Abstract
Objective: To evaluate the efficacy of low-molecular-weight heparin (LMWH) in pregnant women with recurrent spontaneous abortion (RSA) and to investigate the correlation with changes in the gestational sac and embryo development, and miscarriage prevention as outcomes. Methods: A retrospective analysis was conducted on 100 pregnant women with RSA treated at Hulunbeier People’s Hospital between January 2022 and January 2023. Among them, 52 patients received LMWH therapy (observation group), while 48 received routine treatment (control group). Serum levels of human chorionic gonadotropin (hCG), progesterone (P), estradiol (E2), and coagulation markers (D-dimer [D-D], plasminogen activator inhibitor 1 [PAI-1], and fibrinogen), as well as gestational sac diameter (GSD), embryo length (EL), and uterine artery blood flow resistance, were assessed before and after treatment. Pregnancy outcome and adverse reaction rate were recorded and compared. Patients were categorized into successful and unsuccessful groups based on miscarriage prevention outcomes, and differences in GSD and EL were analyzed. Logistic regression was performed to identify factors influencing miscarriage prevention.Results: Baseline hCG, P, E2, D-D, PAI-1, fibrinogen, GSD, EL, and uterine artery blood flow resistance were comparable between groups (all P>0.05). After treatment, these measuremnts improved significantly, with greater changes in the observation group (all P<0.05). The term pregnancy rate was significantly higher in the observation group than in the control group (P<0.05), while adverse reaction rates were similar (P>0.05). The successful group exhibited significantly greater GSD and EL compared to the unsuccessful group (P<0.05). Logistic regression identified age, number of miscarriages, and LMWH use as independent factors influencing miscarriage prevention outcome (P<0.05). Conclusions: LMWH therapy significantly improves hormone levels, coagulation status, GSD, and EL in pregnant women with RSA, enhancing miscarriage prevention outcomes with minimal adverse reactions, making it a safe and effective treatment option for clinical practice.
Keywords: Recurrent spontaneous abortion, low-molecular-weight heparin, gestational sac, embryo, miscarriage prevention outcomes
Introduction
Recurrent spontaneous abortion (RSA) is a prevalent condition in obstetrics and gynecology, with an incidence of approximately 4% among women of childbearing age. A history of spontaneous abortion is recognized as an independent risk factor for RSA. The incidence of clinical spontaneous abortion is 15%-25%, with 80% occurring as early abortions before 12 weeks of gestation [1,2]. RSA is a multifactorial condition characterized by complex pathogenesis and nonspecific symptoms. Key contributing factors include low progesterone (P) levels, chromosomal abnormalities, cervical incompetence, reproductive system infections, and uterine microthrombosis. RSA severely harms patients’ physical and mental health, as well as family well-being, and clinical treatments are often suboptimal [3].
Anticoagulation therapy has demonstrated efficacy in managing RSA. Among anticoagulants, low-molecular-weight heparin (LMWH) sodium plays a critical role by reducing blood viscosity, preventing thrombosis, promoting thrombolysis, and increasing placental blood flow [4]. LMWH also has anti-inflammatory effects, modulating the complement and immune systems, inhibiting excessive complement activation, enhancing trophoblast proliferation and invasion, improving fetoplacental microcirculation, and increasing uteroplacental perfusion, thereby reducing RSA incidence [5,6].
The outcome of RSA is influenced not only by placental microcirculation but also by the condition of the gestational sac and embryo. The gestational sac serves as the structure for embryonic development within the uterus, while the embryo resides within the sac [7]. Studies have shown that the size, morphology, and development of the gestational sac and embryo are closely associated with miscarriage prevention outcome in early threatened abortion [8].
A review of RSA cases treated with LMWH sodium suggested that this therapy may improve gestational sac and embryo conditions, leading to better miscarriage prevention outcomes. Although LMWH sodium has shown promising results in RSA patients with prethrombotic status, few studies have explored its effect on gestational sac and embryo changes and their correlation with miscarriage prevention outcomes.
Materials and methods
Clinical data
This retrospective study analyzed 100 RSA cases treated at Hulunbeier People’s Hospital from January 2022 to January 2023. Among them, 52 patients treated with LMWH formed the observation group, while 48 patients receiving routine treatment constituted the control group.
Inclusion criteria: ① Patients with at least two previous spontaneous abortions (including biochemical pregnancies and embryo arrests) meeting RSA diagnostic criteria [9]. ② Normal karyotypes of both partners. ③ Complete clinical data.
Exclusion criteria: ① History of cesarean section or significant intrauterine manipulation. ② Reproductive organ abnormalities (e.g., uterine malformations, fibroids, cervical incompetence). ③ Severe liver or kidney dysfunction or malignancy. ④ Other infectious or autoimmune diseases.
Treatment methods
All patients received routine hormonal therapy for miscarriage prevention following a positive urine or blood test for human chorionic gonadotropin (hCG). Dydrogesterone was administered orally at an initial dose of 40 mg, followed by 10 mg every 8 hours. Additionally, 40 mg of progesterone was injected intramuscularly twice daily. Patients were advised to maintain bed rest and consume a nutritious diet.
In the observation group, LMWH (GlaxoSmithKline Tianjin Co., Ltd.) was administered at a dose of 4100 IU once daily in addition to routine treatment. All patients received treatment for two weeks.
Outcome measures
Fasting venous blood samples were collected from patients and centrifuged to obtain the supernatant. Serum levels of hCG, P, and estradiol (E2) were measured using electrochemiluminescence immunoassay.
Coagulation markers, including D-dimer (D-D), plasminogen activator inhibitor 1 (PAI-1), and fibrinogen, were quantified using an automatic biochemical analyzer before and after treatment for comparative analysis.
Gestational sac diameter (GSD) and embryo length (EL) were measured by B-ultrasound before and after treatment.
After routine abdominal ultrasound, Doppler ultrasound was used to measure transvaginal blood flow parameters of the cervical and uterine arteries. Using a probe frequency of 3.5-5.0 MHz, the average of three measurements was recorded. Parameters included the systolic/diastolic velocity ratio (S/D), pulsatility index (PI), and resistance index (RI) of the artery at the end of contraction.
Pregnancy outcomes, including term pregnancy, premature birth, and miscarriage, were recorded and compared between the groups.
Adverse reactions, such as gastrointestinal disturbances, rashes, gingival bleeding, and ecchymosis, were documented and compared.
Patients were divided into successful and unsuccessful groups based on pregnancy outcomes to compare GSD and EL changes between the groups.
Statistical methods
Data analysis was performed using SPSS 20.0, and visualizations were created using GraphPad Prism 8. Sample size calculation was based on N=Z2×(P×(1-P))/E2, where Z is the statistic (z=1.64 at 90% confidence), E is the error value, and P is the probability value. Independent sample t-tests were employed for inter-group comparisons, and paired t-tests were used for intra-group analyses of measured data. Chi-square tests were applied to analyze categorical data. A significance level of P<0.05 was considered significant.
Results
Comparison of general information
The observation and control groups were comparable in terms of age, body mass index, number of miscarriages, and other baseline characteristics (all P>0.05; Table 1).
Table 1.
Comparison of general data
| Factor | Observation group (n=52) | Control group (n=48) | t/χ2 | P |
|---|---|---|---|---|
| Age (years) | 0.000 | 0.987 | ||
| ≥26 | 27 (51.92) | 25 (52.08) | ||
| <26 | 25 (48.08) | 23 (47.92) | ||
| Body mass index (kg/m2) | 0.001 | 0.974 | ||
| ≥23 | 28 (53.85) | 26 (54.17) | ||
| <23 | 24 (46.15) | 22 (45.83) | ||
| Number of miscarriages | 0.107 | 0.744 | ||
| 2 times | 20 (38.46) | 20 (41.67) | ||
| >2 times | 32 (61.54) | 28 (58.33) | ||
| Gestational week | 11.04±1.17 | 11.13±1.18 | 0.383 | 0.703 |
| Duration of vaginal bleeding (d) | 0.000 | 0.987 | ||
| ≤3 | 27 (51.92) | 25 (52.08) | ||
| >3 | 25 (48.08) | 23 (47.92) |
Comparison of hormonal levels
No significant inter-group differences in P, E2, or hCG levels were observed before treatment (P>0.05). After treatment, these levels significantly increased in both groups, with more pronounced elevations in the observation group compared to the control group (all P<0.05; Figure 1).
Figure 1.
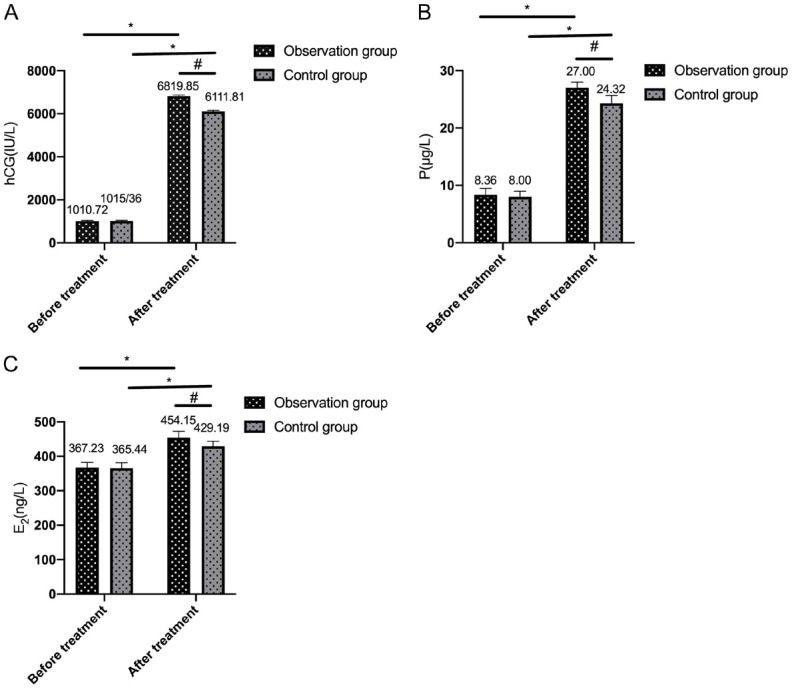
Comparison of progesterone levels. A: Comparison of human chorionic gonadotropin (hCG) levels between the two groups before and after treatment; B: Comparison of progesterone (P) levels between the two groups before and after treatment; C: Comparison of estradiol (E2) levels between the two groups before and after treatment. Note: * denotes P<0.05 in the intra-group comparison before and after treatment; # denotes P<0.05 in the inter-group comparison after treatment.
Comparison of coagulation function indexes
Baseline levels of D-D, PAI-1, and fibrinogen showed no significant differences between groups (all P>0.05). Post-treatment levels decreased significantly in both groups, with greater reductions in the observation group (all P<0.05; Figure 2).
Figure 2.
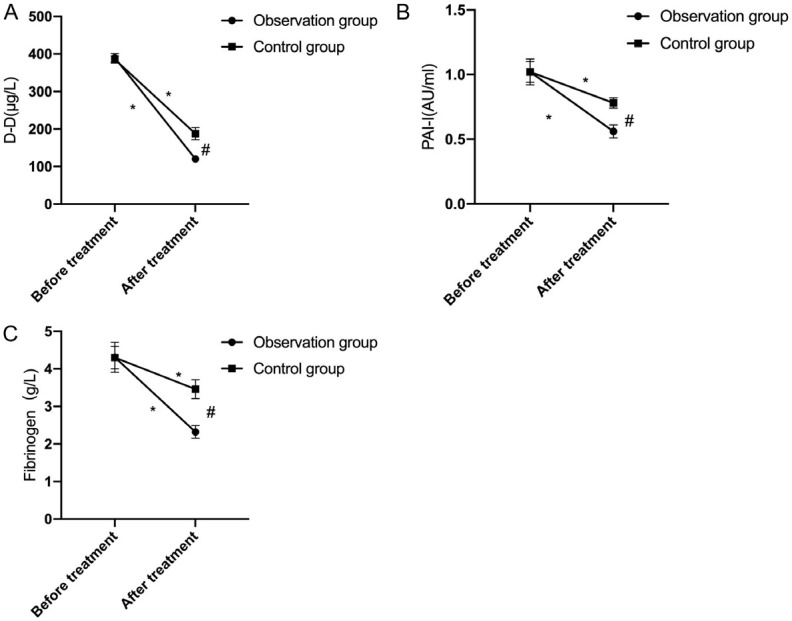
Comparison of coagulation function. A: Comparison of D-dimer (D-D) between the two groups before and after treatment; B: Comparison of plasminogen activator inhibitor 1 (PAI-1) between the two groups before and after treatment; C: Comparison of fibrinogen between the two groups before and after treatment. Note: * denotes P<0.05 in the intra-group comparison before and after treatment; # denotes P<0.05 in the inter-group comparison after treatment.
Comparison of GSD and EL
Pre-treatment GSD and EL values were similar between groups (both P>0.05). Post-treatment, both values increased significantly in both groups, with greater increases observed in the observation group (both P<0.05; Figure 3).
Figure 3.
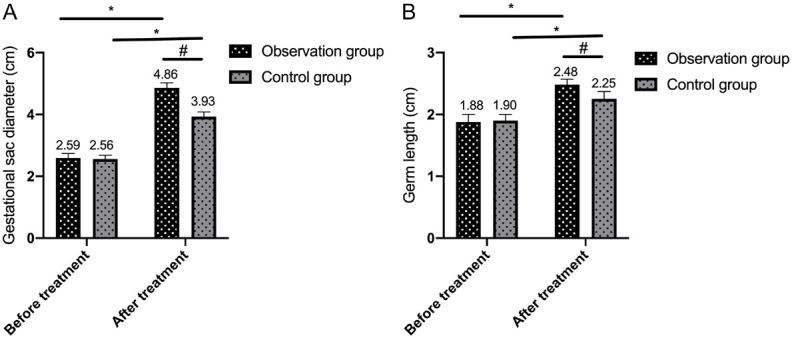
Comparison of gestational sac diameter and embryo length. A: Comparison of gestational sac diameter between the two groups before and after treatment; B: Comparison of embryo length between the two groups before and after treatment. Note: * denotes P<0.05 in the intra-group comparison before and after treatment; # denotes P<0.05 in the inter-group comparison after treatment.
Comparison of uterine artery blood flow resistance before and after treatment
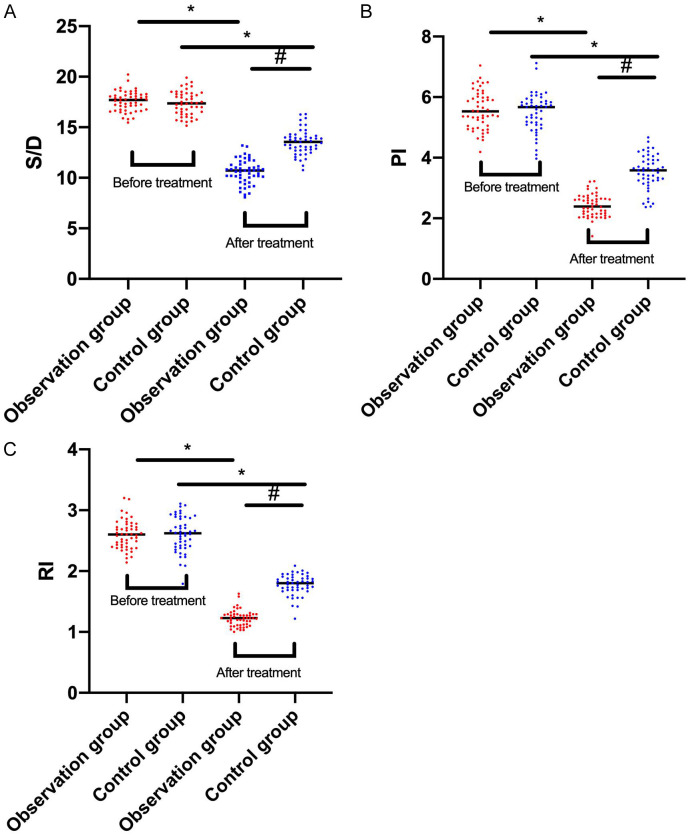
The arterial blood flow resistance parameters S/D, PI, and RI were significantly higher in both the observation and control groups compared to normal values before treatment (P<0.05). After treatment, these values decreased in both groups, with the observation group showing greater reductions than the control group (all P<0.05; Figure 4).
Figure 4.
Comparison of uterine artery blood flow resistance before and after treatment between the two groups of patients. A: Comparison of peak/end-diastolic velocity value (S/D) between the two groups of patients before and after treatment; B: Comparison of pulsatility index (PI) between the two groups of patients before and after treatment; C: Comparison of resistance index (RI) between the two groups of patients before and after treatment. Note: * indicates that P<0.05 in comparing between the two groups of patients before and after treatment, and # indicates that P<0.05 in comparing between the two groups of patients after treatment.
Comparison of pregnancy outcomes
The premature birth rate, miscarriage rate, and term pregnancy rate were 7.69%, 9.62%, and 82.69% in the observation group, respectively, compared to 16.67%, 27.07%, and 56.25% in the control group. The observation group had a significantly lower miscarriage rate and a higher term pregnancy rate than the control group (both P<0.05; Table 2).
Table 2.
Comparison of pregnancy outcomes
| Category | Observation group (n=52) | Control group (n=48) | χ2 | P |
|---|---|---|---|---|
| Premature birth rate | 4 (7.69) | 8 (16.67) | 1.904 | 0.167 |
| Miscarriage rate | 5 (9.62) | 13 (27.08) | 5.160 | 0.023 |
| Term pregnancy rate | 43 (82.69) | 27 (56.25) | 8.310 | 0.004 |
Comparison of adverse reactions
In the observation group, 3 patients experienced gastrointestinal reactions, 1 had a rash, 1 had gingival bleeding, and 1 had ecchymosis, resulting in an overall incidence of 11.54%. In the control group, 3 patients experienced gastrointestinal reactions, 2 had rashes, 1 had gingival bleeding, and 1 had ecchymosis, with a total incidence of 14.58%. The difference in adverse reaction rate between the two groups was not significant (P>0.05; Table 3).
Table 3.
Comparison of adverse reactions [n, (%)]
| Adverse reaction | Observation group | Control group | χ2 | P |
|---|---|---|---|---|
| n=52 | n=48 | |||
| Gastrointestinal reactions | 3 (5.77) | 3 (6.25) | - | - |
| Rashes | 1 (1.92) | 2 (4.17) | - | - |
| Gingival bleeding | 1 (1.92) | 1 (2.08) | - | - |
| Ecchymosis | 1 (1.92) | 1 (2.08) | - | - |
| Incidence of adverse reactions | 6 (11.54) | 7 (14.58) | 0.205 | 0.651 |
Comparison of GSD and EL in patients with different miscarriage prevention outcomes
Based on treatment outcome, patients were divided into two groups: 70 cases with successful miscarriage prevention and 30 cases with unsuccessful outcome. Patients in the successful group had significantly longer GSD and EL compared to those in the unsuccessful group (both P<0.05; Table 4).
Table 4.
Comparison of gestational sac diameter and embryo length in patients with different miscarriage prevention outcomes
| Category | Successful group | Unsuccessful group | t | P |
|---|---|---|---|---|
| n=70 | n=30 | |||
| Gestational sac diameter | 4.54±0.47 | 4.13±0.41 | 4.147 | <0.001 |
| Embryo length | 2.43±0.12 | 2.23±0.14 | 7.260 | <0.001 |
Comparison of clinical characteristics of patients with different miscarriage prevention outcomes
To further investigate factors influencing miscarriage prevention outcome, clinical characteristics were analyzed (Table 5). Univariate analysis revealed that age, number of miscarriages, and heparin use were associated with miscarriage prevention. Multivariate logistic regression confirmed these variables as independent factors affecting miscarriage prevention (Table 6).
Table 5.
Univariate analysis
| Factor | Successful group | Unsuccessful group | χ2 | P |
|---|---|---|---|---|
| n=70 | n=30 | |||
| Age (years) | 8.310 | 0.004 | ||
| ≤26 (n=52) | 43 (61.43) | 9 (30.00) | ||
| >26 (n=48) | 27 (38.57) | 21 (70.00) | ||
| Body mass index (kg/m2) | 0.928 | 0.335 | ||
| ≥23 (n=54) | 40 (57.14) | 14 (46.67) | ||
| <23 (n=46) | 30 (42.86) | 16 (53.33) | ||
| Number of miscarriages (times) | 16.07 | <0.001 | ||
| ≤2 (n=40) | 37 (52.86) | 3 (10.00) | ||
| >2 (n=60) | 33 (47.14) | 27 (90.00) | ||
| Duration of vaginal bleeding (d) | 0.374 | 0.541 | ||
| ≤3 (n=52) | 35 (50.00) | 17 (56.67) | ||
| >3 (n=48) | 35 (50.00) | 13 (43.33) | ||
| Use of low-molecular-weight heparin | 14.11 | <0.001 | ||
| With (n=52) | 45 (64.29) | 7 (23.33) | ||
| Without (n=48) | 25 (35.71) | 23 (76.67) |
Table 6.
Multivariate analysis
| Factor | B | S.E. | Wals | P | Exp (B) | 95% C.I. | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Lower bound | Upper bound | ||||||
| Age | 1.723 | 0.594 | 8.408 | 0.004 | 5.600 | 1.748 | 17.945 |
| Body mass index (kg/m2) | 0.649 | 0.576 | 1.270 | 0.261 | 1.913 | 0.619 | 5.912 |
| Number of miscarriages | 2.547 | 0.738 | 11.905 | 0.001 | 12.775 | 3.005 | 54.301 |
| Duration of vaginal bleeding | -0.523 | 0.563 | 0.862 | 0.353 | 0.593 | 0.197 | 1.787 |
| Use of low-molecular-weight heparin | 1.634 | 0.581 | 7.916 | 0.005 | 5.123 | 1.642 | 15.988 |
Discussion
In recent years, the incidence of RSA has been increasing, affecting 1%-3% of women of childbearing age [10]. RSA is characterized by a complex etiology, with endogenous P deficiency being one of the primary causes. Current clinical treatments often involve bed rest and P supplementation [11]; however, improving miscarriage prevention outcome remains a significant clinical challenge.
Dydrogesterone, a natural progesterone analog, binds to P receptors to exert progestational effects by inhibiting uterine contractions and improving the uterine environment to support embryo implantation. Additionally, it enhances nitric oxide synthesis in placental blood vessel walls, dilates microvessels, and increases uterine blood flow, making it a common treatment for threatened abortion [12]. LMWH, known for its strong anticoagulant effect through selective inhibition of coagulation factor X and its minimal impact on coagulation factor II, offers high safety [13]. LMWH effectively prevents thrombosis, improves local microcirculation, promotes blastocyst adhesion and endometrial invasion, stimulates trophoblast proliferation, enhances placental microcirculation, and shields embryos from maternal immune attacks, thereby reducing the incidence of RSA [14].
This study evaluated the effects of LMWH in RSA patients. Our findings revealed that the observation group demonstrated significantly greater improvement in serum hormone levels compared to the control group. hCG maintains luteal function [15]. P is essential for conception and pregnancy maintenance [16], and E2 supports intrauterine growth and development [17]. These results suggest that LMWH enhances P and estrogen levels, benefiting fetal growth. Previous studies [18] have shown that LMWH promotes trophoblast proliferation, enhances invasiveness, and increases hCG secretion. However, its impact on E2 and P remained unconfirmed.
We hypothesize that the improved hormone levels observed in this study are influenced by concurrent hormone therapy. Nonetheless, the mechanisms underlying the more pronounced improvement in the observation group compared to the control group warrant further investigation.
We compared coagulation function between the two groups. Serum D-D, PAI-1, and fibrinogen levels significantly decreased in patients receiving LMWH compared to those receiving routine treatment. D-D is a specific product of fibrinolytic enzyme activity and serves as a serum marker for hypercoagulability and fibrinolysis [19]. PAI-1 reflects the body’s thrombotic tendency, with elevated levels promoting local thrombosis formation and progression [20]. Fibrinogen contributes to platelet aggregation, increased blood viscosity, and vascular endothelial injury, thereby enhancing erythrocyte adhesion and thrombosis [21]. These findings suggest that LMWH alleviates hypercoagulability, prevents thrombosis, and maintains blood supply to the uterus and placenta.
Subsequently, GSD, EL, and pregnancy outcomes were assessed. The observation group demonstrated significantly greater GSD and EL, along with a higher term pregnancy rate than the control group. These findings indicate that LMWH therapy improves GSD and EL, enhancing pregnancy outcomes. The gestational sac, characterized by the “double decidual sac sign”, is a key marker for diagnosing intrauterine pregnancy before the yolk sac becomes visible [22]. Studies have shown that a gestational sac diameter >20 mm without an embryo suggests an empty sac. At 5 weeks of pregnancy, the yolk sac is the first anatomic structure within the gestational sac that ultrasound can detect. It serves, as a hallmark of intrauterine pregnancy [22]. Yolk sac diameters >10 mm or <3 mm are associated with poor pregnancy outcomes [23]. These findings highlight the strong correlation between GSD, EL, and pregnancy outcome.
We further analyzed GSD and EL in the successful and unsuccessful groups. Patients in the successful group had significantly greater GSD and EL, indicating that these metrics are closely associated with miscarriage prevention outcomes and can serve as indicators for evaluating the effectiveness of LMWH therapy in RSA patients.
Analysis of adverse reactions showed no significant difference between the groups, confirming that LMWH is safe for RSA treatment. Previous studies [24] have demonstrated the favorable anticoagulant effects of LMWH and its low bleeding risk, consistent with our findings.
In conclusion, LMWH therapy significantly improves hormone levels, coagulation status, GSD, and EL in pregnant women with RSA, leading to better miscarriage prevention outcomes. The treatment is associated with minimal adverse reactions and high safety, making it a valuable option for clinical practice.
Disclosure of conflict of interest
None.
References
- 1.Duckitt K, Qureshi A. Recurrent miscarriage. BMJ Clin Evid. 2011;2011:1409. [PMC free article] [PubMed] [Google Scholar]
- 2.van Wely M. Series of overviews on miscarriage and recurrent miscarriage. Fertil Steril. 2023;120:932–933. doi: 10.1016/j.fertnstert.2023.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Melo P, Dhillon-Smith R, Islam MA, Devall A, Coomarasamy A. Genetic causes of sporadic and recurrent miscarriage. Fertil Steril. 2023;120:940–944. doi: 10.1016/j.fertnstert.2023.08.952. [DOI] [PubMed] [Google Scholar]
- 4.Mu F, Wang M, Huang J, Wang F. Pregnancy outcomes and adverse events in patients with recurrent miscarriage receiving fondaparinux versus low molecular-weight heparin: a meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2023;287:29–35. doi: 10.1016/j.ejogrb.2023.05.031. [DOI] [PubMed] [Google Scholar]
- 5.Jiang F, Hu X, Jiang K, Pi H, He Q, Chen X. The role of low molecular weight heparin on recurrent pregnancy loss: a systematic review and meta-analysis. Taiwan J Obstet Gynecol. 2021;60:1–8. doi: 10.1016/j.tjog.2020.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Ni L, Sun XQ, Zhao DX, Zhu ZW. Low molecular weight heparin monotherapy for recurrent abortion with antiphospholipid system: a protocol of a systematic review. Medicine (Baltimore) 2019;98:e14619. doi: 10.1097/MD.0000000000014619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hou X, Zeb A, Dil S, Zhou J, Zhang H, Shi B, Muhammad Z, Khan I, Zaman Q, Shah WA, Jiang X, Wu L, Ma H, Shi Q. A homozygous KASH5 frameshift mutation causes diminished ovarian reserve, recurrent miscarriage, and non-obstructive azoospermia in humans. Front Endocrinol (Lausanne) 2023;14:1128362. doi: 10.3389/fendo.2023.1128362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim YY, Kim H, Suh CS, Liu HC, Rosenwaks Z, Ku SY. Effects of natural progesterone and synthetic progestin on germ layer gene expression in a human embryoid body model. Int J Mol Sci. 2020;21:769. doi: 10.3390/ijms21030769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Practice Committee of the American Society for Reproductive Medicine. Electronic address: asrm@asrm.org. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril. 2020;113:533–535. doi: 10.1016/j.fertnstert.2019.11.025. [DOI] [PubMed] [Google Scholar]
- 10.Scarrone M, Villanacci R, Canti V, Bordoli S, Pasi F, Quaranta L, Candiani M, Rovere-Querini P, Vanni VS. Low-molecular-weight heparin for prevention of unexplained recurrent miscarriage. Eur J Obstet Gynecol Reprod Biol. 2021;260:235–236. doi: 10.1016/j.ejogrb.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Guo H, Lu Q. Efficacy of dydrogesterone on treating recurrent miscarriage and its influence on immune factors: a systematic review and meta-analysis. Ann Palliat Med. 2021;10:10971–10985. doi: 10.21037/apm-21-2605. [DOI] [PubMed] [Google Scholar]
- 12.Demir SC, Gedikbasi A, Timur H, Cetin C, Gursoy Pala H, Gulumser C. Threatened miscarriage and recurrent miscarriage: expert opinions on progesterone therapy and treatment challenges. Turk J Obstet Gynecol. 2023;20:242–248. doi: 10.4274/tjod.galenos.2023.66789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaaban OM, Abbas AM, Zahran KM, Fathalla MM, Anan MA, Salman SA. Low-molecular-weight heparin for the treatment of unexplained recurrent miscarriage with negative antiphospholipid antibodies: a randomized controlled trial. Clin Appl Thromb Hemost. 2017;23:567–572. doi: 10.1177/1076029616665167. [DOI] [PubMed] [Google Scholar]
- 14.Cetin O, Karaman E, Cim N, Dirik D, Sahin HG, Kara E, Esen R. The impact of low molecular weight heparin on obstetric outcomes among unexplained recurrent miscarriages complicated with methylenetetrahydrofolate reductase gene polymorphism. Ginekol Pol. 2017;88:260–265. doi: 10.5603/GP.a2017.0049. [DOI] [PubMed] [Google Scholar]
- 15.Liu L, Wang Y, Chen X, Tian Y, Li TC, Zhao L, Chen Q, Wei M, Zhang S. Evidence from three cohort studies on the expression of MUC16 around the time of implantation suggests it is an inhibitor of implantation. J Assist Reprod Genet. 2020;37:1105–1115. doi: 10.1007/s10815-020-01764-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang R, Yu J, Yan Z, Cheng X, Chen J, Guo Y. Cluster-based immunotherapy for patients with recurrent abortion caused by antiphospholipid syndrome. J Healthc Eng. 2021;2021:4581900. doi: 10.1155/2021/4581900. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Peng L, Chelariu-Raicu A, Ye Y, Ma Z, Yang H, Ishikawa-Ankerhold H, Rahmeh M, Mahner S, Jeschke U, von Schonfeldt V. Prostaglandin E2 receptor 4 (EP4) affects trophoblast functions via activating the cAMP-PKA-pCREB signaling pathway at the maternal-fetal interface in unexplained recurrent miscarriage. Int J Mol Sci. 2021;22:9134. doi: 10.3390/ijms22179134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Wu XX, Tan JP, Liu ML, Liu YL, Zhang JP. Effects of low molecular weight heparin and heparin-binding epidermal growth factor on human trophoblast in first trimester. Fertil Steril. 2012;97:764–770. doi: 10.1016/j.fertnstert.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Cang R, Hu Z, Tian Z, Xia T, Gao Y, Fu Z, He M, Ma S, Ding X. Efficacy and safety of the Bushen-Shugan method in pregnancy outcomes in patients with recurrent miscarriage complicated by anxiety and depression: a prospective randomized trial. Altern Ther Health Med. 2022;28:124–131. [PubMed] [Google Scholar]
- 20.Barlik M, Seremak-Mrozikiewicz A, Drews K, Klejewski A, Kurzawinska G, Lowicki Z, Wolski H. Correlation between factor VII and PAI-1 genetic variants and recurrent miscarriage. Ginekol Pol. 2016;87:504–509. doi: 10.5603/GP.2016.0034. [DOI] [PubMed] [Google Scholar]
- 21.Sucker C, Geisen C, Schmitt U, Zawislak B. Hypofibrinogenemia and miscarriage: report of a first successful pregnancy under fibrinogen substitution and short review of the literature. Arch Clin Cases. 2022;9:100–103. doi: 10.22551/2022.36.0903.10211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adibi JJ, Layden AJ, Birru RL, Miragaia A, Xun X, Smith MC, Yin Q, Millenson ME, O’Connor TG, Barrett ES, Snyder NW, Peddada S, Mitchell RT. First trimester mechanisms of gestational sac placental and foetal teratogenicity: a framework for birth cohort studies. Hum Reprod Update. 2021;27:747–770. doi: 10.1093/humupd/dmaa063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stabile G, Cracco F, Nappi L, Sorrentino F, Vitale SG, Angioni S, Carlucci S, Ricci G. Hysteroscopic removal of intrauterine device in pregnancy: a scoping review to guide personalized care. Medicina (Kaunas) 2022;58:1688. doi: 10.3390/medicina58111688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu ZL, Wang Q, Wang M, Wang B, Huang LN. Low molecular weight heparin in treating patients with lung cancer received chemotherapy: a meta-analysis. J Cancer Res Ther. 2018;14:S437–S443. doi: 10.4103/0973-1482.176174. [DOI] [PubMed] [Google Scholar]