Abstract
Objective: To evaluate the clinical efficacy of early tracheotomy in neurologic critical care patients. Methods: A retrospective analysis was conducted on 100 patients with severe craniocerebral injury (SCI) who underwent percutaneous tracheotomy at The First Affiliated Hospital, Zhejiang University School of Medicine from January 2021 to February 2022. Of them, 52 cases (observation group) received the procedure within 24 hours of injury, while 48 patients (control group) received the procedure after 24 hours. Therapeutic efficacy was assessed using the National Institutions of Health Stroke Scale (NIHSS) criteria. The utilization rates of antibiotics, muscle relaxants, and sedatives during hospitalization, along with mechanical ventilation duration, and the length of intensive care were recorded for comparative analyses. Additionally, blood gas indicators, Glasgow Coma Scale (GCS) score, and Disability Rating Scale (DRS) score were compared before and after treatment. Pulmonary infection and case fatality rates were also recorded and compared. The early prognosis of patients was assessed based on their GCS scores during a 3-month postoperative follow-up, and risk factors for adverse prognosis were identified. Results: The effective rate was evidently higher in the observation group compared to the control group. No statistical inter-group difference was identified in the utilization rate of antibiotics or sedatives during hospitalization (all P>0.05), but the observation group had a lower utilization rate of muscle relaxants than the control group (P<0.05). The observation group showed markedly shorter mechanical ventilation duration and length of intensive care compared with the control group (all P<0.05). Both groups demonstrated significant improvements in blood gas indices, GCS and DRS scores after treatment, with significantly greater improvement in the observation group (all P<0.05). The observation group exhibited lower pulmonary infection and case fatality rates than the control group (all P<0.05). GCS score >3 upon admission, DRS score >15 upon admission, and tracheotomy after 24 h of injury were all independent risk factors for poor early prognosis in patients with SCI. Conclusions: Tracheotomy for SCI patients within 24 hours of injury can effectively improve therapeutic efficacy, enhance neurologic function, and reduce the risk of disability and pulmonary infection.
Keywords: Neurocritical care patients, early tracheotomy, neurological function, pulmonary infection
Introduction
As the economy develops, the number patients with stroke and craniocerebral injury that requires neurologic critical care has been rising steadily each year [1]. These patients typically present with clinical symptoms such as glossoptosis, dyspnea, and coma, with critical conditions and high disability and mortality rates [2]. Due to neurological impairment, the patient experience weakened or absent cough and swallowing reflexes, leading to an inability to clear respiratory secretions effectively. This can result in respiratory obstruction and airway gas exchange disorders, further inducing dyspnea and exacerbating their condition [3,4].
In the emergency clinical treatment of neurologic critical care patients, particularly those with severe craniocerebral injury (SCI), a simple, fast, and reliable procedure for establishing artificial airways is essential to ensure unobstructed breathing. Such patients often require a tracheotomy to maintain airway patency and reduce the risk of associated complications [5,6]. However, despite its effectiveness in improving ventilation, tracheotomy can also increase the risk of lower respiratory tract infections, adversely affecting patient recovery [7]. In addition, there is still controversy over the optimal timing of tracheostomy for SCI patients in current clinical practice. Research [8] has shown that performing tracheotomy more than 7 days after injury is an independent risk factor for post-tracheotomy pulmonary infection in patients with craniocerebral injury. Therefore, determining the optimal timing for tracheotomy in SCI patients is critical for accelerating their brain function recovery and improving their outcomes.
This study analyzed 100 SCI patients who underwent tracheotomy at our hospital between January 2021 and February 2022 to evaluate the influence of early tracheotomy on the clinical efficacy and the incidence of pulmonary infections.
Materials and methods
Clinical data
The clinical data of 100 SCI patients who underwent percutaneous tracheotomy in The First Affiliated Hospital, Zhejiang University School of Medicine from January 2021 to February 2022 were analyzed retrospectively. Among them, 52 patients who received the procedure within 24 hours of injury were included in the observation group, and the other 48 patients who received the procedure more than 24 hours after SCI were included in the control group.
Inclusion criteria: Patients who met the diagnostic criteria for SCI [9] and were confirmed by computerized tomography (CT) or magnetic resonance imaging (MRI); Patients with a Glasgow Coma Scale (GCS) [10] score ranging from 3 to 9; and Patients with complete case records. Exclusion criteria: Those with severe multiple injuries, malignant tumor(s), or severe functional defects of the heart, liver, or kidneys were excluded. This study was approved by the First Affiliated Hospital, Zhejiang University School of Medicine Ethics Committee, and all procedures followed the principles of the Declaration of Helsinki.
Treatment methods
Upon admission, both groups were treated with dehydrating agents (mannitol, Shanghai Baxter Medical Supplies Co. Ltd., SFDA Approval No. H20003300) to reduce intracranial pressure, neurotrophic agent (vitamin B) to supplement brain cells, and antibiotics [penicillins (Harbin Pharmaceutical Group Pharmaceutical Factory, National Medicine Approval No. H23021439) and cephalosporins (CSPC Pharmaceutical Group Zhongnuo Pharmaceutical Co., Ltd., National Medicine Approval No. H13020765)] to prevent infection. In addition, intracranial hematoma evacuation was also performed to remove necrotic brain tissue. During the procedure, sedatives (slow intravenous injection of 50 µg·kg-1 of midazolam, Jiangsu Enhua Pharmaceutical Co., Ltd., National Medicine Approval No. H20143222) were administered to patients with hypothermia and those who could not cooperate with the surgery. Muscle relaxants (0.12 mg/kg of vecuronium bromide, Zhejiang Xianjun Pharmaceutical Co., Ltd., National Medicine Approval No. H19991116) could be given if the sedation failed.
Percutaneous tracheotomy was performed within 24 hours of craniocerebral injury in the observation group and more than 24 hours after craniocerebral injury in the control group. The specific surgical operations are as follows:
The operation was performed under general or local anesthesia. The skin and subcutaneous tissue were incised along the anterior midline of the neck, from the lower edge of the cricoid cartilage to just above the suprasternal fossa. After a blunt separation of the pretracheal tissue and the determination of the trachea, two tracheal rings (from the 2nd to 4th) were incised using a surgical blade, followed by the insertion of the tracheal cannula and fixation. Finally, a piece of gauze was placed between the wound and the cannula. A ventilator was then connected for mechanical ventilation. The patient’s vital signs were continuously monitored for pulmonary infections. If an infection occurred, empirical anti-infection treatment was given first, followed by targeted antibiotic therapy based on the etiological diagnosis and drug sensitivity to complete the anti-infection treatment.
Outcome measures
(1) Therapeutic efficacy was evaluated using the National Institutions of Health Stroke Scale (NIHSS) [11]. Cure: NIHSS score decreased by more than 90% compared with the pretreatment level, with no disability; Marked effectiveness: NIHSS score reduction by 46%-90%, with mild disability; Effective: NIHSS score reduced by 18%-45%, with some level of disability; Ineffective: No improvement in neurological function, with severe disability or even death. Total clinical effective rate = cure rate + marked effectiveness rate + effective rate. (2) The utilization rates of antibiotics, muscle relaxants, and sedatives during hospitalization were recorded and comparatively analyzed. (3) Mechanical ventilation duration and length of intensive care were recorded and compared between the two groups. (4) Blood gas indexes before and after treatment were compared, including arterial partial pressure of oxygen (PaO2), arterial partial pressure of carbon dioxide (PaCO2), and blood oxygen saturation (SpO2). (5) GCS scores [10] were evaluated and compared between the two groups before and after treatment. (6) The Disability Rating Scale (DRS) [12] was used to evaluate the degree of disability on the day of injury and one week after injury. The total DRS score is 30, with 0 points indicating no disability and 30 points indicating death. Higher scores indicates more severe disability. (7) The pulmonary infection rate and case fatality rate were recorded and compared between the two groups. (8) Early prognosis was evaluated using GCS score during a 3-month postoperative follow-up. A favorable prognosis was defined as a GCS score of 1-3 at the end of 3-month postoperative follow-up. Risk factors contributing to the poor prognosis of patients were analyzed.
Statistical methods
SPSS20.0 software and GraphPad Prism 8 software were used for data processing, analysis, and visualization. Measured data were expressed as mean ± SD; An independent sample t-test was used for inter-group comparisons of the measured data, and a paired t-test was used for intra-group comparisons before and after treatment (expressed by t). Counted data were expressed as number (%), and chi-square test was used for the analysis of counted data. A P-value less than 0.05 was considered statistically significant.
Results
Comparison of baseline characteristics between the two groups
As shown in Table 1, the two groups were comparable in baseline characteristics, such as gender, age, and body mass index (BMI) (all P>0.05).
Table 1.
Comparison of baseline characteristics between the two groups
| Factor | Observation group n=52 | Control group n=48 | χ2 | P |
|---|---|---|---|---|
| Sex | 0.007 | 0.935 | ||
| Male | 31 (59.62) | 29 (60.42) | ||
| Female | 21 (40.38) | 19 (39.58) | ||
| Age (years) | 0.575 | 0.448 | ||
| ≤40 | 21 (40.38) | 23 (47.92) | ||
| >40 | 31 (59.62) | 25 (52.08) | ||
| Body mass index (kg/m2) | 0.050 | 0.822 | ||
| ≤23 | 25 (48.08) | 22 (45.83) | ||
| >23 | 27 (51.92) | 26 (54.17) | ||
| Smoking history | 0.014 | 0.907 | ||
| Yes | 33 (63.46) | 31 (64.58) | ||
| No | 19 (36.54) | 17 (35.42) | ||
| Cause of injury | 1.159 | 0.560 | ||
| Traffic accident | 20 (38.46) | 20 (41.67) | ||
| Fall from heights | 21 (40.38) | 19 (39.58) | ||
| Others | 11 (21.15) | 9 (18.75) |
Comparison of clinical efficacy between the two groups
The clinical efficacy were compared between two groups of patients after treatment. As shown in Table 2, the total clinical effective rate in the observation group was 92.31%, which was higher than the 75.00% of the control group (P<0.05).
Table 2.
Comparison of clinical efficacy between the two groups
| Group | Observation group n=52 | Control group n=48 | χ2 | P |
|---|---|---|---|---|
| Cure | 25 (48.08) | 18 (37.50) | - | - |
| Marked effectiveness | 14 (26.92) | 12 (25.00) | ||
| Effectiveness | 9 (17.31) | 6 (12.50) | - | - |
| Ineffectiveness | 4 (7.69) | 12 (25.00) | - | - |
| Total effective rate | 48 (92.31) | 36 (75.00) | 5.563 | 0.018 |
Comparison of drug use during hospitalization between the two groups
The observation and control groups were not markedly different in the utilization rate of antibiotics and sedatives (all P>0.05). However, fewer muscle relaxants were used in the observation group compared to the control group (P<0.05, Table 3).
Table 3.
Comparison of drug utilization during hospitalization between the two groups
| Group | Observation group n=52 | Control group n=48 | χ2 | P |
|---|---|---|---|---|
| Antibiotic utilization rate | 43 (82.69) | 44 (91.67) | 1.777 | 0.183 |
| Sedative utilization rate | 48 (92.31) | 46 (95.83) | 0.550 | 0.458 |
| Utilization rate of muscle relaxants | 15 (28.85) | 30 (62.50) | 11.42 | <0.001 |
Comparison of mechanical ventilation duration and length of intensive care between the two groups
As shown in Table 4, the observation group had statistically shorter mechanical ventilation duration and length of intensive care than the control group (all P<0.05, Table 4).
Table 4.
Comparison of mechanical ventilation duration and length of intensive care between the two groups
| Group | Observation group n=52 | Control group n=48 | χ2 | P |
|---|---|---|---|---|
| Mechanical ventilation duration (d) | 7.64±1.24 | 10.86±1.09 | 13.74 | <0.001 |
| Length of intensive care (d) | 11.73±1.35 | 15.56±1.04 | 15.80 | 0.017 |
Comparison of blood gas indices between the two groups before and after treatment
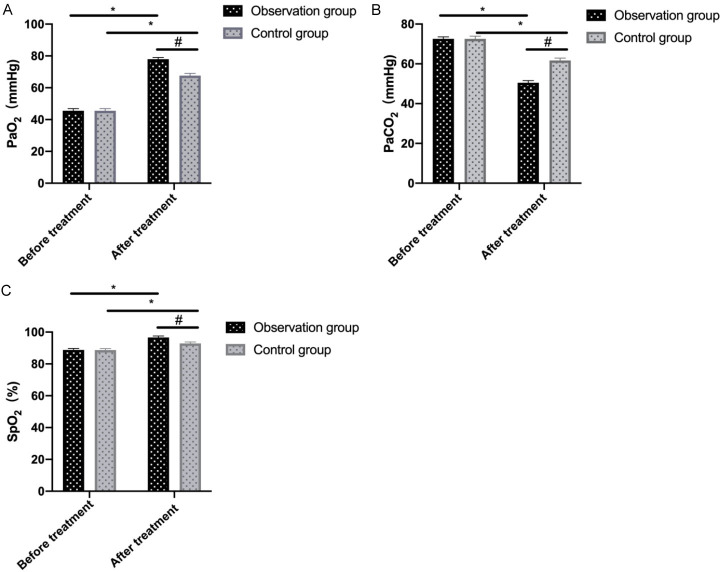
No notable inter-group difference was found in pretreatment blood gas indexes (all P>0.05). However, after treatment, significantly higher PaO2 and SpO2 and lower PaCO2 were observed in the observation group compared to the control group (all P<0.05, Figure 1).
Figure 1.
Comparison of blood gas parameters between two groups before and after treatment. A: PaO2; B: PaCO2; C: SpO2. Note: * denotes P<0.05 in the intra-group comparison before and after treatment; # denotes P<0.05 in the inter-group comparison after treatment. PaO2, arterial partial pressure of oxygen; PaCO2, arterial partial pressure of carbon dioxide; SpO2, blood oxygen saturation.
Comparison of GCS and DRS scores between the two groups before and after treatment
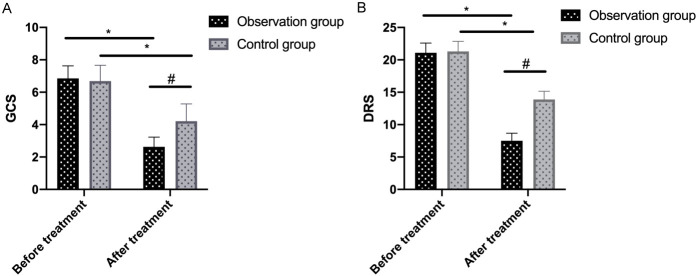
The two groups showed similar GCS and DRS scores before treatment (all P>0.05). After treatment, GCS and DRS scores decreased greatly in both groups after treatment, with the observation group showing significantly lower scores than the control group (all P<0.05), as shown in Figure 2.
Figure 2.
Comparison of GCS (A) and DRS (B) scores between the two groups before and after treatment. Note: * denotes P<0.05 in the intra-group comparison before and after treatment; # denotes P<0.05 in the inter-group comparison after treatment. GCS, Glasgow Coma Scale; DRS, Dis ability Rating Scale.
Comparison of pulmonary infection rate and case fatality rate between the two groups
The pulmonary infection rate and case fatality rate in the observation group were 25.00% and 5.77%, both of which were significantly lower compared to the control group (52.08% and 22.92%) (all P<0.05), as shown in Table 5.
Table 5.
Comparison of pulmonary infection rate and case fatality rate between the two groups
| Group | Observation group n=52 | Control group n=48 | χ2 | P |
|---|---|---|---|---|
| Pulmonary infection rate | 13 (25.00) | 25 (52.08) | 8.614 | 0.003 |
| Case fatality rate | 3 (5.77) | 11 (22.92) | 6.096 | 0.014 |
Multivariate analysis of factors influencing patient prognosis
Patients were divided into a poor prognosis group (38 cases) and a good prognosis group (62 cases) based on their GCS score during the 3-month postoperative follow-up. Univariate analysis identified that age, GCS score upon admission, DRS score upon admission, and the timing of tracheotomy were associated with prognosis in patients with SCI (Table 6). Multivariate logistic regression analysis, incorporating these four significant variables, identified that GCS score >3 upon admission, DRS score >15 upon admission, and delayed tracheotomy after 24 hour of injury were all independent risk factors for a poor early prognosis in SCI patients (Table 7).
Table 6.
Univariate analysis of factors affecting patient prognosis
| Factor | Good prognosis group (n=62) | Poor prognosis group (n=38) | χ2 | P |
|---|---|---|---|---|
| Age | 27.81 | <0.001 | ||
| ≤40 years old (n=44) | 40 (64.52) | 4 (10.53) | ||
| >40 years old (n=56) | 22 (35.48) | 34 (89.47) | ||
| Smoking history | 0.004 | 0.947 | ||
| With (n=64) | 40 (64.52) | 24 (63.16) | ||
| Without (n=36) | 22 (35.48) | 14 (36.84) | ||
| GCS score upon admission | 28.98 | <0.001 | ||
| ≤3 points (n=60) | 50 (80.65) | 10 (26.32) | ||
| >3 points (n=40) | 12 (19.35) | 28 (73.68) | ||
| DRS score upon admission | 33.13 | <0.001 | ||
| ≤15 points (n=55) | 48 (77.42) | 7 (18.42) | ||
| >15 points (n=45) | 14 (22.58) | 31 (81.58) | ||
| Timing of tracheotomy | 63.49 | <0.001 | ||
| Percutaneous tracheotomy within 24 hours (n=52) | 50 (80.65) | 2 (5.26) | ||
| Percutaneous tracheotomy after 24 hours (n=48) | 12 (19.35) | 36 (94.74) |
Note: GCS, Glasgow Coma Scale; DRS, Disability Rating Scale.
Table 7.
Multivariate analysis of factors affecting patient prognosis
| Factor | B | S.E. | Wals | P | OR | 95% C.I. | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Lower bound | Upper bound | ||||||
| Age | 1.043 | 0.808 | 1.665 | 0.197 | 2.838 | 0.582 | 13.839 |
| GCS score upon admission | 1.705 | 0.797 | 4.570 | 0.033 | 5.501 | 1.152 | 26.257 |
| DRS score upon admission | 1.356 | 0.595 | 5.189 | 0.023 | 3.880 | 1.208 | 12.458 |
| Timing of tracheotomy | 2.318 | 0.589 | 15.510 | <0.001 | 10.155 | 3.204 | 32.185 |
Note: GCS, Glasgow Coma Scale; DRS, Disability Rating Scale.
Discussion
Patients with severe craniocerebral injury (SCI) are susceptible to airway obstruction due to damage to cranial nerve tissue, impaired coughing and swallowing reflexes, and inability to clear secretions from the oral cavity and airways, which compromises airway patency and ventilation, leading to dyspnea [13]. Clinically, craniotomy hematoma removal is commonly used to treat craniocerebral trauma and cerebral hemorrhage, but patients often experience symptoms such as respiratory tract obstruction even after the control of bleeding. To clear the airway, facilitate the early restoration of spontaneous breathing, effectively improve airway ventilation, and increase alveolar ventilation, tracheal intubation is essential [14]. However, tracheotomy may lead to long-term complications, such as tracheomalacia, tracheal stenosis, and bleeding. Therefore, for patients with SCI, determining the appropriate timing for tracheostomy is a critical clinical challenge. Although it is clinically recognized that percutaneous tracheotomy should be performed no later than 7 days after injury in SCI patients, evidence [15] suggests that performing this procedure within 24 hours of injury can more effectively ameliorate pulmonary infections.
Therefore, in this study, we analyzed the effect of percutaneous tracheotomy in SCI patients within 24 hours of injury. The results indicated that patients treated with percutaneous tracheotomy within 24 hours of injury had a higher overall clinical effective rate, less mechanical ventilation time, and a shorter duration of intensive care than those undergoing the procedure 24 hours after injury. It is suggested that earlier percutaneous tracheotomy enhances therapeutic efficacy and shortens the recovery process in SCI patients. We then compared blood gas indices and found that early percutaneous tracheotomy significantly improved these outcome measures. The purpose of tracheotomy is to relieve respiratory obstruction, maintain airway patency, and restore adequate oxygen supply to the brain tissue. Early tracheotomy within 24 hours can effectively dredge the respiratory tract, relieve hypoxemia, minimize neurological damage, and reduce the risk of clinical disability. Therefore, earlier treatment provides better control of the patient’s condition and enhanced quality of life [16,17]. Subsequently, we comparatively analyzed GCS and DRS scores. Although both groups showed improvement after treatment, the observation group experienced significantly greater improvement. This suggests that earlier percutaneous tracheotomy helps restore normal blood oxygenation to brain tissue more quickly, prevents the exacerbation of neurological damage, accelerates patient recovery from coma, and reduces the risk of disability, exerting a positive impact on patients’ treatment and recovery [18], consistent with our overall results.
It is important to note that, in addition to evaluating the curative effect, reducing the risk of post-tracheostomy infections is also a clinical concern. Clinical studies have confirmed that several factors contribute to pulmonary infections in SCI patients. First, neurogenic pulmonary edema often occurs in the early stage of craniocerebral injury, leading to a rapid increase in pulmonary inflammatory factors, which can trigger pulmonary infections [19]. Second, long-term bed rest is required after craniocerebral injury, and patients in a coma experience impaired or absent cranial nerve function and cough reflexes. This results in the accumulation of oral saliva, blood, and respiratory secretions, which cannot be swallowed, leading to lung hypostasis and infection [20]. Third, mechanical ventilation can damage the respiratory mucosa, compromising the natural physiological barrier function [21]. Based on the above factors, it is important to choose the appropriate timing for tracheostomy for patient recovery. In this study, we found that tracheotomy within 24 hours of injury significantly reduced the risk of pulmonary infection. The reason behind this may be that the early tracheostomy alleviates airway obstruction, preventing brain tissue hypoxia, and facilitates the clearance of secretions from the respiratory tract and deep trachea. This improves lung oxygenation, effectively reduces intracranial pressure, and helps prevent lung infections caused by factors such as low vital capacity, reduced cough reflex, and impaired expectoration ability [22,23]. Similarly, previous research [24] has also found that early tracheotomy can significantly reduce the risk of pulmonary infection in patients.
In conclusion, tracheotomy for SCI patients within 24 hours of injury can effectively improve therapeutic effects, improve neurological function, and reduce the risk of disability and pulmonary infections, making it valuable for clinical practice. However, this study also has certain limitations. First, due to the relatively small sample size, the research results still need to be further verified by multi-center and large-sample studies in the future. Second, it remains to be explored whether the timing of early tracheotomy can be further refined by further time stratification according to patients’ conditions in subsequent studies. Nevertheless, it is still expected that the determination of the timing of tracheotomy can be more precise in the future.
Disclosure of conflict of interest
None.
References
- 1.Salluh JI, Wang H, Schneider EB, Nagaraja N, Yenokyan G, Damluji A, Serafim RB, Stevens RD. Outcome of delirium in critically ill patients: systematic review and meta-analysis. BMJ. 2015;350:h2538. doi: 10.1136/bmj.h2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romeo DM, Ricci D, Brogna C, Mercuri E. Use of the Hammersmith infant neurological examination in infants with cerebral palsy: a critical review of the literature. Dev Med Child Neurol. 2016;58:240–245. doi: 10.1111/dmcn.12876. [DOI] [PubMed] [Google Scholar]
- 3.Fan Y, Lv X, Chen Z, Peng Y, Zhang M. m6A methylation: critical roles in aging and neurological diseases. Front Mol Neurosci. 2023;16:1102147. doi: 10.3389/fnmol.2023.1102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones S, Schwartzbauer G, Jia X. Brain monitoring in critically neurologically impaired patients. Int J Mol Sci. 2016;18:43. doi: 10.3390/ijms18010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michelutti A, D’Angelo M, Szulin M, Stroppolo G, Bargellesi S, Giorgini T, Quattrin R, Biasutti E. The tracheotomy tube weaning in patients with severe acquired brain injury: comparison of two operative procedures in a postacute rehabilitation hospital. Eur J Phys Rehabil Med. 2021;57:347–355. doi: 10.23736/S1973-9087.21.06342-5. [DOI] [PubMed] [Google Scholar]
- 6.Zhong X, Shan A, Xu J, Liang J, Long Y, Du B. Hyperbaric oxygen for severe traumatic brain injury: a randomized trial. J Int Med Res. 2020;48:300060520939824. doi: 10.1177/0300060520939824. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Dong M, Zhou Y, Yang J, Yang J, Liao X, Kang Y. Compare the effect of noninvasive ventilation and tracheotomy in critically ill mechanically ventilated neurosurgical patients: a retrospective observe cohort study. BMC Neurol. 2019;19:79. doi: 10.1186/s12883-019-1297-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chorath K, Hoang A, Rajasekaran K, Moreira A. Association of early vs late tracheostomy placement with pneumonia and ventilator days in critically Ill patients: a meta-analysis. JAMA Otolaryngol Head Neck Surg. 2021;147:450–459. doi: 10.1001/jamaoto.2021.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trouillet JL, Collange O, Belafia F, Blot F, Capellier G, Cesareo E, Constantin JM, Demoule A, Diehl JL, Guinot PG, Jegoux F, L’Her E, Luyt CE, Mahjoub Y, Mayaux J, Quintard H, Ravat F, Vergez S, Amour J, Guillot M French Intensive Care Society; French Society of Anaesthesia and Intensive Care. Tracheotomy in the intensive care unit: guidelines from a French expert panel: The French Intensive Care Society and the French Society of Anaesthesia and Intensive Care Medicine. Anaesth Crit Care Pain Med. 2018;37:281–294. doi: 10.1016/j.accpm.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Liu C, Xie J, Xiao X, Li T, Li H, Bai X, Li Z, Wang W. Clinical predictors of prognosis in patients with traumatic brain injury combined with extracranial trauma. Int J Med Sci. 2021;18:1639–1647. doi: 10.7150/ijms.54913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwah LK, Diong J. National institutes of health stroke scale (NIHSS) J Physiother. 2014;60:61. doi: 10.1016/j.jphys.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 12.McCrea MA, Giacino JT, Barber J, Temkin NR, Nelson LD, Levin HS, Dikmen S, Stein M, Bodien YG, Boase K, Taylor SR, Vassar M, Mukherjee P, Robertson C, Diaz-Arrastia R, Okonkwo DO, Markowitz AJ, Manley GT TRACK-TBI Investigators. Adeoye O, Badjatia N, Bullock MR, Chesnut R, Corrigan JD, Crawford K, Duhaime AC, Ellenbogen R, Feeser VR, Ferguson AR, Foreman B, Gardner R, Gaudette E, Goldman D, Gonzalez L, Gopinath S, Gullapalli R, Hemphill JC, Hotz G, Jain S, Keene CD, Korley FK, Kramer J, Kreitzer N, Lindsell C, Machamer J, Madden C, Martin A, McAllister T, Merchant R, Ngwenya LB, Noel F, Nolan A, Palacios E, Perl D, Puccio A, Rabinowitz M, Rosand J, Sander A, Satris G, Schnyer D, Seabury S, Sherer M, Toga A, Valadka A, Wang K, Yue JK, Yuh E, Zafonte R. Functional outcomes over the first year after moderate to severe traumatic brain injury in the prospective, longitudinal TRACK-TBI study. JAMA Neurol. 2021;78:982–992. doi: 10.1001/jamaneurol.2021.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson TN, Hwang J, Munar M, Papa L, Hinson HE, Vaughan A, Rowell SE. Blood-based biomarkers for prediction of intracranial hemorrhage and outcome in patients with moderate or severe traumatic brain injury. J Trauma Acute Care Surg. 2020;89:80–86. doi: 10.1097/TA.0000000000002706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jha RM, Mondello S, Bramlett HM, Dixon CE, Shear DA, Dietrich WD, Wang KKW, Yang Z, Hayes RL, Poloyac SM, Empey PE, Lafrenaye AD, Yan HQ, Carlson SW, Povlishock JT, Gilsdorf JS, Kochanek PM. Glibenclamide treatment in traumatic brain injury: operation brain trauma therapy. J Neurotrauma. 2021;38:628–645. doi: 10.1089/neu.2020.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang L, Wu Y, Zhang Y, Lu D, Yan K, Gao J. Effects of intraoperative lung-protective ventilation on clinical outcomes in patients with traumatic brain injury: a randomized controlled trial. BMC Anesthesiol. 2021;21:182. doi: 10.1186/s12871-021-01402-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahn JM, Le T, Angus DC, Cox CE, Hough CL, White DB, Yende S, Carson SS ProVent Study Group Investigators. The epidemiology of chronic critical illness in the United States*. Crit Care Med. 2015;43:282–287. doi: 10.1097/CCM.0000000000000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bossers SM, Schwarte LA, Loer SA, Twisk JW, Boer C, Schober P. Experience in prehospital endotracheal intubation significantly influences mortality of patients with severe traumatic brain injury: a systematic review and meta-analysis. PLoS One. 2015;10:e0141034. doi: 10.1371/journal.pone.0141034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knapp J, Doppmann P, Huber M, Meuli L, Albrecht R, Sollid S, Pietsch U. Pre-hospital endotracheal intubation in severe traumatic brain injury: ventilation targets and mortality-a retrospective analysis of 308 patients. Scand J Trauma Resusc Emerg Med. 2023;31:46. doi: 10.1186/s13049-023-01115-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gravesteijn BY, Sewalt CA, Nieboer D, Menon DK, Maas A, Lecky F, Klimek M, Lingsma HF CENTER-TBI collaborators. Tracheal intubation in traumatic brain injury: a multicentre prospective observational study. Br J Anaesth. 2020;125:505–517. doi: 10.1016/j.bja.2020.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson J, Ebeid A, Stallwood-Hall C. Pre-hospital tracheal intubation in severe traumatic brain injury: a systematic review and meta-analysis. Br J Anaesth. 2022;129:977–984. doi: 10.1016/j.bja.2022.07.033. [DOI] [PubMed] [Google Scholar]
- 21.Denninghoff KR, Griffin MJ, Bartolucci AA, Lobello SG, Fine PR. Emergent endotracheal intubation and mortality in traumatic brain injury. West J Emerg Med. 2008;9:184–189. [PMC free article] [PubMed] [Google Scholar]
- 22.Villemure-Poliquin N, Costerousse O, Lessard Bonaventure P, Audet N, Lauzier F, Moore L, Zarychanski R, Turgeon AF. Tracheostomy versus prolonged intubation in moderate to severe traumatic brain injury: a multicentre retrospective cohort study. Can J Anaesth. 2023;70:1516–1526. doi: 10.1007/s12630-023-02539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marra A, Vargas M, Buonanno P, Iacovazzo C, Coviello A, Servillo G. Early vs. late tracheostomy in patients with traumatic brain injury: systematic review and meta-analysis. J Clin Med. 2021;10:3319. doi: 10.3390/jcm10153319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu W, Wu T, Cui P, Zhang J, Sheng X, Ding Z. Timing of tracheotomy in patients with severe traumatic brain injury. J Craniofac Surg. 2019;30:2168–2170. doi: 10.1097/SCS.0000000000005721. [DOI] [PubMed] [Google Scholar]