Abstract
Objective: Cushing’s syndrome increases the risk of cardiovascular disease. The triglyceride-glucose (TyG) index has been linked to an increased risk of cardiometabolic disorders. Whether patients with non-functioning adrenal incidentaloma (NFAI) or cortisol-secreting adrenal incidentaloma (CSAI) have altered TyG index is unknown. Therefore, we aimed to investigate the TyG index between adrenal incidentaloma patients and controls. Materials and methods: This cross-sectional study retrospectively included patients with NFAI, CSAI and healthy controls admitted to a tertiary endocrinology service. Subjects receiving hormone replacement, or having cancer, diabetes mellitus, alcoholism, psychiatric disorders, hepatic and/or renal insufficiency or morbid obesity were excluded. TyG index was calculated using the following formula: (Ln [fasting triglyceride (mg/dL) × fasting glucose (mg/dL)]/2). The primary endpoint was the difference in TyG index between patients with NFAI, CSAI and healthy controls. Results: A total of 142 patients with incidentaloma [NFAI, n=95, age: 60.9±10.6 years, women: 75.8%; CSAI, n=47, age: 59.6±13.7 years, women: 63.8%] and 116 age and sex matched healthy controls (age: 59.8±13.4 years, women: 76.7%) were evaluated. Compared with healthy controls, patients with overall incidentaloma, NFAI and CSAI had increased TyG index (6.39±1.87 vs. 8.85±0.52, 8.76±0.25, and 8.81±0.51, respectively, P<0.001 for all). There was no difference in TyG index between patients with NFAI and CSAI (8.76±0.25 vs. 8.81±0.51), and with possible autonomous cortisol secretion, autonomous cortisol secretion and Cushing’s syndrome (8.80±0.54, 8.86±0.52 and 9.31±0.37). Conclusion: This study showed increased TyG index in patients with NFAI or CSAI, emphasizing the importance of cardiometabolic risk assessment in patients with adrenal incidentaloma even if it is non-functioning.
Keywords: Triglyceride-glucose index, adrenal incidentaloma, hypercortisolism, cardiometabolic risk
Introduction
Previous studies have shown heightened morbidity and mortality among individuals with overt Cushing’s syndrome (CS). Metabolic and cardiovascular complications cause most of the increased morbidity in these patients [1-3]. In these individuals with hypercortisolism, cardiovascular events resulting from the profound metabolic effects of glucocorticoid excess are the primary cause of increased mortality [2]. The most common cause is a corticotropic pituitary adenoma in most cases. However, it can also be caused by an extrapituitary tumor or an adrenal adenoma, or carcinoma [4].
Detection of mild hypercortisolism in patients with adrenal incidentaloma is defined as autonomous cortisol secretion (ACS) [5]. Distinct clinical manifestations of overt CS (e.g., myopathy, skin and bone changes) are usually absent in these patients [5]. ACS is defined as a serum cortisol level >5.0 mcg/dL after a 1 mg dexamethasone suppression test (1 mg DST), while a level between 1.9-5.0 mcg/dL is defined as possible ACS [5]. The progression from ACS to overt CS is relatively rare [6,7]. However, even in the absence of specific clinical manifestations of hypercortisolism and typically normal levels of urinary and nocturnal cortisol, corticosteroid excess in these patients has been shown to increase the risk of cardiometabolic conditions, including diabetes, hypertension, osteoporosis, and mortality [8]. Moreover, studies have found that even a non-functioning adrenal incidentaloma (NFAI) is associated with a higher risk of cardiometabolic disorders and mortality compared to the general population [9]. Therefore, hypercortisolism is a continuum between normal hormone levels, where the cardiometabolic risk is lower, and apparent clinical and laboratory findings of higher risk [5]. At the bottom of the spectrum are mild cases of NFAI. However, these patients face a higher risk of cardiometabolic issues when compared to the general population [9].
The triglyceride-glucose (TyG) index, computed from fasting glucose and triglyceride levels, has been introduced as a novel marker for insulin resistance [10-12]. Studies have reported a strong association between elevated TyG index and cardiometabolic disorders, including diabetes mellitus [13], hypertension [14], fatty liver disease [15] and cardiovascular diseases [16]. Therefore, there is a potential correlation between the TyG index and an elevated cardiometabolic risk in these patients. Nevertheless, the available data regarding the TyG index in individuals with hypercortisolism is scarce [17] and limited to only NFAI [18]. Therefore, this study aimed to assess the TyG index in patients with NFAI and cortisol-secreting adrenal incidentaloma (CSAI) compared with healthy controls.
Material and methods
Study type, setting and subjects
This retrospective cross-sectional study included patients with NFAI and CSAI attending scheduled outpatient visits at the Endocrinology and Metabolic Diseases Clinic of Ankara Gulhane Training and Research Hospital. Patients with a diagnosis of pheochromocytoma, hyperaldosteronism, or adrenal or non-adrenal malignancies were excluded. The hospital registration system and the social security institution system were screened and patients receiving hormone replacement therapy (e.g., oral estrogen and exogenous glucocorticoids), having diabetes mellitus or antidiabetic medication use for other reasons, psychiatric disorders, hepatic and/or renal insufficiency, morbid obesity, dyslipidemia or hypolipidemic users for other reasons, using medications that could affect the screening tests for primary hyperaldosteronism and pheochromocytoma (e.g., antihypertensive drugs) before the screening were further excluded. The control group consisted of age, sex, and body mass index (BMI) matched healthy volunteers with a documented lack of adrenal masses on abdominal imaging and normal hormone levels. This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Ankara Gulhane Training and Research Hospital (2023/168).
Definition of incidentaloma and hypercortisolism
The institutional algorithm for incidentaloma evaluation, defined as the incidental discovery of an adrenal mass during investigation for other conditions [19], included the following course for all patients in this study. Patients in whom adrenal incidentaloma was detected by searching the hospital registry system and in whom benign-malignant adenoma was differentiated with functionality tests routinely performed in the approach to adrenal incidentaloma patients were included in the study. Patients with pheochromocytoma and aldosteronism other than hypercortisolemia and patients with malignant adrenal masses were excluded from the study. In all patients included in the study, the functionality of adrenal adenoma was evaluated by 24-hour urinary catecholamine excretion for pheochromocytoma, plasma aldosterone and plasma renin activity for primary aldosteronism, and hypercortisolemia was evaluated by 1 mg DST, basal plasma adrenocorticotropic hormone (ACTH), 24-hour urinary free cortisol and nocturnal serum/salivary cortisol measurement [20].
The patients were categorized into four groups according to the outcomes of adrenal tests [6]: 1. NFAI (normal 1 mg DST results [<1.8 mcg/dL], 24-hour urinary catecholamine excretion, aldosterone, and plasma renin activity). 2. Possible ACS (serum cortisol levels between 1.8-5.0 mcg/dL after 1 mg DST, suppressed basal plasma ACTH levels [<10 pg/mL], and low 24-hour urinary free cortisol levels). 3. ACS (serum cortisol levels >5.0 mcg/dL after 1 mg DST, suppressed basal plasma ACTH levels [<10 pg/mL], and low 24-hour urinary free cortisol levels). 4. CS (overt hypercortisolism who meet two out of three criteria: serum cortisol level >1.8 mcg/dL after 1 mg DST, high 24-hour urinary cortisol levels, nocturnal serum cortisol level >7.5 mcg/dL or high nocturnal salivary cortisol levels). Adrenal incidentaloma groups in which cortisol secretion was detected (sum of possible ACS, ACS and CS groups) were classified as the CSAI group.
Biochemical measurements and TyG Index
Age, BMI, systolic blood pressure (SBP), diastolic blood pressure (DBP), fasting plasma glucose (FPG), creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), plasma triglyceride, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) levels in the patient files before performing 1 mg DST were recorded in the current study. The TyG index was computed using FPG and triglyceride level (Ln [fasting triglyceride (mg/dL) × fasting glucose (mg/dL)]/2).
Study endpoints
The primary endpoint was the difference in TyG index between patients with NFAI, CSAI and healthy controls. The secondary endpoints were the TyG index in patients with NFAI vs. CSAI and between patients with possible ACS, ACS and CS.
Statistical analyses
The data were analyzed using the SPSS (IBM SPSS Statistics for Windows, version 22, IBM Corp., Armonk, N.Y., USA). Normality of distribution of continuous variables was tested by the Shapiro-Wilk test. Results were reported as mean ± standard deviation (SD) for continuous variables and n (%) for categorical variables. The demographic and clinical variables were compared using the student’s t-test or Mann-Whitney U test for normally distributed or skewed variables, respectively. The Chi-square test was used in the comparison of ratio variables between the groups. The One-way Analysis of Variance (ANOVA) was used to determine differences between results from three or more groups. Post-hoc comparisons were performed using a Bonferroni adjustment to identify the between-group differences. Pearson correlation coefficients were calculated to analyze the correlations between the TyG index and continuous variables. P<0.05 was considered significant.
Power analysis
We conducted three post-hoc power analyses for the comparison of Tyg index of total adrenal incidentaloma subjects vs. controls, NFAI subjects vs. controls and CSAI subjects vs. controls. The first calculation for the comparison of Tyg levels between total adrenal incidentaloma and control subjects showed that with our sample size in each group, there was an observed standard deviation of 0.52 for the Tyg index in the adrenal incidentaloma group and 1.87 for controls, and an observed difference of 2.46 between the groups should be detectable at a two-sided 0.05 confidence level with a power at least 0.95. The second calculation for the comparison of Tyg index between total NFAI and control subjects showed that with our sample size in each group, there was an observed standard deviation of 0.25 for the Tyg index in the NFAI group and 1.87 for controls, and an observed difference of 2.37 between the groups should be detectable at a two-sided 0.05 confidence level with a power at least 0.95. The third calculation for the comparison of the Tyg index between total CSAI and control subjects showed that with our sample size in each group, the observed standard deviation was 0.51 for the Tyg index in the NFAI group and 1.87 for controls, and an observed difference of 2.42 between the groups should be detectable at a two-sided 0.05 confidence level with a power at least 0.95.
Results
Basic clinical and laboratory characteristics
The study included 142 patients with incidentaloma [NFAI, n=95, age, mean ± SD: 60.9±10.6 years, women: 75.8%; CSAI, n=47, age, mean ± SD: 59.6±13.7 years, women: 63.8%]. The control group comprised 116 healthy controls (age, mean ± SD: 59.8±13.4 years, women: 76.7%). The clinical and laboratory characteristics of the patients and controls are displayed in Table 1. Mean age, sex ratio and mean BMI were similar between the total patient and control groups. Mean FPG and triglyceride levels were significantly higher in the incidentaloma group vs. controls, whereas mean blood pressure, LDL-C level and HDL-C level were similar.
Table 1.
Demographic and metabolic variables in patients with adrenal incidentaloma and healthy controls
| Variable | Adrenal incidentaloma (n=142) | Healthy controls (n=116) | p1 | p2 | ||
|---|---|---|---|---|---|---|
|
| ||||||
| NFAI (n=95) | CSAI (n=47) | Total (n=142) | ||||
| Age, years, mean ± SD | 60.9±10.6 | 59.6±13.7 | 60.5±11.7 | 59.8±13.4 | 0.571 | 0.506 |
| Sex, women, n (%) | 72 (75.8) | 30 (63.8) | 102 (71.8) | 89 (76.7) | 0.375 | 0.209 |
| BMI, kg/m2, mean ± SD | 29.1±4.8 | 29.6±5.6 | 29.3±5.0 | 29.3±4.5 | 0.940 | 0.876 |
| SBP, mmHg, mean ± SD | 130.0±13.0 | 134.3±17.0 | 131.4±14.5 | 131.1±15.0 | 0.865 | 0.031a |
| DBP, mmHg, mean ± SD | 79.1±8.3 | 81.7±11.8 | 80.0±9.6 | 79.3±8.8 | 0.103 | 0.077 |
| FPG, mg/dL, mean ± SD | 96.6±12.7 | 100.2±15.0 | 100.4±19.4 | 94.6±18.8 | 0.016 | 0.004b |
| Creatinine, mg/dL, mean ± SD | 0.86±0.16 | 0.92±0.22 | 0.89±0.18 | 0.84±0.15 | 0.096 | |
| AST, mg/dL, mean ± SD | 20.5±5.53 | 20.7±6.67 | 20.60±5.76 | 21.64±7.68 | 0.534 | |
| ALT, mg/dL, mean ± SD | 20.1±9.79 | 18.4±11.01 | 19.80±10.03 | 18.22±8.85 | 0.501 | |
| TG, mg/dL, mean ± SD | 142.987±4 | 160.23±85.2 | 157.2±78.6 | 122.9±51.6 | <0.001 | <0.001 |
| LDL-C, mg/dL, mean ± SD | 119.3±36.1 | 119.9±29.0 | 123.5±35.4 | 123.5±35.9 | 0.988 | 0.706 |
| HDL-C (mg/dL) | 47.8±11.0 | 50.2±12.8 | 48.6±11.6 | 48.0±13.5 | 0.713 | 0.072 |
| ACTH, pg/mL, mean ± SD | 19.65±11.29 | 11.49±8.44 | - | - | 0.002c | |
| Post 1 mg-DST cortisol, mcg/dL, mean ± SD | 1.02±0.33 | 4.96±0.73 | - | - | <0.001c | |
| Tumour size, mm, mean ± SD | 22.5±8.04 | 29.8±11.49 | <0.001e | |||
| TyG index, mean ± SD | 8.76±0.25 | 8.81±0.51 | 8.85±0.52 | 6.39±1.87 | <0.001 | <0.001d |
p1: total incidentaloma vs. healthy controls, eCSAI vs. NFAI (student’s t-test or the Chi-square test). p2: NFAI vs. CSAI vs. healthy controls vs. (ANOVA). Post hoc tests were significant aCSAI vs. healthy controls and b,dNFAI and CSAI vs. healthy controls. cstudent’s t-test. NFAI: non-functioning adrenal incidentaloma; CSAI: cortisol-secreting adrenal incidentaloma; SBP: systolic blood pressure; DBP: diastolic blood pressure; SD: standard deviation; BMI: body mass index; FPG: fasting plasma glucose; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; TG: triglycerides; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; DST: dexamethasone suppression test; ACTH: adrenocorticotropic hormone; TyG: triglyceride-glucose.
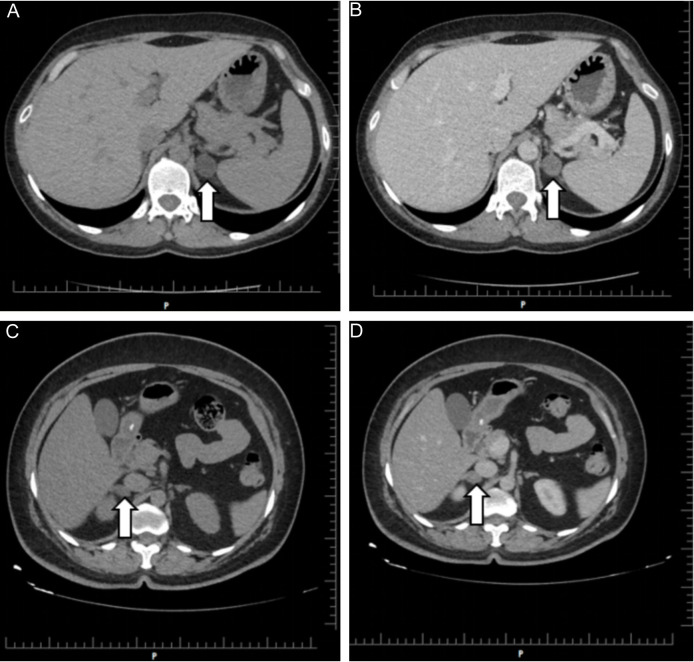
Patients with CSAI showed a significant decrease in ACTH levels compared to those with NFAI. The 1 mg DST results showed a significant increase in mean cortisol levels in patients with CSAI vs. NFAI. The maximum adrenal adenoma size (mm) in patients with CSAI was larger copared to patients with NFAI (29.87±11.49 vs. 22.53±8.04, P<0.001). Computed tomography examples of adrenal adenoma in NFAI and CSAI cases are shown in Figure 1. Mean SBP was higher in CSAI patients compared with the controls. Mean FPG and triglyceride levels were higher in patients with NFAI and CSAI vs. controls. Age, sex, BMI, DBP, creatinine, AST, ALT, LDL and HDL measurements did not differ significantly according to hypercortisolism status (Table 1).
Figure 1.
Unenhanced (A) and enhanced (B) axial CT images of an incidentally detected 18×21 mm left adrenal mass (arrows) in a 42-year-old woman, CSAI case. Unenhanced (C) and enhanced (D) axial CT images of a 17×21 mm right adrenal mass (arrows) incidentally detected in a 52-year-old woman, NFAI case. CT: Computed tomography; NFAI: nonfunctioning adrenal incidentaloma; CSAI: cortisol secreting adrenal incidentaloma.
Primary and secondary endpoints
Mean TyG index was significantly higher in the total incidentaloma group compared with the healthy controls (8.85±0.52 vs. 6.39±1.87, P<0.001). Compared with the controls, the mean TyG index was higher in patients with NFAI (8.76±0.25 vs. 6.39±1.87, P<0.001) and in patients with CSAI (8.81±0.51, 6.39±1.87, P<0.001). The mean TyG did not show a statistically significant difference between patients with NFAI vs. CSAI (P=0.302) (Figure 2). Although the TyG index showed a gradual increase from possible ACS to ACS and CS, the difference did not reach statistical significance (Table 2). Post hoc analyses were also not significant.
Figure 2.
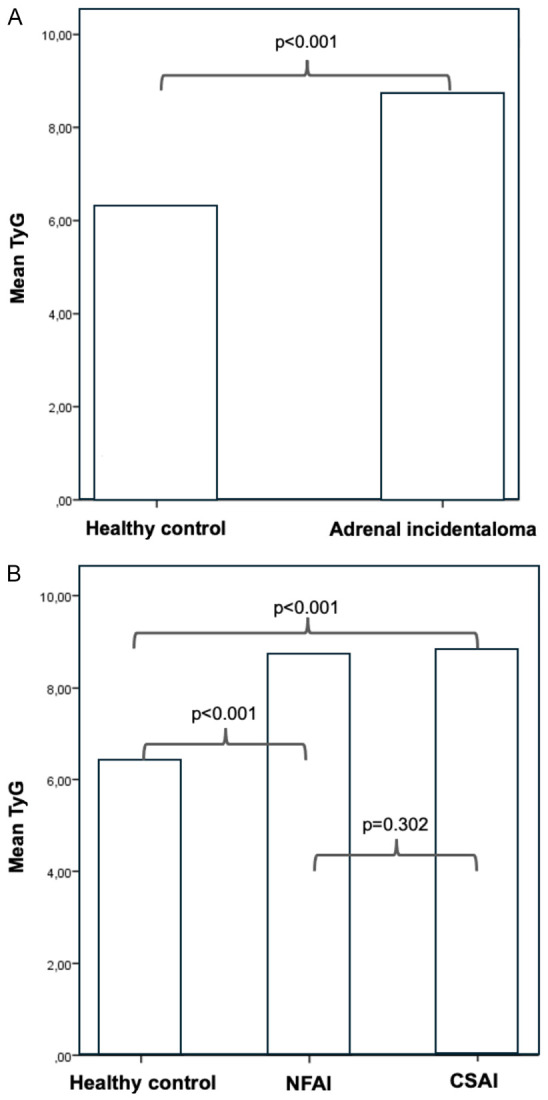
Comparison of TyG index between healthy control and adrenal incidentaloma patients (A), comparison of TyG index between healthy controls, NFAI and CSAI (B). NFAI: nonfunctioning adrenal incidentaloma; CSAI: cortisol secreting adrenal incidentaloma; TyG: triglyceride glucose index.
Table 2.
TyG index from non-functioning adrenal incidentaloma to functioning patterns of cortisol-secreting adrenal incidentalomas
| NFAI (n=95) | CSAI (n=47) | p | |||
|---|---|---|---|---|---|
|
| |||||
| Possible ACS (n=35) | ACS (n=8) | CS (n=4) | |||
| TyG index, mean ± SD | 8.76±0.25 | 8.80±0.54 | 8.86±0.52 | 9.31±0.37 | 0.076 |
NFAI: non-functioning adrenal incidentaloma; CSAI: cortisol-secreting adrenal incidentaloma; ACS: autonomous cortisol secretion; CS: Cushing’s syndrome; TyG: triglyceride glucose index; SD: standard deviation.
Correlation analyses
The TyG index correlated significantly with SBP and HDL-C levels in the total incidentaloma group. There was no correlation between the TyG index and age, BMI, diastolic blood pressure, LDL-C level, ACTH level and 1 mg DST cortisol levels (Table 3). Because FPG and TG are used to calculate the TyG index, they were not included in the correlation analyses.
Table 3.
Correlations (Pearson) between TyG index and clinical and biochemical parameters in patients with adrenal incidentaloma
| Variable | TyG index | |
|---|---|---|
|
| ||
| r | p | |
| Age | 0.153 | 0.069 |
| BMI | 0.082 | 0.187 |
| SBP | 0.201 | 0.001 |
| DBP | 0.110 | 0.077 |
| LDL-C | 0.039 | 0.535 |
| HDL-C | -0.347 | <0.001 |
| Post 1 mg-DST cortisol | 0.076 | 0.368 |
| ACTH | -0.121 | 0.339 |
TyG: triglyceride-glucose; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; DST: dexamethasone suppression test; ACTH: adrenocorticotropic hormone.
Discussion
This study identified a significant elevation in the TyG index among patients with adrenal incidentaloma compared to healthy controls. Interestingly, TyG index was significantly higher not only in patients with CSAI but also with NFAI. Although the TyG index level increased with the degree of hypercortisolism, no statistically significant difference was observed. To our knowledge, this is the first study to investigate the relationship between the severity of hypercortisolism and the TyG index. These findings suggest that the TyG index may be a practical measure for assessing cardiometabolic risk, even in patients with mild hypercortisolism.
Numerous studies have reported that cardiovascular and metabolic disorders associated with hypercortisolism are frequently observed in patients with possible or confirmed ACS, even in the absence of clinical signs of CS (e.g., abdominal obesity, proximal myopathy, skin atrophy, striae, and hirsutism) [5,6,21]. Increased mortality, hypertension, diabetes, obesity, dyslipidemia, and osteoporosis have been reported in patients with hypercortisolism [22,23]. Moreover, these patients have been identified with cardiovascular disease as the primary cause of mortality [24,25]. Therefore, an accurate evaluation of increased morbidity and mortality in patients with hypercortisolism is of great importance. Determination of cardiometabolic risk in hypercortisolism by simple and easy-to-use methods is also of great importance. Several methods and markers can be useful in assessing cardiometabolic risk in various diseases. The TyG index has recently emerged as a simple method that has gained significant attention [12-16]. Elevated TyG index has been linked to the occurrence of metabolic diseases like diabetes [13], hypertension [14], hepatosteatosis [15], and cardiovascular diseases [16].
Patients with NFAI are at greater cardiometabolic risk compared to individuals without adrenal tumors [9]. Furthermore, improvements in cardiometabolic parameters have been observed in patients with seemingly non-functional adrenal tumors following adrenalectomy [26,27]. These results collectively indicate that even mild cortisol secretion or other adrenal steroid hormone metabolites, which may not be detected through conventional tests, can adversely affect cardiometabolic health. This increased cardiometabolic risk in patients with adrenal incidentaloma has been demonstrated in various studies using different indices of insulin resistance. Using the HOMA-IR (homeostatic model assessment), QUICKI (Quantitative insulin sensitivity check index) and MATSUDA (Matsuda DeFronzo insulin sensitivity index) indices, Peppa et al. found that insulin resistance was higher in patients with NFAI compared to healthy controls [28]. Using the same insulin resistance indices, Papanastasiou et al. found that insulin resistance was higher in patients with ACS than in patients with NFAI [29]. In addition, Karatas et al. found that the Visceral Adiposity Index, which has been shown to be associated with increased cardiovascular events, glycemic disorders and metabolic syndrome, was higher in patients with NFAI compared to healthy control group [30]. However, available data on the TyG index in patients with adrenal incidentaloma and ACS are scarce [17,18]. Our observation of a higher TyG index in patients with NFAI compared to the healthy controls is consistent with a previous study by Miomira et al. who reported a significantly higher TyG index in patients with NFAI compared to healthy controls (8.792±0.062 vs. 8.433±0.078, P<0.05) [27]. As a novel finding, we have shown a gradual increase in the TyG index with increasing degrees of hypercortisolism among patients with hypercortisolism. Hypercortisolism is known to be associated with cardiometabolic abnormalities such as cardiovascular disease, hypertension, diabetes mellitus, metabolic syndrome and even increased mortality [1,2]. This association with cardiometabolic abnormalities increases in parallel with the degree of hypercortisolism [5]. The risk of cardiometabolic disease and mortality is higher in NFAI patients than in the general population without adrenal lesions [9,28,31]. This risk is higher in patients with ACS without specific signs of hypercortisolemia compared with patients with NFAI [32,33]. Patients with overt CS have an extremely high cardiovascular and mortality risk [3,24]. In our study, the TyG index increased gradually with the degree of hypercortisolemia. Although the difference was nonsignificant, this finding may be a reference for future work as the numbers of our patients with ACS and CS subgroups were low, suggesting that confirmatory works with sufficient power are required.
We observed correlations between the TyG index and SBP and HDL-C levels in the total incidentaloma group. Excess of glucocorticoids affects various metabolic pathways associated with metabolic syndrome and other comorbidities [5]. Studies have reported a higher prevalence of masked and resistant hypertension in patients with NFAI compared to those without adrenal adenoma [9,34]. In CS, hypertension is more common and found in approximately 50-93% of patients. Consequently, the prevalence of hypertension has been reported to be higher in patients with possible ACS, ACS and CS in whom the level of hypercortisolism is higher compared to healthy controls [3,35]. For instance, Petrova et al. reported that patients with cortisol levels >1.8 mcg/dL after a DST were more prone to cardiovascular disease and hypertension [33]. Similarly, Rossi et al. reported higher prevalence and more severe hypertension in patients with cortisol levels >5 mcg/dL during the 1 mg-DST [36]. CS is also associated with dyslipidemia, including elevated total cholesterol, LDL-C, and triglyceride levels, and reduced HDL-C levels, which contribute to increased cardiovascular risk [3,37]. Studies have shown an increased frequency of dyslipidemia in patients with possible ACS, ACS and NFAI, as well as in CS [38]. Collectively, the TyG index, which is associated with most of the metabolic syndrome components, showed a correlation with SBP, FPG, triglyceride and HDL-C levels in our study in patients with adrenal incidentaloma [11-14]. However, we observed no significant correlation between the TyG index and ACTH level, post 1 mg DST cortisol level. This could be explained by the limited number of patients in the hypercortisolism subgroups and similar average ages in patient groups. All these findings suggest that the TyG index is a simple method that can be useful in assessing cardiometabolic risk in patients with functional and non-functional adrenal incidentalomas.
Tumor size has been considered a risk factor for malignancy and hypercortisolism in adrenal lesions in many studies [39-42]. One study also showed that the probability of inadequate suppression of serum cortisol in DST increased with tumor size (OR 1.93, P<0.001) [40]. Araujo-Castro et al. showed that tumor size was a good predictor of ACS (OR=1.1 per mm, P<0.001) and that a cut-off value of 25 mm offered good diagnostic accuracy in predicting ACS (sensitivity 69.4%, specificity 74.1%) [43]. Consistent with the literature, we found that the maximum size of adrenal adenoma was larger in CSAI patients compared to NFAI patients.
Several limitations of the present study should be acknowledged. The mean age and sex ratio suggest that our study population represented a true patient population with incidentaloma. However, the number of patients with ACS and CS remained low. Another limitation was that a cross-sectional study design could not establish a cause-and-effect relationship between the TyG index and future complications. Finally, a retrospective study may not effectively control confounders and potential biases in subject selection. The strengths of our study include it being a single-center, systematic diagnosis and follow-up of patients through the same clinical algorithm. Also, confounders for TyG calculation were effectively managed thanks to the high-quality registry of our referral endocrinology unit.
In conclusion, this study found that patients with adrenal incidentaloma have elevated TyG index levels. Furthermore, patients with NFAI also have increased TyG index, suggesting the importance of monitoring these individuals for cardiometabolic risk. However, long-term prospective follow-up studies are necessary to elucidate the role of the TyG index in determining the occurrence of cardiometabolic disorders in patients with adrenal incidentaloma.
Disclosure of conflict of interest
None.
References
- 1.Ferraù F, Korbonits M. Metabolic comorbidities in Cushing’s syndrome. Eur J Endocrinol. 2015;173:M133–57. doi: 10.1530/EJE-15-0354. [DOI] [PubMed] [Google Scholar]
- 2.Etxabe J, Vazquez JA. Morbidity and mortality in Cushing’s disease: an epidemiological approach. Clin Endocrinol (Oxf) 1994;40:479–84. doi: 10.1111/j.1365-2265.1994.tb02486.x. [DOI] [PubMed] [Google Scholar]
- 3.Pivonello R, Isidori AM, De Martino MC, Newell-Price J, Biller BM, Colao A. Complications of Cushing’s syndrome: state of the art. Lancet Diabetes Endocrinol. 2016;4:611–29. doi: 10.1016/S2213-8587(16)00086-3. [DOI] [PubMed] [Google Scholar]
- 4.Newell-Price J, Bertagna X, Grossman AB, Nieman LK. Cushing’s syndrome. Lancet. 2006;367:1605–17. doi: 10.1016/S0140-6736(06)68699-6. [DOI] [PubMed] [Google Scholar]
- 5.Araujo-Castro M, Pascual-Corrales E, Lamas C. Possible, probable, and certain hypercortisolism: a continuum in the risk of comorbidity. Ann Endocrinol (Paris) 2023;84:272–284. doi: 10.1016/j.ando.2023.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, Tabarin A, Terzolo M, Tsagarakis S, Dekkers OM. Management of adrenal incidentalomas: European Society of Endocrinology clinical practice guideline in collaboration with the european network for the study of adrenal tumors. Eur J Endocrinol. 2016;175:G1–G34. doi: 10.1530/EJE-16-0467. [DOI] [PubMed] [Google Scholar]
- 7.Araujo-Castro M, Parra Ramírez P, Robles Lázaro C, García Centeno R, Gracia Gimeno P, Fernández-Ladreda MT, Sampedro Núñez MA, Marazuela M, Escobar-Morreale HF, Valderrabano P. Predictors of tumour growth and autonomous cortisol secretion development during follow-up in non-functioning adzrenal ıncidentalomas. J Clin Med. 2021;10:5509. doi: 10.3390/jcm10235509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Araujo-Castro M, Sampedro Núñez MA, Marazuela M. Autonomous cortisol secretion in adrenal incidentalomas. Endocrine. 2019;64:1–13. doi: 10.1007/s12020-019-01888-y. [DOI] [PubMed] [Google Scholar]
- 9.Kim JH, Kim MJ, Lee JH, Yoon JW, Shin CS. Nonfunctioning adrenal ıncidentalomas are not clinically silent: a longitudinal cohort study. Endocr Pract. 2020;26:1406–1415. doi: 10.4158/EP-2020-0182. [DOI] [PubMed] [Google Scholar]
- 10.Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6:299–304. doi: 10.1089/met.2008.0034. [DOI] [PubMed] [Google Scholar]
- 11.Vasques AC, Novaes FS, de Oliveira Mda S, Souza JR, Yamanaka A, Pareja JC, Tambascia MA, Saad MJ, Geloneze B. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. 2011;93:e98–e100. doi: 10.1016/j.diabres.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 12.Guerrero-Romero F, Villalobos-Molina R, Jiménez-Flores JR, Simental-Mendia LE, Méndez-Cruz R, Murguía-Romero M, Rodríguez-Morán M. Fasting triglycerides and glucose ındex as a diagnostic test for ınsulin resistance in young adults. Arch Med Res. 2016;47:382–387. doi: 10.1016/j.arcmed.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Zhang M, Wang B, Liu Y, Sun X, Luo X, Wang C, Li L, Zhang L, Ren Y, Zhao Y, Zhou J, Han C, Zhao J, Hu D. Cumulative increased risk of incident type 2 diabetes mellitus with increasing triglyceride glucose index in normal-weight people: The Rural Chinese Cohort Study. Cardiovasc Diabetol. 2017;16:30. doi: 10.1186/s12933-017-0514-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jian S, Su-Mei N, Xue C, Jie Z, Xue-Sen W. Association and interaction between triglyceride-glucose index and obesity on risk of hypertension in middle-aged and elderly adults. Clin Exp Hypertens. 2017;39:732–739. doi: 10.1080/10641963.2017.1324477. [DOI] [PubMed] [Google Scholar]
- 15.Zhang S, Du T, Zhang J, Lu H, Lin X, Xie J, Yang Y, Yu X. The triglyceride and glucose index (TyG) is an effective biomarker to identify nonalcoholic fatty liver disease. Lipids Health Dis. 2017;16:15. doi: 10.1186/s12944-017-0409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sánchez-Íñigo L, Navarro-González D, Fernández-Montero A, Pastrana-Delgado J, Martínez JA. The TyG index may predict the development of cardiovascular events. Eur J Clin Invest. 2016;46:189–97. doi: 10.1111/eci.12583. [DOI] [PubMed] [Google Scholar]
- 17.Ivović M, Marina LV, Vujović S, Tančić-Gajić M, Stojanović M, Radonjić NV, Gajić M, Soldatović I, Micić D. Nondiabetic patients with either subclinical Cushing’s or nonfunctional adrenal incidentalomas have lower insulin sensitivity than healthy controls: clinical implications. Metabolism. 2013;62:786–92. doi: 10.1016/j.metabol.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Krzyżewska K, Niemczuk E, Myśliwiec BJ, Junik R. Glucose metabolism disorders in patients with non-functioning adrenal adenomas - single-centre experience. Endokrynol Pol. 2017;68:416–421. doi: 10.5603/EP.a2017.0034. [DOI] [PubMed] [Google Scholar]
- 19.Cuthbertson DJ, Alam U, Davison AS, Belfield J, Shore SL, Vinjamuri S. Investigation and assessment of adrenal incidentalomas. Clin Med (Lond) 2023;23:135–140. doi: 10.7861/clinmed.2023-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fassnacht M, Tsagarakis S, Terzolo M, Tabarin A, Sahdev A, Newell-Price J, Pelsma I, Marina L, Lorenz K, Bancos I, Arlt W, Dekkers OM. European Society of Endocrinology clinical practice guidelines on the management of adrenal incidentalomas, in collaboration with the European network for the study of adrenal tumors. Eur J Endocrinol. 2023;189:G1–G42. doi: 10.1093/ejendo/lvad066. [DOI] [PubMed] [Google Scholar]
- 21.Pelsma ICM, Fassnacht M, Tsagarakis S, Terzolo M, Tabarin A, Sahdev A, Newell-Price J, Marina L, Lorenz K, Bancos I, Arlt W, Dekkers OM. Comorbidities in mild autonomous cortisol secretion and the effect of treatment: systematic review and meta-analysis. Eur J Endocrinol. 2023;189:S88–S101. doi: 10.1093/ejendo/lvad134. [DOI] [PubMed] [Google Scholar]
- 22.Debono M, Bradburn M, Bull M, Harrison B, Ross RJ, Newell-Price J. Cortisol as a marker for increased mortality in patients with incidental adrenocortical adenomas. J Clin Endocrinol Metab. 2014;99:4462–70. doi: 10.1210/jc.2014-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salcuni AS, Morelli V, Eller Vainicher C, Palmieri S, Cairoli E, Spada A, Scillitani A, Chiodini I. Adrenalectomy reduces the risk of vertebral fractures in patients with monolateral adrenal incidentalomas and subclinical hypercortisolism. Eur J Endocrinol. 2016;174:261–9. doi: 10.1530/EJE-15-0977. [DOI] [PubMed] [Google Scholar]
- 24.Limumpornpetch P, Morgan AW, Tiganescu A, Baxter PD, Nyawira Nyaga V, Pujades-Rodriguez M, Stewart PM. The effect of endogenous cushing syndrome on all-cause and cause-specific mortality. J Clin Endocrinol Metab. 2022;107:2377–2388. doi: 10.1210/clinem/dgac265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Haalen FM, Broersen LH, Jorgensen JO, Pereira AM, Dekkers OM. Management of endocrine disease: mortality remains increased in Cushing’s disease despite biochemical remission: a systematic review and meta-analysis. Eur J Endocrinol. 2015;172:R143–9. doi: 10.1530/EJE-14-0556. [DOI] [PubMed] [Google Scholar]
- 26.Bernini G, Moretti A, Iacconi P, Miccoli P, Nami R, Lucani B, Salvetti A. Anthropometric, haemodynamic, humoral and hormonal evaluation in patients with incidental adrenocortical adenomas before and after surgery. Eur J Endocrinol. 2003;148:213–9. doi: 10.1530/eje.0.1480213. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Zhu Y, Wang Z, Liu C, Liu S, Li X, Chen R, Zhan Y, Wang S, Zeng X. Hypertension Resolution after laparoscopic adrenal tumor resection in patients of adrenal ıncidentaloma with normal hormone levels. Urol Int. 2023;107:193–201. doi: 10.1159/000524803. [DOI] [PubMed] [Google Scholar]
- 28.Peppa M, Boutati E, Koliaki C, Papaefstathiou N, Garoflos E, Economopoulos T, Hadjidakis D, Raptis SA. Insulin resistance and metabolic syndrome in patients with nonfunctioning adrenal incidentalomas: a cause-effect relationship? Metabolism. 2010;59:1435–41. doi: 10.1016/j.metabol.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Papanastasiou L, Alexandraki KI, Androulakis II, Fountoulakis S, Kounadi T, Markou A, Tsiavos V, Samara C, Papaioannou TG, Piaditis G, Kaltsas G. Concomitant alterations of metabolic parameters, cardiovascular risk factors and altered cortisol secretion in patients with adrenal incidentalomas during prolonged follow-up. Clin Endocrinol (Oxf) 2017;86:488–498. doi: 10.1111/cen.13294. [DOI] [PubMed] [Google Scholar]
- 30.Karatas S, Hacioglu Y, Beysel S. Metabolic syndrome and Visceral Adiposity Index in non-functional adrenal adenomas. Arch Endocrinol Metab. 2023;67:323–329. doi: 10.20945/2359-3997000000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Athanasouli F, Georgiopoulos G, Asonitis N, Petychaki F, Savelli A, Panou E, Angelousi A. Nonfunctional adrenal adenomas and impaired glucose metabolism: a systematic review and meta-analysis. Endocrine. 2021;74:50–60. doi: 10.1007/s12020-021-02741-x. [DOI] [PubMed] [Google Scholar]
- 32.Di Dalmazi G, Vicennati V, Rinaldi E, Morselli-Labate AM, Giampalma E, Mosconi C, Pagotto U, Pasquali R. Progressively increased patterns of subclinical cortisol hypersecretion in adrenal incidentalomas differently predict major metabolic and cardiovascular outcomes: a large cross-sectional study. Eur J Endocrinol. 2012;166:669–77. doi: 10.1530/EJE-11-1039. [DOI] [PubMed] [Google Scholar]
- 33.Patrova J, Kjellman M, Wahrenberg H, Falhammar H. Increased mortality in patients with adrenal incidentalomas and autonomous cortisol secretion: a 13-year retrospective study from one center. Endocrine. 2017;58:267–275. doi: 10.1007/s12020-017-1400-8. [DOI] [PubMed] [Google Scholar]
- 34.Arruda M, Mello Ribeiro Cavalari E, Pessoa de Paula M, Fernandes Cordeiro de Morais F, Furtado Bilro G, Alves Coelho MC, de Oliveira E Silva de Morais NA, Choeri D, Moraes A, Vieira Neto L. The presence of nonfunctioning adrenal incidentalomas increases arterial hypertension frequency and severity, and is associated with cortisol levels after dexamethasone suppression test. J Hum Hypertens. 2017;32:3–11. doi: 10.1038/s41371-017-0011-4. [DOI] [PubMed] [Google Scholar]
- 35.Valassi E. Clinical presentation and etiology of Cushing’s syndrome: data from ERCUSYN. J Neuroendocrinol. 2022;34:e13114. doi: 10.1111/jne.13114. [DOI] [PubMed] [Google Scholar]
- 36.Rossi R, Tauchmanova L, Luciano A, Di Martino M, Battista C, Del Viscovo L, Nuzzo V, Lombardi G. Subclinical Cushing’s syndrome in patients with adrenal incidentaloma: clinical and biochemical features. J Clin Endocrinol Metab. 2000;85:1440–8. doi: 10.1210/jcem.85.4.6515. [DOI] [PubMed] [Google Scholar]
- 37.Salehidoost R, Korbonits M. Glucose and lipid metabolism abnormalities in Cushing’s syndrome. J Neuroendocrinol. 2022;34:e13143. doi: 10.1111/jne.13143. [DOI] [PubMed] [Google Scholar]
- 38.Moraes AB, Cavalari EMR, de Paula MP, Arruda M, Curi DSC, Leitão RA, de Mendonça LMC, Farias MLF, Madeira M, Vieira Neto L. Evaluation of body composition using dual-energy X-ray absorptiometry in patients with non-functioning adrenal incidentalomas and an intermediate phenotype: is there an association with metabolic syndrome? J Endocrinol Invest. 2019;42:797–807. doi: 10.1007/s40618-018-0985-y. [DOI] [PubMed] [Google Scholar]
- 39.Araujo-Castro M, Robles Lázaro C, Parra Ramírez P, Cuesta Hernández M, Sampedro Núñez MA, Marazuela M. Cardiometabolic profile of non-functioning and autonomous cortisol-secreting adrenal incidentalomas. Is the cardiometabolic risk similar or are there differences? Endocrine. 2019;66:650–659. doi: 10.1007/s12020-019-02066-w. [DOI] [PubMed] [Google Scholar]
- 40.Olsen H, Nordenström E, Bergenfelz A, Nyman U, Valdemarsson S, Palmqvist E. Subclinical hypercortisolism and CT appearance in adrenal incidentalomas: a multicenter study from Southern Sweden. Endocrine. 2012;42:164–73. doi: 10.1007/s12020-012-9622-2. [DOI] [PubMed] [Google Scholar]
- 41.Vassilatou E, Vryonidou A, Michalopoulou S, Manolis J, Caratzas J, Phenekos C, Tzavara I. Hormonal activity of adrenal incidentalomas: results from a long-term follow-up study. Clin Endocrinol (Oxf) 2009;70:674–9. doi: 10.1111/j.1365-2265.2008.03492.x. [DOI] [PubMed] [Google Scholar]
- 42.Falcetta P, Orsolini F, Benelli E, Agretti P, Vitti P, Di Cosmo C, Tonacchera M. Clinical features, risk of mass enlargement, and development of endocrine hyperfunction in patients with adrenal incidentalomas: a long-term follow-up study. Endocrine. 2021;71:178–188. doi: 10.1007/s12020-020-02476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Araujo-Castro M, Robles Lázaro C, Parra Ramírez P, García Centeno R, Gracia Gimeno P, Fernández-Ladreda MT, Sampedro Núñez MA, Marazuela M, Escobar-Morreale HF, Valderrabano P. Maximum adenoma diameter, regardless of uni- or bilaterality, is a risk factor for autonomous cortisol secretion in adrenal incidentalomas. J Endocrinol Invest. 2021;44:2349–2357. doi: 10.1007/s40618-021-01539-y. [DOI] [PubMed] [Google Scholar]