Abstract
Introduction: Hepatolithiasis (HL) is a complex liver and biliary disorder characterized by high rates of recurrence. This study aimed to evaluate the efficacy of Twelve Shugan Lidan Granules (TSLG), a compound herbal traditional Chinese formulation, in the treatment of HL, as well as to investigate its underlying mechanism. Methods: A retrospective analysis was conducted involving 157 patients diagnosed with HL, who were divided into two groups: the control group and the research group. In the control group, no treatment was given postoperatively, while in the research group, TSLG was orally administered three times a day postoperatively for two months. Both groups were followed up by telephone at 1 month, 2 months, and 3 months postoperatively. Liver function indicators were measured before and after surgery, and miRNA expression profiling was analyzed using high-throughput sequencing (HTS). Additionally, the expression levels of related proteins were assessed through western blots. Results: Postoperative liver function indicators were significantly lower in the research group compared to the control group (P < 0.05). Additionally, 64 miRNAs were differentially expressed in HL patients. Further analysis of 64 miRNAs revealed their abnormal targeting of the Hippo signaling pathway. Further experimental results indicate that TAZ protein expression is elevated in HL patients, reflecting abnormal activation of the Hippo signaling pathway in these patients. TSLG treatment significantly reduced the expression of YAP, TAZ, and SREBP-2 proteins, while increasing the expression of p-YAP and p-TAZ proteins (all P < 0.05). Furthermore, TSLG inhibited the Extracellular Acidification Rate (ECAR) in LPS-induced WRL68 cells. Conclusion: TSLG effectively improved postoperative liver function by downregulating sterol regulatory element-binding protein-2 (SREBP-2) and inhibiting the Hippo signaling pathway.
Keywords: Hepatolithiasis, Twelve Shugan Lidan Granules, Hippo signaling pathway, cholesterol metabolism, miRNAs
Introduction
Hepatolithiasis (HL), also known as intrahepatic calculi, is a condition characterized by the formation of stones within the intrahepatic bile ducts, which are located beyond the bifurcation of the right and left hepatic ducts [1-5]. This disease is more prevalent in Southeast and East Asian countries but remains relatively uncommon in western nations [2,3,5,6]. However, with increasing immigration from Asia, hepatolithiasis has emerged as a clinical challenge in western countries [2,3,5,6]. The incidence of hepatolithiasis varies significantly across China, with higher occurrence rates primarily observed in North China, Southwest China, South China, and the Yangtze River Basin region [3,7,8].
Most biliary stones associated with hepatolithiasis are soft and prone to fragmentation, commonly referred to as brown pigment stones or calcium bilirubinate stones. These stones consist of a complex mixture of cholesterol and cholesterol-based components, indicating a complicated pathogenesis that involves both calcium bilirubinate deposition and cholesterol solubility [9]. Several key factors contributing to pathogenic changes in the hepatobiliary system that facilitate stone formation have been identified. These factors include disorders of bile metabolism, infections, bile duct injury, and cholestasis [1-3,10,11]. However, the precise pathogenesis of hepatolithiasis remains incompletely understood.
Hepatolithiasis presents a significant challenge for patients due to its high recurrence rate and the necessity for repeated surgical intervention, including hepatectomy, endoscopic lithotomy, and lithotripsy [4-6,12-16]. This highlights the urgent need for effective treatment options, since failure to address the condition promptly and appropriately may result in serious complications, including intrahepatic cholangiocarcinoma [17,18]. The optimal treatment strategy for hepatolithiasis depends on individual patient characteristics, and various therapies have been developed in recent years, including pharmacologic treatments, endoscopic lithotomy, and other surgical interventions [12-16,19].
Traditional Chinese medicine has also been utilized in the treatment of hepatobiliary diseases, including hepatolithiasis. The in-hospital preparation from our institution, Twelve Shugan Lidan Granules (TSLG), is a traditional Chinese herbal medicine used to enhance hepatobiliary and enteric function. It is employed as an empirical prescription for the clinical treatment of postoperative recurrence of intrahepatic bile duct stones at our hospital [18-21]. TSLG comprises several key ingredients: Bupleurum, Curcuma aromatica, Fructus aurantii immaturus, Mag-nolia officinalis, raw rhubarb, Scutellaria baicalensis, red peony root, Herba Lysimachiae, plantain, Folium Pyrrosiae, Salvia miltiorrhiza, and white peony root [22]. Among these, Radix bupleuri has been reported to promote hepatic function [23-25], which is able to prevent stone formation. Additionally, Turmeric supplementation can also improve liver function both in patients and animals [26-28]. Moreover, Radix paeoniae is found to protect against hepatic damage [29-31]. Several ingredients of TSLG have also shown hepatoprotective effects when extracted from their respective components.
For instance, dihydrotanshinone I, a natural monomeric compound isolated from Salvia miltiorrhiza, has been shown to improve liver function and mitigate liver fibrosis [32]. The results of the mass spectrometry analysis for each component are presented in Annex 1. These liver protective effects may provide a foundation for the therapeutic efficacy of TSLG in in the treatment of hepatolithiasis. Therefore, in this study, we investigated the clinical efficacy of Twelve Shugan Lidan Granules (TSLG) in treating hepatolithiasis and its underlying mechanism.
Methods
Clinical efficacy of Twelve Shugan Lidan Granules
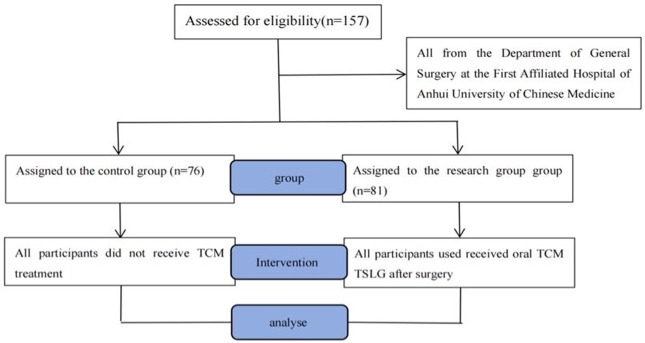
Data sources: Retrospective analysis of patients admitted to the Department of General Surgery of Anhui Hospital of Traditional Chinese Medicine from January 2021 to December 2022, Sample size estimation was based on previous observations of clinical efficacy by our team. The study design is illustrated in Figure 1. Ethical approval number for this study: 2022AH-41.
Figure 1.
Flow chart for patient assignment.
Inclusion criteria: Diagnosis of hepatolithiasis by color ultrasound, CT, or MRCP followed by surgical treatment. Post-treatment confirmation of no residual stones by CT, MRCP, cholangiography or other imaging examinations.
Exclusion criteria: Patients with lower common bile duct stenosis and sphincter of the duodenal papilla insufficiency. Patients with concurrent hepatobiliary malignancy. Patients with other organ dysfunctions that make them unable to tolerate surgery.
Details of study interventions
Both groups underwent surgery performed by the same team of surgeons. Laparoscopic common bile duct exploration with T-tube drainage was conducted as follows: a pneumoperitoneum was established using the four-port method, allowing visualization of the common bile duct, cystic duct, and left and right hepatic ducts. Bile was extracted from the common bile duct puncture site, followed by vertical dissection of the common bile duct. A choledochoscope was then inserted to locate the stones, which were subsequently removed using a net basket. After confirming that no residual stones remained, a T-tube was placed, and absorbable sutures were used to close the anterior wall of the common bile duct. Postoperative treatment included antibiotics, nutritional support, liver protection, and other supportive measures. After surgery, patients in the research group received TSLG orally. On the second day post-surgery, patients began taking Twelve Shugan Lidan Granules (Anhui Medicine Z20080011, prepared by the Preparation Center of the First Affiliated Hospital of Anhui University of Traditional Chinese Medicine). Each dose consisted of one bag (6 g) dissolved in 100 ml of warm water, administered orally three times a day. The duration of treatment was determined by subsequent ultrasound and MRCP examinations for stones, continuing until the stones were cleared and liver function returned to normal levels.
Observation indicators and methods
The diameters and numbers of stones of patients in the two groups were collected. Stone properties: FTIR spectroscopy (Bruker ALPHA II) was used to analyze the stone composition and record the data. Liver function indicators were assessed by extracting 5 ml of venous blood at 7:00 a.m. on the 1st, 3rd, and 7th days post-surgery. The blood samples were processed using an automatic biochemical analyzer (Hitachi 7600-020) and analyzed using the circulating enzyme rate method. The levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TB), and albumin (ALB) were measured and recorded.
Tissue samples
Hepatic biopsies were obtained from five patients diagnosed with hepatolithiasis and five patients with hepatic hemangioma (HH) from the Department of General Surgery at the First Affiliated Hospital of Anhui University of Chinese Medicine, with informed consent from all participants. The liver biopsies from the hepatolithiasis patients were designated as the HL group, while those from the HH patients were designated as the normal group (NOR), as their hepatobiliary function is nearly normal and they do not exhibit stone formation.
RNA extraction and sequencing
Total RNA was isolated from each hepatic sample using TRIzol (Thermo Fisher Scientific, USA) and the RNeasy Mini Kit (Qiagen, Germany). The quantity and quality of the RNA were assessed using a Qubit 4.0 Fluorometer (Thermo Fisher Scientific, USA), while RNA integrity was evaluated through agarose gel electrophoresis.
The miRNA library was prepared using the Illumina TruSeq Stranded Total RNA Sample Preparation Kits (Illumina, San Diego, CA) with approximately 1 μg of total RNA. Subsequent quantification was performed using a Qubit 4.0 Fluorometer (Thermo Fisher Scientific, USA). Additionally, reverse transcription-polymerase chain reaction (RT-PCR) was conducted to create clusters, and the target band of 145-160 bp was recovered by PAGE electrophoresis. The clusters were then sequenced on the Illumina HiSeq (Illumina, USA). All sequencing was performed by Genergy Biotechnology Inc. (Shanghai, China).
Prediction of conserved and novel miRNAs
The Fastx-Toolkit software (Version 0.0.14, http://hannonlab.cshl.edu/fastx_toolkit/) was employed to remove splice sequences and low-quality fragments from the 3’ end of the sequencing data. Sequences containing 14-40 nucleotides were selected for downstream analysis. Bowtie software (Version 1.2.2) was used to map the miRNAs against the reference genome, Rfam sequence database, RepBase sequence database, and miRBase sequence database. Novel miRNAs were predicted using miRDeep 2 software (Version 2.0.0.5), while conserved miRNAs were identified by searching miRBase (http://www.mirbase.org/).
Differential expression analysis of miRNAs
The differential expression of miRNAs between the HL and NOR groups was analyzed using DESeq2 software (Version 1.22.1), with counts per million (CPM) serving as the measurement index. A difference between the two groups was considered significant when the p-value was ≤ 0.05 and the log2 (fold change) was ≥ 1.
Target gene prediction and functional analysis
The target genes of the differentially expressed miRNAs were predicted using miRanda software (Version 3.3a). Gene Ontology (GO) analysis was performed for the functional annotation and classification of the target genes using TopGO software (http://www.bioconductor.org/packages/release/bioc/html/topGO.html). 54Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis was conducted to analyze the involved pathways. The significance of GO terms and pathways was determined using Fisher’s exact test, with the P-value corrected by the Benjamini-Hochberg method to calculate the false discovery rate (FDR). Only GO terms and pathways with a corrected P-value < 0.05 were selected for further analysis.
Western blot
We prepared liver tissue or cells for western blots. NP40 and 1% PMSF (American Abcam Company) were used as protease inhibitors for the extraction of total protein samples, followed by a facility fee of 15,000 centrifugation for 10 minutes. 20 μg protein samples were separated by 5% concentrated gel and 10% separated gel in the electrophoresis apparatus (Bio Rad Company), and then transferred to 0.45 μm NC membrane using the membrane converter (Bio Rad Company). The membrane was blocked in TBS/Tween-20 containing 5% skimmed milk powder for 1 hour, after which the primary antibody was incubated overnight at 4°C. Following the incubation with the secondary antibody, visual detection was performed. The antibodies used for immunoblots included GAPDH (ab263962), SREBP-2 (ab30682), phospho-YAP (ab76252), and TAZ (ab119373) from Abcam, as well as YAP (No. 14074) and phospho-TAZ (No. 75275) from Cell Signaling Technology. The expression of GAPDH was used as the reference band.
Cell culture
LX-2 human hepatic stellate cells, MHCC97-H human metastatic HCC cells, and WRL68 human hepatic cells (abbreviated as LX-2, 97H, and WRL68, respectively) were purchased from the Shanghai Institute of Cell Science, China. These cell lines were cultured in flasks containing Dulbecco’s Modified Eagle’s Minimal Essential Medium (DMEM), supplemented with 10% (v/v) fetal bovine serum, 100 IU/mL penicillin, and 100 mg/mL streptomycin (purchased from Israel Biological Industries Company and Gibco Corporation, USA). These cells were cultured in a 5% carbon dioxide incubator maintained at 37°C (Abcam) to simulate physiologic conditions.
Selection of drug concentration
To investigate whether the efficacy of TSLG in treating HL is based on the regulation of the abnormally activated Hippo signaling pathway, we conducted cell line studies. Based on prior research conducted by our group, TSLG was dissolved in DMEM at a concentration of 100 mg/mL, creating concentration gradients of 0, 1.25, 2, 2.5, and 5 mg/mL. LX-2, WRL68, and MHCC97-H cells were treated with TSLG. After 48 hours, CCK-8 reagent (purchased from Gibco, USA) was added to the cultured cells, and the reading at 450OD was detected using an enzyme-linked immunosorbent assay (ELISA) reader. The cell proliferation was determined based on the reading results to determine the optimal drug concentration.
Drug intervention and western blot
TGFβ-141 (5 ng/mL) (purchased from R&D Systems, USA) was added to LX-2 cells to activate the Hippo signaling pathway, resulting in the upregulation of YAP and TAZ and the downregulation of p-YAP and p-TAZ. Subsequently, traditional Chinese medicine TSLG was added for intervention. For MHCC97-H and WRL68 cells, the drug intervention was performed directly. Forty-eight hours later, cell lysates were collected, and protein expression was analyzed by western blot.
Seahorse XF analysis
To investigate the impact of TSLG intervention on glucose metabolism in WRL68 cells, the extracellular acidification rate (ECAR) was measured using Seahorse XF glycolysis stress tests. TSLG was dissolved in DMEM at a concentration of 100 mg/mL, and an optimal concentration was selected. WRL68 cells were induced with LPS42 (100 ug/mL) (Purchased from R&D Systems, USA) for 6 hours, followed by treatment with TSLG. Glucose metabolism was evaluated after 24 hours. GraphPad Prism 9.0 software was utilized to measure ECAR as an assessment of key measures of glycolytic flux.
Statistical methods
Statistical analysis was performed using SPSS 21.0 software. Measured data were expressed as mean ± standard deviation (SD). An independent samples t-test was used for comparisons between two independent samples, while repeated measured data were analyzed using repeated measures analysis of variance. Counted data were presented as counts or percentages, and the χ2 test was used for comparisons. A p-value of less than 0.05 was considered significant.
Results
Clinical efficacy of Twelve Shugan Lidan Granules in treating hepatolithiasis
As shown in Table 1, there were no significant differences in the general conditions of the two groups. However, after surgery, significant differences were observed in the levels of ALT (F = 10.35, P = 0.002), AST (F = 15.71, P = 0.001), TB (F = 27.62, P = 0.001), and TBA (F = 4.72, P = 0.031) between the two groups. Notably, ALT and TBA levels increased on the third day after surgery and decreased by the seventh day. In contrast, AST and TB levels declined over time following surgery. There were no significant differences in ALT, AST, TB, and TBA levels between the two groups one day before surgery (all P > 0.05). On the third and seventh days postoperatively, ALT, AST, TB, and TBA levels in the control group were significantly higher than those in the TSLG group (all P < 0.05). Additionally, there were no significant differences in preoperative and postoperative ALB levels between the two groups (all P > 0.05), as shown in Table 2. These results indicate that liver function in the TSLG group was significantly improved compared to the control group, suggesting a favorable therapeutic effect. This improvement may be attributed to a deceleration of bilification, which may help prevent stone recurrence.
Table 1.
Comparison of general conditions between two groups
| Project | Control group (n = 76) | TLSG group (n = 81) | Statistics | P value |
|---|---|---|---|---|
| Gender [n (%)] | ||||
| Male | 37 (48.68%) | 41 (50.62%) | ||
| Female | 39 (51.32%) | 40 (49.38%) | 0.058 | 0.80 |
| Age (years) | 60.85±15.91 | 59.55±16.26 | 0.50 | 0.61 |
| BMI | 24.59±5.91 | 23.90±5.88 | 0.72 | 0.46 |
| Basic disease [n (%)] | ||||
| Viral hepatitis | 9 (11.84%) | 11 (13.58%) | 0.10 | 0.74 |
| Fatty liver | 15 (19.74%) | 20 (24.69%) | 3.84 | 0.45 |
| The number of gallstones [n (%)] | ||||
| 1 | 10 (13.16%) | 15 (18.52%) | ||
| ≥ 2 | 66 (86.84%) | 66 (81.48%) | 0.84 | 0.35 |
| The gallstone diameter | ||||
| ≥ 10 mm | 36 (47.37%) | 42 (51.85%) | ||
| < 10 mm | 40 (52.63%) | 39 (48.15%) | 0.31 | 0.57 |
| Properties of gallstone [n (%)] | ||||
| Pigmental stones | 48 (63.16%) | 55 (67.90%) | ||
| Mixed stones | 28 (36.84%) | 26 (32.10%) | 0.40 | 0.52 |
Table 2.
Comparison of changes in liver function between the two groups
| Groups | Control group (n = 76) | TSLG group (n = 81) | PN |
|---|---|---|---|
| ALT | |||
| Preoperative 1 day | 88.93±17.94 | 89.59±17.20 | 0.815 |
| 3 days after operation | 135.68±68.02Δ | 103.15±57.40Δ | ≤ 0.001 |
| 7 days after operation | 52.96±22.17Δ | 44.24±22.22Δ | ≤ 0.001 |
| FM | PM | 10.35 | 0.002 |
| AST | |||
| Preoperative 1 day | 99.18±67.78 | 95.61±56.93 | 0.800 |
| 3 days after operation | 68.65±28.87Δ | 52.46±27.71Δ | ≤ 0.001 |
| 7 days after operation | 38.98±17.46Δ | 35.63±17.01Δ | ≤ 0.001 |
| FM | PM | 15.71 | ≤ 0.001 |
| TB | |||
| Preoperative 1 day | 32.73±13.20 | 30.10±14.12 | 0.230 |
| 3 days after operation | 40.86±14.42Δ | 27.80±10.84Δ | ≤ 0.001 |
| 7 days after operation | 24.59±10.55Δ | 18.75±7.65Δ | ≤ 0.001 |
| FM | PM | 27.62 | ≤ 0.001 |
| ALB | |||
| Preoperative 1 day | 36.40±5.32 | 36.70±5.13 | 0.537 |
| 3 days after operation | 35.36±4.42 | 36.40±5.32 | 0.186 |
| 7 days after operation | 37.95±4.93 | 39.35±4.01 | 0.051 |
| FM | PM | 3.50 | 0.063 |
| TBA | |||
| Preoperative 1 day | 20.54±11.38 | 20.98±9.28 | 0.791 |
| 3 days after operation | 33.18±20.76 | 27.90±13.01 | 0.056 |
| 7 days after operation | 16.23±5.90 | 11.82±6.55 | ≤ 0.001 |
| FM | PM | 4.72 | 0.031 |
Note: 1. PN indicates the difference between the research group and the control group at each time point when the treatment time of in vivo effect in the group × the group;
2. FM , PM refer to the difference between the research group and the control group at each time point when the treatment time of in vivo effect between the group × the group;
3. Compared with preoperative 1 d;
P < 0.05;
4. ALT, alanine aminotransferase; AST, aspartate aminotransferase; TB, total bilirubin; ALB, albumin; TBA, total bile acids.
Differentially expressed miRNAs and abnormal activation of Hippo signaling pathway in HL patients
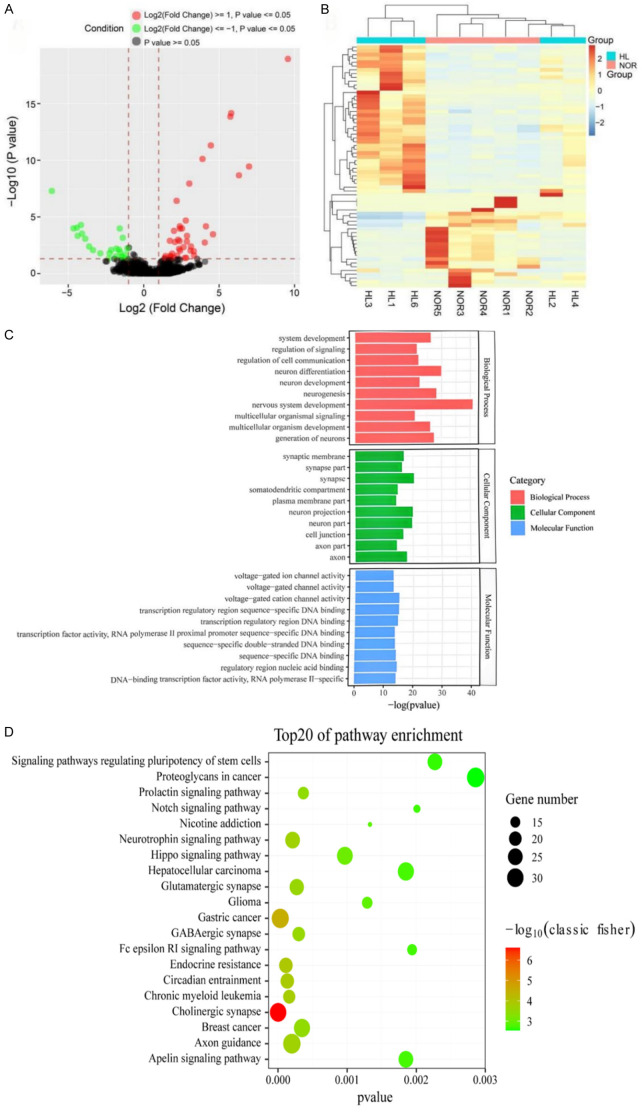
We compared the miRNA expression profiles between the HL group (hepatolithiasis) and the NOR group (hepatic hemangioma). As shown in Figure 2A, the volcano plot displays all detected miRNAs, while Figure 2B presents the heatmap of cluster analysis, illustrating significant differences in miRNA expression patterns between the two groups. A total of 64 miRNAs (10 novel miRNAs and 54 conserved miRNAs) were identified as differentially expressed. Among these, 40 miRNAs were up-regulated and 24 miRNAs were down-regulated. The detailed results of the top 20 up-regulated and down-regulated miRNAs are shown in Table 3 (see Annex 2 for details). To explore possible mechanisms of HL based on these differentially expressed miRNAs, we conducted GO and KEGG analyses on the target genes to evaluate possible signaling pathways and related functions. A total of 1,458 GO terms underwent enrichment and were categorized into three main GO categories: biological processes (BP), cellular components (CC), and molecular functions (MF). As illustrated in Figure 2C, the top ten GO terms in BP, CC, and MF were highlighted. Notably, the biological processes primarily involved were nervous system development (GO: 0007399), neuron differentiation (GO: 0030182), and neurogenesis (GO: 0022008). In the cellular component category, synapse (GO: 0045202), neuron projection (GO: 0043005), and neuron part (GO: 0097458) were the most prominent.
Figure 2.
The miRNAs expression profiling of the two groups. A. Volcano of all detected miRNAs. B. Hierarchical clustering of differentially expressed miRNAs. HL, hepatolithiasis patient group; NOR, normal group. C. GO classification of differential expression genes in the liver tissue of HLD and NOR. D. Top 20 signaling pathways predicted to be regulated by differentially expressed genes. GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; BP, biological processes; CC, cellular components; MF, molecular functions.
Table 3.
Top 20 differentially expressed miRNAs between the HL group and the NOR group
| miRNA | Log2FC | Type of regulation | miRNA | Log2FC | Type of regulation |
|---|---|---|---|---|---|
| hsa-miR-100-5p | 2.797269941 | Up | hsa-miR-106a-3p | -1.432959407 | Down |
| hsa-miR-10b-5p | 2.875506356 | Up | hsa-miR-122-5p | -1.585796479 | Down |
| hsa-miR-125b-1-3p | 4.055534677 | Up | hsa-miR-1307-5p | -2.309855263 | Down |
| hsa-miR-132-3p | 2.652076697 | Up | hsa-miR-378a-5p | -1.557662644 | Down |
| hsa-miR-135b-5p | 4.599912842 | Up | hsa-miR-4488 | -3.609415544 | Down |
| hsa-miR-141-3p | 5.807977068 | Up | hsa-miR-4497 | -4.156749613 | Down |
| hsa-miR-155-5p | 3.906377546 | Up | hsa-miR-4508 | -3.355808988 | Down |
| hsa-miR-181a-5p | 2.8670737 | Up | hsa-miR-4516 | -3.86941589 | Down |
| hsa-miR-181b-5p | 3.145548071 | Up | hsa-miR-4686 | -2.155278225 | Down |
| hsa-miR-199b-5p | 4.457554438 | Up | hsa-miR-4707-5p | -1.432959407 | Down |
| hsa-miR-200c-3p | 5.751097681 | Up | hsa-miR-483-5p | -1.634350528 | Down |
| hsa-miR-20b-5p | 2.725650281 | Up | hsa-miR-7641 | -4.373458396 | Down |
| hsa-miR-223-3p | 3.10820275 | Up | hsa-miR-7704 | -2.872125177 | Down |
| hsa-miR-23a-3p | 2.827084392 | Up | hsa-miR-885-3p | -1.846194664 | Down |
| hsa-miR-342-3p | 3.029913409 | Up | Nov_10_17372 | -6.081664997 | Down |
| hsa-miR-451a | 3.029913409 | Up | Nov_10_17632 | -1.807354922 | Down |
| hsa-miR-708-5p | 6.32089723 | Up | Nov_10_17639 | -4.422064766 | Down |
| Nov_1_300 | 9.570045424 | Up | Nov_3_6560 | -4.077242999 | Down |
| Nov_6_11375 | 6.991303801 | Up | Nov_8_15029 | -4.654310547 | Down |
| Nov_7_12460 | 3.346802764 | Up | Nov_9_15656 | -2.263034406 | Down |
Notably, KEGG analysis indicated that the target genes were significantly enriched in several signaling pathways possibly related to the occurrence of hepatolithiasis (HL). As shown in Figure 2D, the top 20 involved signaling pathways include the Cholinergic synapse pathway (hsa04725), Hippo signaling pathway (hsa04390), Gastric cancer pathway (hsa05226), Axon guidance pathway (hsa04360), Signaling pathways regulating pluripotency of stem cells (hsa04550), and Hepatocellular carcinoma pathway (hsa05225). In particular, Hippo signaling pathway has been reported to be related to fibrosis formation [37,38], which is also well recognized as playing a critical role in controlling organ size by regulating cell proliferation, apoptosis, and the self-renewal of stem cells [39,40].
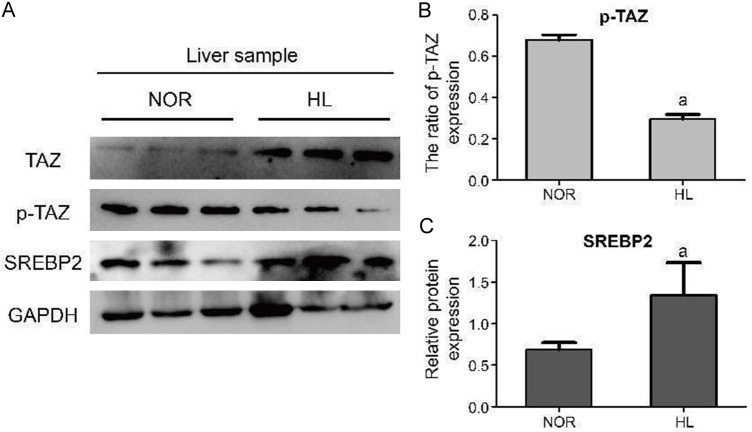
GO and KEGG analyses revealed the abnormal activation of the Hippo signaling pathway in hepatolithiasis (HL) patients, which may contribute to the pathogenesis of intrahepatic bile duct calculi formation. As shown in Figure 3A, we conducted western blot analysis of liver samples from the HL and NOR groups. Figure 3B and 3C demonstrate that the expression of p-TAZ is significantly down-regulated in the HL group compared to the NOR group, while the expression of SREBP2 is markedly up-regulated in the HL group. The elevated levels of SREBP2 in the HL group suggest that the Hippo signaling pathway is indeed abnormally activated, aligning with our omics analysis.
Figure 3.
WB analysis between the HL group and the NOR group. A. WB results of the liver sample between the HL group and the NOR group. B. The ratio of p-TAZ expression in total TAZ expression (p-TAZ + TAZ). C. Relative protein expression of SREBP2. aP < 0.01, compared with the NOR group. HL group, hepatolithiasis patient group; NOR, normal group; SREBP2, sterol regulatory element-binding protein-2.
Down-regulation of SREBP2 and suppression of Hippo signaling pathway intervened by TSLG
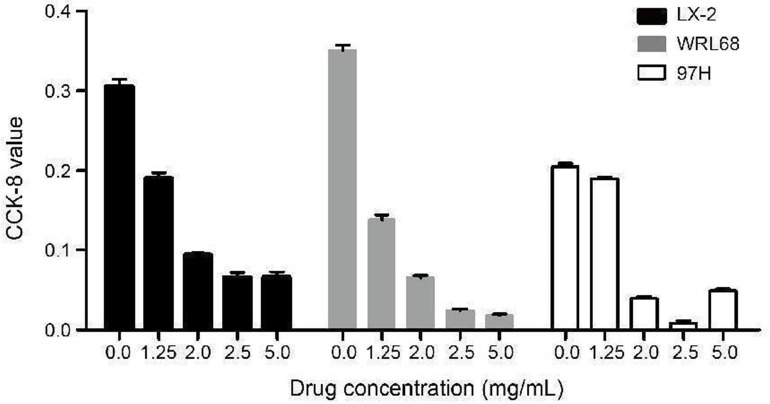
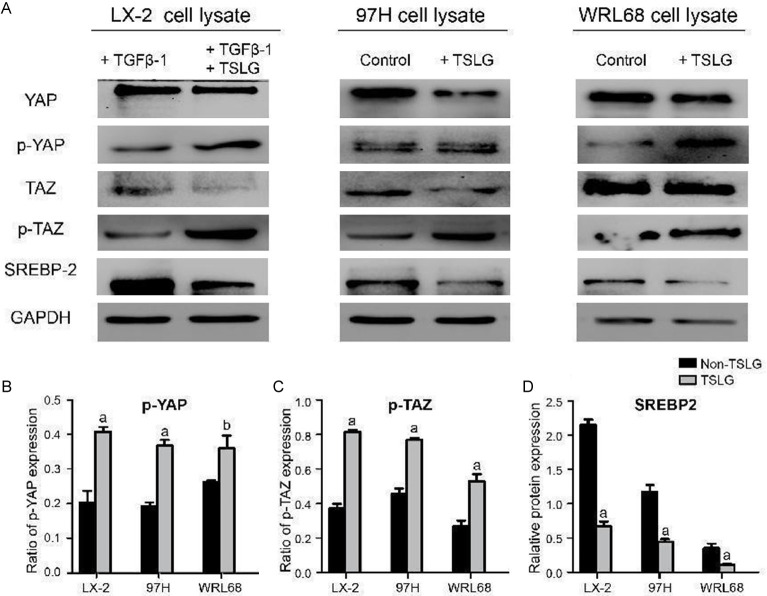
After intervention with TSLG at various concentrations, the CCK-8 values were measured 48 hours later. As shown in Figure 4, the optimal drug concentration for cell line studies in LX-2, MHCC97-H, and WRL68 cells was determined to be 1.25 mg/mL. The results from the cell line studies, illustrated in Figure 5, show the protein expression levels of YAP, p-YAP, TAZ, p-TAZ, SREBP2, and GAPDH following the treatment with TGFβ-1 and TSLG in different cell lines. The findings indicated that TSLG treatment led to a down-regulation of YAP and TAZ proteins, an up-regulation of p-YAP and p-TAZ proteins, and a down-regulation of SREBP2 in LX-2, MHCC97-H, and WRL68 cells. This suggests that TSLG may suppress the Hippo signaling pathway and inhibit the expression of SREBP2. Therefore, we speculate that TSLG may prevent the formation of intrahepatic bile duct calculi by inhibiting the Hippo signaling pathway.
Figure 4.
Intervention using TSLG at different drug concentration in cells.
Figure 5.
Cell line studies to detect different protein expressions after the intervention of TGFβ-1 and TSLG in LX-2, MHCC97-H and WRL68 cells. A. The WB results for different cell lines. B. The ratio of p-YAP expression in total YAP expression (p-YAP + YAP). C. The ratio of p-TAZ expression in total TAZ expression (p-TAZ + TAZ). D. Relative protein expression of SREBP2. aP < 0.01, compared with the non-TSLG group. bP < 0.05, compared with the non-TSLG group. SREBP2, sterol regulatory element-binding protein-2.
LPS induced glycolysis and glycolysis capacity are inhibited after TSLG intervention
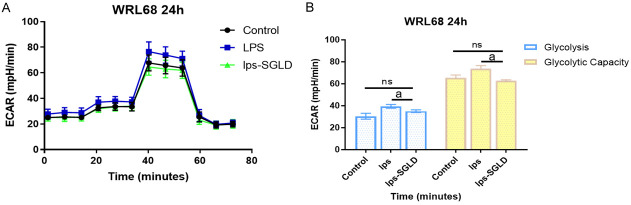
Seahorse XF technology was used to detect glycolysis and glycolytic capacity in WRL68 cells after LPS induction and TSLG intervention. The cells were incubated in a glycolysis stress test solution without glucose and pyruvate, and the extracellular acidification rate (ECAR) was measured to assess the relative glycolytic function of live cells following LPS and TSLG treatment. Key measures for a comprehensive evaluation of the glycolytic pathway were obtained, including glycolysis and glycolytic capacity. The experimental results indicated that LPS induction significantly increased glycolysis and glycolytic capacity in WRL68 cells. However, following TSLG intervention, both glycolysis and glycolytic capacity were significantly reduced compared to the LPS induction group, though they did not return to baseline levels. These results are shown in Figure 6.
Figure 6.
Effect of TSLG on glycolysis after LPS induction of WRL68. A. SeahorseXF measures the ECAR values of cells from three different groups at various time points. B. Quantification of ECAR values of cells from three groups under emergency conditions. aP < 0.01 compared with the LPS-induced group. P > 0.05, compared with the Control group. ECAR, Extracellular Acidification Rate; ns, not significant.
Discussion
The precise pathogenesis of hepatic bile duct stones remains elusive and may involve various factors, including cholestasis, bile duct stenosis, infections, disorders of bile metabolism, and genetic mutations. The obstruction of small bile ducts caused by these stones can lead to bile duct inflammation, stenosis, and liver fibrosis, which may result in liver atrophy or cirrhosis [33,34]. These complications can lead to severe local biliary issues and are a common cause of mortality associated with benign biliary diseases [35,36]. The therapeutic principles for managing hepatic bile duct stones include stone removal, alleviating stenosis, ensuring smooth drainage, and preventing recurrence. Research indicates that intrahepatic bile duct exploration and lithotomy, when combined with liver resection, represent a safe and effective approach for treating complex bilateral primary hepatobiliary stones, resulting in satisfactory outcomes [37]. However, there remains a high postoperative recurrence rate of stones and a significant rate of residual stones, influenced by factors such as bacterial infection and cholestasis [38]. The primary goal of clinical practice is to implement effective preventive measures to reduce the recurrence of stones and enhance patient outcome.
In recent years, the combination of double mirror techniques and the hospital-prepared Twelve Shugan Lidan Granules (TSLG) has shown significant efficacy in the treatment of intrahepatic bile duct stones. Modern pharmacological studies have demonstrated that Bupleurum can enhance bile secretion [36], and accelerate bile excretion, significantly improving the dissolution capacity of cholesterol in bile, thereby reducing stone formation. Curcuma aromatica is known for its ability to promote qi and alleviate depression, benefit bile, and reduce jaundice [39]. Modern research has demonstrated that Curcuma aromatica offers protective effects on the liver, lowers blood lipids, and possesses antibacterial and anti-inflammatory properties. The active component of Scutellaria baicalensis, baicalin, has been found to effectively inhibit inflammatory responses and protect the liver by preventing carbon tetrachloride-induced damage [40].
Raw rhubarb attack accumulation, wet retreat yellow, has the effect of liver and gallbladder [41]. Studies have shown that Raw rhubarb can reduce the serum level of glutamate gamma transaminase, reduce hepatocyte swelling, degeneration and necrosis, and promote liver synthesis capacity and hepatocyte regeneration. Herba Lysimachiae is known for its ability to alleviate dampness and reduce jaundice by enhancing bile secretion while simultaneously decreasing total bile acid, free bile acid, and total cholesterol levels in the blood [42]. The combined effects of this prescription can enhance microcirculation in the hepatic bile duct, protect liver enzymes, improve bile flow, and contribute to maintaining homeostasis within the body. This approach minimizes the surgical effect to patients’ bodies, improves surgical outcomes, and enhances overall quality of life. Indeed, the results indicate that levels of ALT, AST, and total bilirubin (TB) were significantly reduced in the research group (hepatolithiasis patients administered TSLG postoperatively) compared to the control group (patients without any traditional Chinese medicine treatment). This provides strong experimental evidence for the hepatoprotective effects of TSLG.
To gain a deeper understanding of the pathogenesis of hepatolithiasis (HL) and the efficacy of TSLG, we conducted high-throughput sequencing (HTS) to analyze miRNA expressions in five HL patients and five hepatic hemangioma (HH) patients, with the latter serving as a control group due to their nearly normal liver function (without HL or cirrhosis). The HTS data revealed that 64 miRNAs were differentially expressed in HL patients (P < 0.05, fold change ≥ 1), comprising 40 up-regulated and 24 down-regulated miRNAs. Additionally, we performed Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) functional enrichment analyses, which highlighted the abnormal activation of the Hippo signaling pathway in the HL group. This finding was further validated by western blot (WB) testing. The activation of the Hippo signaling pathway in HL patients suggests a link to stone formation. This pathway is a crucial regulator of cell proliferation and apoptosis, with its central components including the tumor suppressor kinases MST1, MST2, LATS1, and LATS2, along with the adaptor proteins SAV1 and MOB1/2 [43-45]. These conserved kinase cassettes inhibit tissue growth (organ size) and progenitor cell proliferation by phosphorylating and inactivating the transcriptional coactivators YAP and TAZ. Notably, LATS2 can bind to the endoplasmic reticulum (ER)-tethered precursors of SREBP1 and SREBP2 (P-SREBP), thereby limiting their transcriptional and biologic activities. The SREBPs (sterol regulatory element-binding proteins), particularly SREBP1 and SREBP2, play a crucial role in regulating cholesterol and lipid metabolism [46-51]. In particular, SREBP1 primarily regulates lipogenic processes, while SREBP2 mainly activates genes related to cholesterol synthesis. Additionally, the downstream effectors YAP and TAZ have been reported to promote cancer through their reliance on the activity of cholesterol and the SREBP-mevalonate pathway. As a signaling hub for metabolism and proliferation, the activities of SREBPs must be finely tuned by Hippo and other cellular pathways. Our cell line studies revealed that protein expression of SREBP2 is significantly downregulated following TSLG intervention, suggesting that TSLG suppresses the Hippo signaling pathway. Further investigation showed that the LPS-induced inflammation model increased both glycolysis and glycolytic capacity in hepatocytes, but these effects were inhibited by TSLG intervention. This finding corroborates our previous results regarding the pathway intervention. Collectively, we propose that the aberrant activation of the Hippo signaling pathway in HL patients disrupts the activities of LAST2 and YAP/TAZ, thereby disturbing the lipid and cholesterol metabolic balance regulated by SREBPs, which may ultimately lead to the formation of intrahepatic bile duct calculi.
Conclusion
Our study evaluated the clinical efficacy of Twelve Shugan Lidan Granules (TSLG), in the treatment of patients with hepatolithiasis. The results demonstrated that TSLG exerts a hepatoprotective effect by improving liver function post-surgery. Omics analysis revealed abnormal activation of the Hippo signaling pathway in HL patients. The underlying mechanism for the clinical efficacy of TSLG in treating HL appears to be associated with the downregulation of SREBP2 and the inhibition of the Hippo signaling pathway, which may help prevent the formation of intrahepatic bile duct calculi.
Acknowledgements
We thank our alma mater Anhui University of Traditional Chinese Medicine for the experimental platform provided for this study. Thank you all for your support and help. This work was supported by the 12th Five-Year national key clinical specialist construction project ([2013] 239).
Disclosure of conflict of interest
None.
Abbreviations
- HL
Hepatolithiasis
- TSLG
Twelve Shugan Lidan Granules
- WB
Western blot
- HTS
high-throughput sequencing
- ECAR
Extracellular Acidification Rate
- SREBP2
sterol regulatory element-binding protein-2
- HH
hepatic hemangioma
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- TB
total bilirubin
- ALB
albumin
- NOR
normal group
- RT-PCR
reverse transcription-polymerase chain reaction
- CPM
counts per million
- GO
Gene Ontology
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- FDR
false discovery rate
- SD
standard deviation
- BP
biological processes
- CC
cellular components
- MF
molecular functions
- UDC
ursodeoxycholate
References
- 1.Nakayama F, Koga A. Hepatolithiasis: present status. World J Surg. 1984;8:9–14. doi: 10.1007/BF01658357. [DOI] [PubMed] [Google Scholar]
- 2.Uchiyama K, Onishi H, Tani M, Kinoshita H, Ueno M, Yamaue H. Indication and procedure for treatment of hepatolithiasis. Arch Surg. 2002;137:149–153. doi: 10.1001/archsurg.137.2.149. [DOI] [PubMed] [Google Scholar]
- 3.Shoda J, Tanaka N, Osuga T. Hepatolithiasis--epidemiology and pathogenesis update. Front Biosci. 2003;8:e398–e409. doi: 10.2741/1091. [DOI] [PubMed] [Google Scholar]
- 4.Vetrone G, Ercolani G, Grazi GL, Ramacciato G, Ravaioli M, Cescon M, Varotti G, Del Gaudio M, Quintini C, Pinna AD. Surgical therapy for hepatolithiasis: a Western experience. J Am Coll Surg. 2006;202:306–312. doi: 10.1016/j.jamcollsurg.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 5.Lorio E, Patel P, Rosenkranz L, Patel S, Sayana H. Management of hepatolithiasis: review of the literature. Curr Gastroenterol Rep. 2020;22:30. doi: 10.1007/s11894-020-00765-3. [DOI] [PubMed] [Google Scholar]
- 6.Sakpal SV, Babel N, Chamberlain RS. Surgical management of hepatolithiasis. HPB (Oxford) 2009;11:194–202. doi: 10.1111/j.1477-2574.2009.00046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakayama F, Soloway RD, Nakama T, Miyazaki K, Ichimiya H, Sheen PC, Ker CG, Ong GB, Choi TK, Boey J. Hepatolithiasis in East Asia. Retrospective study. Dig Dis Sci. 1986;31:21–26. doi: 10.1007/BF01347905. [DOI] [PubMed] [Google Scholar]
- 8.Nakayama F, Koga A, Ichimiya H, Todo S, Shen K, Guo RX, Zeng XJ, Zhang ZH. Hepatolithiasis in East Asia: comparison between Japan and China. J Gastroenterol Hepatol. 1991;6:155–158. doi: 10.1111/j.1440-1746.1991.tb01457.x. [DOI] [PubMed] [Google Scholar]
- 9.Yang T, Lau WY, Lai EC, Yang LQ, Zhang J, Yang GS, Lu JH, Wu MC. Hepatectomy for bilateral primary hepatolithiasis: a cohort study. Ann Surg. 2010;251:84–90. doi: 10.1097/SLA.0b013e3181b2f374. [DOI] [PubMed] [Google Scholar]
- 10.Kim HJ, Kim JS, Joo MK, Lee BJ, Kim JH, Yeon JE, Park JJ, Byun KS, Bak YT. Hepatolithiasis and intrahepatic cholangiocarcinoma: a review. World J Gastroenterol. 2015;21:13418–13431. doi: 10.3748/wjg.v21.i48.13418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tabata M, Nakayama F. Bacteriology of hepatolithiasis. Prog Clin Biol Res. 1984;152:163–174. [PubMed] [Google Scholar]
- 12.Chen DW, Tung-Ping Poon R, Liu CL, Fan ST, Wong J. Immediate and long-term outcomes of hepatectomy for hepatolithiasis. Surgery. 2004;135:386–393. doi: 10.1016/j.surg.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Lee TY, Chen YL, Chang HC, Chan CP, Kuo SJ. Outcomes of hepatectomy for hepatolithiasis. World J Surg. 2007;31:479–482. doi: 10.1007/s00268-006-0441-6. [DOI] [PubMed] [Google Scholar]
- 14.Otani K, Shimizu S, Chijiiwa K, Ogawa T, Morisaki T, Sugitani A, Yamaguchi K, Tanaka M. Comparison of treatments for hepatolithiasis: hepatic resection versus cholangioscopic lithotomy. J Am Coll Surg. 1999;189:177–182. doi: 10.1016/s1072-7515(99)00109-x. [DOI] [PubMed] [Google Scholar]
- 15.Tan J, Tan Y, Chen F, Zhu Y, Leng J, Dong J. Endoscopic or laparoscopic approach for hepatolithiasis in the era of endoscopy in China. Surg Endosc. 2015;29:154–162. doi: 10.1007/s00464-014-3669-5. [DOI] [PubMed] [Google Scholar]
- 16.Su CH, Shyr YM, Lui WY, P’Eng FK. Hepatolithiasis associated with cholangiocarcinoma. Br J Surg. 1997;84:969–973. doi: 10.1002/bjs.1800840717. [DOI] [PubMed] [Google Scholar]
- 17.Chen MF, Jan YY, Wang CS, Hwang TL, Jeng LB, Chen SC, Chen TJ. A reappraisal of cholangiocarcinoma in patient with hepatolithiasis. Cancer. 1993;71:2461–2465. doi: 10.1002/1097-0142(19930415)71:8<2461::aid-cncr2820710806>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 18.Chen Q, Zhang Y, Li S, Chen S, Lin X, Li C, Asakawa T. Mechanisms underlying the prevention and treatment of cholelithiasis using traditional Chinese medicine. Evid Based Complement Alternat Med. 2019;2019:2536452. doi: 10.1155/2019/2536452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jan YY, Chen MF, Wang CS, Jeng LB, Hwang TL, Chen SC. Surgical treatment of hepatolithiasis: long-term results. Surgery. 1996;120:509–514. doi: 10.1016/s0039-6060(96)80071-7. [DOI] [PubMed] [Google Scholar]
- 20.Fan N, Meng K, Zhang Y, Hu Y, Li D, Gao Q, Wang J, Li Y, Wu S, Cui Y. The effect of ursodeoxycholic acid on the relative expression of the lipid metabolism genes in mouse cholesterol gallstone models. Lipids Health Dis. 2020;19:158. doi: 10.1186/s12944-020-01334-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma Z, Zhang B, Fan Y, Wang M, Kebebe D, Li J, Liu Z. Traditional Chinese medicine combined with hepatic targeted drug delivery systems: a new strategy for the treatment of liver diseases. Biomed Pharmacother. 2019;117:109128. doi: 10.1016/j.biopha.2019.109128. [DOI] [PubMed] [Google Scholar]
- 22.Liang Z, Chen X, Shi J, Hu H, Xue Y, Ung COL. Efficacy and safety of traditional Chinese medicines for non-alcoholic fatty liver disease: a systematic literature review of randomized controlled trials. Chin Med. 2021;16:9. doi: 10.1186/s13020-020-00422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao X, Bu Y, Jia Q. Traditional Chinese medicine as supportive care for the management of liver cancer: past, present, and future. Genes Dis. 2019;7:370–379. doi: 10.1016/j.gendis.2019.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han W, Li H, Jiang H, Xu H, Lin Y, Chen J, Bi C, Liu Z. Progress in the mechanism of autophagy and traditional Chinese medicine herb involved in alcohol-related liver disease. PeerJ. 2023;11:e15977. doi: 10.7717/peerj.15977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao CQ, Zhou Y, Ping J, Xu LM. Traditional Chinese medicine for treatment of liver diseases: progress, challenges and opportunities. J Integr Med. 2014;12:401–408. doi: 10.1016/S2095-4964(14)60039-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang JZ, Zhang Xl, Liang XQ, Gu HG, Zhu Pt. Effects of different Chinese herbal medicines on biochemical parameters in guinea-pig with pigment gallstones. Zhong Xi Yi Jie He Xue Bao. 2008;6:856–859. doi: 10.3736/jcim20080816. [DOI] [PubMed] [Google Scholar]
- 27.Wu Y, Liu L, Zhao Y, Zhao R. Polysaccharides of vinegar-baked radix bupleuri promote the hepatic targeting effect of oxymatrine by regulating the protein expression of HNF4α, Mrp2, and OCT1. J Ethnopharmacol. 2021;267:113471. doi: 10.1016/j.jep.2020.113471. [DOI] [PubMed] [Google Scholar]
- 28.Wang YX, Du Y, Liu XF, Yang FX, Wu X, Tan L, Lu YH, Zhang JW, Zhou F, Wang GJ. A hepatoprotection study of Radix Bupleuri on acetaminophen-induced liver injury based on CYP450 inhibition. Chin J Nat Med. 2019;17:517–524. doi: 10.1016/S1875-5364(19)30073-1. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Q, Zhang L, Liu K, Shang H, Ruan J, Yu Z, Meng S, Liang F, Wang T, Zhang H, Peng W, Wang Y, Chen J, Xiao T, Wang B. A network pharmacology study on the active components and targets of the radix ginseng and radix bupleuri herb pair for treating nonalcoholic fatty liver disease. Evid Based Complement Alternat Med. 2022;2022:1638740. doi: 10.1155/2022/1638740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dehzad MJ, Ghalandari H, Amini MR, Askarpour M. Effects of curcumin/turmeric supplementation on liver function in adults: a GRADE-assessed systematic review and dose-response meta-analysis of randomized controlled trials. Complement Ther Med. 2023;74:102952. doi: 10.1016/j.ctim.2023.102952. [DOI] [PubMed] [Google Scholar]
- 31.Abd El-Hack ME, El-Saadony MT, Swelum AA, Arif M, Abo Ghanima MM, Shukry M, Noreldin A, Taha AE, El-Tarabily KA. Curcumin, the active substance of turmeric: its effects on health and ways to improve its bioavailability. J Sci Food Agric. 2021;101:5747–5762. doi: 10.1002/jsfa.11372. [DOI] [PubMed] [Google Scholar]
- 32.Luber RP, Rentsch C, Lontos S, Pope JD, Aung AK, Schneider HG, Kemp W, Roberts SK, Majeed A. Turmeric induced liver injury: a report of two cases. Case Reports Hepatol. 2019;2019:6741213. doi: 10.1155/2019/6741213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li R, Guo W, Fu Z, Ding G, Zou Y, Wang Z. Hepatoprotective action of Radix Paeoniae Rubra aqueous extract against CCl4-induced hepatic damage. Molecules. 2011;16:8684–8694. doi: 10.3390/molecules16108684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan YQ, Chen HW, Li J, Wu QJ. Efficacy, chemical constituents, and pharmacological actions of radix paeoniae rubra and radix paeoniae alba. Front Pharmacol. 2020;11:1054. doi: 10.3389/fphar.2020.01054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagappan A, Kim JH, Jung DY, Jung MH. Cryptotanshinone from the salvia miltiorrhiza bunge attenuates ethanol-induced liver injury by activation of AMPK/SIRT1 and Nrf2 signaling pathways. Int J Mol Sci. 2019;21:265. doi: 10.3390/ijms21010265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim CL, Choi SH, Mo JS. Role of the hippo pathway in fibrosis and cancer. Cells. 2019;8:468. doi: 10.3390/cells8050468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J, Lyu Z, Li B, You Z, Cui N, Li Y, Li Y, Huang B, Chen R, Chen Y, Peng Y, Fang J, Wang Q, Miao Q, Tang R, Gershwin ME, Lian M, Xiao X, Ma X. P4HA2 induces hepatic ductular reaction and biliary fibrosis in chronic cholestatic liver diseases. Hepatology. 2023;78:10–25. doi: 10.1097/HEP.0000000000000317. [DOI] [PubMed] [Google Scholar]
- 38.Zheng Y, Pan D. The hippo signaling pathway in development and disease. Dev Cell. 2019;50:264–282. doi: 10.1016/j.devcel.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lammert F, Gurusamy K, Ko CW, Miquel JF, Méndez-Sánchez N, Portincasa P, van Erpecum KJ, van Laarhoven CJ, Wang DQ. Gallstones. Nat Rev Dis Primers. 2016;2:16024. doi: 10.1038/nrdp.2016.24. [DOI] [PubMed] [Google Scholar]
- 40.Chen G, Wu J, Xiao L, Wen Y, Yang T, Wang S. Right posteroinferior bile duct angulation correlates with bile duct stone occurrence in patients with hepatolithiasis. Abdom Radiol (NY) 2020;45:3103–3108. doi: 10.1007/s00261-020-02444-3. [DOI] [PubMed] [Google Scholar]
- 41.Tazuma S. Gallstone disease: Epidemiology, pathogenesis, and classification of biliary stones (common bile duct and intrahepatic) Best Pract Res Clin Gastroenterol. 2006;20:1075–1083. doi: 10.1016/j.bpg.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 42.Manti M, Shah J, Papaefthymiou A, Facciorusso A, Ramai D, Tziatzios G, Papadopoulos V, Paraskeva K, Papanikolaou IS, Triantafyllou K, Arvanitakis M, Archibugi L, Vanella G, Hollenbach M, Gkolfakis P. Endoscopic management of difficult biliary stones: an evergreen issue. Medicina (Kaunas) 2024;60:340. doi: 10.3390/medicina60020340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li EL, Yuan RF, Liao WJ, Feng Q, Lei J, Yin XB, Wu LQ, Shao JH. Intrahepatic bile duct exploration lithotomy is a useful adjunctive hepatectomy method for bilateral primary hepatolithiasis: an eight-year experience at a single centre. BMC Surg. 2019;19:16. doi: 10.1186/s12893-019-0480-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xia H, Zhang H, Xin X, Liang B, Yang T, Liu Y, Wang J, Meng X. Surgical management of recurrence of primary intrahepatic bile duct stones. Can J Gastroenterol Hepatol. 2023;2023:5158580. doi: 10.1155/2023/5158580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun P, Li Y, Wei S, Zhao T, Wang Y, Song C, Xue L, Wang F, Xiao L, Wu J, Qiao M. Pharmacological effects and chemical constituents of bupleurum. Mini Rev Med Chem. 2019;19:34–55. doi: 10.2174/1871520618666180628155931. [DOI] [PubMed] [Google Scholar]
- 46.Kim H, Hong J, Lee J, Jeon W, Yeo C, Lee Y, Baek S, Ha I. Curcuma aromatica Salisb. protects from acetaminophen-induced hepatotoxicity by regulating the Sirt1/HO-1 signaling pathway. Nutrients. 2023;15:808. doi: 10.3390/nu15040808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soleimani V, Sahebkar A, Hosseinzadeh H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: review. Phytother Res. 2018;32:985–995. doi: 10.1002/ptr.6054. [DOI] [PubMed] [Google Scholar]
- 48.Wang T, Lu Z, Qu XH, Xiong ZY, Wu YT, Luo Y, Zhang ZY, Han XJ, Xie CF. Chrysophanol-8-O-glucoside protects mice against acute liver injury by inhibiting autophagy in hepatic stellate cells and inflammatory response in liver-resident macrophages. Front Pharmacol. 2022;13:951521. doi: 10.3389/fphar.2022.951521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jeong YH, Kim TI, Oh YC, Ma JY. Lysimachiae herba inhibits inflammatory reactions and improves lipopolysaccharide/D-Galactosamine-induced hepatic injury. Antioxidants (Basel) 2021;10:1387. doi: 10.3390/antiox10091387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shu Z, Gao Y, Zhang G, Zhou Y, Cao J, Wan D, Zhu X, Xiong W. A functional interaction between Hippo-YAP signalling and SREBPs mediates hepatic steatosis in diabetic mice. J Cell Mol Med. 2019;23:3616–3628. doi: 10.1111/jcmm.14262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aylon Y, Oren M. The Hippo pathway, p53 and cholesterol. Cell Cycle. 2016;15:2248–2255. doi: 10.1080/15384101.2016.1207840. [DOI] [PMC free article] [PubMed] [Google Scholar]