Abstract
Objective: To investigate the expression and prognostic value of neutrophil-to-lymphocyte ratio (NLR) and fibrinogen-to-albumin ratio (FAR) in patients with locally advanced or metastatic pancreatic cancer (PC). Methods: This retrospective study included 118 cases diagnosed with metastatic or locally advanced PC who received systemic chemotherapy at People’s Hospital of Deyang City from January 2018 to February 2021. Data and blood indicators were collected from patients, and the platelet-to-lymphocyte ratio (PLR), NLR, and FAR were calculated. Receiver operating characteristic (ROC) curves were used to determine optimal cutoff values. Kaplan-Meier survival curves were plotted, and the Log-rank test was employed for intergroup survival analysis. Prognostic factors affecting the prognosis of PC patients were identified using multiple Cox regression analysis. Results: The optimal cutoff values of FAR and NLR were 0.1 and 3.28 respectively, with areas under the curve (AUC) of 0.776 and 0.804, respectively. Significant differences were observed between the low FAR and high FAR groups in terms of tumor invasion into large blood vessels, distant metastasis, and pre-treatment NLR (all P < 0.05). Similarly, significant differences were observed between the low NLR and high NLR groups in terms of distant metastasis, pre-treatment FAR, and pre-treatment PLR (all P < 0.05). At the end of the follow-up, 65 patients died and 53 survived. The 24-month survival rate was 97.62% in the high FAR group, significantly higher than 31.58% in the low FAR group (P < 0.001). The 24-month survival rate for the high NLR group was 91.30%, also significantly higher than 31.94% in the low NLR group (P < 0.001). In the Cox regression model, both high FAR and high NLR were identified as independent risk factors for poor prognosis in PC patients (all P < 0.05). The AUC for FAR combined with NLR in predicting the prognosis of PC patients was 0.946 (95% CI: 0.905-0.986), with a specificity of 92.30% and a sensitivity of 92.40%. Conclusion: Both FAR and NLR are correlated with prognosis in patients suffering from locally advanced or metastatic PC, and their combined detection may precisely predict prognosis in PC patients undergoing systemic chemotherapy.
Keywords: Pancreatic cancer, neutrophil/lymphocyte ratio, prognostic value of fibrinogen/albumin ratio, prognosis
Introduction
Pancreatic cancer (PC) is a malignant tumor with an insidious onset, rapid development, and poor therapeutic outcome. The median survival time for PC ranges from 6 to 12 months, with a 5-year survival rate of only 10% [1-3]. Currently, surgical treatment remains the primary choice for PC, but only 20% of patients are eligible for radical resection at the time of diagnosis [4,5]. With the continuous advancements in multidisciplinary collaboration and updated treatment guidelines, the diagnosis and treatment of PC are increasingly standardized [6]. For patients with metastatic or locally advanced PC, systemic chemotherapy is the mainstay of treatment. Chemotherapy regimens based on gemcitabine or 5-fluorouracil (5-FU) have demonstrated greater survival benefits and are recommended as first-line treatment for advanced PC patients, yet some patients still have a poor prognosis [7-10]. Over the years, studies have sought to identify factors influencing the prognosis of PC and to explore simple and cost-effective molecular markers to help clinicians better evaluate patient prognosis.
With the continuous development and improvement of molecular biomarkers, the role of the inflammatory microenvironment in tumorigenesis and metastasis has become widely recognized. The evaluation of tumor prognosis through whole blood cell analysis and blood biochemical indices is clinically promising [11,12]. Studies have indicated that inflammatory markers such as the lymphocyte-to-monocyte ratio (LMR), platelet-to-lymphocyte ratio (PLR), peripheral blood neutrophil-to-lymphocyte ratio (NLR), and C-reactive protein-to-albumin ratio are significantly correlated with the overall survival (OS) in tumor patients, making them valuable markers [13-17].
On the other hand, tumors frequently lead to a hypercoagulable state. As a key pro-coagulant factor, fibrinogen plays a central role in balancing anticoagulation and coagulation processes and facilitating thrombosis [18]. It has been reported that fibrinogen is also implicated in the inflammatory response and immune regulation of the body, and its elevation can directly contribute to the occurrence, invasion, and metastasis of tumors, suggesting that fibrinogen may serve as a prognostic marker [19-21]. Serum albumin, the most abundant plasma protein, is synthesized by liver cells and plays a role in visceral protein synthesis, serving as an indicator of the body’s nutritional status. Studies have found that the plasma fibrinogen/albumin ratio (FAR) in cancer patients can undergo significant changes, and it has been utilized as a crucial indicator for evaluating prognosis of patients with gastric cancer, colorectal cancer, and bladder cancer [22-24]. However, there is limited research on FAR and NLR in PC patients, as well as their prognostic value. Furthermore, existing studies on FAR in PC patients have primarily focused on those undergoing pancreatic cancer resection [25,26], with few addressing its prognostic value in patients receiving systemic chemotherapy for PC. Therefore, this retrospective study aimed to investigate the relations between FAR, NLR and prognosis in patients with locally advanced or metastatic PC patients undergoing chemotherapy. Furthermore, it sought to determine whether these biomarkers can be used to assess prognosis in this population.
Materials and methods
Basic information
Patients diagnosed with PC and receiving first-line chemotherapy in People’s Hospital of Deyang City from June 2018 to November 2021 were included in this study. Inclusion criteria: ① PC confirmed by histopathology or cytologic biopsy, with locally advanced or metastatic PC as defined by the NCCN Clinical Practice Guidelines for Oncology, based on relevant clinical imaging data [10]; ② Completion of at least one cycle of first-line systemic chemotherapy; ③ Availability of complete clinicopathologic data, laboratory examination results, and follow-up information. Exclusion criteria: ① Malignant tumors of other systems; ② Blood system diseases or acute or chronic infections; ③ Patients who had received anticoagulation therapy before treatment; ④ Patients with hepatic or renal insufficiency; ⑤ Follow-up time less than 1 month.
Based on reference studies, the 2-year survival rate of PC patients following chemotherapy ranges from 20% to 50% [27,28]. Assuming a survival rate of 30% for this study, it was anticipated that three variables would be encompassed in the multivariate regression model. The sample size was calculated using the events per variable (EPV) method, where EPV = 10. The sample size formula: sample size = number of included variables * EPV/incidence = 3 * 10/30% = 100 cases. Considering an additional 15% to 20% for loss to follow-up or refusal, 118 patients were ultimately included. This study was approved by the ethics committee of People’s Hospital of Deyang City.
Data collection
By consulting the electronic medical record system, the general information (age, gender) clinicopathologic data (differentiation degree, tumor location, etc.), and chemotherapy regimens [FOLFIRINOX (oxaliplatin + irinotecan + fluorouracil + calcium folinate), AG (gemcitabine + albumin-bound paclitaxel), GS (gemcitabine + Teggio), and AS (albumin-bound + Teggio)] of the included subjects were collected. The duration of the FOLFIRINOX regimen was defined as 2 weeks, while the duration of the other chemotherapy regimens was defined as 3 weeks.
Fasting venous blood was collected from all patients for testing within 1 week prior to treatment. The tests included blood routine, biochemical indicators, and coagulation routine indicators. The specific indicators collected included peripheral blood neutrophils, lymphocytes, platelet count, plasma albumin level, plasma fibrinogen level, and calculation of peripheral blood NLR, PLR, and FAR before treatment.
Treatment and follow-up
All patients with pancreatic cancer received first-line chemotherapy treatment based on gemcitabine or 5-FU. Patients were followed up for a period of two years (until February 2023) through outpatient consultations, telephone visits, and reviews of inpatient medical records to assess their survival status. The follow-up period began at the time of diagnosis with pancreatic cancer. Overall survival (OS) was the primary prognostic indicator, defined as the time from diagnosis to the last follow-up or death (measured in months). No cases were lost during the follow-up period.
Statistical methods
Data analysis was performed using SPSS version 24.0. Measured data following a normal distribution were described as mean ± standard deviation (mean ± SD) and analyzed using the t-test. Categorical data were expressed as percentage (%) and analyzed using χ2 tests. The cutoff values for FAR and NLR were determined byusing receiver operating characteristics (ROC) curve. Survival curves were plotted using the Kaplan-Meier (K-M) method, and prognostic factors were assessed using Cox regression analysis. A P value of less than 0.05 was considered statistically significant.
Results
Patient baseline data
A total of 118 patients were included in this study, including 63 men and 55 women. The age ranged from 42 to 82 years old, with a median age of 60 years old. Degree of differentiation: 32 cases of high differentiation tumor, and 86 cases of low differentiation; Tumor location: 71 cases of pancreatic head, 47 cases of pancreatic body and tail; Tumor invasion of large blood vessels: invasion in 58 cases, no-invasion in 60 cases; Distant metastasis: metastasis in 46 cases, no-metastasis in 72 cases; Chemotherapy regimen: FOLFIRINOX regimen in 46 cases, AG in 39 cases, GS in 19 cases, AS in 14 cases. The baseline data are shown in Table 1.
Table 1.
Baseline data of the included 118 patients
| Variables | Group | n (%) |
|---|---|---|
| Sex | ||
| Male | 63 | 53.39 |
| Female | 55 | 46.61 |
| Age (years) | ||
| > 60 | 69 | 58.47 |
| ≤ 60 | 49 | 41.53 |
| Differentiation extent | ||
| Moderate/high differentiation | 32 | 27.12 |
| Poor differentiation | 86 | 72.88 |
| Tumor location | ||
| Head of pancreas | 71 | 60.17 |
| Pancreatic body and tail | 47 | 39.83 |
| Tumor invasion of large blood vessels | ||
| Yes | 58 | 49.15 |
| No | 60 | 50.85 |
| Distant metastasis | ||
| Yes | 46 | 41.53 |
| No | 72 | 61.01 |
| Chemotherapy regimen | ||
| FOLFIRINOX | 46 | 38.98 |
| AG | 39 | 33.05 |
| GS | 19 | 16.10 |
| AS | 14 | 11.86 |
Note: AG, gemcitabine + albumin-bound paclitaxel; GS, gemcitabine + Teggio; AS, albumin-bound + Teggio; FAR, fibrinogen/albumin ratio; NLR, neutrophil/lymphocyte ratio.
Optimal cutoff values and ROC curves of FAR and NLR
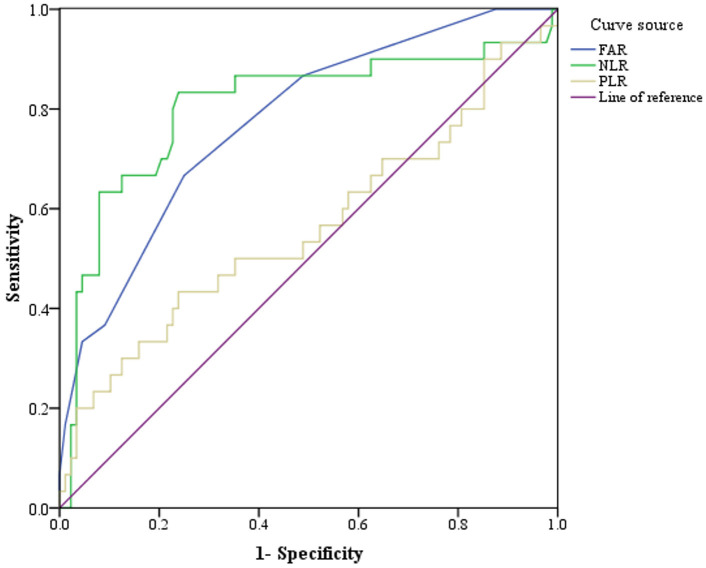
Before treatment, the average fibrinogen level in 118 patients was (3.87 ± 0.65) g/L, and the plasma albumin level was (39.57 ± 4.73) g/L, with a calculated FAR of (0.10 ± 0.21). The peripheral blood neutrophil count was (4.75 ± 1.58) × 109/mL, lymphocyte count was (1.62 ± 0.52) × 109/mL, platelet count was (211.81 ± 68.54) × 109/mL, NLR was (3.35 ± 1.80), PLR was (146.48 ± 74.01). The curve analysis results of FAR, NLR and PLR are shown in Table 2 and Figure 1.
Table 2.
Curve analysis results of FAR, NLR and PLR
| Indicators | AUC (90% CI) | P | Sensitivity (%) | Specificity (%) | Cut-off value |
|---|---|---|---|---|---|
| FAR | 0.822 (0.747-0.898) | < 0.001 | 75.80 | 63.50 | 0.10 |
| NLR | 0.808 (0.728-0.889) | < 0.001 | 83.30 | 76.10 | 3.28 |
| PLR | 0.587 (0.484-0.690) | 0.105 | 43.30 | 76.10 | 170.06 |
Note: FAR, fibrinogen/albumin ratio; NLR, neutrophil/lymphocyte ratio; PLR, platelet/lymphocyte ratio.
Figure 1.
ROC curves for pre-treatment FAR, NLR and PLR in predicting prognosis of PC patients. PC, Pancreatic cancer; FAR, fibrinogen/albumin ratio; NLR, neutrophil/lymphocyte ratio.
Relationship between pre-treatment FAR and clinical data of PC patients
Based on the optimal pre-treatment cutoff value for FAR, 118 patients were divided into a high FAR group (> 0.1, n = 42) and a low FAR group (≤ 0.1, n = 76). Significant differences were observed between the two groups in terms of tumor invasion of large blood vessels, distant metastasis, and pre-treatment NLR (all P < 0.05), as shown in Table 3.
Table 3.
Relationship between FAR and clinical data of PC patients
| Index | Low FAR group (≤ 0.1, n = 76) | High FAR group (> 0.1, n = 42) | t/χ2 | P |
|---|---|---|---|---|
| Gender (male/female) | 39/37 | 24/18 | 0.369 | 0.543 |
| Age (years) | 0.316 | 0.574 | ||
| > 60 | 43 (56.58) | 26 (61.90) | ||
| ≤ 60 | 33 (43.42) | 16 (38.10) | ||
| Differentiation extent | 1.068 | 0.301 | ||
| Moderate/high differentiation | 23 (30.26) | 9 (21.43) | ||
| Poor differentiation | 53 (69.74) | 33 (78.57) | ||
| Tumor location | 2.814 | 0.093 | ||
| Head of pancreas | 50 (65.79) | 21 (50.00) | ||
| Pancreatic body and tail | 26 (34.21) | 21 (50.00) | ||
| Tumor invasion of large blood vessels | 8.003 | 0.005 | ||
| Yes | 30 (39.47) | 28 (66.67) | ||
| No | 46 (60.53) | 14 (33.33) | ||
| Distant metastasis | 21.001 | < 0.001 | ||
| Yes | 18 (23.68) | 28 (66.67) | ||
| No | 58 (76.32) | 14 (33.33) | ||
| Chemotherapy regimen | 2.691 | 0.442 | ||
| FOLFIRINOX | 27 (35.53) | 19 (45.24) | ||
| AG | 29 (38.16) | 10 (23.81) | ||
| GS | 11 (14.47) | 8 (19.05) | ||
| AS | 9 (11.84) | 5 (11.90) | ||
| Pre-treatment NLR | 6.826 | 0.009 | ||
| ≤ 3.28 | 53 (69.74) | 19 (45.24) | ||
| > 3.28 | 23 (30.26) | 23 (54.76) | ||
| Pre-treatment PLR | 0.796 | 0.372 | ||
| ≤ 170.06 | 52 (68.42) | 32 (76.19) | ||
| > 170.06 | 24 (31.58) | 10 (23.81) |
Note: AG, gemcitabine + albumin-bound paclitaxel; GS, gemcitabine + Teggio; AS, albumin-bound + Teggio; PC, pancreatic cancer; FAR, fibrinogen/albumin ratio; NLR, neutrophil/lymphocyte ratio; PLR, platelet/lymphocyte ratio.
Relationship between pre-treatment NLR and clinical data of PC patients
According to the optimal pre-treatment cutoff value for NLR, 118 patients were divided into high NLR (> 3.28, n = 46) and low NLR groups (≤ 3.28, n = 72). Significant differences were found between the two groups in terms of distant metastasis, pre-treatment FAR, and PLR (all P < 0.05), as shown in Table 4.
Table 4.
Relationship between NLR and clinical data of PC patients
| Index | Low NLR group (≤ 3.28, n = 72) | High NLR group (> 3.28, n = 46) | t/χ2 | P |
|---|---|---|---|---|
| Gender (male/female) | 39/33 | 24/22 | 0.045 | 0.832 |
| Age (years) | 3.819 | 0.051 | ||
| > 60 | 37 (51.39) | 14 (30.43) | ||
| ≤ 60 | 35 (48.61) | 32 (69.57) | ||
| Differentiation extent | 0.041 | 0.840 | ||
| Moderate/high differentiation | 20 (27.78) | 12 (26.09) | ||
| Poor differentiation | 52 (72.22) | 34 (80.95) | ||
| Tumor location | 2.011 | 0.156 | ||
| Head of pancreas | 47 (65.28) | 24 (52.17) | ||
| Pancreatic body and tail | 25 (34.72) | 22 (47.83) | ||
| Tumor invasion of large blood vessels | 0.053 | 0.818 | ||
| Yes | 36 (50.00) | 24 (52.17) | ||
| No | 36 (50.00) | 22 (47.83) | ||
| Distant metastasis | 5.515 | 0.019 | ||
| Yes | 50 (69.44) | 22 (47.83) | ||
| No | 22 (30.56) | 24 (52.17) | ||
| Chemotherapy regimen | 5.940 | 0.115 | ||
| FOLFIRINOX | 28 (38.89) | 18 (39.13) | ||
| AG | 20 (27.78) | 19 (41.30) | ||
| GS | 16 (22.22) | 3 (6.52) | ||
| AS | 8 (11.11) | 6 (13.04) | ||
| Pre-treatment FAR | 5.984 | 0.014 | ||
| ≤ 0.1 | 52 (72.22) | 23 (50.00) | ||
| > 0.1 | 20 (27.78) | 23 (50.00) | ||
| Pre-treatment PLR | 18.169 | < 0.001 | ||
| ≤ 170.06 | 62 (86.11) | 23 (50.00) | ||
| > 170.06 | 10 (13.89) | 23 (50.00) |
Note: AG, gemcitabine + albumin-bound paclitaxel; GS, gemcitabine + Teggio; AS, albumin-bound + Teggio; PC, pancreatic cancer; FAR, fibrinogen/albumin ratio; NLR, neutrophil/lymphocyte ratio; PLR, platelet/lymphocyte ratio.
Correlation between pre-treatment FAR, NLR and the survival rate of PC patients
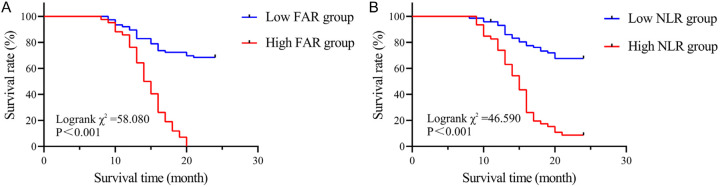
At the end of the follow-up, 65 patients died and 53 survived. The 24-month survival rate in the high FAR group was 97.62% (41/42), significantly higher than the 31.58% (24/76) in the low FAR group (Log rank χ2 = 58.080, P < 0.001, Figure 2A). Similarly, the 24-month survival rate in the high NLR group was 91.30% (42/46), significantly higher compared to 31.94% (23/72) in the low NLR group (Log rank χ2 = 46.590, P < 0.001, Figure 2B).
Figure 2.
Survival curve of PC patients. A. Survival curves of PC patients in high FAR group NLR and low FAR group; B. Survival curves of PC patients in high NLR group and low NLR group. PC, Pancreatic cancer; FAR, fibrinogen/albumin ratio; NLR, neutrophil/lymphocyte ratio.
Univariate and multivariate Cox regression analysis of prognostic factors in PC patients
Univariate analysis revealed that tumor invasion of large blood vessels, distant metastasis, pre-treatment FAR, pre-treatment NLR, and pre-treatment PLR were associated with poor prognosis in PC patients (all P < 0.05). Upon incorporating these variables into the Cox regression model, high FAR and high NLR emerged as significant risk factors for poor prognosis in PC patients (all P < 0.05), as shown in Table 5.
Table 5.
Univariate and multivariate Cox regression analysis of prognostic factors in PC patients
| Variable | Single factor analysis | Multiple-factor analysis | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P | HR (95% CI) | P | |
| Gender | 0.890 (0.549-1.444) | 0.637 | - | - |
| Age | 1.226 (0.742-2.025) | 0.426 | - | - |
| Differentiation extent | 1.529 (0.847-2.760) | 0.158 | - | - |
| Tumor location | 1.444 (0.889-2.345) | 0.138 | - | - |
| Tumor invasion of large blood vessels | 1.375 (0.847-2.235) | 0.198 | - | - |
| Distant metastasis | 1.993 (1.227-3.238) | 0.005 | 0.665 (0.372-1.189) | 0.169 |
| Chemotherapy regimen | 0.970 (0.766-1.228) | 0.798 | - | - |
| Pre-treatment FAR | 6.062 (3.548-10.357) | < 0.001 | 4.972 (2.670-9.261) | < 0.001 |
| Pre-treatment NLR | 4.757 (2.836-7981) | < 0.001 | 3.308 (1.876-5.835) | < 0.001 |
| Pre-treatment PLR | 1.510 (0.909-2.507) | 0.111 | - | - |
Note: AG, gemcitabine + albumin-bound paclitaxel; GS, gemcitabine + Teggio; AS, albumin-bound + Teggio; PC, pancreatic cancer; FAR, fibrinogen/albumin ratio; NLR, neutrophil/lymphocyte ratio; PLR, platelet/lymphocyte ratio.
Value of FAR combined with NLR in evaluating the prognosis of PC patients
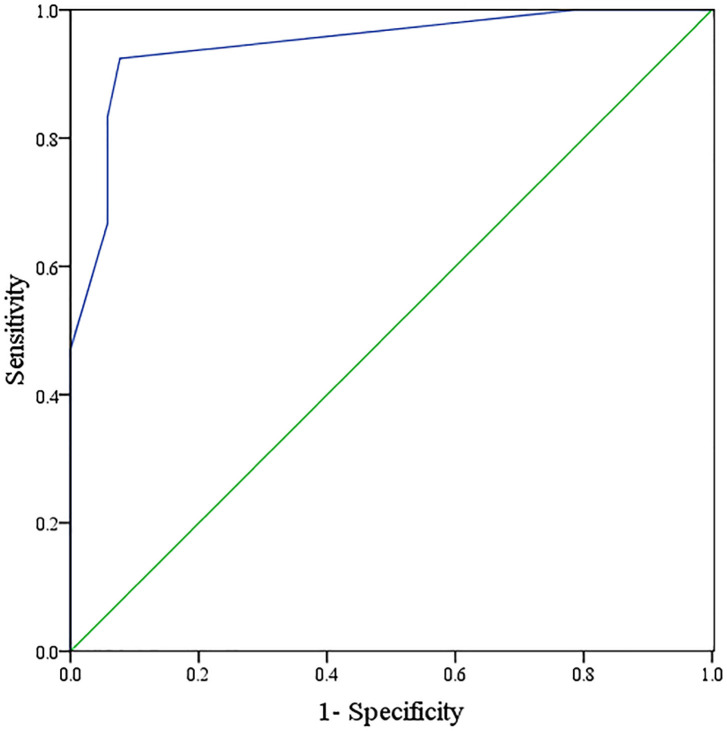
ROC curve analysis demonstrated that the AUC for FAR combined with NLR in assessing the prognosis of PC patients was 0.946 (95% CI: 0.905-0.986), with a specificity of 92.30% and a sensitivity of 92.40%, respectively. Results are shown in Figure 3.
Figure 3.
ROC curve of FAR combined with NLR in predicting the prognosis of PC patients. PC, Pancreatic cancer; FAR, fibrinogen/albumin ratio; NLR, neutrophil/lymphocyte ratio.
Survival curve analysis of FAR and NLR for evaluating the prognosis of PC patients
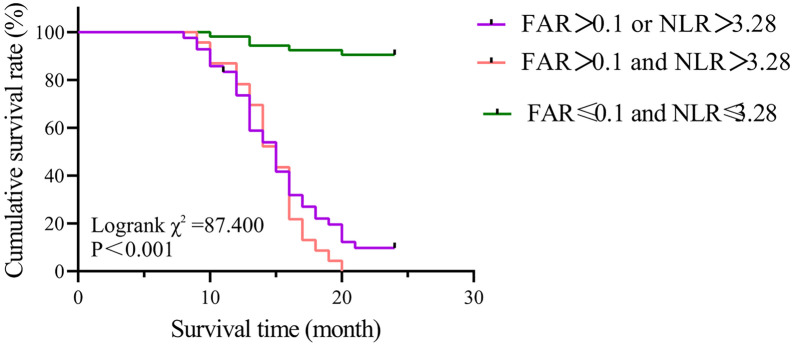
Survival curve analysis showed that FAR combined with NLR accurately stratified the prognosis of patients with locally advanced or metastatic PC in this study, as shown in Figure 4. The median survival time (MST) of patients with FAR ≤ 0.1 and NLR ≤ 3.28 was not reached, as less than half of the patients had died by the end of the trial. However, the MST for patients with either FAR > 0.1 or NLR > 3.28 was 14 months, while the MST for those with both FAR > 0.1 and NLR > 3.28 was 10 months. The prognosis was significantly different between groups (χ2 = 29.594, P < 0.001).
Figure 4.
Survival curves of FAR combined with NLR for predicting the prognosis of PC patients. PC, Pancreatic cancer; FAR, fibrinogen/albumin ratio; NLR, neutrophil/lymphocyte ratio.
Discussion
Previous studies have affirmed that systemic inflammatory response and the body’s autoimmune nutritional status are implicated in the development and progression of tumors and are important factors affecting the prognosis of tumor patients [11]. As a newly discovered inflammatory indicator, peripheral blood FAR has been identified to be associated with the prognosis in various malignant tumors. However, its role in the prognostic assessment of PC, particularly in locally advanced or metastatic PC, remains ambiguous. In this study, the clinicopathologic data and follow-up data of patients with locally advanced or metastatic PC undergoing first-line chemotherapy were retrospectively analyzed, and the correlations between FAR, NRP and the clinicopathologic features and prognosis of the patients were evaluated. The optimal cutoff values for FAR in predicting the prognosis of patients with metastatic or locally advanced PC were determined according to relevant research reports [26,29,30]. This study found that FAR and NLR were related to tumor invasion of large blood vessels and distant metastasis, suggesting that FAR and NLR reflect the aggressive nature of tumor cells. This may be due to the increase in inflammatory markers, which induces the production of bioactive substances that promote angiogenesis and promote tumor metastasis. More importantly, our study showed that the OS of PC patients decreased with the increase of FAR, suggesting that FAR can serve as an independent predictor of prognosis in patients with locally advanced or metastatic PC. Some studies have reported the value of FAR in the prognosis evaluation. One study analyzed the survival data of 282 patients undergoing R0 resection for PC, and found that elevated pre-operative plasma FAR (> 0.08) was significantly associated with poor prognosis [26]. Fang et al. [20] discovered that FAR, NLR, and PLR, and other hematologic values were superior to NLR and PLR in assessing prognosis in patients with resectable, locally advanced or metastatic PC. These findings are in line with the results of this study and validate the significance of FAR in the prognosis evaluation of PC patients.
It has been reported that fibrinogen can be synthesized by hepatocytes or malignant tumor cells and released into the bloodstream, with the systemic inflammatory response further enhancing its release [19]. In addition to its crucial role in coagulation process, studies have revealed that in the tumor microenvironment, fibrinogen is involved in the tumor microenvironment by contributing to the formation of extracellular matrix and inducing epithelial-mesenchymal transition as well as the synthesis of inflammatory factors, thereby promoting tumor cell proliferation, metastasis, and angiogenesis [20,31-33]. In addition, fibrinogen can facilitate the bonding of platelets to tumor cells, protecting tumor cells from the attacks by natural killer cells [34]. Current evidence shows that hyperfibrinogenemia exists in patients with various malignant tumors, including PC, and is significantly associated with poor prognosis [35-37]. Additionally, inflammatory cytokines (such as interleukin-1 or 6, and interferon-γ) synthesized and released by tumor cells can disrupt normal albumin synthesis in the liver, resulting in hypoproteinemia [20]. Plasma albumin level not only reflects the nutritional state of the host but are also important factors influencing the prognosis of cancer patients. Therefore, FAR, as a comprehensive indicator of systemic inflammatory-immune response, may help to provide a more accurate prognostic stratification for PC patients.
Peripheral blood NLR is one of the most investigated indicators of systemic inflammatory response. Neutrophils can facilitate tumor proliferation and angiogenesis by secreting cytokines and vascular endothelial growth factor (VEGF), while lymphocytes, as key players in cell-mediated immune response, play a crucial role in tumor immune surveillance [11,38]. Previous studies have affirmed the association between NLR and the prognosis of PC patients [39,40]. In this study, the ROC curve analysis determined that the optimal cutoff value of NLR for predicting the death of patients with metastatic or locally advanced PC was 3.28. This cutoff was used as the threshold for evaluating the prognostic value of NLR before treatment in these patients, and it was found that NLR is a reliable indicator of prognosis.
Based on the significance of FAR and NLR in the prognosis evaluation of patients with locally advanced or metastatic PC, this study further explored the role of their combination in the prognosis evaluation. The results showed that FAR combined with NLR had a better predictive effect, with an AUC of 0.946 (95% CI: 0.905-0.986), and specificity and sensitivity of 92.30% and 92.40%, respectively. FAR combined with NLR could further classify the prognosis of these patients, suggesting that this combination could serve as a valuable tool for assessing prognosis in PC patients undergoing first-line chemotherapy.
Nevertheless, there are some limitations to this study: First, as a retrospective study with a relatively small sample size, there may have been bias. Second, the analysis of data from a single center cannot fully represent the overall population. Therefore, it is necessary to carry out multi-center, large-scale, prospective studies to further validate these conclusions.
Conclusion
FAR and NLR are both correlated with an unfavorable prognosis in patients with locally advanced or metastatic PC. Their combined detection might offer a more precise prognostic assessment for PC patients undergoing systemic chemotherapy.
Disclosure of conflict of interest
None.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 3.Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun (Lond) 2019;39:22. doi: 10.1186/s40880-019-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pedrazzoli S. Surgical treatment of pancreatic cancer: currently debated topics on morbidity, mortality, and lymphadenectomy. Surg Oncol. 2022;45:101858. doi: 10.1016/j.suronc.2022.101858. [DOI] [PubMed] [Google Scholar]
- 5.Dumitrascu T, Popescu I. Pancreatico-duodenectomy for pancreatic ductal adenocarcinoma: from artery-first approaches to TRIANGLE operation. Chirurgia (Bucur) 2022;117:377–384. doi: 10.21614/chirurgia.2771. [DOI] [PubMed] [Google Scholar]
- 6.Grossberg AJ, Chu LC, Deig CR, Fishman EK, Hwang WL, Maitra A, Marks DL, Mehta A, Nabavizadeh N, Simeone DM, Weekes CD, Thomas CR Jr. Multidisciplinary standards of care and recent progress in pancreatic ductal adenocarcinoma. CA Cancer J Clin. 2020;70:375–403. doi: 10.3322/caac.21626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang X, Ma Y, Wang T, Zhou H, Wang K, Shi W, Qin L, Guan J, Li L, Long B, Wang J, Guan X, Ye H, Yang J, Yu Z, Jiao Z. Targeting UBE2T potentiates gemcitabine efficacy in pancreatic cancer by regulating pyrimidine metabolism and replication stress. Gastroenterology. 2023;164:1232–1247. doi: 10.1053/j.gastro.2023.02.025. [DOI] [PubMed] [Google Scholar]
- 8.Kunzmann V, Siveke JT, Algul H, Goekkurt E, Siegler G, Martens U, Waldschmidt D, Pelzer U, Fuchs M, Kullmann F, Boeck S, Ettrich TJ, Held S, Keller R, Klein I, Germer CT, Stein H, Friess H, Bahra M, Jakobs R, Hartlapp I, Heinemann V German Pancreatic Cancer Working Group (AIO-PAK) and NEOLAP investigators. Nab-paclitaxel plus gemcitabine versus nab-paclitaxel plus gemcitabine followed by FOLFIRINOX induction chemotherapy in locally advanced pancreatic cancer (NEOLAP-AIO-PAK-0113): a multicentre, randomised, phase 2 trial. Lancet Gastroenterol Hepatol. 2021;6:128–138. doi: 10.1016/S2468-1253(20)30330-7. [DOI] [PubMed] [Google Scholar]
- 9.Zong Y, Yuan J, Peng Z, Lu M, Wang X, Shen L, Zhou J. Nab-paclitaxel plus S-1 versus nab-paclitaxel plus gemcitabine as first-line chemotherapy in patients with advanced pancreatic ductal adenocarcinoma: a randomized study. J Cancer Res Clin Oncol. 2021;147:1529–1536. doi: 10.1007/s00432-020-03442-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, Chiorean EG, Chung V, Czito B, Del Chiaro M, Dillhoff M, Donahue TR, Dotan E, Ferrone CR, Fountzilas C, Hardacre J, Hawkins WG, Klute K, Ko AH, Kunstman JW, LoConte N, Lowy AM, Moravek C, Nakakura EK, Narang AK, Obando J, Polanco PM, Reddy S, Reyngold M, Scaife C, Shen J, Vollmer C, Wolff RA, Wolpin BM, Lynn B, George GV. Pancreatic adenocarcinoma, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:439–457. doi: 10.6004/jnccn.2021.0017. [DOI] [PubMed] [Google Scholar]
- 11.Min L, Ziyu D, Xiaofei Z, Shunhe X, Bolin W. Analysis of levels and clinical value of CA19-9, NLR and SIRI in patients with pancreatic cancer with different clinical features. Cell Mol Biol (Noisy-le-grand) 2022;67:302–308. doi: 10.14715/cmb/2021.67.4.41. [DOI] [PubMed] [Google Scholar]
- 12.Huang H, Sun J, Jiang Z, Zhang X, Li Z, Zhu H, Yu X. Risk factors and prognostic index model for pancreatic cancer. Gland Surg. 2022;11:186–195. doi: 10.21037/gs-21-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin K, Jung EK, Park SJ, Jeong S, Kim IH, Lee MA. Neutrophil-to-lymphocyte ratio and carbohydrate antigen 19-9 as prognostic markers for advanced pancreatic cancer patients receiving first-line chemotherapy. World J Gastrointest Oncol. 2021;13:915–928. doi: 10.4251/wjgo.v13.i8.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neumann CCM, Schneider F, Hilfenhaus G, Vecchione L, Felsenstein M, Ihlow J, Geisel D, Sander S, Pratschke J, Stintzing S, Keilholz U, Pelzer U. Inflammation-based prognostic scores in pancreatic cancer patients-a single-center analysis of 1294 patients within the last decade. Cancers (Basel) 2023;15:2367. doi: 10.3390/cancers15082367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maloney S, Pavlakis N, Itchins M, Arena J, Mittal A, Hudson A, Colvin E, Sahni S, Diakos C, Chan D, Gill AJ, Samra J, Clarke SJ. The prognostic and predictive role of the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and lymphocyte-to-monocyte ratio (LMR) as biomarkers in resected pancreatic cancer. J Clin Med. 2023;12:1989. doi: 10.3390/jcm12051989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takano S, Yoshitomi H, Kagawa S, Furukawa K, Takayashiki T, Kuboki S, Suzuki D, Sakai N, Mishima T, Nakadai E, Miyazaki M, Ohtsuka M. Long-term outcomes and significance of preoperative lymphocyte-to-monocyte ratio as a prognostic indicator in patients with invasive pancreatic neoplasms after repeat pancreatectomy. BMC Cancer. 2020;20:111. doi: 10.1186/s12885-020-6602-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hajibandeh S, Hajibandeh S, Romman S, Parente A, Laing RW, Satyadas T, Subar D, Aroori S, Bhatt A, Durkin D, Athwal TS, Roberts KJ. Preoperative C-reactive protein-to-albumin ratio and its ability to predict outcomes of pancreatic cancer resection: a systematic review. Biomedicines. 2023;11:1983. doi: 10.3390/biomedicines11071983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campello E, Ilich A, Simioni P, Key NS. The relationship between pancreatic cancer and hypercoagulability: a comprehensive review on epidemiological and biological issues. Br J Cancer. 2019;121:359–371. doi: 10.1038/s41416-019-0510-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li C, Fan Z, Guo W, Liang F, Mao X, Wu J, Wang H, Xu J, Wu D, Liu H, Wang L, Li F. Fibrinogen-to-prealbumin ratio: a new prognostic marker of resectable pancreatic cancer. Front Oncol. 2023;13:1149942. doi: 10.3389/fonc.2023.1149942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang L, Yan FH, Liu C, Chen J, Wang D, Zhang CH, Lou CJ, Lian J, Yao Y, Wang BJ, Li RY, Han SL, Bai YB, Yang JN, Li ZW, Zhang YQ. Systemic inflammatory biomarkers, especially fibrinogen to albumin ratio, predict prognosis in patients with pancreatic cancer. Cancer Res Treat. 2021;53:131–139. doi: 10.4143/crt.2020.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arakawa Y, Miyazaki K, Yoshikawa M, Yamada S, Saito Y, Ikemoto T, Imura S, Morine Y, Shimada M. Value of the fibrinogen-platelet ratio in patients with resectable pancreatic cancer. J Med Invest. 2021;68:342–346. doi: 10.2152/jmi.68.342. [DOI] [PubMed] [Google Scholar]
- 22.Lin GT, Ma YB, Chen QY, Zhong Q, Zheng CH, Li P, Xie JW, Wang JB, Lin JX, Huang CM. Fibrinogen-albumin ratio as a new promising preoperative biochemical marker for predicting oncological outcomes in gastric cancer: a multi-institutional study. Ann Surg Oncol. 2021;28:7063–7073. doi: 10.1245/s10434-021-10027-9. [DOI] [PubMed] [Google Scholar]
- 23.Ying HQ, Sun F, Liao YC, Cai D, Yang Y, Cheng XX. The value of circulating fibrinogen-to-pre-albumin ratio in predicting survival and benefit from chemotherapy in colorectal cancer. Ther Adv Med Oncol. 2021;13:17588359211022886. doi: 10.1177/17588359211022886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Claps F, Rai S, Mir MC, van Rhijn BWG, Mazzon G, Davis LE, Valadon CL, Silvestri T, Rizzo M, Ankem M, Liguori G, Celia A, Trombetta C, Pavan N. Prognostic value of preoperative albumin-to-fibrinogen ratio (AFR) in patients with bladder cancer treated with radical cystectomy. Urol Oncol. 2021;39:835.e9–835.e17. doi: 10.1016/j.urolonc.2021.04.026. [DOI] [PubMed] [Google Scholar]
- 25.Yu H, Wang M, Wang Y, Yang J, Deng L, Bao W, He B, Lin Z, Chen Z, Chen K, Zhang B, Liu F, Yu Z, Ye L, Jin B, Chen G. The prognostic value of sarcopenia combined with preoperative fibrinogen-albumin ratio in patients with intrahepatic cholangiocarcinoma after surgery: a multicenter, prospective study. Cancer Med. 2021;10:4768–4780. doi: 10.1002/cam4.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang LP, Ren H, Du YX, Wang CF. Prognostic value of the preoperative fibrinogen-to-albumin ratio in pancreatic ductal adenocarcinoma patients undergoing R0 resection. World J Gastroenterol. 2020;26:7382–7404. doi: 10.3748/wjg.v26.i46.7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Satoi S, Fujii T, Yanagimoto H, Motoi F, Kurata M, Takahara N, Yamada S, Yamamoto T, Mizuma M, Honda G, Isayama H, Unno M, Kodera Y, Ishigami H, Kon M. Multicenter phase II study of intravenous and intraperitoneal paclitaxel with S-1 for pancreatic ductal adenocarcinoma patients with peritoneal metastasis. Ann Surg. 2017;265:397–401. doi: 10.1097/SLA.0000000000001705. [DOI] [PubMed] [Google Scholar]
- 28.Shen ZT, Zhou H, Li AM, Ji XQ, Jiang CC, Yuan X, Li B, Zhu XX, Huang GC. Clinical outcomes and prognostic factors of stereotactic body radiation therapy combined with gemcitabine plus capecitabine for locally advanced unresectable pancreatic cancer. J Cancer Res Clin Oncol. 2020;146:417–428. doi: 10.1007/s00432-019-03066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomita K, Ochiai S, Gunji T, Hikita K, Kobayashi T, Sano T, Chiba N, Kawachi S. Prognostic significance of plasma fibrinogen/serum albumin ratio in the postoperative outcome of pancreatic ductal adenocarcinoma. Anticancer Res. 2020;40:7017–7023. doi: 10.21873/anticanres.14727. [DOI] [PubMed] [Google Scholar]
- 30.Cao X, Cui J, Yu T, Li Z, Zhao G. Fibrinogen/albumin ratio index is an independent prognosis predictor of recurrence-free survival in patients after surgical resection of gastrointestinal stromal tumors. Front Oncol. 2020;10:1459. doi: 10.3389/fonc.2020.01459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang F, Wang Y, Sun P, Wang ZQ, Wang DS, Zhang DS, Wang FH, Fu JH, Xu RH, Li YH. Fibrinogen promotes malignant biological tumor behavior involving epithelial-mesenchymal transition via the p-AKT/p-mTOR pathway in esophageal squamous cell carcinoma. J Cancer Res Clin Oncol. 2017;143:2413–2424. doi: 10.1007/s00432-017-2493-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Igarashi Y, Shirai Y, Tanji Y, Hamura R, Yanagaki M, Abe K, Onda S, Furukawa K, Matsumoto M, Tsunematsu M, Ikegami T. The impact of fibrinogen to prognostic nutritional index rate on prognosis of pancreatic ductal adenocarcinoma. Am Surg. 2023;89:4255–4261. doi: 10.1177/00031348231204912. [DOI] [PubMed] [Google Scholar]
- 33.Yoneyama T, Ohtsuki S, Ono M, Ohmine K, Uchida Y, Yamada T, Tachikawa M, Terasaki T. Quantitative targeted absolute proteomics-based large-scale quantification of proline-hydroxylated alpha-fibrinogen in plasma for pancreatic cancer diagnosis. J Proteome Res. 2013;12:753–762. doi: 10.1021/pr3008144. [DOI] [PubMed] [Google Scholar]
- 34.Zheng S, Shen J, Jiao Y, Liu Y, Zhang C, Wei M, Hao S, Zeng X. Platelets and fibrinogen facilitate each other in protecting tumor cells from natural killer cytotoxicity. Cancer Sci. 2009;100:859–865. doi: 10.1111/j.1349-7006.2009.01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo Q, Zhang B, Dong X, Xie Q, Guo E, Huang H, Wu Y. Elevated levels of plasma fibrinogen in patients with pancreatic cancer: possible role of a distant metastasis predictor. Pancreas. 2009;38:e75–79. doi: 10.1097/MPA.0b013e3181987d86. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki T, Shimada H, Nanami T, Oshima Y, Yajima S, Washizawa N, Kaneko H. Prognostic significance of hyperfibrinogenemia in patients with esophageal squamous cell carcinoma. Int J Clin Oncol. 2017;22:461–468. doi: 10.1007/s10147-016-1087-5. [DOI] [PubMed] [Google Scholar]
- 37.Deng S, Fan Z, Xia H, Gong Y, Qian Y, Huang Q, Cheng H, Jin K, Xiao Z, Luo G, Yu X, Liu C. Fibrinogen/albumin ratio as a promising marker for predicting survival in pancreatic neuroendocrine neoplasms. Cancer Manag Res. 2021;13:107–115. doi: 10.2147/CMAR.S275173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 39.Iwai N, Okuda T, Sakagami J, Harada T, Ohara T, Taniguchi M, Sakai H, Oka K, Hara T, Tsuji T, Komaki T, Kagawa K, Yasuda H, Naito Y, Itoh Y. Neutrophil to lymphocyte ratio predicts prognosis in unresectable pancreatic cancer. Sci Rep. 2020;10:18758. doi: 10.1038/s41598-020-75745-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kubo H, Murakami T, Matsuyama R, Yabushita Y, Tsuchiya N, Sawada Y, Homma Y, Kumamoto T, Endo I. Prognostic impact of the neutrophil-to-lymphocyte ratio in borderline resectable pancreatic ductal adenocarcinoma treated with neoadjuvant chemoradiotherapy followed by surgical resection. World J Surg. 2019;43:3153–3160. doi: 10.1007/s00268-019-05159-9. [DOI] [PubMed] [Google Scholar]