Abstract
Objective: To investigate the characteristics of reperfusion arrhythmia during direct percutaneous coronary intervention (PCI) in elderly patients with acute coronary syndrome (ACS) and its impact on prognosis. Methods: A total of 286 elderly ACS patients admitted to Kweichow Moutai Hospital from January 2019 to February 2023 were included in this retrospective study, with 200 patients used for model development and 86 for validation. Patients were selected based on predefined inclusion and exclusion criteria applied to existing medical records. Data were retrospectively collected, including demographics (age, gender, BMI), clinical history (smoking, alcohol use, hypertension, diabetes), laboratory results (white blood cell count [WBC], hemoglobin [Hb], high-sensitivity C-reactive protein [hs-CRP]), imaging parameters (left atrial diameter [LA], left ventricular end-systolic diameter [LVESD], left ventricular end-diastolic diameter [LVEDD], and left ventricular ejection fraction [LVEF]), and PCI-specific details (time from symptom onset to PCI, pre-infarction angina, and TIMI grade). Statistical analysis was performed to identify risk factors for reperfusion arrhythmia during PCI in elderly ACS patients, and a prediction model was constructed and evaluated for its accuracy. Results: The prevalence of reperfusion arrhythmia in the model group was 74%. Risk factors for post-PCI reperfusion arrhythmia included multivessel disease, presence of pre-infarction angina, preprocedural TIMI grade 0 flow, and shorter time from onset to PCI. A predictive model was developed using the number of vascular lesions, presence of pre-infarction angina, TIMI grade, and time from onset to PCI, and visualized with a nomogram, showing a C-index of 0.841. The calibration curves indicated good agreement between observed and predicted outcomes, while Decision Curve Analysis (DCA) demonstrated a standardized net benefit for risk thresholds above 0.05. Validation with an independent dataset yielded an ROC AUC of 0.837, a Hosmer-Lemeshow goodness-of-fit test χ2 value of 4.280 (P = 0.747), with a specificity of 90.62% and a sensitivity of 68.18%. Conclusion: Elderly ACS patients with multivessel disease, pre-infarction angina, preprocedural TIMI grade 0 flow, and shorter time from symptom onset to PCI are at higher risk of reperfusion arrhythmia during PCI. Early identification and preventive strategies should be implemented to improve patient prognosis.
Keywords: Elderly acute coronary syndrome, percutaneous coronary intervention, reperfusion arrhythmia, predictive model
Introduction
Atherosclerosis is the most common type of arteriosclerosis, and the aging population and rising prevalence of metabolic diseases are significant factors contributing to the increasing burden of coronary heart disease prevention and control [1-4]. The pathophysiology involves the adhesion and deposition of lipid-like substances on the arterial endothelium, accumulation of complex sugars, the formation of vascular plaques, fibrous tissue proliferation, and calcification, accompanied by medial arterial lesions. Rupture or erosion of these plaques can lead to thrombosis, significantly increasing the risk of acute coronary syndrome (ACS) [5-8]. The main symptoms of ACS include dyspnea, profuse sweating, and chest pain, characterized by rapid onset, fast progression, and high mortality rates, posing a serious threat to patient lives [9,10].
Percutaneous coronary intervention (PCI) is an effective treatment for revascularizing occluded vessels and improving cardiac function, making it one of the primary therapeutic approaches for ACS [11,12]. However, post-PCI patients with ACS are at a higher risk of developing reperfusion arrhythmias, which increase the likelihood of reinfarction or even fatal outcomes [13,14]. For high-risk ACS patients, a loading dose of Rosuvastatin administered prophylactically before PCI has been shown to reduce the risk of reperfusion arrhythmias [15]. It has been reported that the occurrence of reperfusion arrhythmias during PCI in myocardial infarction patients is influenced by the number of affected vessels, the infarct site, and other factors [16]. Additionally, the plasma atherogenic index is a significant predictor of adverse outcomes in ACS patients undergoing PCI with low-density lipoprotein cholesterol (LDL-C) levels below 1.8 mmol/L [17]. The non-HDL-C/HDL-C ratio also serves as an effective predictor of nonculprit coronary lesion progression in ACS patients undergoing PCI [18]. Preoperative serum levels of HIF-1α and VEGF are recognized as risk factors for developing reperfusion arrhythmias post-PCI in ACS patients [19].
Therefore, identifying preoperative risk factors for reperfusion arrhythmias during PCI is crucial for guiding preventive measures and clinical interventions. In this study, we retrospectively analyzed clinical data from elderly ACS patients, developed a predictive model for intraoperative reperfusion arrhythmias, and validated its clinical value. This study introduces a novel predictive model that integrates multiple risk factors to accurately assess the risk of reperfusion arrhythmias during PCI in elderly atherosclerosis patients. Unlike previous studies that focused on individual risk factors, our model provides a comprehensive risk assessment tool and demonstrates robustness through validation with an independent patient dataset. Moreover, the study specifically addresses elderly patients, filling a critical gap in the literature, and employs advanced statistical methods, including nomogram construction and Decision Curve Analysis (DCA), to enhance clinical utility. These innovations aim to improve clinical decision-making and patient outcomes by offering a reliable tool for assessing the risk of reperfusion arrhythmias during PCI.
Materials and methods
General information
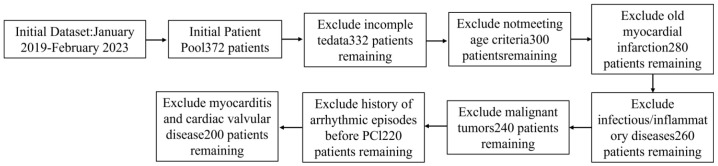
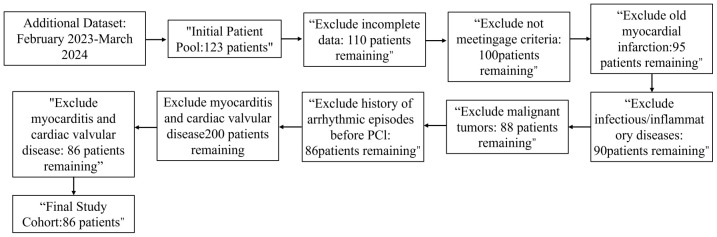
A total of 286 elderly patients with acute coronary syndromes (ACS) admitted to Kweichow Moutai Hospital between January 2019 and February 2023 were retrospectively analyzed. Of these, 70% (n = 200) were randomly assigned to the modeling group, which was further divided into the reperfusion arrhythmia group (RA group, n = 148) and the no reperfusion arrhythmia group (NRA group, n = 52) based on the presence or absence of intraoperative reperfusion arrhythmias. The remaining 30% (n = 86) constituted the external validation group (see Figures 1 and 2 for the case inclusion process).
Figure 1.
lnitial Dataset case flowchart. PCI: Percutaneous Coronary Intervention.
Figure 2.
Additional Dataset case flowchart. PCI: Percutaneous Coronary Intervention.
Inclusion criteria: (1) Patients meeting the diagnostic criteria for ACS [20]. (2) Undergoing first-time PCI. (3) Age ≥ 60 years. (4) Availability of complete clinical, laboratory, and imaging data.
Exclusion criteria: (1) History of old myocardial infarction. (2) Presence of infectious or inflammatory diseases. (3) Presence of malignant tumors. (4) History of arrhythmic episodes prior to PCI. (5) Coexisting cardiac diseases, such as myocarditis or valvular heart disease.
This study was approved by the Ethics Committee of Kweichow Moutai Hospital.
Methods
Sample size calculation (Cross-Validation)
When establishing a prediction model, it is recommended that the sample size should be 5 to 10 times the total number of factors to be analyzed. In this study, the anticipated number of factors to be included in the model is approximately 31. Based on a minimum requirement of 5 times the number of factors, at least 155 patients who developed the outcome should be enrolled. Given an expected reperfusion arrhythmia incidence of 50-75%, a total sample size of 206 to 310 cases would be adequate. Following the inclusion and exclusion criteria, data from 286 cases were collected. Of these, 70% (n = 200) were randomly selected for model development, while the remaining 30% (n = 86) were used for model validation.
Clinical data collection
General demographic information was collected from patient medical records, including age, gender, BMI, history of smoking, alcohol abuse, hypertension, diabetes, coronary artery disease, number of vascular lesions, location of diseased vessels, pre-infarction angina, cardiogenic shock, and Thrombolysis in Myocardial Infarction (TIMI) classification.
Laboratory indices
White blood cell count (WBC), neutrophil count (N), hemoglobin (Hb), high-sensitivity C-reactive protein (hs-CRP), glucose (GLU), red blood cell distribution width (RDW), total cholesterol (TC), triglycerides (TG), uric acid (UA), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and creatine kinase-MB (CK-MB).
Imaging parameters
Left atrial diameter (LA), left ventricular end-systolic diameter (LVESD), left ventricular end-diastolic diameter (LVEDD), and left ventricular ejection fraction (LVEF).
Other indicators
Time from symptom onset to PCI, and time to surgery.
Definitions
Pre-infarction Angina: Typical chest pain occurring within three months prior to the onset of the disease.
Cardiogenic shock: Defined by a cardiac index < 2.2 L/(min·m2), mean arterial pressure (MAP) < 65 mmHg or the need for vasoactive medications to maintain MAP > 65 mmHg, lactate > 4 mmol/L, and signs of oliguria and hypoperfusion.
TIMI classification: Grade 0 indicates no blood flow distal to the occluded vessel; grade I indicates that some contrast passes across the occlusion but fails to opacify the distal vessel completely.
Data extraction was conducted using electronic medical records and manual chart reviews by trained research personnel. All extracted data were cross-checked against original records and validated by an independent reviewer to ensure accuracy.
Outcome measures
The primary outcome was the occurrence of reperfusion arrhythmias during PCI. Secondary outcomes included the incidence of specific types of reperfusion arrhythmias and long-term clinical outcomes, such as mortality and major adverse cardiac events (MACE) during follow-up.
Evaluation of reperfusion arrhythmias
Reperfusion arrhythmias were diagnosed by the sudden onset of marked transient arrhythmias upon reperfusion of the coronary artery, following balloon dilatation or guidewire passage through the occluded vessel during direct PCI.
Statistical analysis
Statistical analyses were performed using SPSS version 22.0. The Shapiro-Wilk test was used to assess the normality of data distribution. Normally distributed data were expressed as mean ± standard deviation (x̅ ± sd) and compared between groups using independent t-tests. Categorical data were expressed as rates (%) and analyzed using the chi-square (χ2) test. ROC analysis for continuous variables with significant univariate results was performed using MedCalc software. Logistic regression was used to analyze influencing factors. Model construction and internal validation were conducted using R software. Model performance was evaluated by the area under the ROC curve (AUC) and the Hosmer-Lemeshow goodness-of-fit test, with statistical significance set at P < 0.05.
Results
Comparison of baseline data
There were no statistically significant differences between the two groups in terms of age, gender, BMI, or other clinical parameters (all P > 0.05). However, patients in the reperfusion arrhythmia group had a higher prevalence of multivessel disease, a greater incidence of pre-infarction angina, a higher proportion of TIMI grade 0 flow, and a shorter time from symptom onset to PCI compared to the non-reperfusion arrhythmia group (all P < 0.05), as shown in Table 1.
Table 1.
Comparison of baseline information between the two groups (x̅ ± sd)
| Data | Reperfusion arrhythmia group (n = 148) | Non-reperfusion arrhythmia group (n = 52) | t/x2/Z | P |
|---|---|---|---|---|
| Age (years) | 68.64±4.09 | 68.31±4.54 | 0.483 | 0.630 |
| Gender [n (%)] | 0.433 | 0.510 | ||
| Male | 79 (53.38) | 25 (48.08) | ||
| Female | 69 (46.62) | 27 (51.92) | ||
| BMI (kg/m2) | 23.02±3.64 | 23.81±3.85 | -1.330 | 0.185 |
| Smoking history [n (%)] | 0.975 | 0.324 | ||
| Yes | 94 (63.51) | 29 (55.77) | ||
| No | 54 (36.49) | 23 (44.23) | ||
| Alcohol abuse history [n (%)] | 1.259 | 0.262 | ||
| Yes | 76 (51.35) | 22 (42.31) | ||
| No | 72 (48.65) | 30 (57.69) | ||
| Hypertension history [n (%)] | 0.433 | 0.511 | ||
| Yes | 53 (35.81) | 16 (30.77) | ||
| No | 95 (64.19) | 36 (69.23) | ||
| Diabetes mellitus history [n (%)] | 2.222 | 0.136 | ||
| Yes | 86 (58.11) | 24 (46.15) | ||
| No | 62 (41.89) | 28 (53.85) | ||
| Coronary heart disease history [n (%)] | 0.449 | 0.503 | ||
| Yes | 59 (39.86) | 18 (34.62) | ||
| No | 89 (60.14) | 34 (65.38) | ||
| Number of vascular lesions [n (%)] | 19.378 | < 0.001 | ||
| Single | 36 (24.32) | 30 (57.69) | ||
| Multiple | 112 (75.68) | 22 (42.31) | ||
| Site of diseased vessels [n (%)] | 0.358 | 0.836 | ||
| Left anterior descending branch | 36 (24.33) | 14 (26.92) | ||
| Left-hand branch | 34 (22.97) | 10 (19.23) | ||
| Right coronary artery | 78 (52.70) | 28 (53.85) | ||
| Preinfarction angina [n (%)] | 8.609 | 0.004 | ||
| Yes | 86 (58.11) | 18 (34.62) | ||
| No | 62 (41.89) | 34 (65.38) | ||
| Cardiogenic shock [n (%)] | 0.137 | 0.712 | ||
| Yes | 67 (45.27) | 22 (42.31) | ||
| No | 81 (54.73) | 30 (57.69) | ||
| TIMI classification [n (%)] | 10.230 | 0.001 | ||
| Grade 0 | 92 (62.16) | 19 (36.54) | ||
| Grade I | 56 (37.84) | 33 (63.46) | ||
| WBC (×109/L) | 12.35±4.27 | 11.73±4.56 | 0.775 | 0.439 |
| N (×109/L) | 9 (7, 12) | 8 (6, 11.75) | -1.271 | 0.204 |
| Hb (g/L) | 135.55±25.82 | 138.62±28.65 | -0.707 | 0.480 |
| hs-CRP (mg/L) | 12.28±4.20 | 11.68±5.06 | 0.692 | 0.490 |
| GLU (mmol/L) | 7.54±3.40 | 6.48±2.88 | 1.880 | 0.062 |
| RDW | 15.68±3.68 | 14.56±4.67 | 1.624 | 0.109 |
| UA (umol/L) | 360.50±66.84 | 354.84±46.45 | 0.573 | 0.567 |
| TC (mmol/L) | 4.46±1.06 | 4.58±1.25 | -0.672 | 0.502 |
| TG (mmol/L) | 1.36±0.42 | 1.58±0.77 | -1.967 | 0.054 |
| HDL-C (mmol/L) | 1.08±0.40 | 1.09±0.51 | -0.112 | 0.911 |
| LDL-C (mmol/L) | 2.86±0.94 | 2.99±0.98 | -0.851 | 0.396 |
| CK-MB (U/L) | 225.97±80.42 | 218.27±72.87 | 0.608 | 0.544 |
| LA (mm) | 36.05±8.62 | 36.71±8.96 | -0.473 | 0.637 |
| LVS (mm) | 36.20±7.45 | 37.58±8.61 | -1.103 | 0.271 |
| LVD (mm) | 50.80±8.62 | 51.50±6.98 | -0.529 | 0.597 |
| LVEF | 0.46±0.28 | 0.54±0.36 | -1.529 | 0.131 |
| Time from onset to PCI (h) | 6 (4.5, 8) | 4 (3, 6) | -4.860 | < 0.001 |
| Surgical time (h) | 2 (1, 2) | 1.5 (1, 2) | -1.431 | 0.152 |
BMI: Body Mass Index; WBC: White Blood Cell Count; N: Neutrophil Count; Hb: Hemoglobin; hs-CRP: High-sensitivity C-reactive Protein; GLU: Glucose; RDW: Red Blood Cell Distribution Width; UA: Uric Acid; TC: Total Cholesterol; TG: Triglycerides; HDL-C: High-Density Lipoprotein Cholesterol; LDL-C: Low-Density Lipoprotein Cholesterol; CK-MB: Creatine Kinase-MB; LA: Left Atrial Diameter; LVS: Left Ventricular End-Systolic Diameter; LVD: Left Ventricular End-Diastolic Diameter; LVEF: Left Ventricular Ejection Fraction; PCI: Percutaneous Coronary Intervention; TIMI: Thrombolysis in Myocardial Infarction.
Binary logistic regression analysis
The number of vascular lesions, pre-infarction angina, TIMI grade, and time from symptom onset to PCI were identified as significant predictors (P < 0.05) of reperfusion arrhythmias during PCI in elderly patients with acute coronary syndrome, as presented in Table 2.
Table 2.
Logistic regression analysis of reperfusion arrhythmias during PCI in elderly patients with acute coronary syndrome
| Data | β | SE | Wald | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| Number of vascular lesions | 1.367 | 0.407 | 11.273 | 0.001 | 3.924 | 1.767-8.717 |
| Pre-infarction angina | 0.837 | 0.399 | 4.403 | 0.036 | 2.310 | 1.057-5.049 |
| TIMI classification | 1.206 | 0.405 | 8.853 | 0.003 | 3.341 | 1.509-7.395 |
| Time from onset to PCI | -0.622 | 0.134 | 21.416 | < 0.001 | 0.537 | 0.413-0.699 |
OR: Odds Ratio; CI: Confidence Interval; SE: Standard Error; Wald: Wald Test Statistic; β: Regression Coefficient; TIMI: Thrombolysis in Myocardial Infarction; PCI: Percutaneous Coronary Intervention.
Construction of a predictive model for reperfusion arrhythmias during PCI
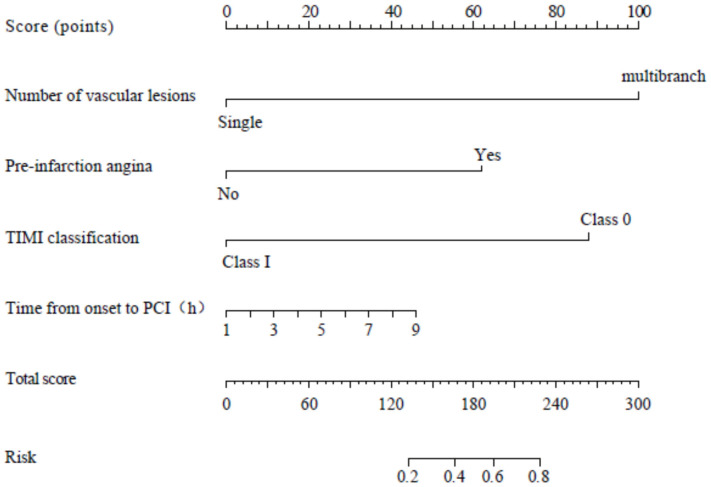
A predictive Nomogram model was constructed using the number of vascular lesions, pre-infarction angina, TIMI classification, and time from symptom onset to PCI as predictors, as shown in Figure 3.
Figure 3.
Nomogram model for predicting intraoperative reperfusion arrhythmias in patients undergoing PCI. PCI: Percutaneous Coronary Intervention; TIMI: Thrombolysis in Myocardial Infarction.
Nomogram model calibration and decision curve analysis (DCA)
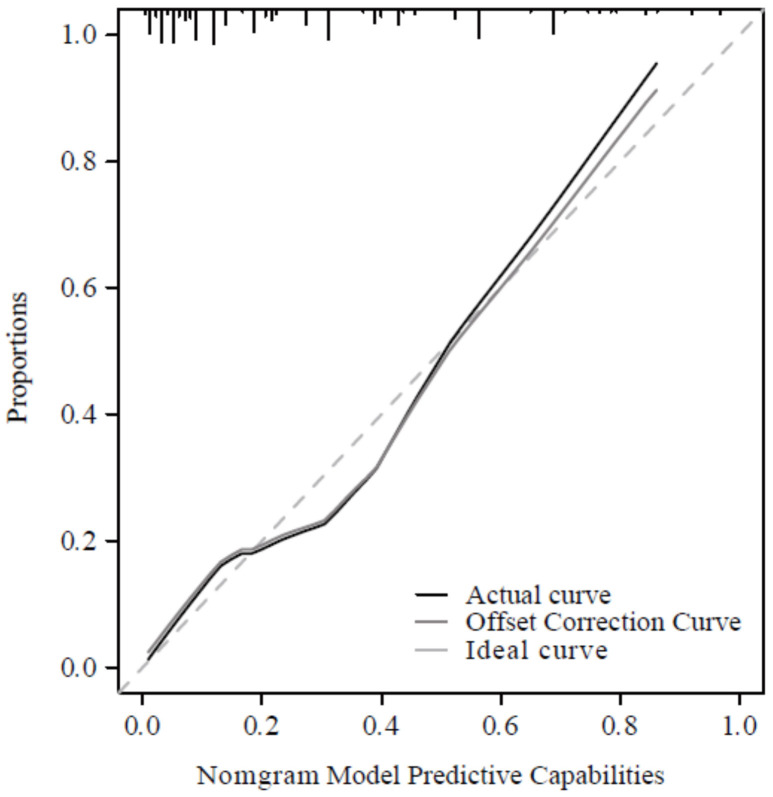
The Nomogram model demonstrated excellent performance for predicting the risk of intraoperative reperfusion arrhythmias, with a C-index of 0.841 (95% CI: 0.779-0.904). The calibration curves showed good agreement between observed and predicted outcomes, as illustrated in Figure 4. The DCA indicated that the model provided a substantial standardized net benefit when the risk threshold was greater than 0.05, outperforming other variables included in the study, as depicted in Figure 5.
Figure 4.
Calibration curve.
Figure 5.
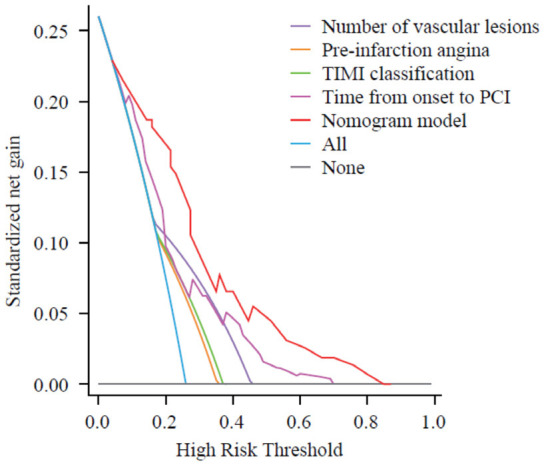
Decision curve. PCI: Percutaneous Coronary Intervention; TIMI: Thrombolysis in Myocardial Infarction.
Predictive model validation
No significant differences (all P > 0.05) were found between the clinical characteristics of the two groups in the model-testing set, as shown in Table 3. The variables from the model-testing group were applied to the predictive model established using the modeling group data, and the risk of reperfusion arrhythmia was calculated. The ROC curve analysis demonstrated an AUC of 0.837 (95% CI: 0.742-0.908), as shown in Figure 6. The Hosmer-Lemeshow goodness-of-fit test yielded a χ2 value of 4.280 with P = 0.747 (P > 0.05), indicating good model discrimination and calibration. The model achieved a specificity of 90.62% and a sensitivity of 68.18%, suggesting that it can accurately predict the occurrence of intraoperative reperfusion arrhythmias in elderly post-PCI patients.
Table 3.
Comparison of clinical data between model and validation groups
| Data | Model group (n = 200) | Validation group (n = 86) | t/x2/Z | P |
|---|---|---|---|---|
| Age (years) | 68.55±4.20 | 68.19±4.07 | 0.678 | 0.498 |
| Gender [n (%)] | 0.053 | 0.817 | ||
| Male | 104 (52.00) | 46 (53.49) | ||
| Female | 96 (48.00) | 40 (46.51) | ||
| BMI (kg/m2) | 23.23±3.70 | 23.25±3.54 | -0.047 | 0.963 |
| Smoking history [n (%)] | 0.042 | 0.837 | ||
| Yes | 123 (61.50) | 54 (62.79) | ||
| No | 77 (38.50) | 32 (37.21) | ||
| Alcohol abuse history [n (%)] | 0.024 | 0.877 | ||
| Yes | 98 (49.00) | 43 (50.00) | ||
| No | 102 (51.00) | 43 (50.00) | ||
| Hypertension history [n (%)] | 0.260 | 0.610 | ||
| Yes | 69 (34.50) | 27 (31.40) | ||
| No | 131 (65.50) | 59 (68.60) | ||
| Diabetes mellitus history [n (%)] | 0.173 | 0.677 | ||
| Yes | 110 (55.00) | 45 (52.33) | ||
| No | 90 (45.00) | 41 (47.67) | ||
| Coronary heart disease history [n (%)] | 0.154 | 0.695 | ||
| Yes | 77 (38.50) | 31 (36.05) | ||
| No | 123 (61.50) | 55 (63.95) | ||
| Number of vascular lesions [n (%)] | 0.096 | 0.757 | ||
| Single | 66 (33.00) | 30 (34.88) | ||
| Multiple | 134 (67.00) | 56 (65.12) | ||
| Site of diseased vessels [n (%)] | 0.013 | 0.993 | ||
| Left anterior descending branch | 50 (25.00) | 22 (25.58) | ||
| Left-hand branch | 44 (22.00) | 19 (22.09) | ||
| Right coronary artery | 106 (53.00) | 45 (52.33) | ||
| Preinfarction angina [n (%)] | 0.017 | 0.897 | ||
| Yes | 104 (52.00) | 44 (51.16) | ||
| No | 96 (48.00) | 42 (48.84) | ||
| Cardiogenic shock [n (%)] | 0.053 | 0.818 | ||
| Yes | 89 (44.50) | 37 (43.02) | ||
| No | 111 (55.50) | 49 (56.98) | ||
| TIMI classification [n (%)] | 0.018 | 0.895 | ||
| Grade 0 | 111 (55.50) | 47 (54.65) | ||
| Grade I | 89 (44.50) | 39 (45.35) | ||
| WBC (×109/L) | 12.19±4.34 | 11.95±4.03 | 0.432 | 0.666 |
| N (×109/L) | 12 (10, 14.75) | 12 (9, 15) | -0.316 | 0.752 |
| Hb (g/L) | 136.35±26.55 | 136.33±26.23 | 0.006 | 0.995 |
| hs-CRP (mg/L) | 12.12±4.44 | 11.90±4.21 | 0.401 | 0.689 |
| GLU (mmol/L) | 7.26±3.30 | 7.04±3.21 | 0.531 | 0.596 |
| RDW | 15.39±3.98 | 15.15±4.61 | 0.446 | 0.656 |
| UA (umol/L) | 359.03±62.13 | 356.35±65.54 | 0.328 | 0.743 |
| TC (mmol/L) | 4.49±1.11 | 4.23±1.01 | 1.892 | 0.059 |
| TG (mmol/L) | 1.42±0.54 | 1.45±0.73 | -0.327 | 0.744 |
| HDL-C (mmol/L) | 1.0±0.43 | 1.12±0.51 | -0.601 | 0.549 |
| LDL-C (mmol/L) | 2.89±0.95 | 2.94±0.99 | -0.363 | 0.717 |
| CK-MB (U/L) | 223.97±78.42 | 2258.07±80.65 | -0.108 | 0.914 |
| LA (mm) | 36.22±8.69 | 36.76±9.08 | -0.472 | 0.638 |
| LVS (mm) | 36.56±7.77 | 37.19±9.06 | -0.598 | 0.550 |
| LVD (mm) | 50.98±8.22 | 52.05±9.68 | -0.893 | 0.374 |
| LVEF | 0.46 (0.33, 0.62) | 0.46 (0.24, 0.77) | -0.894 | 0.372 |
| Time from onset to PCI (h) | 6 (4, 7) | 6 (4, 7) | -0.949 | 0.342 |
| Surgical time (h) | 23 (1, 2) | 2 (1, 2) | -1.260 | 0.208 |
BMI: Body Mass Index; TIMI: Thrombolysis in Myocardial Infarction; WBC: White Blood Cell Count; Hb: Hemoglobin; hs-CRP: High-sensitivity C-reactive Protein; GLU: Glucose; UA: Uric Acid; TC: Total Cholesterol; TG: Triglycerides; HDL-C: High-Density Lipoprotein Cholesterol; LDL-C: Low-Density Lipoprotein Cholesterol; CK-MB: Creatine Kinase-MB; LA: Left Atrial Diameter; LVS: Left Ventricular End-Systolic Diameter; LVD: Left Ventricular End-Diastolic Diameter; LVEF: Left Ventricular Ejection Fraction; PCI: Percutaneous Coronary Intervention.
Figure 6.
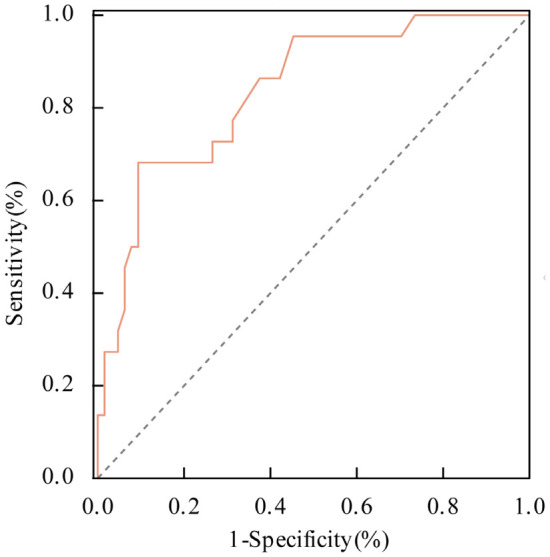
ROC curve analysis of the predictive model for intraoperative reperfusion arrhythmias among post-PCI patients in the validation group. PCI: Percutaneous Coronary Intervention.
Discussion
Arrhythmia is one of the most severe complications of acute myocardial infarction, and various serious reperfusion arrhythmias, such as accelerated idioventricular rhythm, ventricular tachycardia, atrioventricular block, bundle-branch block, sinoatrial block, and transient sinus bradycardia, are frequently observed during direct PCI therapy [21-24]. The mechanism of reperfusion arrhythmias is thought to involve a massive influx of calcium ions into the cells following PCI, leading to mitochondrial calcium overload. This disrupts the electro-mechanical coupling of myocardial contractions, causing intense contraction of ischemic myocardial regions, microvascular spasm, increased vascular resistance, and impaired myocardial blood supply, ultimately resulting in arrhythmias [25,26]. Additionally, excess oxygen free radicals generated during reperfusion may contribute to arrhythmia development. While these free radicals can rapidly restore the action potential of ischemic myocardium, the inconsistency in action potentials between the ischemic and border zones leads to myocardial fiber fibrillation, triggering arrhythmias [27-29]. Reperfusion arrhythmias can have serious consequences, including disturbances in electrical activity and hemodynamics, manifested as severe chest pain, hypotension, and even death [30,31].
In this study, reperfusion arrhythmias occurred in 148 out of 200 elderly patients with acute coronary syndrome undergoing direct PCI in the modeling group, with a prevalence of 74%. Factors associated with reperfusion arrhythmias included multivessel disease, presence of pre-infarction angina, pre-procedural TIMI grade 0, and shorter time from symptom onset to PCI.
The incidence of reperfusion arrhythmia was 24.32% in patients with single-vessel lesions and 75.68% in those with multivessel lesions. This may be attributed to the higher likelihood of ischemic symptoms and greater collateral circulation development in patients with two- or three-vessel disease compared to those with single-vessel disease, providing a certain degree of ischemic preconditioning. The incidence of reperfusion arrhythmia was 58.11% in patients with right coronary artery infarction, higher than that in those with left anterior descending artery (24.33%) or left circumflex artery infarction (22.97%). This could be due to the sinoatrial and atrioventricular nodes receiving blood supply from the right coronary artery, which has a rich vagal innervation, making it more susceptible to bradycardia or hypotension through the Bezold-Jarisch reflex, leading to arrhythmias [32]. Patients with right coronary artery lesions often present with subtle clinical symptoms, higher collateral circulation patency, and full utilization of cardiac functional reserves to maintain myocardial perfusion during progressive occlusion. This results in a reduced ability to tolerate PCI events and difficulty in hemodynamic recovery. Conversely, patients with subtotal occlusion of infarcted vessels experience a smaller extent of myocardial ischemia, less myocardial damage, and a significantly lower incidence of reperfusion arrhythmia compared to those with complete occlusion [33].
In conclusion, timely reperfusion of occluded vessels remains the most effective treatment for acute coronary syndromes, but it also carries a high risk of reperfusion arrhythmias. Reducing the incidence and severity of reperfusion arrhythmias is a critical area for further research. In elderly patients with acute coronary syndromes undergoing direct PCI, reperfusion arrhythmias are closely associated with factors such as the number of vascular lesions, pre-infarction angina, TIMI grade, and time from onset to PCI, and they hold predictive value for the occurrence of reperfusion arrhythmias during PCI. Early detection and management of reperfusion arrhythmias are essential to mitigate reperfusion injury, reduce adverse events, and improve patient outcomes.
Disclosure of conflict of interest
None.
References
- 1.Castillo-Núñez Y, Almeda-Valdes P, González-Gálvez G, Arechavaleta-Granell MDR. Metabolic dysfunction-associated steatotic liver disease and atherosclerosis. Curr Diab Rep. 2024;24:158–166. doi: 10.1007/s11892-024-01542-6. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. The changing landscape of atherosclerosis. Nature. 2021;592:524–533. doi: 10.1038/s41586-021-03392-8. [DOI] [PubMed] [Google Scholar]
- 3.Drexel H, Festa A. Subclinical atherosclerosis: more data - more insights into prevention. Atherosclerosis. 2024;393:117561. doi: 10.1016/j.atherosclerosis.2024.117561. [DOI] [PubMed] [Google Scholar]
- 4.Nayor M, Brown KJ, Vasan RS. The molecular basis of predicting atherosclerotic cardiovascular disease risk. Circ Res. 2021;128:287–303. doi: 10.1161/CIRCRESAHA.120.315890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmadi A, Argulian E, Leipsic J, Newby DE, Narula J. From subclinical atherosclerosis to plaque progression and acute coronary events: JACC state-of-the-art review. J Am Coll Cardiol. 2019;74:1608–1617. doi: 10.1016/j.jacc.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Libby P, Pasterkamp G, Crea F, Jang IK. Reassessing the mechanisms of acute coronary syndromes. Circ Res. 2019;124:150–160. doi: 10.1161/CIRCRESAHA.118.311098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114:1852–66. doi: 10.1161/CIRCRESAHA.114.302721. [DOI] [PubMed] [Google Scholar]
- 8.Dziedzic EA, Gąsior JS, Tuzimek A, Paleczny J, Junka A, Dąbrowski M, Jankowski P. Investigation of the associations of novel inflammatory biomarkers-Systemic Inflammatory Index (SII) and Systemic Inflammatory Response Index (SIRI)-with the severity of coronary artery disease and acute coronary syndrome occurrence. Int J Mol Sci. 2022;23:9553. doi: 10.3390/ijms23179553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crea F, Libby P. Acute coronary syndromes: the way forward from mechanisms to precision treatment. Circulation. 2017;136:1155–1166. doi: 10.1161/CIRCULATIONAHA.117.029870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sagatelyan AA, Konstantinova EV, Bogdanova AA, Svet AV, Pershina ES, Pershukov IV, Gilyarov MY. Atherosclerosis of the carotid and coronary arteries in elderly patients with acute coronary syndrome. Kardiologiia. 2022;62:38–44. doi: 10.18087/cardio.2022.8.n2149. [DOI] [PubMed] [Google Scholar]
- 11.Anayat S, Majid K, Nazir HS, Nizami AA, Mustafa W, Abbasi MSR, Ahsan MN, Jadoon SK, Ullah I, Asghar MS. Meta-analysis on the efficacy of high-dose statin loading before percutaneous coronary intervention in reducing no-reflow phenomenon in acute coronary syndrome. Am J Cardiol. 2023;195:9–16. doi: 10.1016/j.amjcard.2023.02.024. [DOI] [PubMed] [Google Scholar]
- 12.Bhatt DL, Lopes RD, Harrington RA. Diagnosis and treatment of acute coronary syndromes: a review. JAMA. 2022;327:662–675. doi: 10.1001/jama.2022.0358. [DOI] [PubMed] [Google Scholar]
- 13.Khanal RR, Gajurel RM, Shah S, Poudel CM, Shrestha H, Devkota S, Thapa S. Arrhythmias: its occurrence, risk factors, therapy, and prognosis in acute coronary syndrome. J Nepal Health Res Counc. 2023;21:8–14. doi: 10.33314/jnhrc.v21i1.4019. [DOI] [PubMed] [Google Scholar]
- 14.Wongthida T, Lumkul L, Patumanond J, Wongtheptian W, Piyayotai D, Phinyo P. Development of a clinical risk score for prediction of life-threatening arrhythmia events in patients with ST elevated acute coronary syndrome after primary percutaneous coronary intervention. Int J Environ Res Public Health. 2022;19:1997. doi: 10.3390/ijerph19041997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Zhang L, Zheng H, Chen H. Effects of atrial fibrillation on complications and prognosis of patients receiving emergency PCI after acute myocardial infarction. Exp Ther Med. 2018;16:3574–3578. doi: 10.3892/etm.2018.6640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X. Clinical analysis of reperfusion arrhythmias after direct percutaneous coronary intervention for acute myocardial infarction. Chinese Journal for Clinicians. 2017;45:48–50. [Google Scholar]
- 17.Wang Y, Wang S, Sun S, Li F, Zhao W, Yang H, Wu X. The predictive value of atherogenic index of plasma for cardiovascular outcomes in patients with acute coronary syndrome undergoing percutaneous coronary intervention with LDL-C below 1.8mmol/L. Cardiovasc Diabetol. 2023;22:150. doi: 10.1186/s12933-023-01888-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Zhao L, Zhang Y, Wang L, Feng Q, Cui J, Zhang W, Zheng J, Wang D, Zhao F, He J, Chen Y. A higher non-HDL-C/HDL-C ratio was associated with an increased risk of progression of nonculprit coronary lesion in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Clin Cardiol. 2024;47:e24243. doi: 10.1002/clc.24243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li B, Feng Q, Yu C, Yang J, Qin X, Li X, Cao J, Xu X, Yang C, Jin Y. Predictive value of serum HIF-1α and VEGF for arrhythmia in acute coronary syndrome patients. Exp Biol Med (Maywood) 2023;248:685–690. doi: 10.1177/15353702231171902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Branch CMDAEP. 2015 China Clinical Practice Guidelines for Emergency Acute Coronary Syndrome (II)-Diagnosis. Chinese Journal of Critical Care Medicine. 2016:9–11. [Google Scholar]
- 21.Sun Y. Irbesartan combined with amiodarone in the treatment of heart failure combined with ventricular arrhythmias. Chinese Journal of Medicine. 2012;47:63–64. [Google Scholar]
- 22.Rav-Acha M, Shah K, Hasin T, Gumuser E, Tovia-Brodie O, Shauer A, Konstantino Y, Yair E, Wolak A, Sinai E, Ziv-Baran T, Amsalem I, Michowitz Y, Glikson M, Heist K, Ng CY. Incidence and predictors for recurrence of ventricular arrhythmia presenting during acute myocarditis: a multicenter study. JACC Clin Electrophysiol. 2024;10:1161–1174. doi: 10.1016/j.jacep.2024.02.027. [DOI] [PubMed] [Google Scholar]
- 23.Xu X, Wang Z, Yang J, Fan X, Yang Y. Burden of cardiac arrhythmias in patients with acute myocardial infarction and their impact on hospitalization outcomes: insights from China acute myocardial infarction (CAMI) registry. BMC Cardiovasc Disord. 2024;24:218. doi: 10.1186/s12872-024-03889-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiong X, Ye Q, Peng Y. Risk factors for electrical storms following percutaneous coronary intervention in patients with acute myocardial infarction: a meta-analysis. Biomol Biomed. 2024;24:1077–1091. doi: 10.17305/bb.2024.10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng L, Ma KJ, Li X, Ma C. Electrophysiological mechanism of CACNA1C(R1950K) causing L type calcium channel dysfunction in Brugada syndrome. Chinese Journal of Cardiac Arrhythmias. 2016;20:64–68. [Google Scholar]
- 26.Sun M, Qian L, Wang R. Small-conductance calcium-activated potassium channels and the mechanism of arrhythmogenesis. Chinese Journal of Cardiology. 2015;43:187–189. [Google Scholar]
- 27.Wang ZQ, Chen MX, Liu DL, Zheng WX, Cao XZ, Chen H, Huang MF, Luo ZR. The effect on myocardial perfusion and clinical outcome of intracoronary nicorandil injection prior to percutaneous coronary intervention in ST-segment elevation myocardial infarction. Zhonghua Xin Xue Guan Bing Za Zhi. 2017;45:26–33. doi: 10.3760/cma.j.issn.0253-3758.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Sanjuan R, Blasco ML, Martinez-Maicas H, Carbonell N, Miñana G, Nuñez J, Bodí V, Sanchis J. Acute myocardial infarction: high risk ventricular tachyarrhythmias and admission glucose level in patients with and without diabetes mellitus. Curr Diabetes Rev. 2011;7:126–34. doi: 10.2174/157339911794940675. [DOI] [PubMed] [Google Scholar]
- 29.Kumar S, Sivagangabalan G, Thiagalingam A, West EB, Narayan A, Sadick N, Ong AT, Kovoor P. Effect of reperfusion time on inducible ventricular tachycardia early and spontaneous ventricular arrhythmias late after ST elevation myocardial infarction treated with primary percutaneous coronary intervention. Heart Rhythm. 2011;8:493–9. doi: 10.1016/j.hrthm.2010.11.046. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Yang L, Li T, Geng S. Development and validation of nomogram for the prediction of malignant ventricular arrhythmia including circulating inflammatory cells in patients with acute ST-segment elevation myocardial infarction. J Inflamm Res. 2023;27:3185–3196. doi: 10.2147/JIR.S420305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frampton J, Ortengren AR, Zeitler EP. Arrhythmias after acute myocardial infarction. Yale J Biol Med. 2023;96:83–94. doi: 10.59249/LSWK8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang J, Zhou Y, Han H. A case of acute inferior wall myocardial infarction and electrical storm due to right coronary artery spasm. Chinese Journal of Cardiology. 2017;45:64–65. doi: 10.3760/cma.j.issn.0253-3758.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Liu J, Zhang Q, Liu Z, Wang X, Gong Y, Fan F, Zhang B, Jia J, Zhang Y, Liu Y, Zheng B, Li J, Huo Y. Microvascular reperfusion of fibrinolysis followed by percutaneous coronary intervention versus primary percutaneous coronary intervention for ST-segment-elevation acute myocardial infarction. Quant Imaging Med Surg. 2024;14:765–776. doi: 10.21037/qims-23-666. [DOI] [PMC free article] [PubMed] [Google Scholar]