Abstract
Objective: To study the effects of Changtong Paste on gastrointestinal function after colorectal cancer surgery. Methods: A retrospective analysis was conducted on the clinical data of 216 patients who underwent colorectal cancer surgery at the Affiliated Hospital of North Sichuan Medical College from June 2021 to June 2023. Patients were divided into two groups according to their treatment plan: the control group (n=109), who received abdominal multi-source therapy irradiation, and the study group (n=107), who received Changtong Paste in conjunction with the same abdominal irradiation therapy. The two groups were compared in terms of hemorheology, gastrointestinal hormones, immune function, cancer-related fatigue, quality of life, gastrointestinal function, duration of hospital stay, and clinical efficacy. Results: After treatment, both groups demonstrated significant reductions in plasma viscosity, erythrocyte sedimentation rate, high-shear and low-shear whole blood viscosity, somatostatin (SS), vasoactive intestinal peptide (VIP), and scores on the Piper Fatigue Scale-Revised (PFS-R) compared to before treatment, with the study group showing lower levels. Conversely, levels of motilin (MTL), immunoglobulin G (IgG), IgA, IgM, and Quality of Life Questionnaire (QLQ-C30) scores significantly increased after treatment, with the study group showing higher values (P < 0.05). Additionally, indicators for gastrointestinal function recovery and length of hospital stay were shorter in the study group than in the control group (P < 0.05). The total clinical effective rate in the study group (94.39%) was higher than that of the control group (84.40%) (P < 0.05). Conclusion: Changtong Paste therapy can effectively regulate hemorheology, improve gastrointestinal hormone levels, promote gastrointestinal and immune function recovery, alleviate cancer-related fatigue, enhance quality of life, and shorten hospital stays after colorectal cancer surgery.
Keywords: Colorectal cancer, gastrointestinal function, hemorheology, changtong paste, surgery, quality of life
Introduction
As a common malignant tumor of the digestive system, colorectal cancer ranks third in incidence and fourth in mortality among all malignancies, posing a great threat to human health. Colorectal cancer includes both colon and rectal cancer, with a higher prevalence observed in middle-aged and elderly populations [1]. Surgical intervention remains one of the main treatment methods for colorectal cancer, with its efficacy well established [2,3]. Relevant studies have found that the recovery of gastrointestinal function after surgery is closely related to patient prognosis following colorectal cancer surgery [4]. Factors such as systemic conditions, anesthesia, and surgical procedures can adversely affect gastrointestinal function, possibly leading to complications such as intestinal dilation, gut dysbiosis, abdominal distension, anastomotic leakage and intestinal adhesions. These complications can cause malnutrition, prolonged hospital stays, and worsen overall prognosis [5,6]. Therefore, enhancing the recovery of gastrointestinal function after colorectal cancer surgery is critical for improving patient outcomes. Modern medical practices often employ multimodal analgesia and gastrointestinal decompression to promote postoperative gastrointestinal function recovery; however, these methods frequently yield limited efficacy [7]. Therefore, effectively promotion of gastrointestinal function recovery after colorectal cancer surgery is needed for improving patient prognosis.
In recent years, various traditional Chinese medical treatments, such as the external application of herbal medicines, acupuncture, and injections of Chinese patent medicines, have been used as adjunctive therapies after digestive tract surgeries. These treatments demonstrated both effectiveness and safety, showing promising application prospects [8,9]. Traditional Chinese medicine posits that gastrointestinal dysfunction following colorectal cancer surgery primarily stems from impaired spleen and stomach function, resulting in stagnation of qi and blood. Treatment strategies focus on strengthening the spleen, eliminating dampness, promoting blood circulation, and resolving blood stasis. Changtong Paste, a formulation comprising key herbal powders and excipients, has the effects of strengthening the spleen and qi, dispersing cold, unclogging channels, and promoting blood circulation to remove blood stasis, which is speculated to facilitate gastrointestinal recovery after colorectal cancer surgery. Thus, this study explored the effects of Changtong Paste on hemorheology, gastrointestinal function, and quality of life in patients after colorectal cancer surgery.
Materials and methods
General data
A retrospective analysis was conducted on 216 patients who underwent colorectal cancer surgery at the Affiliated Hospital of North Sichuan Medical College from June 2021 to June 2023. These patients were divided into a control group (n=109) and a study group (n=107) based on their treatment plans. This study was approved by the Ethics Committee of North Sichuan Medical College (NSMC42).
Inclusion criteria
(1) Inclusion Criteria: Patients were included if they met the diagnostic criteria for colorectal cancer, confirmed by surgical pathology; had complete relevant clinical data [10]; underwent surgical treatment; experienced gastrointestinal dysfunction after surgery; were diagnosed with traditional Chinese medicine syndrome differentiation of qi deficiency and blood stasis type, characterized by symptoms such as abdominal pain, distension, fatigue, nausea, and vomiting; were aged between 40 and 70 years; and were classified as clinical stages II-III.
(2) Exclusion Criteria: Patients were excluded if they had gastrointestinal dysfunction due to non-surgical causes; demonstrated poor treatment compliance; had distant metastases; had an expected survival time of less than 6 months; had allergic constitutions; presented with other malignant tumors or severe diseases of the heart, liver, kidneys, or brain; had local skin damage; suffered from mechanical intestinal obstruction; or were pregnant or breastfeeding.
Methods
All patients received standard postoperative care, which mainly included the use of antibiotics to prevent infection and routine fasting procedures. The control group was treated using the MF-C02B multi-source therapeutic device (Chengdu Xinbohao Technology Co., Ltd.). Patients were positioned supine with the abdomen fully exposed. The multi-source therapeutic device was positioned 15-20 centimeters above the abdomen to deliver irradiation treatment. Treatments were performed in medium or low temperature mode, lasting 25-30 minutes per session, three times daily, ensuring the patient felt warm and comfortable. The study group, consisting of 107 patients, received Changtong Paste (Preparation Room of Nantong Hospital of Traditional Chinese Medicine, Sichuan Drug Preparation No. Z202002101000) in addition to irradiation with the multi-source therapeutic device. Patients in this group were also positioned supine with the abdomen fully exposed. Changtong Paste was evenly applied to the abdomen, avoiding the surgical incision area to prevent infection or irritation. The application area was adjusted based on the size of the surgical incision and the patient’s body size to maximize the therapeutic effect of the paste. After the application of Changtong Paste, three layers of sterile gauze, thoroughly soaked with physiologic saline, were placed over the treated area. This step aimed to enhance the efficacy of Changtong Paste by maintaining a moist environment, thereby preventing rapid drying of the paste and ensuring continuous release and absorption of the medication. The gauze was applied evenly and adhered closely to the abdomen without applying excessive pressure, avoiding discomfort and ensuring that the medication’s efficacy was not compromised. Subsequently, the multi-source therapeutic device was used for irradiation treatment, following the same protocol as in the control group: 25-30 minutes per session, three times daily. Both groups were treated for 7 days.
Observation indicators
Primary indicators
(1) Gastrointestinal Function Recovery and Hospital Stay: The timing of the first defecation, first flatus, return of bowel sounds, and length of hospital stay were compared between the two groups. (2) Clinical Efficacy: Evaluated based on the “Standards for Diagnosis and Efficacy of TCM Diseases and Syndromes” [11]. The efficacy categories were defined as follows: Significant effect: Symptoms such as abdominal pain, distension, fatigue, nausea, and vomiting were either mostly resolved or significantly reduced, with TCM syndrome scores decreasing by more than 70% compared to before treatment. Effective: Symptoms such as abdominal pain, distension, fatigue, nausea, and vomiting were alleviated, with TCM syndrome scores decreasing by 30%-70% compared to before treatment. Ineffective: Criteria for either significant effect or effective outcomes were not met. The total effective rate was calculated as the sum of the significant effect rate and the effective rate. (3) Quality of life: Assessed using the Quality of Life Questionnaire (QLQ-C30) before and after treatment, covering topics such as emotional and cognitive function, with each domain scoring a maximum of 100. Higher QLQ-C30 scores correlate positively with improved quality of life.
Secondary indicators
(1) Hemorheology: Fasting venous blood samples (3 ml) were collected from both groups before and after treatment. A hemorheology analyzer was used to measure plasma viscosity, erythrocyte sedimentation rate, and high-shear and low-shear whole blood viscosity. (2) Immune Function: Fasting peripheral blood samples (3 ml) were collected from both groups before and after treatment. Immunoturbidimetry was employed to measure immunoglobulin levels (IgG, IgA, IgM). (3) Gastrointestinal Hormones: Levels of somatostatin (SS), vasoactive intestinal peptide (VIP), and motilin (MTL) were measured using enzyme-linked immunosorbent assay (ELISA) before and after treatment. (4) Cancer-Related Fatigue: Evaluated using the Piper Fatigue Scale-Revised (PFS-R) before and after treatment, which includes 24 items covering cognitive and behavioral aspects, with higher PFS-R scores indicating greater levels of cancer-related fatigue.
Statistical methods
SPSS 23.0 software was used for statistical analysis. Measured data were assessed for normality using the Shapiro-Wilk test and expressed as (χ±S). Independent sample t-tests were employed for intergroup comparisons, while paired sample t-tests were used for within-group comparisons. Categorical data were presented as percentages and analyzed using the χ2 test. A P value of < 0.05 was considered significant.
Results
Comparison of baseline data between the two groups
Among the 216 patients who underwent colorectal cancer surgery, there were 126 men and 90 fwomen, with an age range of 42 to 69 years and a mean age of (56.73±5.18) (56.73/pm 5.18) (56.73±5.18) years. Pathological classifications included 140 cases of tubular adenocarcinoma and 76 cases of mucinous adenocarcinoma. Lesion locations comprised 73 cases of colon cancer and 143 cases of rectal cancer. Clinical stages included both stage II and stage III. Baseline data of the two groups were comparable (P > 0.05) (Table 1).
Table 1.
Comparison of baseline data between the two groups [n/(X̅±S)]
| Group | Control group (n=109) | Study group (n=107) | χ2/t | P |
|---|---|---|---|---|
| Gender (Male/female) | 63/46 | 60/47 | 0.065 | 0.798 |
| Age (years) | 56.85±5.63 | 57.12±5.41 | 0.359 | 0.720 |
| Tumor site (colon/rectum) | 60/49 | 61/46 | 0.085 | 0.771 |
| Clinical stage (Stage II/III) | 66/43 | 67/40 | 0.097 | 0.755 |
| Pathologic classification (mucinous adenocarcinoma/tubular adenocarcinoma) | 41/68 | 38/69 | 0.103 | 0.749 |
Comparison of hemorheology between the two groups
There were no significant differences in hemorheological indicators between the two groups before treatment (P > 0.05). After treatment, all hemorheological indicators significantly decreased in both groups compared to their pre-treatment values, with the study group showing lower levels (P < 0.05) (Table 2).
Table 2.
Comparison of hemorheology between the two groups (X̅±S)
| Group | Plasma viscosity (mPa·s) | Erythrocyte sedimentation rate (mm/h) | High-shear whole blood viscosity (mPa·s) | Low-shear whole blood viscosity (mPa·s) | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | |
| Control group (n=109) | 3.02±0.56 | 1.92±0.43* | 28.23±2.36 | 21.66±2.24* | 7.21±1.69 | 4.98±1.12* | 14.51±2.65 | 10.02±2.21* |
| Study group (n=107) | 2.91±0.51 | 1.07±0.36* | 27.98±2.45 | 16.51±1.65* | 7.13±1.58 | 3.78±1.03* | 14.25±2.49 | 8.05±1.89* |
| t | 1.509 | 15.738 | 0.764 | 19.210 | 0.359 | 8.192 | 0.743 | 7.035 |
| P | 0.133 | < 0.001 | 0.446 | < 0.001 | 0.720 | < 0.001 | 0.458 | < 0.001 |
Note: Compared with before treatment in this group;
P < 0.05.
Comparison of gastrointestinal hormone levels between the two groups
There were no significant differences in the levels of SS, VIP, or MTL levels between the two groups before treatment (P > 0.05). After treatment, MTL levels significantly increased in both groups compared to before treatment, with the study group showing higher levels. Conversely, SS and VIP levels significantly decreased in both groups after treatment, with the study group showing lower levels (P < 0.05) (Table 3).
Table 3.
Comparison of gastrointestinal hormone level between the two groups (X±S)
| Group | SS (μg/L) | VIP (ng/L) | MTL (ng/L) | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | |
| Control group (n=109) | 11.98±2.41 | 8.58±1.98* | 237.78±25.08 | 160.57±16.54* | 162.69±12.57 | 197.57±16.74* |
| Study Group (n=107) | 11.45±2.25 | 6.37±1.26* | 239.54±25.21 | 115.47±14.57* | 164.98±11.45 | 237.41±20.13* |
| t | 1.670 | 9.766 | 0.514 | 21.250 | 1.399 | 18.827 |
| P | 0.096 | < 0.001 | 0.608 | < 0.001 | 0.163 | < 0.001 |
Note: Compared to before treatment in this group;
P < 0.05.
SS, somatostatin; VIP, vasoactive intestinal peptide; MTL, motilin.
Comparison of immune function between the two groups
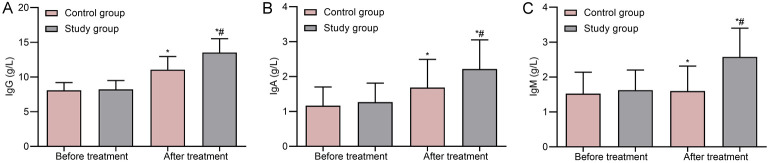
There were no significant differences in immune function-related indicators between the two groups before treatment (P > 0.05). After treatment, IgG, IgA, and IgM levels significantly increased in both groups compared to before treatment, with the study group demonstrating higher levels (P < 0.05) (Figure 1).
Figure 1.
Comparison of immune function between two groups. A. IgG; B. IgA; C. IgM. Note: *P < 0.05 compared to before treatment in this group; #P < 0.05 compared to the control group. IgG, immunoglobulin G.
Comparison of cancer-related fatigue between the two groups
There were no significant differences in PFS-R scores between the two groups before treatment (P > 0.05). After treatment, PFS-R scores significantly decreased in both groups compared to before treatment, with the study group showing lower scores (P < 0.05) (Table 4).
Table 4.
Comparison of cancer-related fatigue (X̅±S, score)
| Group | Cognition | Behavior | Emotion | Sensation | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | |
| Control group (n=109) | 6.52±1.12 | 4.05±1.01* | 6.03±1.08 | 3.75±0.94* | 6.68±1.32 | 4.21±1.02* | 5.99±1.25 | 3.87±1.05* |
| Study Group (n=107) | 6.41±1.05 | 2.53±0.86* | 6.09±1.11 | 2.29±0.81* | 6.81±1.27 | 2.51±0.93* | 6.08±1.29 | 2.37±0.83* |
| t | 0.744 | 11.899 | 0.403 | 12.219 | 0.737 | 12.793 | 0.521 | 11.634 |
| P | 0.458 | < 0.001 | 0.688 | < 0.001 | 0.462 | < 0.001 | 0.603 | < 0.001 |
Note: Compared to before treatment in this group;
P < 0.05.
Comparison of quality of life between the two groups
There were no significant differences in QLQ-C30 scores between the two groups before treatment (P > 0.05). After treatment, QLQ-C30 scores significantly increased in both groups compared to before treatment, with the study group showing higher scores (P < 0.05) (Table 5).
Table 5.
Comparison of quality of life between two groups (χ±S, score)
| Group | Physical Functioning | Role Functioning | Cognitive Functioning | Emotional Functioning | Social Functioning | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||
| Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | |
| Control group (n=109) | 45.82±5.26 | 60.15±6.48* | 48.12±4.38 | 58.06±5.98* | 50.33±4.58 | 61.05±5.39* | 53.25±4.08 | 62.85±5.32* | 55.08±4.78 | 64.28±6.32* |
| Study Group (n=107) | 45.33±5.09 | 70.24±6.83* | 48.37±4.23 | 68.74±6.25* | 50.81±4.92 | 70.07±6.33* | 53.02±4.27 | 72.07±6.08* | 54.81±4.86 | 73.12±6.57* |
| t | 0.696 | 11.140 | 0.427 | 12.833 | 0.742 | 11.283 | 0.405 | 11.867 | 0.412 | 10.079 |
| P | 0.488 | < 0.001 | 0.670 | < 0.001 | 0.459 | < 0.001 | 0.686 | < 0.001 | 0.681 | < 0.001 |
Note: Compared to before treatment in this group;
P < 0.05.
Comparison of gastrointestinal function recovery and length of hospital stay between the two groups
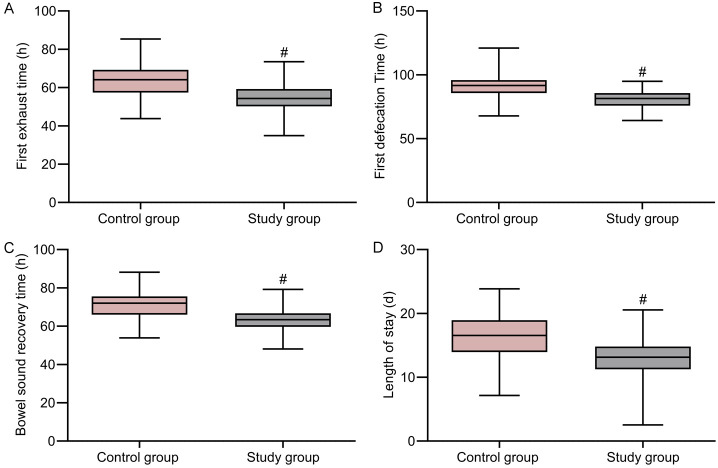
The study group showed significantly shorter recovery times for gastrointestinal function and reduced length of hospital stay compared to the control group (P < 0.05) (Figure 2).
Figure 2.
Comparison of gastrointestinal function recovery and length of hospital stay between the two groups. A. Time to first flatus; B. Time to first bowel movement; C. Time for bowel sound recovery; D. Length of hospital stay. Note: #P < 0.05 compared to the control group.
Comparison of clinical efficacy between the two groups
The total clinical effective rate in the study group (94.39%) was significantly higher than that of the control group (84.40%) (P < 0.05) (Table 6).
Table 6.
Comparison of clinical efficacy between two groups [n (%)]
| Group | Significant effect | Effective | Ineffective | Total effective |
|---|---|---|---|---|
| Control group (n=109) | 64 (58.72) | 28 (25.69) | 17 (15.60) | 92 (84.40) |
| Study Group (n=107) | 71 (66.36) | 30 (28.04) | 6 (5.61) | 101 (94.39) |
| χ2 | 5.663 | |||
| P | 0.017 |
Discussion
At present, surgical intervention remains one of the primary treatments for colorectal cancer, with proven efficacy. However, due to the impact of surgical procedures, electrolyte imbalance, and anesthesia, patients often experience varying degrees of gastrointestinal dysfunction postoperatively. This dysfunction mainly manifests as defecation difficulties, abdominal distension, reduced gastrointestinal motility, and complications like paralytic ileus and intestinal adhesions, all of which negatively influence patient prognosis [12,13]. Current conventional treatments, including gastrointestinal decompression, early functional exercises, and fasting, often yield suboptimal results [14,15]. Therefore, addressing postoperative gastrointestinal dysfunction is critical for improving patient prognosis.
In recent years, various traditional Chinese medicine (TCM) therapies have been gradually emerged as adjuvant treatments after digestive tract tumor surgeries, effectively promoting gastrointestinal recovery and gaining widespread acceptance [16]. TCM classifies postoperative gastrointestinal dysfunction after colorectal cancer surgery into categories of “abdominal distension”, “abdominal pain”, and “pi syndrome”. Surgical trauma can damage internal organs, leading to a deficiency of vital energy, impaired gastrointestinal function, and a diminished capacity for the production of qi and blood, which may result in weakened propulsion, qi stagnation, blood stasis, and the intermingling of phlegm and stasis, manifesting as distension, pain, and pi syndrome. Therefore, TCM treatment principles focus on strengthening the spleen, enhancing qi, activating blood circulation, and promoting the movement of qi to facilitate bowel motility [17]. Given that postoperative patients with colorectal cancer often cannot take oral Chinese medicine due to fasting, this study used Changtong Paste for external application and yielded favorable effects. The efficacy of the paste can be attributed to its key ingredients, including Magnolia bark, Costus root, Atractylodes, and Astragalus. Other components like Evodia rutaecarpa, Fennel, and Artemisia argyi are recognized for their ability to disperse cold and unblock the channels. Magnolia bark, Bitter orange, Areca nut, Clove, Costus root, and Green Tangerine Peel help alleviate pi syndrome and promote qi movement. Astragalus and Atractylodes are effective in strengthening the spleen and enhancing qi, while Rhubarb and Radish seeds can facilitate digestion, reduce distension, and promote qi movement to facilitate bowel motility. The combination of these herbs effectively supports gastrointestinal function recovery. Furthermore, the abdomen contains acupuncture points, such as Qihai, Shenque, Tianshu, Zhongwan, and Xiawan. Stimulation of Tianshu point can promote the movement of qi to clear the bowels and regulate the intestines and stomach. Additionally, the Qihai and Shenque points are associated with the enhancement of qi and blood. Furthermore, activating the Zhongwan and Xiawan points can strengthen the spleen, harmonize the middle jiao, and promote the movement of qi to clear the bowels. The application of Changtong Paste over these acupuncture points, combined with multi-source therapeutic device irradiation, enhances the therapeutic effects through mechanisms akin to moxibustion, thereby accelerating the penetration and absorption of the Chinese medicine, promoting gastrointestinal vascular dilation, improving microcirculation, and promoting the recovery of gastrointestinal function [18]. The results of this study showed that the overall clinical effective rate in the study group (94.39%) was higher than that in the control group (84.40%), and indicators regarding gastrointestinal function recovery and length of hospital stay were also improved in the study group. These results align with those reported by Wang Xianyan [19], highlighting the efficacy of Changtong Paste in enhancing gastrointestinal recovery and reducing the length of hospital stay following surgical procedures.
Clinical observations have found that colorectal cancer patients experience activation of the coagulation system due to anesthesia and surgical interventions. This activation leads to an increased synthesis of fibrinogen by the liver, which ultimately results in a significant increase in blood viscosity [20]. Increased blood viscosity can induce adhesion, exudation, and edema of the surrounding rectal tissues, leading to decreased rectal elasticity and symptoms such as difficulty in defecation and increased frequency of bowel movements, and may even result in fecal incontinence. Moreover, heightened blood viscosity may contribute to an increased risk of postoperative complications such as thrombosis. Patients with colorectal cancer often experience malnutrition, which is exacerbated by the effects of anesthesia and surgical interventions. Malnutrition is linked to gastrointestinal dysfunction and impaired immune function, primarily manifesting as reduced immunoglobulin activity and decreased T lymphocyte expression, which is detrimental to postoperative recovery [21,22]. The results of this study demonstrate that after treatment, plasma viscosity, erythrocyte sedimentation rate, and both high-shear and low-shear whole blood viscosity were significantly lower in both groups compared to before treatment, with the study group exhibiting even greater reductions. In contrast, levels of immunoglobulins IgG, IgA, and IgM were significantly elevated post-treatment, particularly in the study group. These findings suggest that Changtong Paste can effectively regulate hemorheology, reduce blood viscosity, and promote the recovery of immune function after colorectal cancer surgery.
Gastrointestinal dysfunction after colorectal cancer surgery is closely related to the abnormal secretion of gastrointestinal hormones such as SS, VIP, and MTL. This dysfunction may be linked to factors such as brain-gut axis dysregulation and stress responses. Typically, abnormal secretion of gastrointestinal hormones is mainly characterized by elevated levels of SS and VIP, alongside decreased levels of MTL. Furthermore, it is found that cancer-related fatigue is a prevalent symptom in patients with gastrointestinal malignancies, occurring in over 70% of cases, which significantly impacts prognosis and quality of life. The etiology of this fatigue is multifactorial, often stemming from leukopenia, malnutrition, and negative psychological states induced by anesthesia and surgical trauma [23,24]. The results of this study show that, after treatment, the levels of SS, VIP, and PFS-R scores significantly decreased in both groups, while MTL levels and QLQ-C30 scores significantly increased, with the study group exhibiting greater improvement. This suggests that Changtong Paste effectively enhances gastrointestinal hormone levels, alleviates cancer-related fatigue post-surgery, and promotes overall quality of life.
In conclusion, Changtong Paste can effectively regulate hemorheology, improve gastrointestinal hormone levels, and promote the recovery of gastrointestinal and immune functions after colorectal cancer surgery. It can also reduce cancer-related fatigue, enhance quality of life, and shorten hospital stays, exhibiting established therapeutic effects and warranting further promotion. Despite these promising results, this study is limited by its retrospective design, small sample size, and single-source population. Additionally, it did not analyze the molecular mechanisms underlying the effects of Changtong Paste on postoperative colorectal cancer patients. Future large-scale clinical studies are anticipated to explore the mechanisms in greater depth.
Disclosure of conflict of interest
None.
References
- 1.Patel SG, Karlitz JJ, Yen T, Lieu CH, Boland CR. The rising tide of early-onset colorectal cancer: a comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol Hepatol. 2022;7:262–274. doi: 10.1016/S2468-1253(21)00426-X. [DOI] [PubMed] [Google Scholar]
- 2.Ding HB, Wang LH, Sun G, Yu GY, Gao XH, Zheng K, Gong HF, Sui JK, Zhu XM, Zhang W. Evaluation of the learning curve for conformal sphincter preservation operation in the treatment of ultralow rectal cancer. World J Surg Oncol. 2022;20:102. doi: 10.1186/s12957-022-02541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paszat LF, Sutradhar R, Corn E, Luo J, Baxter NN, Tinmouth J, Rabeneck L. Morbidity and mortality following major large bowel resection for colorectal cancer detected by a population-based screening program. J Med Screen. 2021;28:252–260. doi: 10.1177/0969141320957361. [DOI] [PubMed] [Google Scholar]
- 4.Broman KK, Bailey CE, Parikh AA. Sidedness of colorectal cancer impacts risk of second primary gastrointestinal malignancy. Ann Surg Oncol. 2019;26:2037–2043. doi: 10.1245/s10434-019-07326-7. [DOI] [PubMed] [Google Scholar]
- 5.Qin Q, Huang B, Wu A, Gao J, Liu X, Cao W, Ma T, Kuang Y, Guo J, Wu Q, Shao B, Guan Q, Yao H, Zhang X, Wang H Chinese Radiation Intestinal Injury Research Group. Development and validation of a post-radiotherapy prediction model for bowel dysfunction after rectal cancer resection. Gastroenterology. 2023;165:1430–1442. e14. doi: 10.1053/j.gastro.2023.08.022. [DOI] [PubMed] [Google Scholar]
- 6.Yi M, Wu Y, Li M, Zhang T, Chen Y. Effect of remote ischemic preconditioning on postoperative gastrointestinal function in patients undergoing laparoscopic colorectal cancer resection. Int J Colorectal Dis. 2023;38:68. doi: 10.1007/s00384-023-04346-4. [DOI] [PubMed] [Google Scholar]
- 7.Xie A, Zhang X, Ju F, Li W, Zhou Y, Wu D. Effects of the ultrasound-guided stellate ganglion block on hemodynamics, stress response, and gastrointestinal function in postoperative patients with colorectal cancer. Comput Intell Neurosci. 2022;2022:2056969. doi: 10.1155/2022/2056969. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Liu Y, Chan CWH, Chow KM, Zhang B, Zhang X, Wang C, Du G. Nurse-delivered acupressure on early postoperative gastrointestinal function among patients undergoing colorectal cancer surgery. Asia Pac J Oncol Nurs. 2023;10:100229. doi: 10.1016/j.apjon.2023.100229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X, Wang Z, Yao H, Yang Y, Cao H, Toh Z, Zheng R, Ren Y. Effects of acupuncture treatment on postoperative gastrointestinal dysfunction in colorectal cancer: study protocol for randomized controlled trials. Trials. 2022;23:100. doi: 10.1186/s13063-022-06003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cervantes A, Adam R, Roselló S, Arnold D, Normanno N, Taïeb J, Seligmann J, De Baere T, Osterlund P, Yoshino T, Martinelli E ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:10–32. doi: 10.1016/j.annonc.2022.10.003. [DOI] [PubMed] [Google Scholar]
- 11.National Administration of Traditional Chinese Medicine (NATCM) Criteria of curative effect in TCM diagnosis. Nanjing: Nanjing University Press; 1994. [Google Scholar]
- 12.Treasure T. Pulmonarymetastasectomy in colorectal cancer: a nested randomized trial casting doubt ona large survival benefit. ANZ J Surg. 2021;91:1319–1320. doi: 10.1111/ans.16915. [DOI] [PubMed] [Google Scholar]
- 13.Wang N, Zuo H, Xu Y, Zhou Y, Wei A, Li K. Relation of gut microbiota and postoperative gastrointestinal dysfunction in older patients with colon cancer undergoing elective colon resection: a protocol for a prospective, observational cohort study. BMJ Open. 2022;12:e057391. doi: 10.1136/bmjopen-2021-057391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKenna NP, Bews KA, Yost KJ, Cima RR, Habermann EB. Bowel dysfunction after low anterior resection for colorectal cancer: a frequent late effect of surgery infrequently treated. J Am Coll Surg. 2022;234:529–537. doi: 10.1097/XCS.0000000000000085. [DOI] [PubMed] [Google Scholar]
- 15.Tan S, Meng Q, Jiang Y, Zhuang Q, Xi Q, Xu J, Zhao J, Sui X, Wu G. Impact of oral nutritional supplements in post-discharge patients at nutritional risk following colorectal cancer surgery: a randomised clinical trial. Clin Nutr. 2021;40:47–53. doi: 10.1016/j.clnu.2020.05.038. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Wang L, Ni X, Jiang M, Zhao L. Effect of acupuncture therapy for postoperative gastrointestinal dysfunction in gastric and colorectal cancers: an umbrella review. Front Oncol. 2024;14:1291524. doi: 10.3389/fonc.2024.1291524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou T, Wang S, Fan BJ, Zhang LX, Hu SH, Hou W. Clinical efficacy of acupuncture in treating postoperative gastrointestinal dysfunction of colorectal cancer, a systematic review and Meta analysis. Zhen Ci Yan Jiu. 2024;49:208–219. doi: 10.13702/j.1000-0607.20221319. [DOI] [PubMed] [Google Scholar]
- 18.Xu B, Wang X, Wang H, Cao L, Ge Y, Yuan B, Gao R, Li J. Efficacy and safety of herbal formulas with the function of gut microbiota regulation for gastric and colorectal cancer: a systematic review and meta-analysis. Front Cell Infect Microbiol. 2022;12:875225. doi: 10.3389/fcimb.2022.875225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang XY, Lei X, Hou HF, Luo Q, Liao WJ. Observation on the effect of track function recovery about “Changtong Ointment” combined with multi-source spectrum therapy instrument in promoting postoperative bowel in patients with colorectal cancer. J North Sichuan Med Coll. 2019;34:473–476. [Google Scholar]
- 20.Peng D, Li ZW, Liu F, Liu XR, Wang CY. Predictive value of red blood cell distribution width and hematocrit for short-term outcomes and prognosis in colorectal cancer patients undergoing radical surgery. World J Gastroenterol. 2024;30:1714–1726. doi: 10.3748/wjg.v30.i12.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamada K, Saito M, Ando M, Abe T, Mukoyama T, Agawa K, Watanabe A, Takamura S, Fujita M, Urakawa N, Hasegawa H, Kanaji S, Matsuda T, Oshikiri T, Kakeji Y, Yamashita K. Reduced number and immune dysfunction of CD4+ T cells in obesity accelerate colorectal cancer progression. Cells. 2022;12:86. doi: 10.3390/cells12010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang R, Zhu X, Lan T, Ding D, Zheng Z, Chen T, Huang Y, Liu J, Yang X, Shao J, Wei H, Wei B. TIGIT promotes CD8(+)T cells exhaustion and predicts poor prognosis of colorectal cancer. Cancer Immunol Immunother. 2021;70:2781–2793. doi: 10.1007/s00262-021-02886-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Machado P, Morgado M, Raposo J, Mendes M, Silva CG, Morais N. Effectiveness of exercise training on cancer-related fatigue in colorectal cancer survivors: a systematic review and meta-analysis of randomized controlled trials. Support Care Cancer. 2022;30:5601–5613. doi: 10.1007/s00520-022-06856-3. [DOI] [PubMed] [Google Scholar]
- 24.Wang S, Jiang N, Song Y, Ma L, Niu Y, Song J, Jiang X. Correlates of cancer-related fatigue among colorectal cancer patients undergoing postoperative adjuvant therapy based on the theory of unpleasant symptoms. Curr Oncol. 2022;29:9199–9214. doi: 10.3390/curroncol29120720. [DOI] [PMC free article] [PubMed] [Google Scholar]