Abstract
Isoprene is formed in and emitted by plants and the reason for this apparent carbon waste is still unclear. It has been proposed that isoprene stabilizes cell and particularly chloroplast thylakoid membranes. We tested if membrane stabilization or isoprene reactivity with ozone induces protection against acute ozone exposures. The reduction of visible, physiological, anatomical, and ultrastructural (chloroplast) damage shows that clones of plants sensitive to ozone and unable to emit isoprene become resistant to acute and short exposure to ozone if they are fumigated with exogenous isoprene, and that isoprene-emitting plants that are sensitive to ozone do not suffer damage when exposed to ozone. Isoprene-induced ozone resistance is associated with the maintenance of photochemical efficiency and with a low energy dissipation, as indicated by fluorescence quenching. This suggests that isoprene effectively stabilizes thylakoid membranes. However, when isoprene reacts with ozone within the leaves or in a humid atmosphere, it quenches the ozone concentration to levels that are less or non-toxic for plants. Thus, protection from ozone in plants fumigated with isoprene may be due to a direct ozone quenching rather than to an induced resistance at membrane level. Irrespective of the mechanism, isoprene is one of the most effective antioxidants in plants.
Isoprene (C5H8) emission is widespread in plants (Kesselmeier and Staudt, 1999). The biogenic emission of isoprene plays an important role in atmospheric chemistry because of isoprene reactivity with other gases (Fuentes et al., 2000). In the presence of anthropogenic nitrogen oxides and sunlight, isoprene breakdown leads to tropospheric ozone (Chameides et al., 1988). Isoprene can also react directly with ozone. This reaction breaks down isoprene primarily to methyl vinyl ketone methacrolein and formaldehyde, also yielding moderate amounts of hydrogen peroxide and other oxidative species (Sauer et al., 1999; Fuentes et al., 2000; Ruppert and Becker, 2000).
The biochemistry of isoprene formation is now elucidated (Lichtenthaler et al., 1997), but the role of isoprene in plants is unclear (Sharkey and Yeh, 2001). The finding that endogenous and exogenous isoprene increases the thermotolerance of leaves (Sharkey and Singsaas, 1995; Singsaas et al., 1997) suggested that isoprene is formed to protect plants against environmental stresses. This view has been challenged because thermotolerance is often absent in excised leaves (Logan and Monson, 1999), but this view has also recently received additional support by experiments showing a similar protective effect for endogenous and exogenous monoterpenes (Loreto et al., 1998; Delfine et al., 2000) and a regular protective effect in leaves exposed to short and repeated heat bursts rather than to prolonged exposure to heat (Singsaas and Sharkey, 1998; Singsaas et al., 1999). There are several hypotheses as to why isoprene should have such a protective action (Sharkey, 1996; Sharkey and Yeh, 2001). It is likely that isoprene stabilizes the membrane lipid bilayer, which is often denatured by exposure to high temperatures (Gounaris et al., 1984). Moreover, isoprene likely supplies substrates for protein prenylation, one of the main mechanisms that anchors proteins to the lipids in biological membranes (Yalovsky et al., 1999). However, it is not known if this effect is ubiquitous or exclusive of chloroplast (thylakoid) membranes where isoprene is formed and presumably resides (Wildermuth and Fall, 1998).
Ozone and its reaction products (OḢ, O , and H2O2) are toxic to plants (Pell et al., 1997). As for high temperatures, exposure to elevated ozone concentrations causes the peroxidation and denaturation of membrane lipids (Maccarrone et al., 1992; Ranieri et al., 1996; Wellburn and Wellburn, 1996; Ederli et al., 1997; Pell et al., 1997). If isoprene stabilizes membranes, it may avoid, reduce, or delay ozone damage. Because of its reactivity (Fuentes et al., 2000), particularly in wet environments (Sauer et al., 1999), isoprene may also directly scavenge ozone in leaves. Provided that the products of this reaction are less toxic or not sufficiently concentrated in the leaves, this would also enhance plant resistance to ozone. To test if isoprene is involved in ozone resistance mechanisms we exposed leaves of two ozone-sensitive genotypes of tobacco (Nicotiana tabacum cv Bel-W3; Haggestad, 1991) and birch (Betula pendula clone 80; Pääkkonen et al., 1993) to ozone, with or without the addition of exogenous isoprene. These two plant species do not form and emit endogenous isoprene (Kesselmeier and Staudt, 1999), as we also accurately checked (see “Materials and Methods”). As an internal control, we exposed to the same ozone treatment leaves of an ozone-sensitive genotype of poplar (Populus deltoides × maximowiczii clone Eridano; Lorenzini et al., 1999), a plant species that emits isoprene and should, therefore, be naturally protected from ozone damages. We report about visible, physiological, anatomical, and ultrastructural evidence that exogenous isoprene, at levels that are physiological inside the leaves, reduces ozone damage, and we indicate the possible mechanisms by which this resistance to ozone may occur.
, and H2O2) are toxic to plants (Pell et al., 1997). As for high temperatures, exposure to elevated ozone concentrations causes the peroxidation and denaturation of membrane lipids (Maccarrone et al., 1992; Ranieri et al., 1996; Wellburn and Wellburn, 1996; Ederli et al., 1997; Pell et al., 1997). If isoprene stabilizes membranes, it may avoid, reduce, or delay ozone damage. Because of its reactivity (Fuentes et al., 2000), particularly in wet environments (Sauer et al., 1999), isoprene may also directly scavenge ozone in leaves. Provided that the products of this reaction are less toxic or not sufficiently concentrated in the leaves, this would also enhance plant resistance to ozone. To test if isoprene is involved in ozone resistance mechanisms we exposed leaves of two ozone-sensitive genotypes of tobacco (Nicotiana tabacum cv Bel-W3; Haggestad, 1991) and birch (Betula pendula clone 80; Pääkkonen et al., 1993) to ozone, with or without the addition of exogenous isoprene. These two plant species do not form and emit endogenous isoprene (Kesselmeier and Staudt, 1999), as we also accurately checked (see “Materials and Methods”). As an internal control, we exposed to the same ozone treatment leaves of an ozone-sensitive genotype of poplar (Populus deltoides × maximowiczii clone Eridano; Lorenzini et al., 1999), a plant species that emits isoprene and should, therefore, be naturally protected from ozone damages. We report about visible, physiological, anatomical, and ultrastructural evidence that exogenous isoprene, at levels that are physiological inside the leaves, reduces ozone damage, and we indicate the possible mechanisms by which this resistance to ozone may occur.
RESULTS
In treatment 1, acute (300 ppb) and short (3 h) exposure to ozone significantly decreased photosynthesis of tobacco and birch leaves and the effect was exacerbated 12 h after the end of the treatment (Table I). In treatment 2, 3 ppm of gaseous isoprene was mixed with the ozone-enriched air flowing over the leaf. The leaves exposed to this treatment did not show a significant reduction of photosynthesis at the end of ozone exposure. After 12 h, photosynthesis was reduced to a significantly less extent than in leaves only exposed to ozone. No photosynthesis inhibition was observed in the last fully expanded leaves of 2-year-old poplar emitting 30 ± 4 nmol m−2 s−1 of isoprene after exposing them to treatment 1 (Table I).
Table I.
Ozone effect on net photosynthesis of intact leaves of two nonisoprene-emitting plant species (tobacco and birch) and an isoprene-emitting plant species (poplar)
| Time | Tobacco Photosynthesis
|
Birch Photosynthesis
|
Poplar Photosynthesis
|
||
|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | 1 | |
| μmol m−2 s−1 | |||||
| Before O3 fumigation | 12.1 ± 1.5a | 11.9 ± 1.7a | 12.5 ± 1.3a | 12.7 ± 1.1a | 10.4 ± 2.6a |
| After a 3-h O3 fumigation | 6.6 ± 1.0b | 11.4 ± 1.8a | 7.1 ± 1.0b | 10.2 ± 1.7a | 9.9 ± 2.1a |
| After a 12-h recovery from O3 | 3.9 ± 0.9c | 7.9 ± 1.8b | 5.4 ± 0.8c | 12.3 ± 3.6a | 9.7 ± 2.2a |
A 7-cm2 portion of the leaf lamina was enclosed in a Teflon-coated gas-exchange cuvette and fumigated for 3 h with 300 mL L−1 of ozone (1) or with 300 ppm ozone and 3 ppm isoprene (2). In poplar, only treatment 1 was carried out. Photosynthesis was measured before O3 fumigation at the end of the 3-h fumigation and after a 12-h recovery from fumigation. Means (n = 5) ± ses are shown. Values followed by different letters are significantly different within the same column at the 5% level as tested by t test.
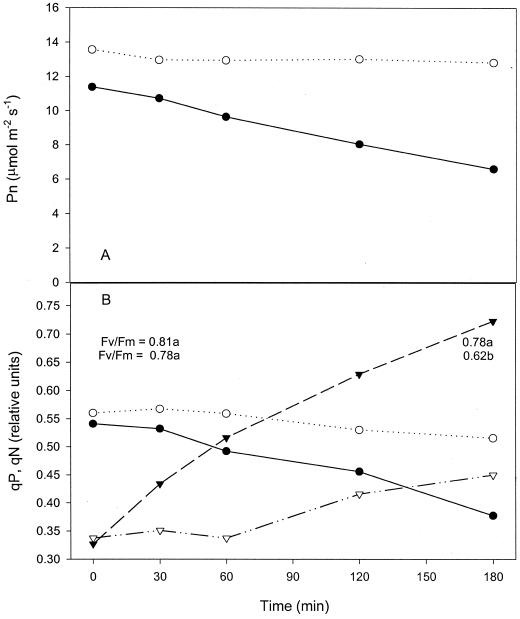
We followed fluorescence and gas exchange during the ozone treatment of tobacco leaves to investigate the origin of the ozone damage and to understand if photosynthesis was constantly protected by the addition of isoprene. Photosynthesis was stable over the whole length of the ozone treatment when isoprene was added, whereas it decreased at a constant rate 30 min after starting the ozone treatment in leaves only exposed to ozone (Fig. 1A). This trend was exactly mirrored by the photochemical quenching of fluorescence (qP; Fig. 1B). The stability of qP and of the Fv/Fm in leaves fumigated with isoprene denotes that the photochemical apparatus remained fully preserved and was not affected by the ozone treatment. In contrast, in leaves treated with ozone only, the Fv/Fm monitored after the exposure was significantly lower than before the exposure. The non-photochemical quenching of fluorescence (qN) increased significantly from the beginning of the ozone treatment in leaves of treatment 1, but in leaves fumigated with isoprene, a moderate increase in qN was observed only after 60 min (Fig. 1B).
Figure 1.
Photosynthesis (Pn, A) and photochemical (qP, ○ and ●) and non-photochemical (qN, ▵ and ▴) fluorescence quenchings (B) of two tobacco leaves. One of the leaves was fumigated for 3 h with 300 ppb of ozone (black symbols) and the other was fumigated with 300 ppb of ozone and 3 ppm of isoprene (white symbols). The mean fluorescence yields (Fv/Fm, relative units) measured before and after the two treatments in dark adapted samples (n = 5) are also reported, and values followed by different letters are significantly different at the 5% level as tested by t test.
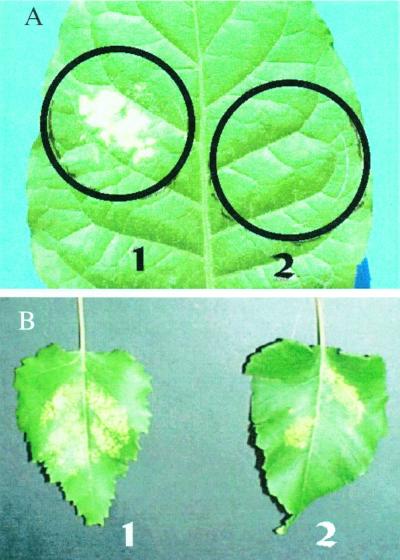
After 3 d, the ozone damage was visible in large areas of the exposed leaves (Fig. 2). However, no damage was observed in tobacco leaves that were also fumigated with isoprene. Isoprene fumigation also dramatically reduced (an average of −60%) the necrotic areas observed in birch leaves after ozone fumigation.
Figure 2.
Ozone damage in tobacco (A) and birch (B) leaves fumigated for 3 h with 300 ppb of ozone (1) or with 300 ppb ozone and 3 ppm isoprene (2). In tobacco, both treatments were carried out in the same leaf to remove possible leaf-to-leaf variability. The tobacco leaf discs exposed to ozone (7 cm2) are circled. In birch, almost all leaf was exposed to the treatment.
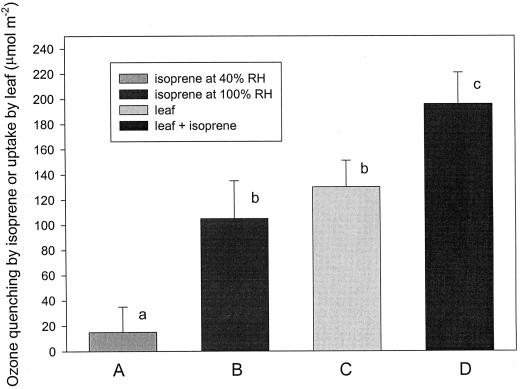
We measured the ozone uptake by the leaf in absence and in presence of isoprene. Over the 3-h exposure, the ozone uptake by the leaf was significantly lower than that observed when the leaf was exposed simultaneously to ozone and isoprene (Fig. 3). Isoprene itself was not able to remove a considerable amount of ozone when the cuvette was empty, but this amount increased remarkably when a saturating humidity, such as in the leaf mesophyll, was reproduced. In this case the ozone removed by isoprene was comparable with the uptake of ozone by the leaf (Fig. 3).
Figure 3.
Ozone quenching by isoprene (3 ppm) in a 7-cm2 cuvette (empty or with a dry paper leaf replica) where the relative humidity was set at 40% (A), and in the same cuvette with a leaf replica made of wet paper, which generated a 100% relative humidity (B). Ozone uptake by 7 cm2 of tobacco leaf fumigated with 300 ppb ozone (C) or simultaneously with 3 ppm of isoprene and 300 ppb ozone (D). The ozone uptake was calculated integrating the instantaneous uptake over the 3-h ozone fumigation and referring it to a 1-m2 surface (of real leaf or of leaf paper replica). Means (n = 5) ± ses are shown. Values followed by different letters are significantly different at the 5% level as tested by t test.
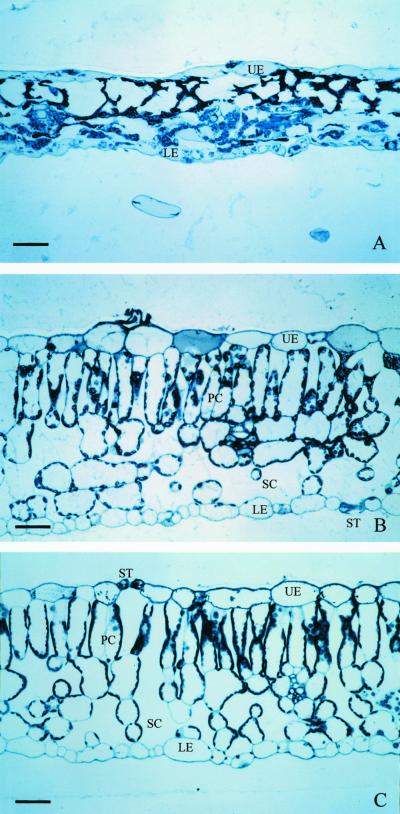
The mesophyll of tobacco leaves exposed to treatment 1 almost completely collapsed with the disappearance of palisade and spongy tissues and the formation of abundant empty spaces (Fig. 4A). The serious damage in the mesophyll of ozone-treated leaves was also indicated by the remarkable reduction in the leaf thickness, whereas epidermal cells collapsed later than mesophyll cells. In a converse manner, the mesophyll of tobacco leaves exposed to ozone and isoprene (Fig. 4B) maintained the anatomy arrangements of control leaves (the leaves that were not exposed to ozone or to ozone and isoprene, Fig. 4C), and only limited damage could be occasionally observed.
Figure 4.
Light micrographs of transverse semi-thin sections of tobacco leaves fumigated with 300 ppb ozone (A), simultaneously with 3 ppm of isoprene and 300 ppb ozone (B), or nonexposed to ozone and isoprene (C). All bars = 40 μm. UE, Upper epidermis; LE, lower epidermis; ST, stomata; PC, palisade cells; SC, spongy cells.
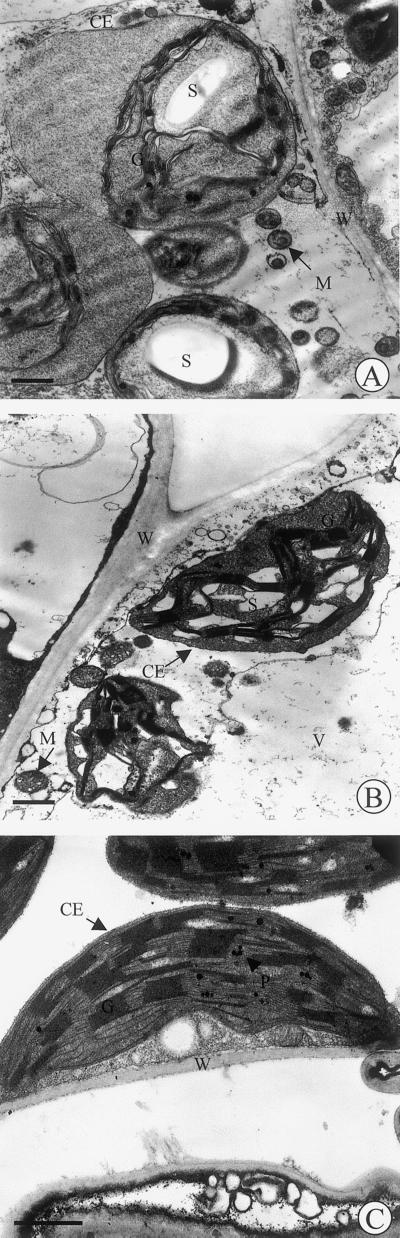
Ultrastructural observations revealed that the chloroplast membranes were often disrupted in leaves exposed to treatment 1 (Fig. 5A). When the chloroplast envelope was still present, the thylakoidal systems shrank and part of chloroplast remained virtually free of thylakoids. The thylakoid membranes were reduced and unstacked and the chloroplasts were swelled, assuming a round shape. In leaves exposed to treatment 2, the chloroplasts maintained their typical elliptical shape and the thylakoids were regularly appressed and stacked (Fig. 5B). Intact mitochondria could also be distinctly observed in these cells. Only the appearance of numerous and small starch grains made the leaves exposed to treatment 2 different from control leaves (Fig. 5C).
Figure 5.
Electron micrographs of transverse ultra-thin sections of tobacco leaves fumigated with 300 ppb ozone (A) or simultaneously with 3 ppm of isoprene and 300 ppb ozone (B,), or nonexposed to ozone and isoprene (C). All bars = 1 μm. CE, Chloroplast envelope; M, mitochondria; S, starch; T, tonoplast; V, vacuole; W, wall.
DISCUSSION
The reason why plants emit isoprene is currently under debate. Several experiments have shown that volatile isoprenoids (isoprene and monoterpenes) protect leaves against bursts of heat (Sharkey and Singsaas, 1995; Singsaas et al., 1997; Loreto et al., 1998; Delfine et al., 2000). An increased thermotolerance, however, was not found in leaf discs when these were detached by the plants (Logan and Monson, 1999). Our experiments demonstrate that isoprene protects leaves exposed to high ozone episodes and suggest that isoprene is an effective antioxidant in leaves.
The isoprene-induced thermotolerance has been attributed to a protective action of isoprene on thylakoid membranes. Membrane lipid bilayers, or the interaction between lipids and protein complexes, would be strengthened by isoprene and, as a result, membranes would resist denaturation (Sharkey, 1996). Membrane strengthening could also be invoked to explain the protective effect of isoprene against ozone. Ozone exposure leads to the peroxidation of membrane lipids (Maccarrone et al., 1992; Ranieri et al., 1996; Wellburn and Wellburn, 1996; Ederli et al., 1997; Pell et al., 1997), which may be counteracted by the presence of isoprene. Logan et al. (1999) reported that isoprene did not protect natural and artificial thylakoids from peroxidation. In our intact leaves exposed to ozone and isoprene, the Fv/Fm revealed a stability of the photochemical apparatus and the qN and qP indicated the maintenance of low energy dissipation status. Thus, we think that isoprene may effectively preserve the photochemical apparatus embedded in the thylakoid membrane, a role also accomplished by another class of isoprenoids, the xanthophylls (Demmig-Adams and Adams, 1996). It should be tested if the low qN maintained by leaves exposed to treatment 2 during the first 60 min of ozone fumigation and its low increase thereafter is associated to the xanthophyll functionality as well as to the isoprene treatment.
If ozone is mainly decomposed at the cell wall and plasma membrane (Laisk et al., 1989), then the negative effect of ozone should be particularly visible on these structures, leading to the loss of membrane semipermeability and eventually to plasmolysis and cell death (Pell et al., 1997). The anatomical and ultrastructural observations clearly indicate that isoprene also effectively preserved mesophyll structure, chloroplast envelopes, and thylakoid assemblage from ozone damage. These observations show that the action of isoprene is eventually able to avoid ozone damage at various levels, but is not able to clarify if the protection occurred at a particular (e.g. chloroplast) structural level.
We wondered if in addition or alternate to its action at the thylakoid level, isoprene may have directly quenched ozone decreasing the ozone pressure over the membranes. In an environment where a moderate relative humidity was maintained, such as in an empty cuvette or in a cuvette with a leaf replica made by dry paper, isoprene removed a small amount of ozone (Fig. 3). This was insufficient to lower ozone to concentrations non-toxic for the plants, but confirmed that isoprene may efficiently react with ozone (Sauer et al., 1999; Fuentes et al., 2000). The residence time of the two gases in the cuvette was very small (about 10 s). The ozone reacting with isoprene would certainly have been higher if the residence time were longer.
Over the 3-h exposure, the ozone uptake by the leaf was significantly lower than that observed when exposing the leaf to ozone and isoprene simultaneously (Fig. 3). If isoprene simply acted as a membrane strengthener, we would have expected a lower ozone uptake, directly associated with a reduced membrane lipid peroxidation, upon exposure to isoprene and ozone. To explain the surprising enhancement of ozone uptake in leaves exposed to isoprene and ozone we monitored the ozone quenching by isoprene in a cuvette in which an environment with saturating humidity, such as in the leaf mesophyll, was reproduced. In this case the ozone removed by isoprene was comparable with the uptake of ozone by the leaf (Fig. 3). We, therefore, conclude that isoprene can directly remove ozone, particularly if the reaction occurs in a humid environment such as in the leaf mesophyll. The enhancement of ozone uptake in a humid environment was expected on the basis of previous analytical reaction experiments of isoprene in air (Sauer et al., 1999).
Whether ozone quenching by exogenous isoprene occurs at the leaf surface, in the intercellular spaces (where the concentration of isoprene was probably one order of magnitude less than in the air because of the stomatal resistances, and close to the concentration expected in leaves naturally emitting isoprene; Singsaas et al., 1997), or within the membranes where isoprene is likely embedded because of its lipophylic properties (Sharkey, 1996), cannot be conclusively clarified with this experiment. However, it can be suggested that endogenous isoprene exerts its protective role in the close proximity of chloroplast membranes where it is presumably formed (Lichtenthaler et al., 1997; Wildermuth and Fall, 1998) and can be found at concentrations similar or even higher than those used in our experiment (Singsaas et al., 1997).
It has been hypothesized that hydroperoxides produced by the reaction between ozone and isoprene may contribute to damage leaves (Hewitt et al., 1990; Sauer et al., 1999). We show an opposite effect. It is possible that the isoprene entering the intercellular spaces was not enough to produce high amounts of hydroperoxides in our experiment, or that they are less toxic or are scavenged more efficiently than ozone in leaves. If hydroperoxides were formed and were toxic, isoprene-emitting plants (e.g. poplar) should suffer ozone damage more than nonemitters. However, we did not find visible damage or photosynthesis inhibition in the leaves of 2-year-old poplar emitting 30 ± 4 nmol m−2 s−1 of isoprene after a 3-h exposure to 300 ppb of ozone (Table I). Photosynthesis was inhibited only 8 h after starting the exposure to ozone. We, therefore, conclude that endogenous isoprene production by leaves has an important antioxidant role.
MATERIALS AND METHODS
Plant Material
Tobacco (Nicotiana tabacum cv Bel-W3), birch (Betula pendula clone 80), and poplar (Populus deltoides × maximowiczii clone Eridano) plants were used. Ten-week-old tobacco plants were grown in 2-L pots and 2-year-old plants of birch and poplar were grown in 20-L pots. All pots were filled with sand:soil (1:1). Plants were watered every day to soil/water capacity and were fertilized once a week with a full strength Hoagland solution. Plants were grown in a greenhouse under the environmental conditions typical of spring in central Italy. Day/night mean temperatures were 28°C/18°C and day light intensity did not exceed 1,000 μmol photons m−2 s−1 because the greenhouse was sheltered with a shading net to reduce daily evapotranspiration.
Ozone and Ozone and Isoprene Treatments
The last fully expanded leaf was used in all plants. A 7-cm2 leaf portion was enclosed in a Teflon-coated gas-exchange cuvette and was exposed to a flow of 0.5 L min−1 of air (80% N2, 20% O2, and 350 ppm CO2). The air was generated from pure gases and did not contain ozone or other contaminants. The leaf temperature was set at 25°C, the relative humidity was set at 40%, and the light intensity was set at 800 μmol photons m−2 s−1 as described previously (Loreto and Delfine, 2000). When photosynthesis was stable, the leaf disc was fumigated for 3 h with 300 ppb of ozone (treatment 1) or with 300 ppb ozone and 3 ppm isoprene (treatment 2). The ozone was generated by flowing the 20% O2 of the air mixture through a UV lamp. The lamp intensity was adjusted with a potentiometer until 300 ppb of ozone at the outlet of the empty cuvette was read by the ozone detector (series 1108, Dasibi, Glendale, CA). Isoprene fumigation was carried out by exploiting the natural evaporation of isoprene from a liquid standard (99% purity, Fluka, Milwaukee, WI). A diffusion tube containing the liquid standard was placed at the cuvette inlet and was maintained at a constant temperature (30°C) to vaporize a constant part of the compound. The concentration of gaseous isoprene mixing with the incoming air was measured on-line by gas chromatography (GC 855, PID detector, Syntech, Groningen, The Netherlands), as described elsewhere (Loreto and Delfine, 2000) and was set at 3 ppm by adjusting the quantity of liquid isoprene in the tube. This concentration should yield, after passing stomatal and mesophyll resistances, an internal concentration about 10-fold lower, and similar to the concentration physiologically contained in the leaves of isoprene-emitting species (Singsaas et al., 1997).
No isoprene and monoterpenes were detected by on-line gas chromatography in the cuvette outlet when tobacco and birch leaves were clamped. The procedure for isoprenoid measurement has been described in detail (Loreto and Delfine, 2000) and was also used to quantify the isoprene emission from poplar leaves. No isoprenoids emission from tobacco and birch leaves were detected by highly sensitive (detection limit = < 0.001 nmol m−2 s−1) gas chromatography-mass spectrometry after concentrating 5 L of air exiting the cuvette in a carbon cartridge (Carbotrap, Supelco, Bellefonte, PA; R. Baraldi, personal communication).
Ozone Damage Evaluation
Net photosynthesis was calculated from the CO2 uptake measured with a gas analyzer (6262 IR, LI-COR, Lincoln, NE), as detailed elsewhere (Loreto and Delfine, 2000). The Fv/Fm in dark-adapted leaves before and after the treatments and the qP and qN, in illuminated leaves were measured as reported by van Kooten and Snel (1990) with a modulated fluorometer (PAM 2000, Walz, Effeltrich, Germany). The fluorescence probe was appressed to the illuminated leaf without shading it, as explained elsewhere (Loreto and Delfine, 2000). The photosynthesis inhibition consequent to ozone was measured immediately after the end of the treatment and after a 12-h overnight recovery. In tobacco leaves, photosynthesis and qN and qP were measured every 30 min during the treatments.
Ozone-visible damage was recorded after 3 d by photography with a digital camera (DC 120, Eastman-Kodak, Rochester, NY). Visible damage was assessed on five leaves per treatment. Ozone-induced necroses of the leaf lamina in presence or in absence of isoprene were compared by separating the damaged areas with computer software (DS 1D Scientific Imaging System, Kodak).
Anatomical and Ultrastructural Observations
Histological observations were carried out in tobacco leaves that had recovered from ozone fumigation 12 h. Tissue pieces (1–2 mm2) were excised from tobacco leaves and were immediately fixed in 3% (w/v) glutaraldehyde in 0.1 m phosphate buffer, pH 7.2, for 3 h. Samples were then washed three times for 15 min each in 0.1 m phosphate buffer, pH 7.2, and were post-fixed in 1% (w/v) OsO4. At this stage, samples were dehydrated in increasing concentrations of ethanol and were then included in resin (Epon, 2-dodecenylsuccinic anhydride, and methylnadic anhydrid mixture). A pre-inclusion at room temperature in increasing concentrations of resin dissolved in propylene oxide was followed by the final inclusion in freshly prepared resin followed by the polymerization at 35°C for 12 h, 45°C for 12 h, and at 60°C for 12 h. Semi-thin (1–2 μm) and ultra-thin (70–90 nm) sections were cut with an ultramicrotome (Reichter, Heidelberg) equipped with a glass blade. The semi-thin sections were stained with toluidine blue and were observed under a light microscope (Dialux 20, Leica, Wetzlar, Germany) equipped with a system to take micrographs. The ultrathin sections were mounted on uncoated copper grids (200 mesh) and were contrasted by adding uranile acetate and an aqueous solution of lead nitrate before observation with a transmission electron microscope (TEM 400 T, Philips, Monza, Italy).
Ozone Quenching by Isoprene and Ozone Uptake by Leaf
A bypass valve was installed to regularly bypass the cuvette and to read the ozone concentration in the air at the cuvette inlet and outlet. The difference between these two values is the instantaneous ozone quenching by isoprene, or ozone uptake by the leaf (treatment 1) or by the leaf and isoprene (treatment 2). The instantaneous uptake was monitored every 15 min and was then integrated over the 3-h ozone treatment to calculate the total ozone uptake shown in Figure 3. In the empty cuvette maintained at the humidity experienced by the leaf (40% relative humidity), isoprene removed a limited amount of ozone (15 ppb). To compensate for this uptake, a slightly higher ozone concentration (315 ppb) was used when fumigating isoprene. To check the ozone uptake by isoprene at saturating humidity, a leaf replica made by wet paper was placed in the cuvette and was fumigated with ozone and isoprene as for treatment 2.
Statistical Analysis
Each treatment was replicated five times. In the table and in Figure 3, means ± se are shown. Values followed by different letters are significantly different at the 5% level as tested by t test.
Footnotes
This work was supported by the European Union-International Cooperation Programme (project no. IC5–CT98–0102).
LITERATURE CITED
- Chameides WL, Lindsay RW, Richardson J, Kiang CS. The role of biogenic hydrocarbons in urban photochemical smog: Atlanta as a case study. Science. 1988;241:1473–1475. doi: 10.1126/science.3420404. [DOI] [PubMed] [Google Scholar]
- Delfine S, Csiky O, Seufert G, Loreto F. Fumigation with exogenous monoterpenes of a non-isoprenoid-emitting oak (Quercus suber): monoterpene acquisition, translocation, and effect on the photosynthetic properties at high temperatures. New Phytol. 2000;146:27–36. [Google Scholar]
- Demmig-Adams B, Adams WW., III The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci. 1996;1:21–26. [Google Scholar]
- Ederli L, Pasqualini S, Batini P, Antonielli M. Photoinhibition and oxidative stress: effects on xanthophyll cycle, scavenger enzymes and abscisic acid content in tobacco plants. J Plant Physiol. 1997;151:422–428. [Google Scholar]
- Fuentes JD, Lerdau M, Atkinson R, Baldocchi D, Botteneheim JW, Ciccioli P, Lamb B, Geron C, Gu L, Guenther A. Biogenic hydrocarbons in the atmosphere boundary layer: a review. Bull Am Met Soc. 2000;81:1537–1575. [Google Scholar]
- Gounaris K, Brain APR, Quinn PJ, Williams WP. Structural reorganization of chloroplast thylakoid membranes in response to heat stress. Biochim Biophys Acta. 1984;766:198–208. [Google Scholar]
- Haggestad HE. Origin of Bel-W3, Bel-C and Bel-B tobacco varieties and their use as indicators of ozone. Environ Pollut. 1991;74:264–291. doi: 10.1016/0269-7491(91)90076-9. [DOI] [PubMed] [Google Scholar]
- Hewitt CN, Kok GL, Fall R. Hydroperoxides inplants exposed to ozone mediate air pollution damage to alkene emitters. Nature. 1990;344:56–58. doi: 10.1038/344056a0. [DOI] [PubMed] [Google Scholar]
- Kesselmeier J, Staudt M. Biogenic volatile organic compounds (VOC): an overview on emission, physiology and ecology. J Atmos Chem. 1999;33:23–88. [Google Scholar]
- Laisk A, Kull O, Moldau H. Ozone concentration in leaf intercellular spaces is close to zero. Plant Physiol. 1989;90:1163–1167. doi: 10.1104/pp.90.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler HK, Schwendler J, Disch A, Rohmer M. Biosynthesis of isoprenoids in higher plant chloroplasts proceeds via a mevalonate-independent pathway. FEBS Lett. 1997;400:271–274. doi: 10.1016/s0014-5793(96)01404-4. [DOI] [PubMed] [Google Scholar]
- Logan BA, Anchordoquy TJ, Monson RK, Pan RS. The effect of isoprene on the properties of spinach thylakoids and phosphatidylcholine liposomes. Plant Biol. 1999;1:602–606. [Google Scholar]
- Logan BA, Monson RK. Thermotolerance of leaf discs from four isoprene-emitting species is not enhanced by exposure to exogenous isoprene. Plant Physiol. 1999;120:821–825. doi: 10.1104/pp.120.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzini G, Guidi L, Nali C, Soldatini GF. Quenching analysis in poplar clones exposed to ozone. Tree Physiol. 1999;19:607–612. doi: 10.1093/treephys/19.9.607. [DOI] [PubMed] [Google Scholar]
- Loreto F, Delfine S. Emission of isoprene from salt-stressed Eucalyptus globulus leaves. Plant Physiol. 2000;123:1605–1610. doi: 10.1104/pp.123.4.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Förster A, Dürr M, Csiky O, Seufert G. On the monoterpene emission under heat stress and on the increased thermotolerance of leaves of Quercus ilex L. fumigated with selected monoterpenes. Plant Cell Environ. 1998;21:101–107. [Google Scholar]
- Maccarrone M, Veldink GA, Vliegenthart JFG. Thermal injury and ozone stress affect soybean lipooxygenase expression. FEBS Lett. 1992;309:225–230. doi: 10.1016/0014-5793(92)80778-f. [DOI] [PubMed] [Google Scholar]
- Pääkkonen E, Paasisalo S, Holopainen T, Kärenlampi L. Growth and stomatal responses of birch (Betula pendula Roth.) to ozone in open-air and chamber fumigations. New Phytol. 1993;125:615–623. doi: 10.1111/j.1469-8137.1993.tb03911.x. [DOI] [PubMed] [Google Scholar]
- Pell EJ, Schlagnhaufer CD, Arteca RN. Ozone-induced oxidative stress: mechanisms of action and reaction. Physiol Plant. 1997;100:264–273. [Google Scholar]
- Ranieri A, D'Urso G, Nali C, Lorenzini G, Soldatini GF. Ozone stimulates apoplastic antioxidant systems in pumpkin leaves. Physiol Plant. 1996;97:381–387. [Google Scholar]
- Ruppert L, Becker KH. A product study of the OH radical-initiated oxidation of isoprene: formation of C-5-unsaturated diols. Atmos Env. 2000;34:1529–1542. [Google Scholar]
- Sauer F, Schafer C, Neeb P, Horie O, Moortgat GK. Formation of hydrogen peroxide in the ozonolysis of isoprene and simple alkenes under humid conditions. Atmos Env. 1999;33:229–241. [Google Scholar]
- Sharkey TD. Isoprene synthesis by plants and animals. Endeavor. 1996;20:74–78. doi: 10.1016/0160-9327(96)10014-4. [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Singsaas EL. Why plants emit isoprene. Nature. 1995;374:769. [Google Scholar]
- Sharkey TD, Yeh S (2001) Isoprene emission from plants. Annu Rev Plant Physiol Plant Mol Biol (in press) [DOI] [PubMed]
- Singsaas EL, Laporte MM, Shi JZ, Monson RK, Bowling DR, Johnson K, Lerdau M, Jasentuliyana A, Sharkey TD. Leaf temperature fluctuation affects isoprene emission from red oak (Quercus rubra) leaves. Tree Physiol. 1999;19:917–924. doi: 10.1093/treephys/19.14.917. [DOI] [PubMed] [Google Scholar]
- Singsaas EL, Lerdau M, Winter K, Sharkey TD. Isoprene increases thermotolerance of isoprene-emitting species. Plant Physiol. 1997;115:1413–1420. doi: 10.1104/pp.115.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singsaas EL, Sharkey TD. The regulation of isoprene emission responses to rapid leaf temperature fluctuations. Plant Cell Environ. 1998;21:1181–1188. [Google Scholar]
- van Kooten O, Snel JFH. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosyn Res. 1990;25:147–150. doi: 10.1007/BF00033156. [DOI] [PubMed] [Google Scholar]
- Wellburn FAM, Wellburn AR. Variable patterns of antioxidant protection but similar ethene emission differences between ozone-fumigated and control treatments in several ozone-sensitive and ozone-tolerant plant selections. Plant Cell Environ. 1996;19:754–760. [Google Scholar]
- Wildermuth MC, Fall R. Biochemical characterization of stromal and thylakoid-bound isoforms of isoprene synthase in willow leaves. Plant Physiol. 1998;116:1111–1123. doi: 10.1104/pp.116.3.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalovsky S, Rodriguez-Concepcion M, Gruissem W. Lipid modifications of proteins: slipping in and out of membranes. Trends Plant Sci. 1999;4:439–445. doi: 10.1016/s1360-1385(99)01492-2. [DOI] [PubMed] [Google Scholar]