf extract of Ficus exasperata P. beauv. (Moraceae) Pharmacognosy Res. 2010;2:89–97. doi: 10.4103/0974-8490.62958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nworu CS, Nwuke HC, Akah PA, Okoye FB, Esimone CO. Extracts of Ficus exasperata leaf inhibit topical and systemic inflammation in rodents and suppress LPS-induced expression of mediators of inflammation in macrophages. J Immunotoxicol. 2013;10:302–10. doi: 10.3109/1547691X.2012.732121. [DOI] [PubMed] [Google Scholar]
- 179.Cheng J, Yi X, Chen H, Wang Y, He X. Anti-inflammatory phenylpropanoids and phenolics from Ficus hirta Vahl. Fitoterapia. 2017;121:229–234. doi: 10.1016/j.fitote.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 180.Ye X, Tian W, Wang G, Zhang X, Zhou M, Zeng D, Liu X, Yao X, Zhang Y, Chen H. Phenolic glycosides from the roots of Ficus hirta Vahl. and their antineuroinflammatory activities. J Agric Food Chem. 2020;68:4196–4204. doi: 10.1021/acs.jafc.9b07876. [DOI] [PubMed] [Google Scholar]
- 181.Li RW, Leach DN, Myers SP, Lin GD, Leach GJ, Waterman PG. A new anti-inflammatory glucoside from Ficus racemosa L. Planta Med. 2004;70:421–6. doi: 10.1055/s-2004-818969. [DOI] [PubMed] [Google Scholar]
- 182.Ferdous M, Rouf R, Shilpi AJ, Uddin SJ. Antinociceptive activity of the ethanolic extract of Ficus racemosa Lin. (Moraceae) Orient Pharm Exp Med. 2008;8:93–96. [Google Scholar]
- 183.Imran M, Rasool N, Rizwan K, Zubair M, Riaz M, Zia-Ul-Haq M, Rana UA, Nafady A, Jaafar HZ. Chemical composition and biological studies of Ficus benjamina. Chem Cent J. 2014;8:12. doi: 10.1186/1752-153X-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Arunachalam K, Parimelazhagan T. Antidiabetic activity of Ficus amplissima Smith. bark extract in streptozotocin induced diabetic rats. J Ethnopharmacol. 2013;147:302–310. doi: 10.1016/j.jep.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 185.Kalaskar MG, Surana SJ. Pharmacognostic and phytochemical studies on Ficus Microcarpa L. fil. Anc Sci Life. 2012;32:107–111. doi: 10.4103/0257-7941.118550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mazumder PM, Farswan M, Parcha V, Singh V. Hypoglycemic and antioxidant activity of an isolated compound from Ficus arnottiana bark. Pharmacol. 2008;3:509–519. [Google Scholar]
- 187.Ojo OA, Ojo AB, Ajiboye B, Fadaka A, Imiere OD, Adeyonu O, Olayide I. Protective influence of Ficus asperifolia Miq leaf extract on carbon tetrachloride (CCl4)-induced testicular toxicity in rat’s testes. J Appl Pharm Sci. 2016;6:37–41. [Google Scholar]
- 188.Patel M, Patel P, Patel M. Aqueous extract of Ficus bengalensis Linn. Bark for inflammatory bowel disease. J Young Pharm. 2010;2:130–6. doi: 10.4103/0975-1483.63149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Singh RK, Mehta S, Jaiswal D, Rai PK, Watal G. Antidiabetic effect of Ficus bengalensis aerial roots in experimental animals. J Ethnopharmacol. 2009;123:110–4. doi: 10.1016/j.jep.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 190.Hasti S, Mora E, Utami R, Yulis LU. Sub-chronic toxicity of Ficus benjamina L. leaves ethanol extract on the liver function of white mice. Procedia Chem. 2014;13:204–208. [Google Scholar]
- 191.Díez-Gómez ML, Quirce S, Aragoneses E, Cuevas M. Asthma caused by Ficus benjamina latex: evidence of cross-reactivity with fig fruit and papain. Ann Allergy Asthma Immunol. 1998;80:24–30. doi: 10.1016/S1081-1206(10)62934-1. [DOI] [PubMed] [Google Scholar]
- 192.Chełmińska M. Latex allergy-part I. Pneumonol Alergol Pol. 2004;72:143–9. [PubMed] [Google Scholar]
- 193.Bircher AJ, Langauer S, Levy F, Wahl R. The allergen of Ficus benjamina in house dust. Clin Exp Allergy. 1995;25:228–33. doi: 10.1111/j.1365-2222.1995.tb01033.x. [DOI] [PubMed] [Google Scholar]
- 194.Antico A, Zoccatelli G, Marcotulli C, Curioni A. Oral allergy syndrome to fig. Int Arch Allergy Immunol. 2003;131:138–42. doi: 10.1159/000070929. [DOI] [PubMed] [Google Scholar]
- 195.Odo GE, Agwu JE, Newze N, Nwadinigwa A, Onyeke CC, Nzekwe U, Ajuziogu GC, Osayi EI, Ikegbunam C. Toxicity and effects of fig (Ficus carica) leaf aqueous extract on haematology and some biochemical indices of wistar albino rats (rattus norvegicus) J Med Plant Res. 2016;10:298–305. [Google Scholar]
- 196.Coloma PM, Avillach P, Salvo F, Schuemie MJ, Ferrajolo C, Pariente A, Fourrier-Réglat A, Molokhia M, Patadia V, van der Lei J, Sturkenboom M, Trifirò G. Reference standard for evaluation of methods for drug safety signal detection using electronic healthcare record databases. Drug Saf. 2013;36:13–23. doi: 10.1007/s40264-012-0002-x. [DOI] [PubMed] [Google Scholar]
- 197.Ippen H. Phototoxic reaction to figs. Hautarzt. 1982;33:337–9. [PubMed] [Google Scholar]
- 198.Polat M, Oztas P, Dikilitas MC, Alli N. Phytophotodermatitis due to Ficus carica. Dermatol Online J. 2008;14:9. [PubMed] [Google Scholar]
- 199.Caiaffa MF, Cataldo VM, Tursi A, Macchia L. Fig and mulberry cross-allergy. Ann Allergy Asthma Immunol. 2003;91:493–5. doi: 10.1016/s1081-1206(10)61520-7. [DOI] [PubMed] [Google Scholar]
- 200.Urbani S, Aruanno A, Nucera E. Adverse reaction to Ficus carica: reported case of a possible cross-reactivity with Der p1. Clin Mol Allergy. 2020;18:9. doi: 10.1186/s12948-020-00125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Awobajo FO, Omorodion-Osagie E, Olatunji-Bello II, Adegoke OA, Adeleke TI. Acute oral toxicity test and phytochemistry of some West African medicinal plants. Nig Q J Hosp Med. 2009;19:53–8. doi: 10.4314/nqjhm.v19i1.50209. [DOI] [PubMed] [Google Scholar]
- 202.Hasanat A, Kabir MSH, Ansari MA, Chowdhury TA, Hossain MM, Islam MN, Ahmed S, Chy MNU, Adnan M, Kamal ATMM. Ficus cunia Buch.-Ham. ex Roxb. (leaves): an experimental evaluation of the cytotoxicity, thrombolytic, analgesic and neuropharmacological activities of its methanol extract. J Basic Clin Physiol Pharmacol. 2019:30. doi: 10.1515/jbcpp-2016-0140. [DOI] [PubMed] [Google Scholar]
- 203.Allahyari S, Delazar A, Najafi M. Evaluation of general toxicity, anti-oxidant activity and effects of ficus carica leaves extract on ischemia/reperfusion injuries in isolated heart of rat. Adv Pharm Bull. 2014;4(Suppl 2):577–82. doi: 10.5681/apb.2014.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Abdel-Atya AM, Hameda MB, Salamaa WH, Alib MM, Fahmya AS, Mohameda SA. Ficus carica, Ficus sycomorus and euphorbia tirucalli latex extracts: phytochemical screening, antioxidant and cytotoxic properties. Biocatal Agric Biotechnol. 2019;20:101199. [Google Scholar]
- 205.Jacob SJP, Prasad VLS, Sivasankar S, Muralidharan P. Biosynthesis of silver nanoparticles using dried fruit extract of Ficus carica - Screening for its anticancer activity and toxicity in animal models. Food Chem Toxicol. 2017;109:951–956. doi: 10.1016/j.fct.2017.03.066. [DOI] [PubMed] [Google Scholar]
- 206.Ghori SS, Khan M, Qureshi MS, Ghori SK. Analgesic and antipyretic effects of Ficus dalhouseae Miq. leaf ethanolic extract. Res J Pharm Technol. 2014;7:1014–1019. [Google Scholar]
- 207.Ilyanie Y, Wong TW, Choo CY. Evaluation of hypoglycemic activity and toxicity profiles of the leaves of Ficus deltoidea in rodents. J Complement Integr Med. 2011:8. doi: 10.2202/1553-3840.1469. [DOI] [PubMed] [Google Scholar]
- 208.Bafor EE, Igbinuwen O. Acute toxicity studies of the leaf extract of Ficus exasperata on haematological parameters, body weight and body temperature. J Ethnopharmacol. 2009;123:302–7. doi: 10.1016/j.jep.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 209.Dhyani PP, Khali MP. Fruit yield and economics of jelly and jam production from fruits of some promising Ficus (fig) tree crops. Ecol Food Nutr. 1993;30:169–178. [Google Scholar]
- 210.Chindo BA, Ya’U J, Danjuma NM, Okhale SE, Gamaniel KS, Becker A. Behavioral and anticonvulsant effects of the standardized extract of Ficus platyphylla stem bark. J Ethnopharmacol. 2014;154:351–60. doi: 10.1016/j.jep.2014.03.061. [DOI] [PubMed] [Google Scholar]
- 211.Heroor SS, Beknal A, Mahurkar N. Ficus bark extract enhances the antihyperglycemic activity of glibenclamide in rats: a herb-drug interaction study. Int J Med Res Rev. 2014;2:395–402. [Google Scholar]
- 212.Barolo MI, Ruiz Mostacero N, López SN. Ficus carica L. (Moraceae): an ancient source of food and health. Food Chem. 2014;164:119–27. doi: 10.1016/j.foodchem.2014.04.112. [DOI] [PubMed] [Google Scholar]
- 213.Cavero RY, Akerreta S, Calvo MI. Medicinal plants used for dermatological affections in Navarra and their pharmacological validation. J Ethnopharmacol. 2013;149:533–542. doi: 10.1016/j.jep.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 214.Rojas P, Jung-Cook H, Ruiz-Sánchez E, Rojas-Tomé IS, Rojas C, López-Ramírez AM, Reséndiz-Albor AA. Historical aspects of herbal use and comparison of current regulations of herbal products between Mexico, Canada and the United States of America. Int J Environ Res Public Health. 2022;19:15690. doi: 10.3390/ijerph192315690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.British Pharmacopoei. 2023. Accessed 13. 10.2023. https://www.pharmacopoeia.com/