Abstract
Objectives: To identify the factors influencing spontaneous preterm birth (SPTB) and develop a prediction model for clinical practice. Methods: This retrospective study included a total of 130 pregnant women with spontaneous preterm birth or full-term delivery at Fujian Maternity and Child Health Hospital between January 2020 and December 2023. The SPTB group consisted of 50 women with spontaneous preterm birth, while the full-term group included 70 women with full-term deliveries. Logistic regression analysis was performed to explore the factors associated with clinical prognosis, and a nomogram prediction model for SPTB risk was constructed and validated. Results: Multivariate logistic regression analysis identified multiple pregnancies (95% CI: 1.415-8.926, P=0.006), abnormal fetal position (95% CI: 1.124-2.331, P=0.008), gestational diabetes (95% CI: 4.918-19.164, P=0.002), mode of conception (95% CI: 1.765-4.285,P=0.002), lower genital tract infection (95% CI: 1.076-2.867, P=0.032), and second trimester cervical length (95% CI: 1.071-2.991, P=0.031) as independent risk factors of SPTB. Using these six variables, a nomogram was developed to predict the incidence of SPTB, with an AUC value of 0.833 (95% CI: 0.665-0.847), demonstrating acceptable agreement between predicted and observed outcomes. Decision curve analysis (DCA) showed a good positive net benefit of the model. Conclusions: Multiple pregnancies, abnormal fetal position, gestational diabetes, mode of conception, lower genital tract infection, and second-trimester cervical length are independent risk factors for the onset of SPTB. In addition, the nomogram prediction model demonstrated good predictive performance, high accuracy, and clinical applicability.
Keywords: Spontaneous preterm birth, risk prediction model, development, validation
Introduction
Spontaneous preterm birth (SPTB) refers to the occurrence of preterm labor or preterm delivery before 37 weeks of gestation, including preterm birth following premature rupture of membranes, accounting for about 70% of all preterm births [1]. Premature infants are prone to underdeveloped organ systems, leading to a higher risk of death and complications compared to full-term infants. Research shows that over one million premature infants die annually due to complications related to prematurity, making it the leading cause of death among children under 5 years of age [2-4]. Common complications of premature infants include respiratory distress syndrome, bronchopulmonary dysplasia, necrotizing enterocolitis, sepsis, periventricular leukomalacia, epileptic seizures, intraventricular hemorrhage, cerebral palsy, infections, feeding difficulties, hypoxic-ischemic encephalopathy, and vision and hearing impairments [5-9]. Therefore, preventing premature birth is one of the most crucial issues in modern healthcare.
The pathogenesis for SPTB is complex. Research has shown that pre-pregnancy body mass index (BMI), age, and social economic status are key contributors to SPTB. Either too low or too high pre-pregnancy BMI, as well as extreme maternal age, can increase the likelihood of spontaneous preterm birth [10-14]. Pregnancies complicated by conditions such as gestational diabetes, hypertension, or intrahepatic cholestasis of pregnancy also elevate the risk of SPTB [15-17]. A history of adverse pregnancy outcomes, including spontaneous abortion [18-20] or preterm birth [21,22], further raises this risk. Therefore, identifying relevant risk factors for SPTB as early and correctly as possible is crucial for implementing effective preventive interventions.
Current studies have developed predictive models for SPTB. Alfirevic Z et al. [23] constructed a predictive model for preterm birth in women following cervical cerclage, incorporating three influencing factors: cervical length, history of cervical conization, and history of cervical cerclage. The area under the ROC curve (AUC) of the model was 0.907. Tranidou A et al. [24] developed a model tailored for high-risk pregnancies, with factors including fetal fibronectin (fFN), cervical length, and history of preterm birth/premature rupture of membranes, yielding an AUC ranging from 0.77 to 0.99. Although these models demonstrate relatively good predictive performance, they do not include Asian populations in their development or validation. Since the factors contributing to SPTB may vary across different racial groups, the applicability of these models is limited. Additionally, the small sample size and single-source samples raise concerns about overfitting, and none of these models have undergone external clinical validation. Therefore, their practical use in clinical settings remains uncertain, and further verification is needed.
Given this, there is still a gap in the availability of an effective and clinically feasible predictive model for SPTB. Thus, more research is required to develop a model that can meet clinical needs. The aim of this study is to identify the relevant factors influencing SPTB and construct a prediction model suitable for clinical practice, providing a useful tool for obstetric healthcare professionals in their assessments.
Methods
Study patients
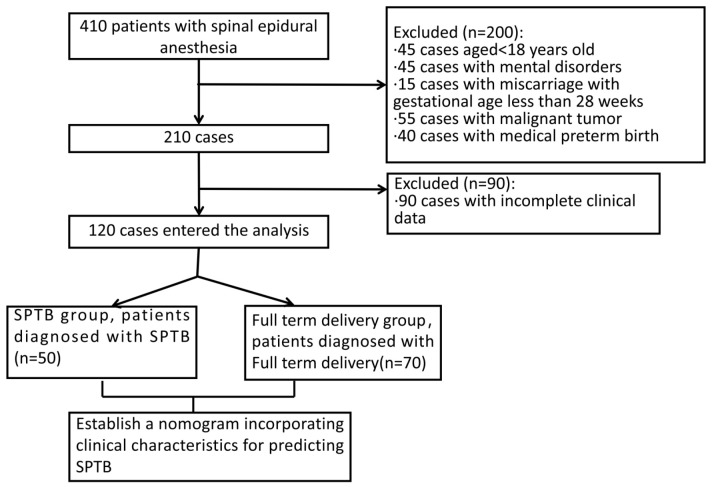
We retrospectively collected and analyzed the medical records of 130 pregnant women with SPTB or full-term delivery at Fujian Maternity and Child Health Hospital between January 2020 and December 2023. The study included 50 women diagnosed with spontaneous preterm birth, comprising the SPTB group, and 70 women who had full-term deliveries, comprising the full-term group. The patient selection process is shown in Figure 1. This study was approved by the Ethics Review Board of Fujian Maternity and Child Health Hospital.
Figure 1.
Flow diagram detailing the selection of patients included in this study.
Inclusion criteria for SPTB subjects: Those who received antenatal check-ups at Fujian Maternity and Child Health Hospital during pregnancy, with regular and standardized antenatal care; Those diagnosed with SPTB [25]: gestational age between 28 and 37 weeks, with neonatal birth weight ≥1000 g; Patients aged ≥18 years.
Inclusion criteria for full-term delivery subjects: Those who received antenatal check-ups at Fujian Maternity and Child Health Hospital during pregnancy, with regular and standardized antenatal care; Those diagnosed with full-term delivery [26]: gestational age ≥37 weeks; Patients aged ≥18 years.
Exclusion criteria: Pregnant women without regular antenatal check-ups during pregnancy; Pregnancies complicated with malignant tumors; Miscarriage before 28 weeks of gestation; Medically indicated preterm birth due to safety considerations such as placental implantation, threatened uterine rupture, chorioamnionitis, gestational hypertension disorders, placental abruption, fetal distress, or fetal growth retardation, where clinical advice recommends early termination of the pregnancy; Pregnancies involving cervical insufficiency or a history of cervical conization or cerclage performed either during or before pregnancy.
Data collection
Two researchers collected demographic data from the patients’ medical records, including age, comorbidities and laboratory results.
The primary outcome was the performance of the predictive model, which was evaluated using the concordance index (c-index), calibration curve, decision curve analysis (DCA), and the area under the receiver operating characteristic (ROC) curve (AUC). The secondary outcome focused on clinical data, including age, pre-pregnancy BMI, history of pre-pregnancy disease, weight change during pregnancy, ethnicity, parity, conception method, history of tobacco and alcohol exposure during pregnancy, nutrient supplementation, physical activity during pregnancy, and pregnancy complications.
Statistical analysis
All statistical analyses were conducted using SPSS V26.0 (SPSS Inc.) and R software v4.0.2 (R Foundation for Statistical Computing, Vienna, Austria). The sample size was calculated using power analysis and corrected for attrition, leading to a final sample size of approximately 130 participants. Categorical variables were expressed as percentage, while continuous variables were expressed as mean ± standard deviation. For comparisons of categorical data between groups, chi-square or Fisher’s exact tests were used as appropriate. For continuous data, if normally distributed, t-tests or analysis of variance (ANOVA) were applied; for non-normally distributed data, the Kruskal-Wallis test was used. A multivariate logistic regression model was used to analyze factors associated with SPTB and to identify risk factors. The nomogram was constructed based on the results of the multivariate logistic regression analysis to calculate the predicted probability of SPTB for each patient. The prognostic performance of the nomogram was measured using the concordance index (c-index), calibration curve, DCA, and AUC. P<0.05 was considered statistically significant.
Results
Comparison of clinical characteristics between the two groups
The characteristics of the two group, including age, pre-pregnancy weight, preconception BMI, parity, and regular physical activity, were comparable (all P>0.05). However, significant differences were observed between the two groups in terms of weight gain, mode of conception, nutritional supplementation during pregnancy, and alcohol/tobacco exposure during pregnancy (all P<0.05) (Table 1).
Table 1.
Comparison of clinical characteristics between the two groups
| Spontaneous preterm birth group (n=50) | Full term delivery group (n=70) | t/χ2 | P | |
|---|---|---|---|---|
| Age | 0.247 | 0.619 | ||
| <35 | 38 (76.00%) | 48 (68.57%) | ||
| ≥35 | 12 (24.00%) | 22 (31.43%) | ||
| Pre-pregnancy weight (Kg) | 56.78±7.12 | 57.23±9.45 | 1.658 | 0.278 |
| Weight gain during pregnancy (Kg) | 11.78±3.39 | 13.53±4.56 | 7.607 | 0.036 |
| Preconception BMI | 22.26±2.84 | 21.83±3.49 | 2.199 | 0.132 |
| Mode of conception | 6.628 | 0.028 | ||
| Spontaneous conception | 45 (90.00%) | 68 (97.14%) | ||
| Assisted reproduction | 5 (10.00%) | 2 (2.86%) | ||
| Parity | 0.022 | 0.997 | ||
| Primipara | 39 (78.00%) | 56 (80.00%) | ||
| Multipara | 11 (22.00%) | 14 (20.00%) | ||
| Nutrient supplementation during pregnancy | 10.01 | 0.007 | ||
| Folic acid in early pregnancy | 39 (78.00%) | 51 (72.86%) | ||
| Multivitamin in second trimester | 40 (80.00%) | 54 (77.14%) | ||
| No supplementation | 4 (8.00%) | 4 (5.71%) | ||
| Alcohol and tobacco exposure during pregnancy | 6.68 | 0.021 | ||
| Yes | 4 (8.00%) | 5 (7.14%) | ||
| No | 46 (92%) | 65 (92.86%) | ||
| Regular physical activity | 0.87 | 0.476 | ||
| Yes | 8 (16.00%) | 11 (15.71%) | ||
| No | 42 (84%) | 59 (84.3%) |
Note: BMI, body mass index.
Comparison of pregnancy complications between the two groups
As shown in Table 2, compared with full-term delivery group, the SPTB group had significantly higher rates of multiple pregnancies, gestational diabetes, abnormal fetal position, abnormal umbilical cord, history of abnormal fetal heart monitoring, and lower genital tract infections (all P<0.05). In contrast, there were no statistically significant differences between the two groups regarding other medical and surgical diseases, abnormal amniotic fluid, placental abnormalities, uterine myoma, history of threatened abortion, thyroid dysfunction, or uterine malformation (all P>0.05).
Table 2.
Comparison of pregnancy complications between the two groups
| Spontaneous preterm birth group (n=50) | Full term delivery group (n=70) | χ2 | P | |
|---|---|---|---|---|
| Multiple pregnancy | 8 (16.00%) | 4 (5.71%) | 10.681 | <0.001 |
| Gestational diabetes | 9 (18.00%) | 4 (5.71%) | 5.681 | <0.001 |
| Uterine myoma | 1 (2.00%) | 1 (1.43%) | -0.233 | 0.816 |
| History of threatened abortion | 8 (16.00%) | 12 (17.14%) | 0.317 | 0.752 |
| Combined with thyroid dysfunction | 5 (10.00%) | 4 (5.71%) | -0.062 | 0.951 |
| Uterine malformation | 1 (2.00%) | 1 (1.43%) | -0.264 | 0.792 |
| Placental abnormality | 3 (6.00%) | 4 (5.71%) | -1.422 | 0.158 |
| Abnormal amniotic fluid | 4 (8.00%) | 5 (7.14%) | 1.677 | 0.097 |
| Abnormal fetal position | 6 (12.00%) | 4 (5.71%) | 6.979 | <0.001 |
| Abnormal umbilical cord | 11 (22.00%) | 8 (11.43%) | 12.182 | <0.001 |
| History of abnormal fetal heart monitoring | 11 (22.00%) | 9 (12.86%) | 14.362 | <0.001 |
| Pregnancy with other medical/surgical diseases | 1 (2.00%) | 1 (1.43%) | -0.758 | 0.451 |
| Lower genital tract infection | 6 (12.00%) | 2 (2.86%) | 10.681 | <0.001 |
Comparison of pregnancy-related laboratory tests between the two groups
As shown in Table 3, significant differences were observed between the two groups in terms of reproductive tract infections, second-trimester cervical length, white blood cell count, and neutrophil percentage (all P<0.05). However, no significant difference was noted in hemoglobin levels (P>0.05).
Table 3.
Comparison of pregnancy-related laboratory indices between the two groups
| Spontaneous preterm birth group (n=50) | Full term delivery group (n=70) | t/χ2 | P | |
|---|---|---|---|---|
| Reproductive tract infection | 12 (24.00%) | 10 (14.29%) | 5.681 | <0.001 |
| Second trimester cervical length (cm) | 24.89±3.12 | 32.98±4.88 | 9.070 | <0.001 |
| Hemoglobin level (g/L) | 113.94±11.87 | 117.23±11.89 | 1.664 | 0.089 |
| White blood cell count (×10^9/L) | 11.98±2.45 | 9.34±2.12 | 11.482 | <0.001 |
| Neutrophil percentage (%) | 0.78±0.03 | 0.87±0.03 | 9.023 | <0.001 |
Multivariate logistic regression analysis
The results of multivariate logistic regression analysis identified multiple pregnancies (95% CI: 1.415-8.926, P=0.006), abnormal fetal position (95% CI: 1.124-2.331, P=0.008), gestational diabetes (95% CI: 4.918-19.164, P=0.002), mode of conception (95% CI: 1.765-4.285, P=0.002), lower genital tract infection (95% CI: 1.076-2.867, P=0.032), and second-trimester cervical length (95% CI: 1.071-2.991, P=0.031) as independent risk factors for SPTB (Table 4).
Table 4.
Multivariate logistic regression analysis
| Factors | Bate | SE | Wald | OR | 95% CI | P |
|---|---|---|---|---|---|---|
| Multiple pregnancy | 1.224 | 0.429 | 7.425 | 3.521 | 1.415-8.926 | 0.006 |
| Abnormal fetal position | 0.536 | 0.128 | 6.633 | 1.538 | 1.124-2.331 | 0.008 |
| Gestational diabetes | 2.218 | 0.617 | 45.812 | 9.816 | 4.918-19.164 | 0.002 |
| Mode of conception | 0.954 | 0.258 | 15.852 | 2.856 | 1.765-4.285 | 0.002 |
| Lower genital tract infection | 0.666 | 0.266 | 4.966 | 1.755 | 1.076-2.867 | 0.032 |
| Second trimester cervical length | 0.451 | 0.251 | 4.581 | 1.741 | 1.071-2.991 | 0.031 |
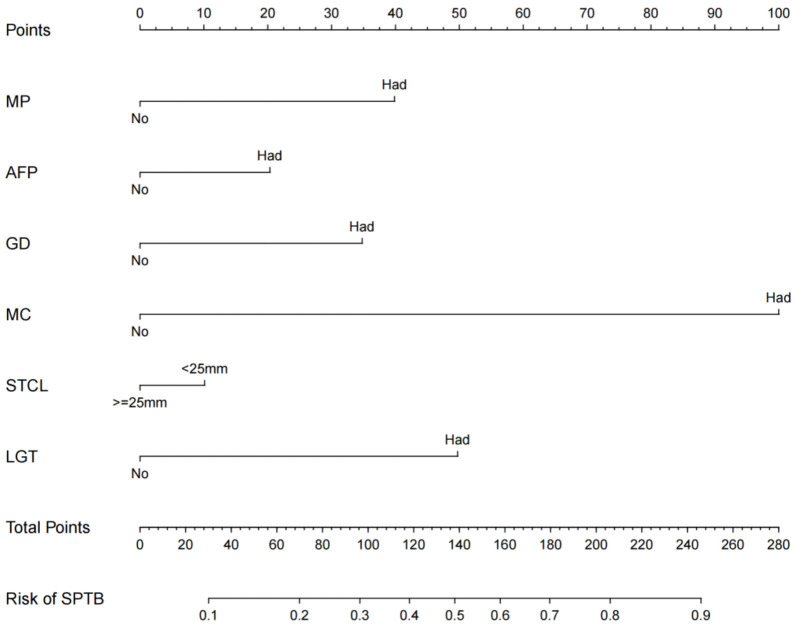
Development and validation of the nomogram
Based on the multivariate logistic regression analysis results, we constructed a nomogram incorporating the independent risk factors (Figure 2). The regression equation was based on these factors:
Figure 2.
The nomogram for predicting the risk of SPTB. MP: Multiple pregnancies; AFP: Abnormal fetal position; GD: Gestational diabetes; MC: Mode of conception; STCL: Second trimester cervical length; LGT: Lower genital tract infection.
logit(P) = -2.120 + 0.521 * multiple pregnancies + 0.538 * abnormal fetal position + 0.816 * gestational diabetes + 0.856 * mode of conception + 0.755 * lower genital tract infection + 0.741 * second-trimester cervical length. To use this nomogram, the corresponding position on each variable axis was located first according to patient’s manifestation. Then, a line was drawn vertically to the points axis above to obtain the respective points. Finally, the points from all six variables were added up, and a line was drawn from the total points axis to the predicted probability axis to estimate the likelihood of SPTB.
The calibration curve (Figure 3) for the training set showed that predicted and actual risks of SPTB are closely aligned, indicating the model’s high prediction accuracy. The AUC was 0.833, demonstrating good predictive performance (Figure 4).
Figure 3.
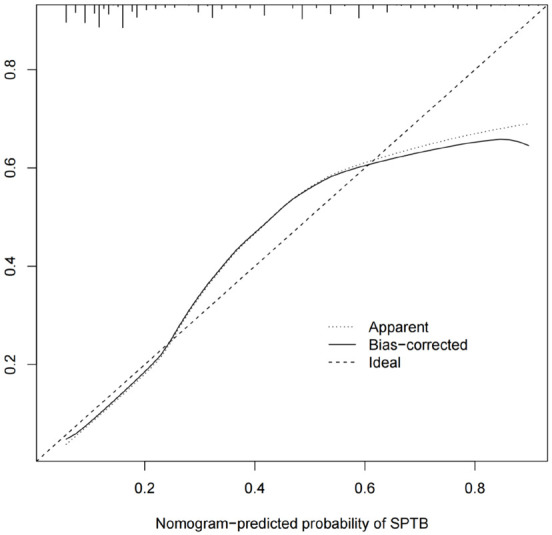
Calibration curve of the nomogram.
Figure 4.
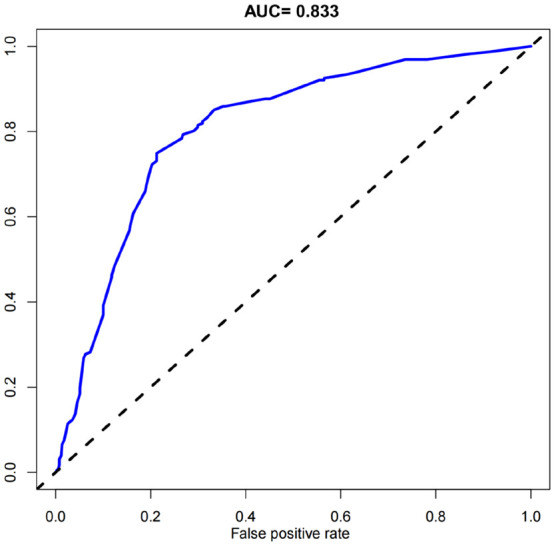
ROC curve analysis of the predictive performance of the nomogram, with an AUC of 0.833 (95% CI: 0.665-0.847).
Clinical utility evaluation and validation
The DCA curve showed that the nomogram provided a high clinical utility (Figure 5). The decision curve indicates that when the threshold probability of SPTB is between 40% and 80%, using this nomogram would provide a clear net benefit.
Figure 5.
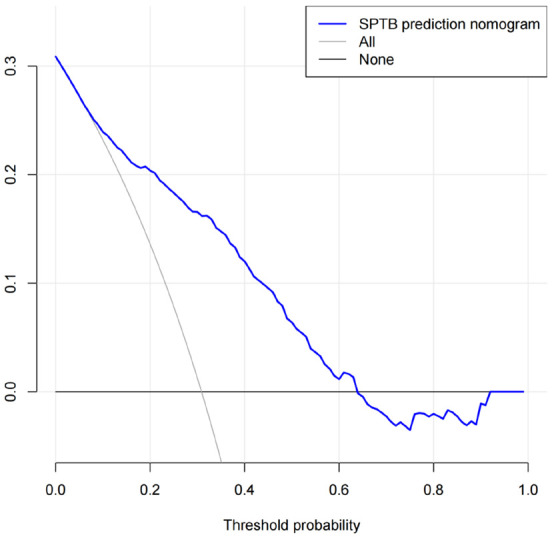
Decision curve analysis of the nomogram model.
Discussion
In this retrospectively study of 130 pregnant women who experienced either spontaneous preterm birth (SPTB) or full-term delivery, we first analyzed the general clinical data and various clinical indicators of the patients, to identify the independent risk factors for SPTB. The results showed that multiple pregnancies, abnormal fetal position, gestational diabetes, mode of conception, lower genital tract infection, and second-trimester cervical length were independent risk factors for SPTB. Using these six identified risk factors, a risk prediction model for SPTB was successfully constructed, which was subsequently evaluated and validated.
In this study, multiple pregnancies was identified as independent risk factors for SPTB, which is consistent with the findings of Murray et al. [27]. In multiple pregnancies, there is an increased demand for nutrients and oxygen to support the growth and development of multiple fetuses. This may lead to an imbalance or insufficiency in the supply, potentially affecting the normal development and stability of the pregnancy, thus increasing the risk of miscarriage [28]. Additionally, the uterine environment in multiple pregnancies is more crowded, leading to greater competition and interaction among fetuses. This can result in complications such as improper implantation or placental abnormalities, which are important contributors to spontaneous abortions [29]. Therefore, pregnant women with multiple pregnancies should be more vigilant about the risk of SPTB and take precautions in advance.
Abnormal fetal position was another independent risk factor for SPTB. An abnormal fetal position may cause changes in the intrauterine environment and increase fetal stress. When the fetus is not in the proper position, it may lead to uneven pressure distribution within the uterus, affecting the stability and function of the placenta and amniotic fluid, which in turn increases the risk of preterm contractions and early rupture of membranes [30]. In addition, an abnormal fetal position may complicate labor, making it more difficult for the fetus to adapt to the process. This can lead to labor complications, more intense uterine contractions, and fetal distress, all of which can contribute to the occurrence of premature birth [31]. In general, an abnormal fetal position can disrupt the normal physiological state of the uterus and fetus through various mechanisms, increasing the likelihood of SPTB.
Gestational diabetes was identified as another independent risk factor for SPTB. Gestational diabetes may cause abnormal glucose metabolism, which may impair placental function. If the placenta does not function properly, it may fail to deliver sufficient oxygen and nutrients to the fetus, potentially triggering preterm contractions and leading to preterm birth [32]. Elevated maternal blood sugar levels can also affect fetal growth, causing fetal overgrowth or other complications that increase the risk of preterm birth [33]. Moreover, gestational diabetes mellitus is associated with increased inflammation, which can contribute to instability in the uterine environment and promote preterm birth [34].
Mode of conception was identified as an independent risk factor for SPTB, particularly in cases involving assisted reproductive technology (ART). The processes involved in ART, including invasive procedures and hormonal interventions, can disrupt the normal physiological state of the uterus and embryo [34]. Hormonal imbalances or fluctuations caused by these procedures may negatively affect the stability and progression of the pregnancy [35]. Additionally, ART involves culturing and manipulating embryos in vitro, which may bring some uncertainties and potential damage [36]. These artificial processes can impact the quality and adaptability of embryos, increasing the risk of complications during implantation and subsequent development [37]. Furthermore, women undergoing ART often have underlying fertility issues or other health conditions, which themselves may increase pregnancy risks [38]. These pre-existing factors, in combination with the ART procedures, contribute to a higher likelihood of spontaneous abortion.
Lower genital tract infection can cause an inflammatory response, releasing inflammatory mediators and cytokines that adversely affect the uterine environment and fetal membranes [39]. This inflammatory response may lead to premature activation and weakening of the fetal membrane, increasing uterine contractions and the risk of preterm birth [40]. Certain pathogens can also directly invade the amniotic cavity and placenta, causing damage and dysfunction, further contributing to preterm birth [41]. Moreover, persistent or severe infections can disrupt the normal physiological and immune function in the reproductive tract, heightening the risk of SPTB. Therefore, pregnant women diagnosed with lower genital tract infection should take preventive measures to reduce the risk of SPTB.
During the second trimester, an insufficient cervical length may be less capable of withstanding the increasing pressure and mechanical stress within the uterus as pregnancy progresses [42]. This can make the cervix more prone to premature dilation and weakening, increasing the risk of preterm birth. Additionally, issues related to the connective tissue and collagen composition of the cervix may affect its stability and ability to remain closed [43].
In conclusion, based on the identified risk factors for SPTB, this study constructed a nomogram prediction model with good predictive performance, high accuracy, and clinical applicability. The model is straightforward and user-friendly in clinical practice, providing a safe and non-invasive screening method that is easily accepted by both doctors and patients. This model aids in the early identification of high-risk populations for SPTB, improving the detection rate while reducing complications associated with excessive invasive examinations. By offering a cost-effective approach for SPTB screening in clinical practice, it is of great medical and social significance.
Disclosure of conflict of interest
None.
References
- 1.Feyaerts D, Marić I, Arck PC, Prins JR, Gomez-Lopez N, Gaudillière B, Stelzer IA. Predicting spontaneous preterm birth using the immunome. Clin Perinatol. 2024;51:441–459. doi: 10.1016/j.clp.2024.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Hillier TA, Pedula KL, Ogasawara KK, Vesco KK, Oshiro CES, Lubarsky SL, Van Marter J. A pragmatic, randomized clinical trial of gestational diabetes screening. N Engl J Med. 2021;384:895–904. doi: 10.1056/NEJMoa2026028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Biasi S, Neroni A, Nasi M, Lo Tartaro D, Borella R, Gibellini L, Lucaccioni L, Bertucci E, Lugli L, Miselli F, Bedetti L, Neri I, Ferrari F, Facchinetti F, Berardi A, Cossarizza A. Healthy preterm newborns: altered innate immunity and impaired monocyte function. Eur J Immunol. 2023;53:e2250224. doi: 10.1002/eji.202250224. [DOI] [PubMed] [Google Scholar]
- 4.Nic Lochlainn LM, de Gier B, van der Maas N, Strebel PM, Goodman T, van Binnendijk RS, de Melker HE, Hahné SJM. Immunogenicity, effectiveness, and safety of measles vaccination in infants younger than 9 months: a systematic review and meta-analysis. Lancet Infect Dis. 2019;19:1235–1245. doi: 10.1016/S1473-3099(19)30395-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He J, Sun X, Xu X, Luo H, Tang J, Xiong T, Zhao J, Shi J. Effects of the feeding protocol during blood transfusion on splanchnic tissue oxygenation and complications in very premature infants. Front Nutr. 2024;11:1408717. doi: 10.3389/fnut.2024.1408717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarda SP, Vanya M, Schwartz EJ, Sorrells K, Namba F, Hirano S, McNulty A, Han L, Mangili A. Burden of treatments for respiratory complications in extremely premature infants: interviews with caregivers. Biomed Hub. 2023;8:15–24. doi: 10.1159/000527375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kemelbekov K, Musaev Y, Bektenova G, Datkayeva G, Tuktibayeva S, Bekenov N, Khatamov F, Ilyassova G, Gatauova M, Zhumabekov Z, Kuandykov Y, Tazhiyeva A, Abdrakhmanova Z, Omarova B, Tasbulatov N. Comparative results of treatment and complications: Pedea in open ductus arteriosus in premature infants. J Cardiovasc Pharmacol. 2021;78:e722–e728. doi: 10.1097/FJC.0000000000001094. [DOI] [PubMed] [Google Scholar]
- 8.Al-Mouqdad MM, Khalil TM, Asfour SS. Retrospective study of short-term complications associated with early morphine use in intubated premature infants. Sci Rep. 2020;10:10874. doi: 10.1038/s41598-020-67891-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.López-Hernández ÁM, Padilla-Muñoz EM, Duque-Sánchez C, Lanzarote-Fernández MD. Influence of perinatal complications on the development of a sample of 36-month-old premature infants. Infant Behav Dev. 2021;62:101507. doi: 10.1016/j.infbeh.2020.101507. [DOI] [PubMed] [Google Scholar]
- 10.He W, Sparén P, Fang F, Sengpiel V, Strander B, Czene K. Pregnancy outcomes in women with a prior cervical intraepithelial neoplasia grade 3 diagnosis: a nationwide population-based cohort study with sibling comparison design. Ann Intern Med. 2022;175:210–218. doi: 10.7326/M21-2793. [DOI] [PubMed] [Google Scholar]
- 11.Suresh S, Freedman A, Adams M, Hirsch E, Ernst LM. Placental histology for targeted risk assessment of recurrent spontaneous preterm birth. Am J Obstet Gynecol. 2024;230:452.e1–452.e11. doi: 10.1016/j.ajog.2023.09.018. [DOI] [PubMed] [Google Scholar]
- 12.Kindschuh WF, Baldini F, Liu MC, Liao J, Meydan Y, Lee HH, Heinken A, Thiele I, Thaiss CA, Levy M, Korem T. Preterm birth is associated with xenobiotics and predicted by the vaginal metabolome. Nat Microbiol. 2023;8:246–259. doi: 10.1038/s41564-022-01293-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boelig RC, Cahanap TJ, Ma L, Zhan T, Berghella V, Chan JSY, Kraft WK, Mckenzie SE. Platelet protease activated receptor 4 (PAR 4) receptor genotype is associated with an increased risk of preterm birth. J Thromb Haemost. 2022;20:2419–2428. doi: 10.1111/jth.15814. [DOI] [PubMed] [Google Scholar]
- 14.Akhter T, Hedeland M, Bergquist J, Ubhayasekera K, Larsson A, Byström L, Kullinger M, Skalkidou A. Elevated plasma levels of arginines during labor among women with spontaneous preterm birth: a prospective cohort study. Am J Reprod Immunol. 2024;91:e13889. doi: 10.1111/aji.13889. [DOI] [PubMed] [Google Scholar]
- 15.Sarker M, Zamudio AR, DeBolt C, Ferrara L. Beyond stillbirth: association of intrahepatic cholestasis of pregnancy severity and adverse outcomes. Am J Obstet Gynecol. 2022;227:517.e1–517.e7. doi: 10.1016/j.ajog.2022.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Jiang Y, Zhang Y, Yang Q, Zeng D, Zhao K, Ma X, Yin W. The association between fetal fraction and pregnancy-related complications among Chinese population. PLoS One. 2022;17:e0271219. doi: 10.1371/journal.pone.0271219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin D, Li P, Fan D, Chen G, Wu S, Ye S, Ma H, Rao J, Zhou Z, Zeng M, Huang Z, Guo X, Liu Z. Association between IVF/ICSI treatment and preterm birth and major perinatal outcomes among dichorionic-diamnionic twin pregnancies: a seven-year retrospective cohort study. Acta Obstet Gynecol Scand. 2021;100:162–169. doi: 10.1111/aogs.13981. [DOI] [PubMed] [Google Scholar]
- 18.Elmaraghy AM, Shaaban SMA, Elsokkary MS, Elshazly ISMA. Uterocervical angle versus cervical length in the prediction of spontaneous preterm birth in women with history of spontaneous preterm birth: a prospective observational study. BMC Pregnancy Childbirth. 2023;23:658. doi: 10.1186/s12884-023-05977-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun Y, Lian F, Deng Y, Liao S, Wang Y. Development and validation of a nomogram to predict spontaneous preterm birth in singleton gestation with short cervix and no history of spontaneous preterm birth. Heliyon. 2023;9:e20453. doi: 10.1016/j.heliyon.2023.e20453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conde-Agudelo A, Romero R. Vaginal progesterone does not prevent recurrent preterm birth in women with a singleton gestation, a history of spontaneous preterm birth, and a midtrimester cervical length >25 mm. Am J Obstet Gynecol. 2022;227:923–926. doi: 10.1016/j.ajog.2022.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heikkilä K, Metsälä J, Pulakka A, Nilsen SM, Kivimäki M, Risnes K, Kajantie E. Preterm birth and the risk of multimorbidity in adolescence: a multiregister-based cohort study. Lancet Public Health. 2023;8:e680–e690. doi: 10.1016/S2468-2667(23)00145-7. [DOI] [PubMed] [Google Scholar]
- 22.Athanasiou A, Veroniki AA, Efthimiou O, Kalliala I, Naci H, Bowden S, Paraskevaidi M, Arbyn M, Lyons D, Martin-Hirsch P, Bennett P, Paraskevaidis E, Salanti G, Kyrgiou M. Comparative effectiveness and risk of preterm birth of local treatments for cervical intraepithelial neoplasia and stage IA1 cervical cancer: a systematic review and network meta-analysis. Lancet Oncol. 2022;23:1097–1108. doi: 10.1016/S1470-2045(22)00334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alfirevic Z, Owen J, Carreras Moratonas E, Sharp AN, Szychowski JM, Goya M. Vaginal progesterone, cerclage or cervical pessary for preventing preterm birth in asymptomatic singleton pregnant women with a history of preterm birth and a sonographic short cervix. Ultrasound Obstet Gynecol. 2013;41:146–151. doi: 10.1002/uog.12300. [DOI] [PubMed] [Google Scholar]
- 24.Tranidou A, Tsakiridis I, Apostolopoulou A, Xenidis T, Pazaras N, Mamopoulos A, Athanasiadis A, Chourdakis M, Dagklis T. Prediction of gestational diabetes mellitus in the first trimester of pregnancy based on maternal variables and pregnancy biomarkers. Nutrients. 2023;16:120. doi: 10.3390/nu16010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Egorov V, Rosen T, Hill J, Khandelwal M, Kurtenoks V, Francy B, Sarvazyan N. Evaluating the efficacy of cervical tactile ultrasound technique as a predictive tool for spontaneous preterm birth. Open J Obstet Gynecol. 2024;14:832–846. doi: 10.4236/ojog.2024.145067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi S, Katsumata M, Mizuguchi Y, Toda M. A case of moyamoya disease diagnosed as cerebral infarction in the early postpregnancy period and surgically treated by bilateral revascularization after term delivery. Clin Neurol Neurosurg. 2023;231:107859. doi: 10.1016/j.clineuro.2023.107859. [DOI] [PubMed] [Google Scholar]
- 27.Murray SR, Stock SJ, Cowan S, Cooper ES, Norman JE. Spontaneous preterm birth prevention in multiple pregnancy. Obstet Gynaecol. 2018;20:57–63. doi: 10.1111/tog.12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sferruzzi-Perri AN, Lopez-Tello J, Salazar-Petres E. Placental adaptations supporting fetal growth during normal and adverse gestational environments. Exp Physiol. 2023;108:371–397. doi: 10.1113/EP090442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grantz KL, Kawakita T, Lu YL, Newman R, Berghella V, Caughey A. SMFM special statement: state of the science on multifetal gestations: unique considerations and importance. Am J Obstet Gynecol. 2019;221:B2–B12. doi: 10.1016/j.ajog.2019.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaffer Z, Romero R, Tarca AL, Galaz J, Arenas-Hernandez M, Gudicha DW, Chaiworapongsa T, Jung E, Suksai M, Theis KR, Gomez-Lopez N. The vaginal immunoproteome for the prediction of spontaneous preterm birth: a retrospective longitudinal study. Elife. 2024;13:e90943. doi: 10.7554/eLife.90943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsikouras P, Kotanidou S, Nikolettos K, Kritsotaki N, Bothou A, Andreou S, Nalmpanti T, Chalkia K, Spanakis V, Peitsidis P, Iatrakis G, Nikolettos N. Shoulder dystocia: a comprehensive literature review on diagnosis, prevention, complications, prognosis, and management. J Pers Med. 2024;14:586. doi: 10.3390/jpm14060586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doshani A, Konje JC. Placental dysfunction in obese women and antenatal surveillance. Best Pract Res Clin Obstet Gynaecol. 2023;91:102407. doi: 10.1016/j.bpobgyn.2023.102407. [DOI] [PubMed] [Google Scholar]
- 33.Boutin A, Guerby P, Gasse C, Tapp S, Bujold E. Pregnancy outcomes in nulliparous women with positive first-trimester preterm preeclampsia screening test: the Great Obstetrical Syndromes cohort study. Am J Obstet Gynecol. 2021;224:204.e1–204.e7. doi: 10.1016/j.ajog.2020.08.008. [DOI] [PubMed] [Google Scholar]
- 34.Kaye DK. The ethical justification for inclusion of neonates in pragmatic randomized clinical trials for emergency newborn care. BMC Pediatr. 2019;19:218. doi: 10.1186/s12887-019-1600-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sánchez-Garrido MA, García-Galiano D, Tena-Sempere M. Early programming of reproductive health and fertility: novel neuroendocrine mechanisms and implications in reproductive medicine. Hum Reprod Update. 2022;28:346–375. doi: 10.1093/humupd/dmac005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ata B, La Marca A, Polyzos NP. Free your patients and yourself from day 2-3: start ovarian stimulation any time in freeze-all cycles. Reprod Biomed Online. 2023;47:103305. doi: 10.1016/j.rbmo.2023.103305. [DOI] [PubMed] [Google Scholar]
- 37.Bellver J. BMI and miscarriage after IVF. Curr Opin Obstet Gynecol. 2022;34:114–121. doi: 10.1097/GCO.0000000000000778. [DOI] [PubMed] [Google Scholar]
- 38.Bayefsky MJ, Sampson A, Blakemore JK, Jalili D, Lilly AG, Fino ME, Quinn GP. Experiences and intentions of patients undergoing medically indicated oocyte or embryo cryopreservation: a qualitative study. Hum Reprod. 2024;39:147–153. doi: 10.1093/humrep/dead228. [DOI] [PubMed] [Google Scholar]
- 39.Kim C, Cathey AL, Watkins DJ, Mukherjee B, Rosario-Pabón ZY, Vélez-Vega CM, Alshawabkeh AN, Cordero JF, Meeker JD. Maternal blood metal concentrations are associated with matrix metalloproteinases (MMPs) among pregnant women in Puerto Rico. Environ Res. 2022;209:112874. doi: 10.1016/j.envres.2022.112874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Menon R, Behnia F, Polettini J, Richardson LS. Novel pathways of inflammation in human fetal membranes associated with preterm birth and preterm pre-labor rupture of the membranes. Semin Immunopathol. 2020;42:431–450. doi: 10.1007/s00281-020-00808-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amrit A, Utture A, More K. Caring for the hypertensive newborn: a prospective evaluation of risk factors, clinical profile, management, and predictors of outcome of neonatal hypertension. Eur J Pediatr. 2023;182:5367–5374. doi: 10.1007/s00431-023-05181-z. [DOI] [PubMed] [Google Scholar]
- 42.Hughes K, Ford H, Thangaratinam S, Brennecke S, Mol BW, Wang R. Diagnosis or prognosis? An umbrella review of mid-trimester cervical length and spontaneous preterm birth. BJOG. 2023;130:866–879. doi: 10.1111/1471-0528.17443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horie K, Takahashi K, Mieno M, Nagayama S, Aoki H, Nagamatsu T, Kanatani A, Hyodo H, Terada K, Hayashi M, Nakai A, Yoneda N, Saito S, Matsuda Y, Matsubara S, Ohkuchi A. Uterine contraction may not be an independent risk factor for spontaneous preterm birth before 35 weeks in women with cervical shortening. Int J Gynaecol Obstet. 2023;161:894–902. doi: 10.1002/ijgo.14578. [DOI] [PubMed] [Google Scholar]