Abstract
Objective: To evaluate the efficacy of levofloxacin combined with azithromycin in the treatment of cervicitis and its effect on serum inflammatory markers. Methods: A retrospective analysis was conducted on the clinical records of 102 patients with cervicitis treated at Gansu Provincial Maternity and Child-care Hospital between March 2022 and March 2023. The control group (47 patients) received azithromycin, while the study group (55 patients) was treated with a combination of levofloxacin and azithromycin. Serum levels of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and C-reactive protein (CRP) were measured before treatment and after two weeks. The efficacy, adverse reaction rate, and recurrence of cervicitis over a 6-month follow-up period were analyzed. A multiple logistic regression was performed to identify factors influencing recurrence. Results: Prior to treatment, no significant differences were observed between the two groups in IL-6, TNF-α, or CRP levels (all P>0.05). After treatment, both groups showed significant reductions in these markers (all P<0.0001), with the study group exhibiting more pronounced decreases (P<0.0001). The overall response rate in the study group was significantly higher than that of the control group (P=0.041). No significant difference in the incidence of adverse reactions was found between the groups (P=0.551). The recurrence rate of cervicitis was significantly higher in the control group compared to the study group (P=0.022). Logistic regression analysis identified disease severity (P=0.014, OR: 8.616; CI: 1.543-48.096), post-treatment IL-6 (P=0.003, OR: 17.573; CI: 2.720-113.531), post-treatment CRP (P=0.039, OR: 5.731; CI: 1.089-30.157), and post-treatment TNF-α (P=0.001, OR: 20.547; CI: 3.210-131.518) as independent predictors of recurrence. Conclusion: Levofloxacin combined with azithromycin is more effective than azithromycin monotherapy in treating cervicitis. The combination significantly reduces inflammatory responses and recurrence rates without increasing adverse effects, making it a valuable option for clinical use.
Keywords: Levofloxacin, azithromycin, cervicitis, efficacy, serum inflammatory factors
Introduction
Cervicitis is a common gynecologic condition, particularly prevalent in women of childbearing age [1]. Its clinical manifestations include purulent leukorrhea, tenderness, and cervical hyperemia [2]. In its early stages, cervicitis is often overlooked by patients, but as the disease progresses, it can lead to cervical erosion, polyps, and cysts, significantly affecting quality of life [3,4].
Current treatment strategies for cervicitis primarily involve antibiotics and topical therapies. Antibiotics target bacterial infections, while topical medications reduce inflammation, but both approaches have limitations, such as antibiotic resistance and incomplete treatment of underlying causes [5]. Macrolides and quinolones are commonly used antibiotics in the treatment of cervicitis [5]. Levofloxacin, a quinolone, has broad-spectrum antibacterial activity against Gram-negative and Gram-positive bacteria, as well as Chlamydia pneumoniae [6,7]. Azithromycin, a macrolide, inhibits bacterial protein synthesis and reduces mucus production, effectively controlling disease progression [8,9]. However, monotherapy with antibiotics often yields suboptimal results, requiring prolonged treatment courses that increase the risk of drug resistance and adverse reactions [10,11].
There are limited studies on the combined use of levofloxacin and azithromycin in cervicitis treatment. This study aimed to evaluate the efficacy of the levofloxacin-azithromycin combination and its effect on serum inflammatory markers, providing a reliable reference for cervicitis therapy. The novelty of this research lies in demonstrating the superior efficacy and lower recurrence rates achieved with combination therapy, while identifying disease severity, post-treatment IL-6, post-treatment CRP, and post-treatment TNF-α as independent factors influencing recurrence.
Materials and methods
Sample selection and criteria
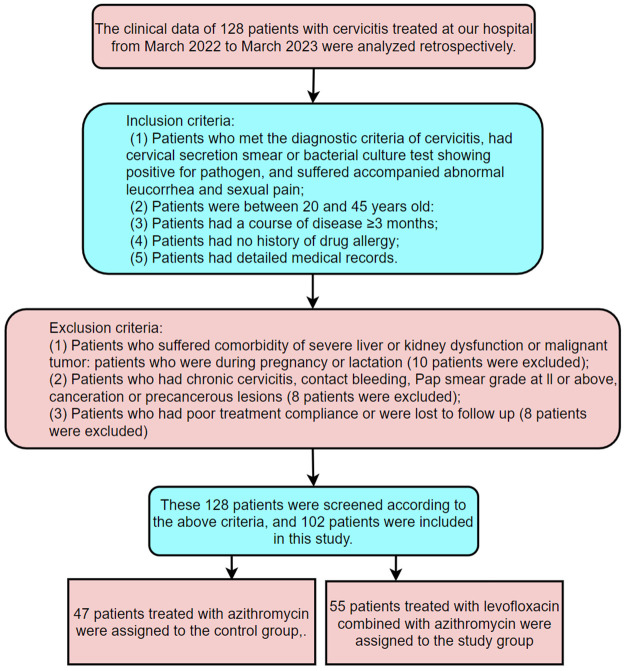
This retrospective study included the records of 128 cervicitis patients treated at Gansu Provincial Maternity and Child-care Hospital between March 2022 and March 2023.
Inclusion criteria: Patients met the diagnostic criteria for cervicitis [12], had a positive cervical secretion smear or bacterial culture for pathogens, exhibited abnormal leucorrhea and sexual pain; patients were aged 20-45 years, had a disease course of ≥3 months; patients had no history of drug allergies, and had complete medical records.
Exclusion criteria: Patients suffered from comorbidities such as severe liver or kidney dysfunction or malignancies; patients were pregnant or lactating; patients had chronic cervicitis, contact bleeding, Pap smear results of grade II or above, cancer, or precancerous lesions; patients had poor treatment compliance or were lost to follow-up.
After screening based on these criteria, 102 patients were included in the study. A total of 47 patients receiving azithromycin were assigned to the control group, while 55 patients receiving levofloxacin combined with azithromycin were assigned to the study group. The screening and grouping process is outlined in Figure 1. This study was approved by the Medical Ethics Committee of Gansu Provincial Maternity and Child-care Hospital.
Figure 1.
Screening and grouping process.
Therapeutic regimen
The control group was treated with oral azithromycin tablets (Tonghua Golden-Horse Pharmaceutical Industry Co., Ltd., SFDA approval no.: H20083813; specification: 0.25 g/tablet) at a dose of 1 g once daily for 2 weeks.
The study group received azithromycin as in the control group, in addition to levofloxacin (Zhejiang Huayuan Pharmaceutical Co., Ltd., SFDA approval no.: H20093894; specification: 0.1 g/capsule) at a dose of 0.3 g twice daily (morning and evening) for 2 weeks. Both groups were followed up for 6 months.
Data collection
Patient data were obtained from the medical record system, including sex, age, body mass index (BMI), disease duration, disease severity, smoking history, alcohol history, place of residence, levels of serum inflammatory factors, treatment efficacy, incidence of adverse reactions, and recurrence of cervicitis within 6 months.
Outcome measures
Main outcome measures
Serum inflammatory factors: Fasting venous blood samples (3 mL) were collected from each patient before treatment and 2 weeks after treatment. The samples were centrifuged at 3,000 rpm for 10 minutes to separate serum. Interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) levels were measured using chemiluminescence kits (IL-6: Derong Medical Technology Co., Ltd., Hunan, China; TNF-α: Tarcine BioMed (Hong Kong) Limited, Hongkong, China). C-reactive protein (CRP) levels were determined by immunoturbidimetry (Nanjing Institute of Environmental Biotechnology, Nanjing, China).
Efficacy: Treatment efficacy was evaluated based on established criteria [12].
Markedly effective: Complete resolution of symptoms such as pruritus vulvae, cervical erosion, edema, and abnormal leukorrhea, with normal cervical morphology and negative pathogen test results.
Effective: Significant symptom improvement with normal cervical morphology and negative or weakly positive pathogen test results.
Ineffective: No improvement or worsening of symptoms after treatment.
Overall response rate = [(markedly effective cases + effective cases)/total cases] × 100%.
Factors influencing recurrence: Multiple logistic regression analysis was used to identify factors affecting recurrence.
Secondary outcome measures
Baseline data: The general baseline characteristics of patients were analyzed. Adverse reactions: Recorded adverse reactions during treatment included nausea, vomiting, diarrhea, and anorexia. Recurrence rate: 2 weeks after treatment, pa-tients were followed up for 6 months to observe for recurrence.
Statistical analyses
Data were analyzed using SPSS 20.0, with GraphPad 8 used for visualization. Continuous data were normally distributed and expressed as mean ± standard deviation (SD). Inter-group and intra-group comparisons were performed using independent-samples t-test and paired t-test, respectively. Categorical data were presented as cases (%), analyzed with the chi-square test (χ2). Multiple logistic regression was used to analyze factors influencing recurrence, with binary outcomes (cervicitis recurrence) examined based on clinical relevance. Models were built using backward elimination, and predictor significance was assessed using odds ratios (OR) and p-values. A p-value of <0.05 indicated significance.
Results
Comparison of baseline data
The control and study groups showed no significant differences in age, disease duration, severity, history of alcoholism, body mass index (BMI), smoking history, or place of residence (all P>0.05, Table 1).
Table 1.
Comparison of baseline data
| Factor | Study group (n=55) | Control group (n=47) | χ2 | P value |
|---|---|---|---|---|
| Age | 0.177 | 0.674 | ||
| ≥30 years old | 20 | 19 | ||
| <30 years old | 35 | 28 | ||
| BMI | 1.933 | 0.164 | ||
| ≥23 kg/m2 | 31 | 20 | ||
| <23 kg/m2 | 24 | 27 | ||
| Course of disease | 1.408 | 0.236 | ||
| ≥1 year | 15 | 18 | ||
| <1 year | 40 | 29 | ||
| Severity | 0.081 | 0.776 | ||
| Mild-moderate erosion | 42 | 37 | ||
| Severe erosion | 13 | 10 | ||
| History of smoking | 0.809 | 0.368 | ||
| Yes | 10 | 12 | ||
| No | 45 | 35 | ||
| History of alcoholism | 0.790 | 0.374 | ||
| Yes | 8 | 10 | ||
| No | 47 | 37 | ||
| Place of residence | 1.585 | 0.208 | ||
| Rural area | 36 | 25 | ||
| Urban area | 19 | 22 |
Note: BMI: Body mass index.
Comparison of inflammatory factors
Prior to therapy, IL-6, CRP, and TNF-α levels were comparable between the two groups (all P>0.05). After therapy, these levels decreased significantly in both groups (all P<0.0001), with the study group showing a more pronounced reduction (P<0.05, Figure 2).
Figure 2.
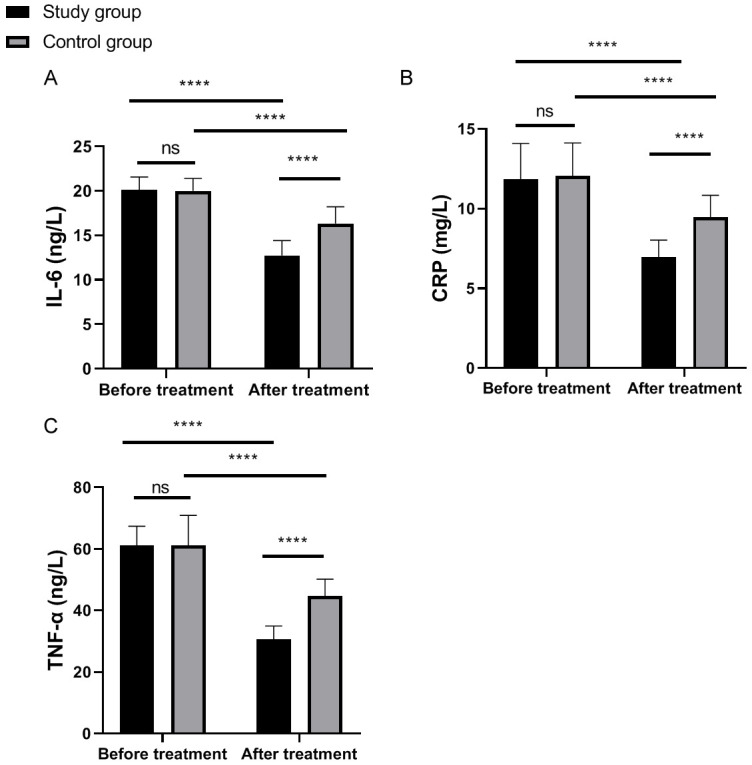
Comparison of inflammatory factors between the two groups before and after treatment. A: Comparison of CRP level between the two groups before and after treatment; B: Comparison of IL-6 level between the two groups before and after treatment; C: Comparison of TNF-α level between the two groups before and after treatment. ns: P>0.05; ****P<0.0001; CRP: C-reactive protein; IL-6: interleukin-6; TNF-α: tumor necrosis factor-α.
Comparison of efficacy
The study group exhibited a significantly higher overall response rate compared to the control group (P=0.041, Table 2).
Table 2.
Comparison of efficacy between the two groups [n (%)]
| Markedly effective | Effective | Ineffective | Overall response rate | |
|---|---|---|---|---|
| Study group (n=55) | 33 (60.00) | 18 (32.73) | 4 (7.27) | 51 (92.73) |
| Control group (n=47) | 24 (51.06) | 13 (27.66) | 10 (21.28) | 37 (78.72) |
| χ2 | 0.821 | 0.308 | 4.197 | 4.197 |
| P value | 0.365 | 0.579 | 0.041 | 0.041 |
Comparison of adverse reactions
There was no significant difference between the two groups in terms of the overall incidence of adverse reactions (P=0.551, Table 3).
Table 3.
Comparison of adverse reactions in the two groups [n (%)]
| Nausea and vomiting | Diarrhea | Anorexia | Total adverse reaction | |
|---|---|---|---|---|
| Study group (n=55) | 2 (3.64) | 1 (1.82) | 2 (3.64) | 5 (9.10) |
| Control group (n=47) | 2 (4.26) | 3 (6.38) | 1 (2.13) | 6 (12.77) |
| χ2 | 0.026 | 1.402 | 0.202 | 0.356 |
| P value | 0.873 | 0.237 | 0.653 | 0.551 |
Comparison of recurrence rate
Six months after treatment, the control group had a significantly higher recurrence rate compared to the study group (P=0.022, Table 4).
Table 4.
Comparison of recurrence rate in the two groups [n (%)]
| Group | Recurrence |
|---|---|
| Study group (n=55) | 4 (7.27) |
| Control group (n=47) | 11 (23.40) |
| χ2 | 5.258 |
| P value | 0.022 |
Analysis of risk factors for cervicitis recurrence
Patients were classified into a recurrence group (n=15) and a non-recurrence group (n=87) based on their recurrence status. Univariate analysis identified disease duration, severity, and post-treatment levels of IL-6, CRP, and TNF-α as factors influencing recurrence (Table 5). These variables were assigned values (Table 6) and subjected to multivariate analysis. Logistic regression revealed that disease severity (P=0.014, OR: 8.616; CI: 1.543-48.096), post-treatment IL-6 (P=0.003, OR: 17.573; CI: 2.720-113.531), post-treatment CRP (P=0.039, OR: 5.731; CI: 1.089-30.157), and post-treatment TNF-α (P=0.001, OR: 20.547; CI: 3.210-131.518) were independent risk factors for recurrence (Table 7).
Table 5.
Univariate analysis of factors influencing patient recurrence of cervicitis
| Recurrence group (n=15) | Non-recurrence group (n=87) | χ2/t | P | |
|---|---|---|---|---|
| Age | 1.470 | 0.225 | ||
| ≥30 years old | 8 | 32 | ||
| <30 years old | 7 | 55 | ||
| BMI | 0.703 | 0.402 | ||
| ≥23 kg/m2 | 9 | 42 | ||
| <23 kg/m2 | 6 | 45 | ||
| Course of disease | 6.142 | 0.013 | ||
| ≥1 year | 9 | 24 | ||
| <1 year | 6 | 63 | ||
| Severity | 14.121 | <0.001 | ||
| Mild-moderate erosion | 6 | 73 | ||
| Severe erosion | 9 | 14 | ||
| Post-treatment IL-6 (ng/L) | 17.32±1.89 | 12.74±1.66 | 11.24 | <0.001 |
| Post-treatment CRP (mg/L) | 10.48±1.36 | 6.87±1.06 | 11.31 | <0.001 |
| Post-treatment TNF-α (ng/L) | 45.82±5.48 | 28.55±4.42 | 15.30 | <0.001 |
| History of smoking | 1.439 | 0.230 | ||
| Yes | 5 | 17 | ||
| No | 10 | 70 | ||
| History of alcoholism | 0.985 | 0.321 | ||
| Yes | 4 | 14 | ||
| No | 11 | 73 | ||
| Place of residence | 0.345 | 0.557 | ||
| Rural area | 10 | 51 | ||
| Urban area | 5 | 36 |
Notes: CRP: C-reactive protein; IL-6: interleukin-6; TNF-α: tumor necrosis factor-α.
Table 6.
Assignment
| Assignment | |
|---|---|
| Course of disease | <1 year =0, ≥1 year =1 |
| Severity | Mild-moderate erosion =0, severe erosion =1 |
| Post-treatment IL-6 | <13.7 =0, ≥13.7 =1 |
| Post-treatment CRP | <7.46 =0, ≥7.46 =1 |
| Post-treatment TNF-α | <32 =0, ≥32 =1 |
| Recurrence | No recurrence =0, recurrence =1 |
Notes: CRP: C-reactive protein; IL-6: interleukin-6; TNF-α: tumor necrosis factor-α.
Table 7.
Multivariable logistic regression analysis
| Factor | B | S.E. | Wals | df | Sig. | Exp (B) | 95% C.I. for EXP (B) | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Lower limit | Upper limit | |||||||
| Course of disease | 0.256 | 0.917 | 0.078 | 1 | 0.780 | 1.291 | 0.214 | 7.783 |
| Severity | 2.154 | 0.877 | 6.025 | 1 | 0.014 | 8.616 | 1.543 | 48.096 |
| Post-treatment IL-6 | 2.866 | 0.952 | 9.067 | 1 | 0.003 | 17.573 | 2.720 | 113.531 |
| Post-treatment CRP | 1.746 | 0.847 | 4.246 | 1 | 0.039 | 5.731 | 1.089 | 30.157 |
| Post-treatment TNF-α | 3.023 | 0.947 | 10.184 | 1 | 0.001 | 20.547 | 3.210 | 131.518 |
Notes: CRP: C-reactive protein; IL-6: interleukin-6; TNF-α: tumor necrosis factor-α.
Discussion
In recent years, changes in lifestyle habits and increased work-related stress have led to a rising incidence of cervicitis [13]. However, due to the personal sensitivity of the disease site and insufficient patient awareness, the condition often progresses unchecked, possibly leading to infertility or malignancy, posing a serious threat to patients’ health and well-being [14,15]. Cervicitis is commonly treated with medications, particularly antibiotics [16]. Azithromycin, a widely used antibiotic, is often employed to treat urogenital infections. However, the efficacy of azithromycin alone is often unsatisfactory, and prolonged use may disrupt the vaginal microbiota [17]. Levofloxacin, a broad-spectrum antibiotic with potent antibacterial activity, has shown enhanced therapeutic effects when combined with azithromycin, as indicated by previous research [18]. This study investigates the efficacy of the combined treatment for cervicitis and its effects on serum inflammatory factors.
CRP and TNF-α levels in cervicitis patients are reportedly higher compared to healthy individuals [19]. IL-6, a multifunctional inflammatory cytokine, plays a crucial role in the inflammatory response. Elevated IL-6 levels can cause inflammatory damage, hindering recovery [20]. CRP, a reactive protein released during inflammation, is a common marker for infection evaluation [21]. TNF-α has dual functions: it regulates immune function and combats inflammation, but excessive production can aggravate inflammation and cause tissue damage [22]. In this study, IL-6, TNF-α, and CRP levels significantly decreased in both groups after treatment, with the study group showing more substantial reductions. These findings suggest that levofloxacin combined with azithromycin effectively lowers serum IL-6, CRP, and TNF-α levels, thereby reducing inflammation and promoting cervical recovery. The study group also had a significantly higher overall response rate compared to the control group, while the incidence of adverse reactions was similar between the two groups. This indicates that levofloxacin combined with azithromycin is more effective than azithromycin alone in treating cervicitis without increasing adverse reactions. The enhanced bioavailability of the combination therapy may explain this improved efficacy. Owais et al. [23] demonstrated that azithromycin and levofloxacin have a favorable synergistic effect on extensively drug-resistant Klebsiella pneumoniae, without antagonistic interactions, supporting the results of this study.
The study also included a 6-month follow-up, showing a significantly lower recurrence rate of cervicitis in the study group. This suggests that the combination of levofloxacin and azithromycin synergistically inhibits bacterial reproduction, effectively eliminates pathogens, and reduces recurrence.
Moreover, the study identified factors influencing cervicitis recurrence, revealing that disease severity, as well as post-treatment IL-6, CRP, and TNF-α levels, were independent predictors of recurrence. Cervicitis severity may be related to pathogen load and the strength of the inflammatory response. More severe cases may leave a residual infection or inflammation after treatment, increasing recurrence risk [24]. Post-treatment IL-6 and CRP levels reflect the degree of inflammation and treatment efficacy [25]. Elevated levels of these markers may indicate persistent inflammation or an inadequate immune response, raising the likelihood of recurrence. High post-treatment TNF-α levels may also indicate an abnormal immune response associated with recurrence.
This study has some limitations. The relatively small sample size may have introduced bias into the conclusions. Additionally, long-term patient outcomes were not tracked, so the impact of levofloxacin plus azithromycin on long-term prognosis remains unknown. Further studies with larger sample sizes and extended follow-up are necessary to provide more comprehensive and convincing evidence about combination therapy for cervicitis.
In conclusion, levofloxacin combined with azithromycin is more effective than azithromycin alone in treating cervicitis. The combination significantly reduces inflammation and recurrence rates without increasing adverse reactions, making it a promising treatment option.
Acknowledgements
This work was supported by Gansu Provincial Administration of Traditional Chinese Medicine (Project Number: GZKP-2021-23).
Disclosure of conflict of interest
None.
References
- 1.Cabellos A, Keim C, Álvarez N, Guzmán C, Vesperinas G. Necrotic cervicitis for co-infection of herpes simplex virus 2 and Mycoplasma genitalium. Rev Chilena Infectol. 2022;39:214–217. doi: 10.4067/S0716-10182022000200214. [DOI] [PubMed] [Google Scholar]
- 2.Chauhan A, Pandey N, Desai A, Raithatha N, Patel P, Choxi Y, Kapadia R, Khandelwal R, Jain N. Association of TLR4 and TLR9 gene polymorphisms and haplotypes with cervicitis susceptibility. PLoS One. 2019;14:e0220330. doi: 10.1371/journal.pone.0220330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hester EE, Middleman AB. A clinical conundrum: chronic cervicitis. J Pediatr Adolesc Gynecol. 2019;32:342–344. doi: 10.1016/j.jpag.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Allen M. Identifying acute cervicitis in an era of less-frequent routine gynecologic examinations. JAAPA. 2018;31:50–53. doi: 10.1097/01.JAA.0000530277.12517.69. [DOI] [PubMed] [Google Scholar]
- 5.Petre I, Sirbu DT, Petrita R, Toma AD, Peta E, Dimcevici-Poesina F. Real-world study of Cerviron(®) vaginal ovules in the treatment of cervical lesions of various etiologies. Biomed Rep. 2023;19:54. doi: 10.3892/br.2023.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deshpande D, Pasipanodya JG, Mpagama SG, Bendet P, Srivastava S, Koeuth T, Lee PS, Bhavnani SM, Ambrose PG, Thwaites G, Heysell SK, Gumbo T. Levofloxacin pharmacokinetics/pharmacodynamics, dosing, susceptibility breakpoints, and artificial intelligence in the treatment of multidrug-resistant tuberculosis. Clin Infect Dis. 2018;67:S293–S302. doi: 10.1093/cid/ciy611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Y, Xie S, Zheng R, Dai Q, Xu Z, Zuo W, Ding J, Zhang Y. Brucellar reproductive system injury: a retrospective study of 22 cases and review of the literature. J Int Med Res. 2020;48:300060520924548. doi: 10.1177/0300060520924548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lusk MJ, Garden FL, Cumming RG, Rawlinson WD, Naing ZW, Konecny P. Cervicitis: a prospective observational study of empiric azithromycin treatment in women with cervicitis and non-specific cervicitis. Int J STD AIDS. 2017;28:120–126. doi: 10.1177/0956462416628319. [DOI] [PubMed] [Google Scholar]
- 9.Giles ML, Krishnaswamy S, Metlapalli M, Roman A, Jin W, Li W, Mol BW, Sheehan P, Said J. Azithromycin treatment for short cervix with or without amniotic fluid sludge: a matched cohort study. Aust N Z J Obstet Gynaecol. 2023;63:384–390. doi: 10.1111/ajo.13648. [DOI] [PubMed] [Google Scholar]
- 10.Zhang S, Xu K, Liu SX, Ye XL, Huang P, Jiang HJ. Retrospective analysis of azithromycin-resistant ureaplasma urealyticum and mycoplasma hominis cervical infection among pregnant women. Infect Drug Resist. 2023;16:3541–3549. doi: 10.2147/IDR.S405286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wood GE, Jensen NL, Astete S, Jensen JS, Kenny GE, Khosropour CM, Gillespie CW, Manhart LE, Totten PA. Azithromycin and doxycycline resistance profiles of U.S. mycoplasma genitalium strains and their association with treatment outcomes. J Clin Microbiol. 2021;59:e0081921. doi: 10.1128/JCM.00819-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ortiz-de la Tabla V, Gutiérrez F. Cervicitis: etiology, diagnosis and treatment. Enferm Infecc Microbiol Clin (Engl Ed) 2019;37:661–667. doi: 10.1016/j.eimc.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Miranda AE, Silveira MFD, Pinto VM, Alves GC, Carvalho NS. Brazilian Protocol for Sexually Transmitted Infections, 2020: infections that cause cervicitis. Rev Soc Bras Med Trop. 2021;54(Suppl 1):e2020587. doi: 10.1590/0037-8682-587-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salmanov AG, Artyomenko V, Koctjuk IM, Mashyr NV, Berestooy OA, Beraia DY. Cervicitis as a cause of preterm birth in women. Wiad Lek. 2022;75:2715–2721. doi: 10.36740/WLek202211201. [DOI] [PubMed] [Google Scholar]
- 15.Nitahara K, Fujita Y, Tanaka D, Magarifuchi N, Taniguchi S, Shimamoto T. Laser vaporization of the cervix is associated with an increased risk of preterm birth and rapid labor progression in subsequent pregnancies. Arch Gynecol Obstet. 2021;304:895–902. doi: 10.1007/s00404-021-06025-7. [DOI] [PubMed] [Google Scholar]
- 16.Bansal S, Bhargava A, Verma P, Khunger N, Panchal P, Joshi N. Etiology of cervicitis: are there new agents in play? Indian J Sex Transm Dis AIDS. 2022;43:174–178. doi: 10.4103/ijstd.ijstd_75_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohammadzadeh F, Dolatian M, Jorjani M, Afrakhteh M, Majd HA, Abdi F, Pakzad R. Urogenital chlamydia trachomatis treatment failure with azithromycin: a meta-analysis. Int J Reprod Biomed. 2019;17:603–620. doi: 10.18502/ijrm.v17i9.5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Izadi M, Dadsetan B, Najafi Z, Jafari S, Mazaheri E, Dadras O, Heidari H, SeyedAlinaghi S, Voltarelli F. Levofloxacin versus ceftriaxone and azithromycin combination in the treatment of community acquired pneumonia in hospitalized patients. Recent Pat Antiinfect Drug Discov. 2018;13:228–239. doi: 10.2174/1574891X13666181024154526. [DOI] [PubMed] [Google Scholar]
- 19.Liu T, Wang R, Liu C, Lu J, Wang Y, Dong L, Zhang X. Active substances from callicarpa nudiflora exert anti-cervicitis effects and regulate NLRP3-associated inflammation. Molecules. 2021;26:6217. doi: 10.3390/molecules26206217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang S, Tanaka T, Narazaki M, Kishimoto T. Targeting interleukin-6 signaling in clinic. Immunity. 2019;50:1007–1023. doi: 10.1016/j.immuni.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Wang MS, Zhou YH, Shi JP, Wang WJ. Prognostic values of LDH and CRP in cervical cancer. Onco Targets Ther. 2020;13:1255–1263. doi: 10.2147/OTT.S235027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hira K, Sajeli Begum A. Methods for evaluation of TNF-α inhibition effect. Methods Mol Biol. 2021;2248:271–279. doi: 10.1007/978-1-0716-1130-2_21. [DOI] [PubMed] [Google Scholar]
- 23.Owais HM, Baddour MM, El-Metwally HAE, Barakat HS, Ammar NS, Meheissen MA. Assessment of the in vitro activity of azithromycin niosomes alone and in combination with levofloxacin on extensively drug-resistant Klebsiella pneumoniae clinical isolates. Braz J Microbiol. 2021;52:597–606. doi: 10.1007/s42770-021-00433-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shroff S. Infectious vaginitis, cervicitis, and pelvic inflammatory disease. Med Clin North Am. 2023;107:299–315. doi: 10.1016/j.mcna.2022.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Iqbal U, Wills C. Cervicitis. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Christina Wills declares no relevant financial relationships with ineligible companies.: StatPearls Publishing Copyright © 2024, StatPearls Publishing LLC.; 2024. [Google Scholar]