Abstract
Objective: To investigate the risk factors for peritonitis in peritoneal dialysis patients and to develop and validate a predictive model. Methods: A total of 219 patients undergoing continuous ambulatory peritoneal dialysis (CAPD) who had their first peritoneal dialysis catheter placement and regular follow-up at Wuhan No. 1 Hospital between April 2020 and August 2023 were included in this study. Patients were categorized into two groups: a peritoneal dialysis-associated peritonitis (PDAP) group and a non-PDAP group, based on the occurrence of PDAP. Univariate and multivariate logistic regression analyses were conducted to identify risk factors for PDAP in peritoneal dialysis patients. A risk prediction model was constructed, and its predictive performance was assessed using the receiver operating characteristic (ROC) curve. Results: Among the study population, 59 patients developed PDAP, with an incidence rate of 26.94%. Univariate and multivariate Logistic regression analyses identified serum albumin, age, hemoglobin, diabetes mellitus, and dialysis duration as independent risk factors for PDAP (all P<0.05). The ROC curve analysis of the predictive model yielded an area under the curve (AUC) of 0.914. A validation cohort consisting of 75 patients who underwent peritoneal dialysis between September 2023 and May 2024 included 22 PDAP. In this validation set, the predictive model achieved an AUC of 0.883 for PDAP. Conclusion: Serum albumin, age, hemoglobin, diabetes, and dialysis duration are independent risk factors for PDAP in peritoneal dialysis patients. The developed predictive model demonstrates strong performance in identifying patients at high risk for PDAP.
Keywords: Peritoneal dialysis, peritonitis, risk factors, predictive modeling, clinical validation
Introduction
Chronic kidney disease (CKD) is characterized by renal damage and a reduced glomerular filtration rate [<60 ml/(min-1.73 m2)] for more than 3 months, resulting from various causes. Renal damage may manifest as pathological abnormalities, abnormal blood or urine composition, or atypical imaging findings of the kidneys [1]. Globally, the incidence of CKD has been steadily increasing, making it a significant public health concern alongside cardiovascular diseases, cancer, and diabetes [2,3]. Replacement therapies such as renal transplantation, hemodialysis, and peritoneal dialysis are primary treatments for end-stage renal disease (ESRD). Among these, kidney transplantation is often limited by high cost, scarcity of donor organs, and risks of post-transplant rejection.
In contrast, hemodialysis and peritoneal dialysis are more widely utilized in clinical practice due to their relative affordability and feasibility. Peritoneal dialysis, in particular, offers the advantages of being accessible and home-based. It provides effective removal of intermediate molecules, better preservation of residual renal function, and a reduced impact on the body’s internal environment, hemodynamics, and cardiovascular stress [4,5]. However, long-term peritoneal dialysis is associated with complications, the most common of which is peritoneal dialysis-related peritonitis (PDAP). PDAP significantly affects the treatment course, often leading to dialysis failure, kidney transplantation, or even mortality [6,7]. Despite advancements in peritoneal dialysis technology and increased awareness, which have contributed to a declining incidence of PDAP, its multifactorial etiology means that there is still no consensus on its risk factors.
To improve understanding of PDAP risk factors in peritoneal dialysis patients, this study conducted a retrospective analysis of patients treated at our hospital and developed a predictive model to identify high-risk individuals, thereby aiding in the prevention of PDAP.
Information and methods
Clinical data
A total of 219 patients on continuous ambulatory peritoneal dialysis (CAPD) who received their first peritoneal dialysis catheter placement and had regular follow-up at Wuhan No. 1 Hospital between April 2020 and August 2023 were included in this study.
Inclusion criteria
(1) All patients underwent initial peritoneal dialysis catheter implantation via open surgical procedure in our nephrology department. (2) Patients and their caregivers received standardized training in peritoneal dialysis practices after catheter placement, using peritoneal dialysis fluid manufactured by Baxter. (3) Patients were 18 years of age or older. (4) Patients were regularly revisited at intervals of three months. (5) Complete clinical profile.
Exclusion criteria
(1) Patients who received hemodialysis treatment in addition to peritoneal dialysis. (2) Patients with concurrent malignancy, heart failure, or stroke. (3) Patients with tuberculous ascites. (4) Patients with dysfunctions of other organs such as heart, liver, lungs. (5) Patients with immune system disorders. (6) Patients on dialysis for less than 3 months.
PDAP diagnostic criteria and grouping
Following the guidelines of the International Society for Peritoneal Dialysis (ISPD) [8], patients were diagnosed with PDAP if they met at least two of the following criteria: (1) Presence of clinical signs and symptoms of peritonitis, such as abdominal pain and/or cloudy peritoneal fluid. (2) A transudate leukocyte count >100 × 106 with neutrophil percentage of >50%. (3) A positive culture for pathogens in the transudative fluid.
Patients were categorized based on PDAP occurrence: those with one episode of PDAP or more were assigned to the PDAP group, and those without PDAP episodes were assigned to the non-PDAP group.
Research method
Collection of clinical data
Clinical data of the patients were collected and recorded, including gender, age, body mass index (BMI), education level, smoking history, alcohol consumption, place of residence, marital status, primary disease, diabetes mellitus, hypertension, dialysis course, blood creatinine, blood urea, serum albumin, hemoglobin, uric acid, blood potassium, blood phosphorus, and C-reactive protein (CRP).
Culture of pathogenic bacteria
Peritoneal dialysis effluent was collected as the sample for bacterial culture. The waste fluid bag was disinfected with iodophor twice. After drying, 10-15 ml of the effluent was drawn with a 20 ml sterile syringe. The sample was injected into a standard blood culture bottle (aerobic and anaerobic) for bacterial culture.
Statistical analysis
SPSS 27.0 was used for statistical analysis. The measurement data were expressed as mean ± standard deviation and compared by t-test; count data were expressed as numbers and percentage and compared by Chi-square test; rank data were expressed as percentile and compared using rank-sum test. Univariate and multivariate logistic regression analyses were performed to identify risk factors for the occurrence of PDAP. A risk prediction model was constructed. The predictive value of the model for PDAP occurrence was evaluated using a receiver operating characteristic (ROC) curve. The difference was statistically significant when P<0.05.
Results
Clinical data
The study included 219 patients on peritoneal dialysis, including 124 males and 95 females, with an average age of (59.11±10.95) years and an average BMI of (23.24±2.91) kg/m2. 132 cases had an educational level of junior high school or below, while 87 cases had a level of high school or above. Among them, 63 patients were smokers, 50 consumed alcohol, 128 resided in urban areas, and 91 in rural areas. Marital status indicated that 176 were married, while 43 were unmarried, divorced, or widowed. The underlying diseases included chronic glomerulonephritis in 95 cases, diabetic nephropathy in 61 cases, hypertension nephropathy in 27 cases, and other causes in 36 cases. Additionally, 69 patients had diabetes, and 71 had hypertension.
Incidence and pathogen distribution of PDAP in peritoneal dialysis patients
PDA occurred in 59 out of 219 peritoneal dialysis patients, with an incidence of 26.94%. Of the 59 patients with PDAP, 38 patients had positive culture results for pathogenic bacteria, yielding a positive detection rate of 64.41%. Each patient was infected with a single strain, with a total of 38 strains identified: 11 gram-negative bacteria (28.95%), 24 gram-positive bacteria (63.16%), and 3 fungi (7.89%) (Table 1).
Table 1.
Distribution of pathogenic bacteria detected in PDAP patients
| Pathogenic bacteria | The number of pathogenic bacteria | Composition ratio (%) |
|---|---|---|
| Gram-negative bacteria | 11 | 28.95 |
| Escherichia coli (E. coli) | 5 | 13.16 |
| Klebsiella pneumonia | 3 | 7.89 |
| Pseudomonas aeruginosa | 1 | 2.63 |
| Acinetobacter baumannii | 1 | 2.63 |
| Aeromonas hydrophilia | 1 | 2.63 |
| Gram-positive bacteria | 24 | 63.16 |
| Staphylococcus aureus | 13 | 34.21 |
| Streptococcus hemolyticus | 7 | 18.42 |
| Staphylococcus epidermidis | 3 | 7.89 |
| Enterococcus faecium | 1 | 2.63 |
| Fungi | 3 | 7.89 |
| Candida albicans | 2 | 5.26 |
| Candida tropicalis | 1 | 2.63 |
| Total | 38 | 100.00 |
Note: PDAP: Peritoneal dialysis-associated peritonitis.
Univariate analysis of factors associated with PDAP
Significant differences were found in age, diabetes, dialysis course, serum albumin, hemoglobin, uric acid, and CRP between the PDAP group and the non-PDAP group (all P<0.05) (Table 2).
Table 2.
Comparison of baseline data between PDAP and non-PDAP groups
| Parameters | PDAP group (n=59) | Non-PDAP group (n=160) | t/χ2 | P |
|---|---|---|---|---|
| Gender [n (%)] | ||||
| Male | 33 (55.93) | 91 (56.88) | 0.016 | 0.901 |
| Female | 26 (44.07) | 69 (43.13) | ||
| Age (years, x̅±s) | 63.14±12.04 | 57.63±10.28 | 3.356 | 0.001 |
| BMI (kg/m2, x̅±s) | 23.43±2.67 | 23.17±2.81 | 0.616 | 0.539 |
| Education level [n (%)] | ||||
| Junior high and below | 35 (59.32) | 97 (60.63) | 0.031 | 0.861 |
| High school or above | 24 (40.68) | 63 (39.38) | ||
| Smoking [n (%)] | ||||
| Yes | 18 (30.51) | 45 (28.13) | 0.120 | 0.730 |
| No | 41 (69.49) | 115 (71.88) | ||
| Alcohol consumption [n (%)] | ||||
| Yes | 13 (22.03) | 37 (23.13) | 0.029 | 0.865 |
| No | 46 (77.97) | 123 (76.88) | ||
| Place of residence [n (%)] | ||||
| Urban | 32 (54.24) | 96 (60.00) | 0.589 | 0.443 |
| Rural | 27 (45.76) | 64 (40.00) | ||
| Marital status [n (%)] | ||||
| Married | 47 (79.66) | 129 (80.63) | 0.025 | 0.873 |
| Unmarried, divorced or widowed | 12 (20.34) | 31 (19.38) | ||
| Uderlying disease [n (%)] | ||||
| Chronic glomerulonephritis | 24 (40.68) | 71 (44.38) | 0.725 | 0.867 |
| Diabetic nephropathy | 16 (27.12) | 45 (28.13) | ||
| Hypertensive nephropathy | 9 (15.25) | 18 (11.25) | ||
| Others | 10 (16.95) | 26 (16.25) | ||
| Diabetes mellitus [n (%)] | ||||
| Yes | 35 (59.32) | 34 (21.25) | 28.953 | <0.001 |
| No | 24 (40.68) | 126 (78.75) | ||
| Hypertension [n (%)] | ||||
| Yes | 20 (33.90) | 51 (31.88) | 0.188 | 0.664 |
| No | 49 (83.05) | 109 (68.13) | ||
| Dialysis course (months, x̅±s) | 34.49±12.05 | 28.66±9.60 | 3.712 | <0.001 |
| Blood creatinine (μmol/L, x̅±s) | 895.64±124.76 | 887.26±159.41 | 0.365 | 0.716 |
| Blood urea (mmol/L, x̅±s) | 15.02±2.97 | 14.85±3.12 | 0.362 | 0.718 |
| Serum albumin (g/L, x̅±s) | 28.94±2.65 | 32.47±2.95 | 8.067 | <0.001 |
| Hemoglobin (g/L, x̅±s) | 81.05±7.62 | 89.92±8.33 | 7.149 | <0.001 |
| Uric acid (μmol/L, x̅±s) | 431.04±58.67 | 407.34±42.16 | 3.301 | 0.001 |
| Potassium (mmol/L, x̅±s) | 4.25±1.36 | 4.42±1.47 | 0.774 | 0.440 |
| Blood phosphorus (mmol/L, x̅±s) | 1.39±0.42 | 1.44±0.36 | 0.871 | 0.385 |
| CRP (mg/L) | 10.65±1.74 | 8.31±1.67 | 9.096 | <0.001 |
Notes: PDAP: Peritoneal dialysis-associated peritonitis; BMI: Body mass index; CRP: C-reactive protein.
Determination of cutoff values for significant variables
ROC analyses were used to determine the cutoff values for significant variables derived from univariate analysis, as shown in Table 3.
Table 3.
Cutoff values for significant variables determined by ROC analysis
| Index | AUC | Cut-off value | Sensitivity | Specificity | P | 95% CI |
|---|---|---|---|---|---|---|
| Age | 0.643 | 61.02 years | 64.40% | 65.00% | 0.001 | 0.557-0.729 |
| Dialysis course | 0.654 | 32.47 months | 44.10% | 88.70% | 0.000 | 0.566-0.742 |
| Serum albumin | 0.817 | 30.97 g/L | 71.20% | 81.20% | 0.000 | 0.751-0.882 |
| Hemoglobin | 0.781 | 87.13 g/L | 79.70% | 62.50% | 0.000 | 0.713-0.849 |
| Uric acid | 0.624 | 420.10 μmol/L | 47.50% | 82.50% | 0.005 | 0.532-0.716 |
| CRP | 0.719 | 9.57 mg/L | 71.20% | 65.60% | 0.000 | 0.644-0.793 |
Notes: ROC: Receiver operating characteristic; AUC: Area under the curve; CRP: C-reactive protein.
Risk factors for PDAP identified by multivariate logistic regression analysis
Values were assigned to variables, as shown in Table 4. Each factor found to be statistically significant in the univariate analysis was further analyzed using multivariate logistic regression. The results showed that serum albumin, age, hemoglobin, diabetes mellitus, and dialysis course were independent risk factors for PDAP (all P<0.05), as shown in Table 5.
Table 4.
Variable assignment
| Variables | Assignment |
|---|---|
| Age | <61.02 years old = 0, ≥61.02 years old = 1 |
| Diabetes | Yes = 0, No = 1 |
| Dialysis course | <32.47 months = 0, ≥32.47 months = 1 |
| Serum albumin | <30.97 g/L = 0, ≥30.97 g/L = 1 |
| Hemoglobin | <87.13 g/L = 0, ≥87.13 g/L = 1 |
| Uric acid | <420.10 μmol/L = 0, ≥420.10 μmol/L = 1 |
| CRP | <9.57 mg/L = 0, ≥9.57 mg/L = 1 |
Note: CRP: C-reactive protein.
Table 5.
Multivariate logistic regression analysis of independent risk factors for PDAP
| Factor | b | S.E | χ2 | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| Serum albumin | -1.856 | 0.475 | 15.268 | 0.000 | 0.156 | 0.062-0.397 |
| Age | 1.664 | 0.512 | 10.563 | 0.001 | 5.280 | 1.936-14.404 |
| Hemoglobin | -1.658 | 0.567 | 8.551 | 0.003 | 0.191 | 0.063-0.579 |
| Diabetes | 1.401 | 0.492 | 8.109 | 0.004 | 4.059 | 1.548-10.647 |
| Dialysis course | 1.388 | 0.576 | 5.807 | 0.016 | 4.007 | 1.296-12.391 |
Note: PDAP: Peritoneal dialysis-associated peritonitis.
Construction of a PDAP prediction model
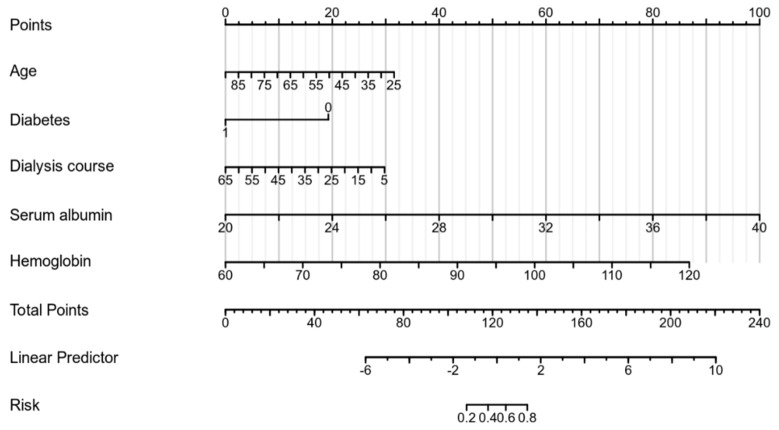
Based on the results of the multivariate Logistic regression analysis, a stepped-wedge plot model, in the form of line chart, was constructed using R 3.6.3 software and rms package, as shown in Figure 1.
Figure 1.
Line chart model.
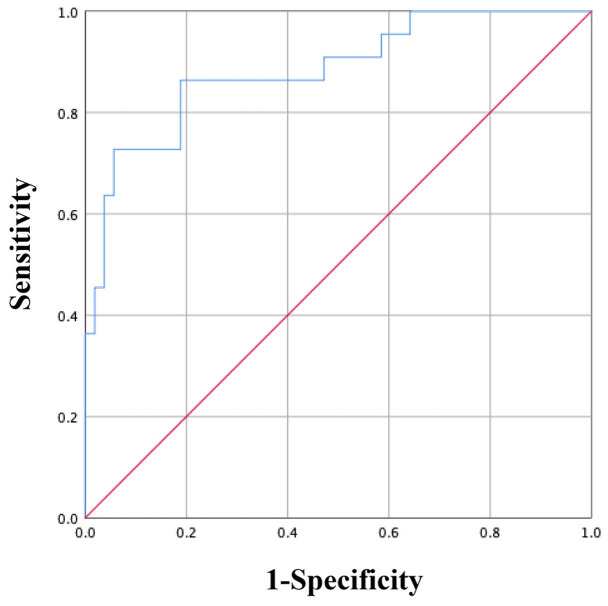
ROC curve was further used to assess the predictive value of the constructed model for PDAP, and the analysis yielded an area under the curve (AUC) of 0.914, indicating high predictive value, as shown in Figure 2.
Figure 2.
Predictive value of the model for PDAP analyzed by ROC curve analysis. Notes: PDAP: Peritoneal dialysis-associated peritonitis; ROC: Receiver operating characteristic.
Model validation
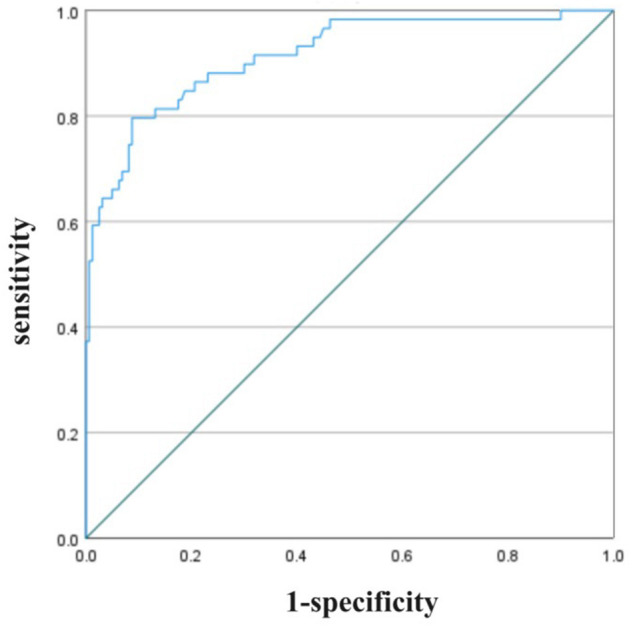
A validation group of 75 patients who underwent peritoneal dialysis from September 2023 to May 2024, meeting the same inclusion and exclusion criteria, was selected. Among these patients, 22 had PDAP. The predictive model demonstrated a predictive value of 0.883 for PDAP in the validation set, as shown in Figure 3.
Figure 3.
Application of the predictive model in the validation set.
Discussion
Peritoneal dialysis-related peritonitis (PDAP) is a specific intraperitoneal infection that arises as a complications of peritoneal dialysis. It is characterized by symptoms such as abdominal pain, fever, and cloudy dialysate, all indicative of peritonitis [9,10]. PDAP is a major complication in peritoneal dialysis patients, serving as a leading cause of treatment failure and mortality in this population. Recurrent episodes of PDAP often result in peritoneal ultrafiltration failure, ultimately leading to peritoneal dialysis technique failure. Additionally, PDAP-related deaths account for approximately 10% to 20% of all peritoneal dialysis-associated fatalities [11-14]. Despite advancements in dialysis technology and improved disinfection protocols, PDAP remains a significant cause of treatment failure in peritoneal dialysis patients, although its incidence has gradually declined in recent years [15-18].
In this study, we analyzed 219 patients from our hospital, using univariate and multivariate logistic regression analyses to identify factors influencing the development of PDAP in peritoneal dialysis patients. Our findings identified serum albumin, age, hemoglobin, diabetes mellitus, and dialysis duration as independent risk factors for the PDAP, consistent with reports from other studies [19-21]. Peritoneal dialysis patients with low serum albumin and hemoglobin levels were found to have a higher risk of developing PDAP. Albumin plays a vital role in maintaining plasma colloid osmotic pressure and in the transport of insoluble small-molecule organic substances. Low albumin levels can lead to malnutrition, protein loss, and systemic inflammatory responses, all of which contribute to the onset of peritonitis [22,23]. Hypoproteinemia is particularly common among peritoneal dialysis patients due to protein leakage during dialysis and poor nutritional intake caused by anorexia [24,25]. Therefore, providing dietary guidance to improve nutritional status is of critical significance in reducing the risk of PDAP.
Aging is associated with a decline in physiological functions and immunity, and elderly patients often have multiple chronic conditions such as hypertension, heart failure, and diabetes, significantly increasing the risk of PDAP [26-28]. Patients with diabetes are particularly susceptible to PDAP, as chronic hyperglycemia impairs immune function, reduces the adhesion capacity of white blood cells, but enhances chemotaxis, all of which contribute to the development of peritonitis. In addition, diabetic patients frequently require insulin administration via dialysis fluid, further elevating the risk of infection [29-31]. Longer durations of peritoneal dialysis predispose patients to peritoneal fibrosis, which impairs peritoneal function and reduces the peritoneal cavity’s defense capacity [32,33]. Furthermore, prolonged exposure to glucose-based dialysate can increase intra-abdominal pressure and intestinal wall tension, leading to intestinal mucosa hypoxia and ischemia. This weakens the mucosal barrier, increases permeability, and facilitates bacterial translocation into peritoneal cavity, thereby heightening the risk of PDAP [34,35].
We identified the risk factors for PDAP through a comprehensive analysis of variables including serum albumin, age, hemoglobin, diabetes mellitus, and dialysis duration. These findings provided valuable insights for clinicians, aiding in the prevention and management of peritonitis and improving the quality of life for peritoneal dialysis patients. In addition to identifing these key risk factors, we developed a risk prediction model for PDAP, which demonstrated strong predictive value with an AUC of 0.914. To validate the model, it was applied to a separate validation set of 75 patients, yielding an AUC of 0.883, These results further support the mopdel’s effectiveness in predicting PDAP risk.
In summary, serum albumin, hemoglobin, age, diabetes mellitus, and dialysis duration are independent risk factors for PDAP in peritoneal dialysis patients. The risk prediction model based on these factors shows strong potential for identifying patients at high clinical risk of PDAP, facilitating proactive and targeted clinical management.
Disclosure of conflict of interest
None.
References
- 1.Chuasuwan A, Pooripussarakul S, Thakkinstian A, Ingsathit A, Pattanaprateep O. Comparisons of quality of life between patients underwent peritoneal dialysis and hemodialysis: a systematic review and meta-analysis. Health Qual Life Outcomes. 2020;18:191. doi: 10.1186/s12955-020-01449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan SF. Updates on Infectious and other complications in peritoneal dialysis: core curriculum 2023. Am J Kidney Dis. 2023;82:481–490. doi: 10.1053/j.ajkd.2023.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Dhoot A, Brown EA, Robinson B, Perl J. Incremental peritoneal dialysis: incremental gains. Perit Dial Int. 2023;43:355–358. doi: 10.1177/08968608231195464. [DOI] [PubMed] [Google Scholar]
- 4.Al Sahlawi M, Zhao J, McCullough K, Fuller DS, Boudville N, Ito Y, Kanjanabuch T, Nessim SJ, Piraino BM, Pisoni RL, Teitelbaum I, Woodrow G, Kawanishi H, Johnson DW, Perl J. Variation in peritoneal dialysis-related peritonitis outcomes in the peritoneal dialysis outcomes and practice patterns study (PDOPPS) Am J Kidney Dis. 2022;79:45–55. e1. doi: 10.1053/j.ajkd.2021.03.022. [DOI] [PubMed] [Google Scholar]
- 5.Perl J, Fuller DS, Bieber BA, Boudville N, Kanjanabuch T, Ito Y, Nessim SJ, Piraino BM, Pisoni RL, Robinson BM, Schaubel DE, Schreiber MJ, Teitelbaum I, Woodrow G, Zhao J, Johnson DW. Peritoneal dialysis-related infection rates and outcomes: results from the peritoneal dialysis outcomes and practice patterns study (PDOPPS) Am J Kidney Dis. 2020;76:42–53. doi: 10.1053/j.ajkd.2019.09.016. [DOI] [PubMed] [Google Scholar]
- 6.Windpessl M, Prischl FC, Prenner A, Vychytil A. Managing hospitalized peritoneal dialysis patients: ten practical points for non-nephrologists. Am J Med. 2021;134:833–839. doi: 10.1016/j.amjmed.2021.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Morales RO, Barbosa F, Farre N. Peritoneal dialysis in heart failure: focus on kidney and ventricular dysfunction. Rev Cardiovasc Med. 2021;22:649–657. doi: 10.31083/j.rcm2203075. [DOI] [PubMed] [Google Scholar]
- 8.Li PK, Szeto CC, Piraino B, de Arteaga J, Fan S, Figueiredo AE, Fish DN, Goffin E, Kim YL, Salzer W, Struijk DG, Teitelbaum I, Johnson DW. ISPD peritonitis recommendations: 2016 update on prevention and treatment. Perit Dial Int. 2016;36:481–508. doi: 10.3747/pdi.2016.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crabtree JH, Shrestha BM, Chow KM, Figueiredo AE, Povlsen JV, Wilkie M, Abdel-Aal A, Cullis B, Goh BL, Briggs VR, Brown EA, Dor FJMF. Creating and maintaining optimal peritoneal dialysis access in the adult patient: 2019 update. Perit Dial Int. 2019;39:414–436. doi: 10.3747/pdi.2018.00232. [DOI] [PubMed] [Google Scholar]
- 10.Haggerty SP, Kumar SS, Collings AT, Alli VV, Miraflor E, Hanna NM, Athanasiadis DI, Morrell DJ, Ansari MT, Abou-Setta A, Walsh D, Stefanidis D, Slater BJ. SAGES peritoneal dialysis access guideline update 2023. Surg Endosc. 2024;38:1–23. doi: 10.1007/s00464-023-10550-8. [DOI] [PubMed] [Google Scholar]
- 11.Kotani A, Oda Y, Hirakawa Y, Nakamura M, Hamasaki Y, Nangaku M. Peritoneal dialysis-related peritonitis caused by streptococcus oralis. Intern Med. 2021;60:3447–3452. doi: 10.2169/internalmedicine.6234-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tunbridge M, Cho Y, Johnson DW. Urgent-start peritoneal dialysis: is it ready for prime time? Curr Opin Nephrol Hypertens. 2019;28:631–640. doi: 10.1097/MNH.0000000000000545. [DOI] [PubMed] [Google Scholar]
- 13.de Vries JC, van Gelder MK, Cappelli G, Bajo Rubio MA, Verhaar MC, Gerritsen KGF. Evidence on continuous flow peritoneal dialysis: a review. Semin Dial. 2022;35:481–497. doi: 10.1111/sdi.13097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Workeneh BT, Mandayam S. Promoting peritoneal dialysis retention in underserved communities. Am J Med Sci. 2021;361:1–2. doi: 10.1016/j.amjms.2020.10.024. [DOI] [PubMed] [Google Scholar]
- 15.Aga Z, Bargman JM. Peritoneal dialysis post-mitrofanoff (appendicovesicostomy) procedure. Perit Dial Int. 2022;42:437–438. doi: 10.1177/08968608221090792. [DOI] [PubMed] [Google Scholar]
- 16.Zimmerman AM. Peritoneal dialysis: increasing global utilization as an option for renal replacement therapy. J Glob Health. 2019;9:020316. doi: 10.7189/jogh.09.020316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lew SQ. Nonsurgical and minimally invasive correction of peritoneal dialysis catheter complications. Nephrol Nurs J. 2021;48:57–63. [PubMed] [Google Scholar]
- 18.Hamidi S, Auguste BL. Continuous quality improvement in peritoneal dialysis: your questions answered. Perit Dial Int. 2023;43:292–300. doi: 10.1177/08968608231156924. [DOI] [PubMed] [Google Scholar]
- 19.Ling CW, Sud K, Patel R, Peterson G, Wanandy T, Yeoh SF, Van C, Castelino R. Culture-directed antibiotics in peritoneal dialysis solutions: a systematic review focused on stability and compatibility. J Nephrol. 2023;36:1841–1859. doi: 10.1007/s40620-023-01716-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shroff R. Peritoneal dialysis in children: reaching milestones but room for growth. Perit Dial Int. 2021;41:137–138. doi: 10.1177/0896860821995385. [DOI] [PubMed] [Google Scholar]
- 21.Calice-Silva V, Nerbass FB. Unplanned-start peritoneal dialysis in Brazil: great results, little application. J Bras Nefrol. 2023;45:3–4. doi: 10.1590/2175-8239-JBN-2023-E002en. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fitzgerald TJ, Brown EA. What assistance does assisted peritoneal dialysis need? Perit Dial Int. 2021;41:519–521. doi: 10.1177/08968608211054374. [DOI] [PubMed] [Google Scholar]
- 23.Roumeliotis S, Dounousi E, Salmas M, Eleftheriadis T, Liakopoulos V. Unfavorable effects of peritoneal dialysis solutions on the peritoneal membrane: the role of oxidative stress. Biomolecules. 2020;10:768. doi: 10.3390/biom10050768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall MR. Non-polyvinyl chloride peritoneal dialysis sets: a double-edged sword? Perit Dial Int. 2021;41:255–260. doi: 10.1177/08968608211001262. [DOI] [PubMed] [Google Scholar]
- 25.Mert M, Ceri M, Dursun B. Peritoneal dialysis-related peritonitis with streptococcus mitis. Saudi J Kidney Dis Transpl. 2021;32:1180–1181. doi: 10.4103/1319-2442.338296. [DOI] [PubMed] [Google Scholar]
- 26.Liu R, Ye H, Peng Y, Yi C, Lin J, Wu H, Diao X, Mao H, Huang F, Yang X. Incremental peritoneal dialysis and survival outcomes: a propensity-matched cohort study. J Nephrol. 2023;36:1907–1919. doi: 10.1007/s40620-023-01735-4. [DOI] [PubMed] [Google Scholar]
- 27.Tan R, Sieunarine K. Peritoneal dialysis catheter intraluminal fibrin cast: a complication after prolonged placement. Case series with a review of literature and the management of this complication. J Vasc Access. 2020;21:1029–1033. doi: 10.1177/1129729820917855. [DOI] [PubMed] [Google Scholar]
- 28.Ng JK, Chan GC, Li PK. Icodextrin in peritoneal dialysis: implications on clinical practice and survival outcome. Kidney360. 2022;3:793–795. doi: 10.34067/KID.0001902022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leal-Escobar G, Osuna-Padilla IA, Vásquez-Jiménez E, Cano-Escobar KB. Nutrition and peritoneal dialysis: fundaments and practical aspects for dietary prescription. Rev Med Inst Mex Seguro Soc. 2021;59:330–338. [PubMed] [Google Scholar]
- 30.Rasmussen SK. An overview of pediatric peritoneal dialysis and renal replacement therapy in infants: a review for the general pediatric surgeon. Semin Pediatr Surg. 2022;31:151193. doi: 10.1016/j.sempedsurg.2022.151193. [DOI] [PubMed] [Google Scholar]
- 31.Lin M, Gao M, Zhang L, Liu W, Ruan Y, Wei Y, Cao F, Hong F. Scrotal abscess complicating peritoneal dialysis-associated peritonitis. Perit Dial Int. 2023;43:110–111. doi: 10.1177/08968608221125594. [DOI] [PubMed] [Google Scholar]
- 32.Jin H, Lv S, Wang L, Zhang M, Wang Q, Fang W, Lin X, Che X, Yan H, Yu Z, Jiang N, Li Z, Che M, Ding L, Huang J, Zhou Y, Ni Z. Automated peritoneal dialysis in urgent-start dialysis ESRD patients: safety and dialysis adequacy. Ther Apher Dial. 2023;27:464–470. doi: 10.1111/1744-9987.13943. [DOI] [PubMed] [Google Scholar]
- 33.Esagian SM, Sideris GA, Bishawi M, Ziogas IA, Lehrich RW, Middleton JP, Suhocki PV, Pappas TN, Economopoulos KP. Surgical versus percutaneous catheter placement for peritoneal dialysis: an updated systematic review and meta-analysis. J Nephrol. 2021;34:1681–1696. doi: 10.1007/s40620-020-00896-w. [DOI] [PubMed] [Google Scholar]
- 34.Trincianti C, Meleca V, La Porta E, Bruschi M, Candiano G, Garbarino A, Kajana X, Preda A, Lugani F, Ghiggeri GM, Angeletti A, Esposito P, Verrina E. Proteomics and extracellular vesicles as novel biomarker sources in peritoneal dialysis in children. Int J Mol Sci. 2022;23:5655. doi: 10.3390/ijms23105655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watnick S. Peritoneal dialysis challenges and solutions for continuous quality improvement. Perit Dial Int. 2023;43:283–285. doi: 10.1177/08968608231160009. [DOI] [PubMed] [Google Scholar]